Anticancer Activity of a Hexapeptide from Skate (Raja porosa) Cartilage Protein Hydrolysate in HeLa Cells
Abstract
:1. Introduction
2. Results and Discussion
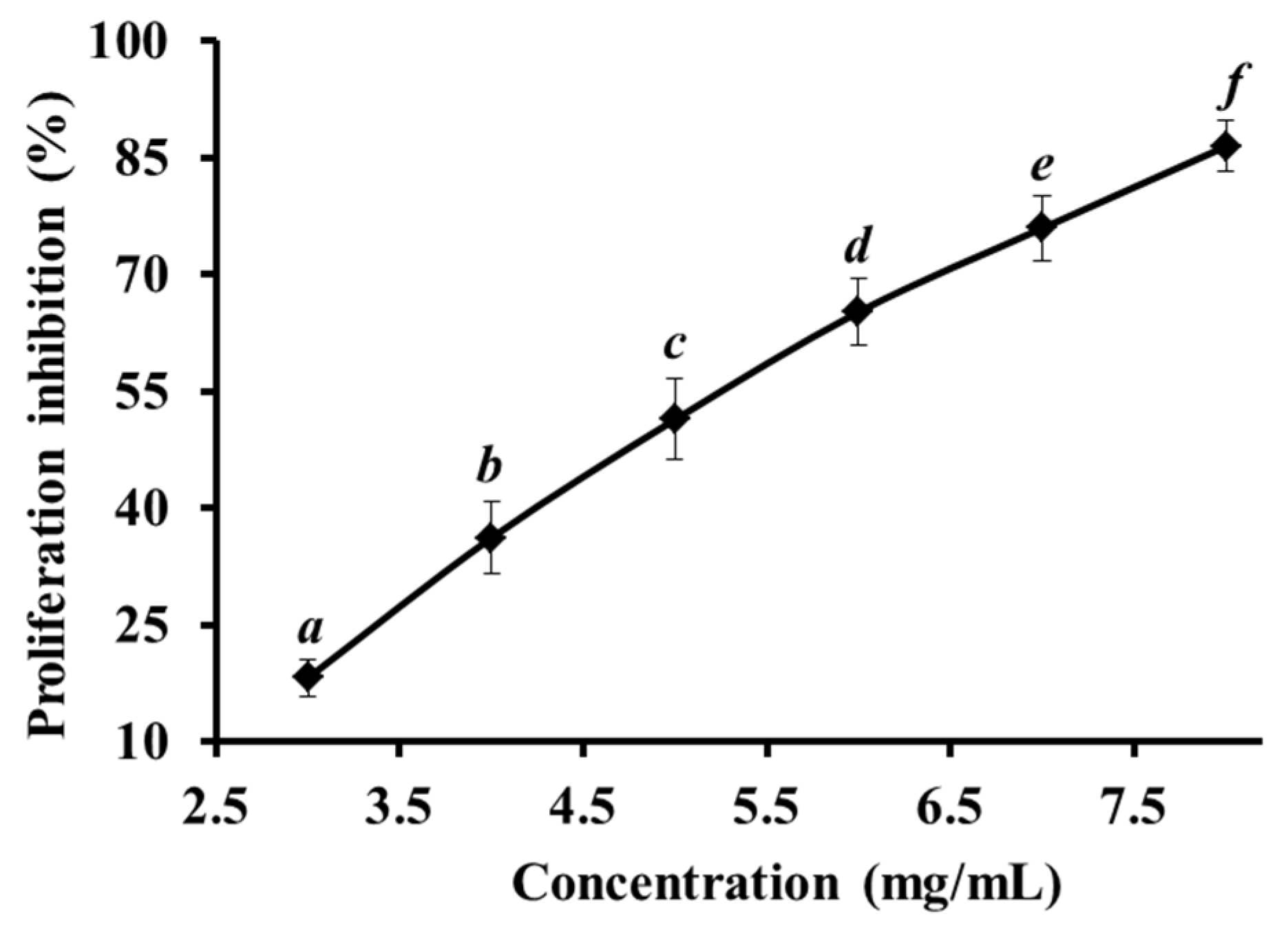
2.1. Proliferation Inhibition of HeLa Cell Lines
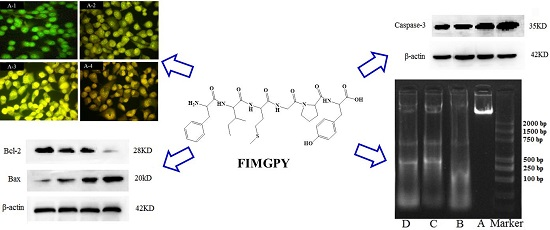
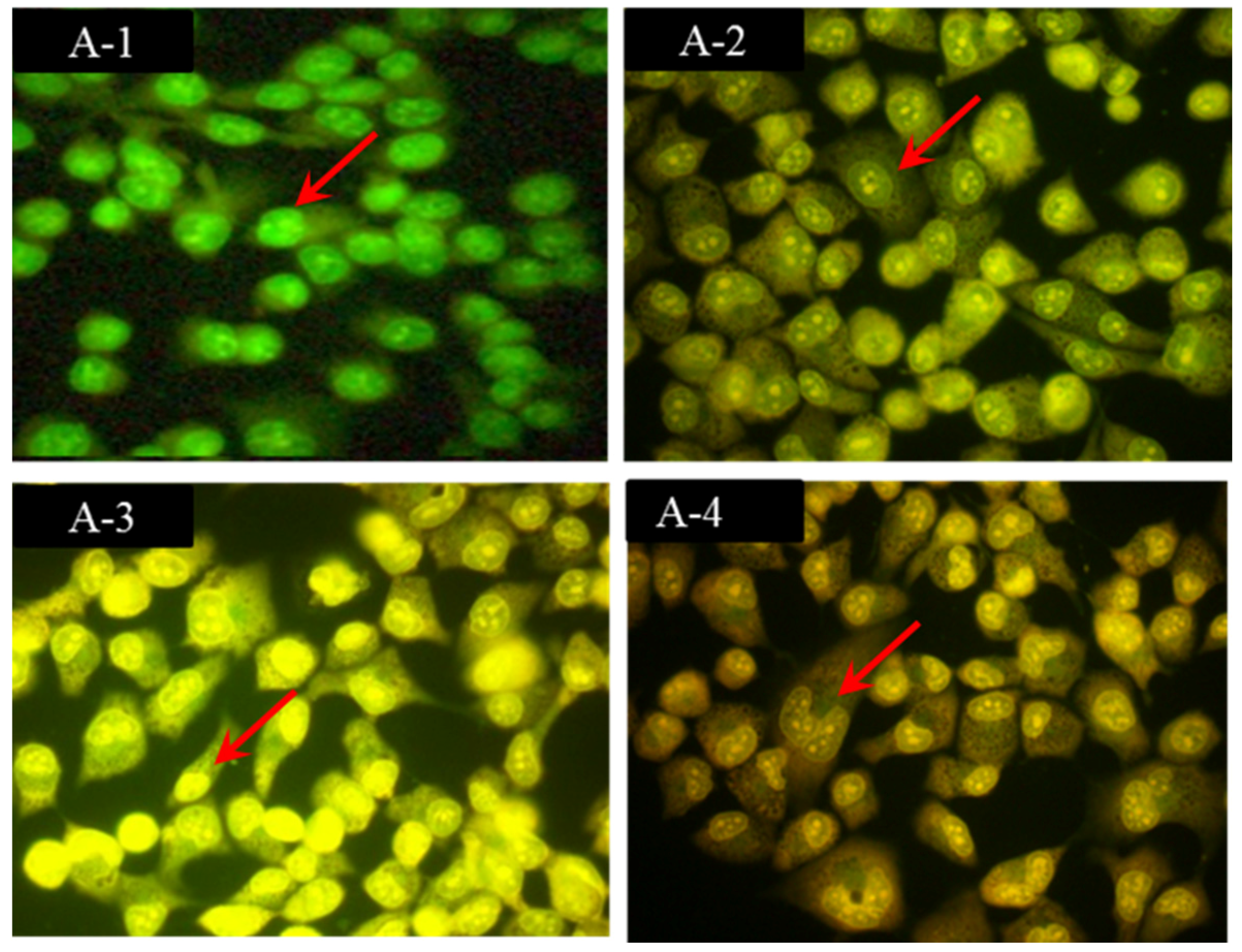
2.2. Morphological Observations by Acridine Orange/Ethidium Bromide (AO/EB) Staining
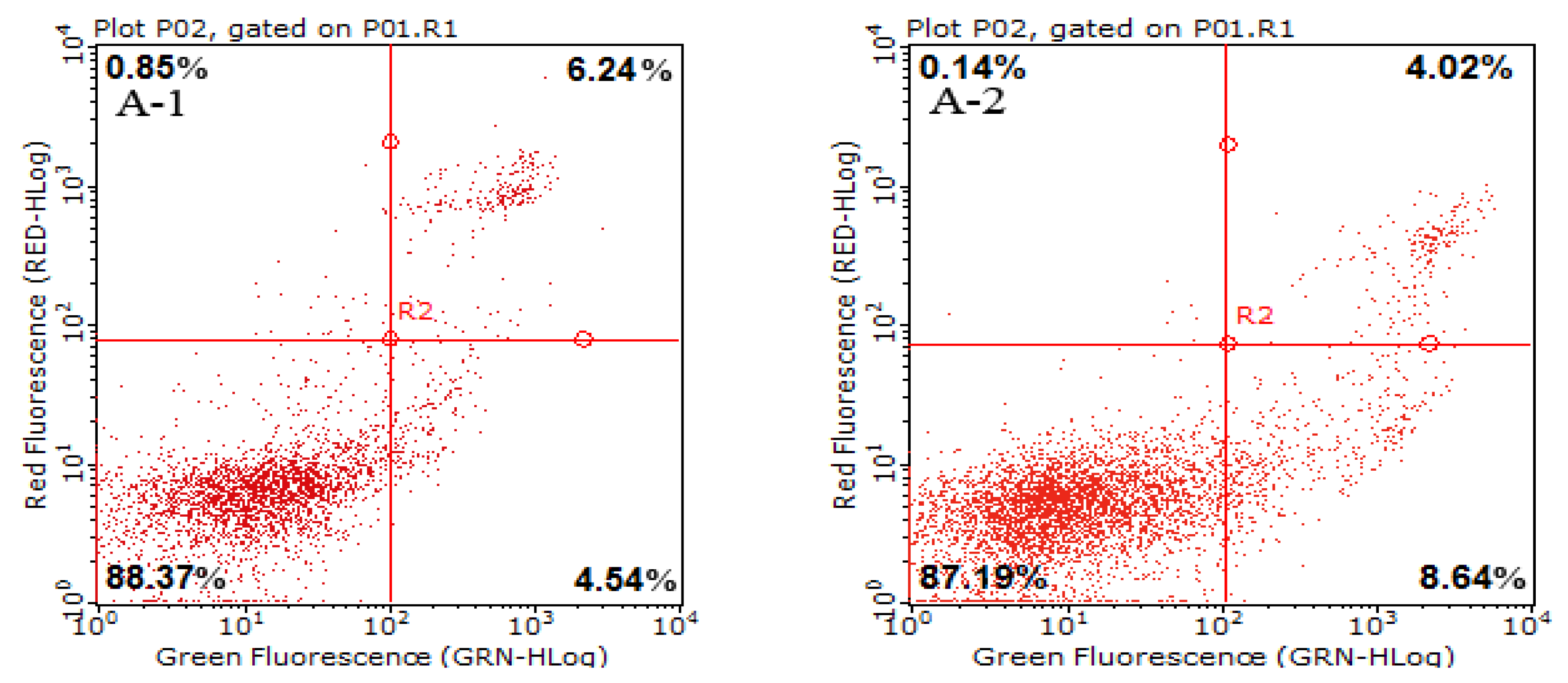
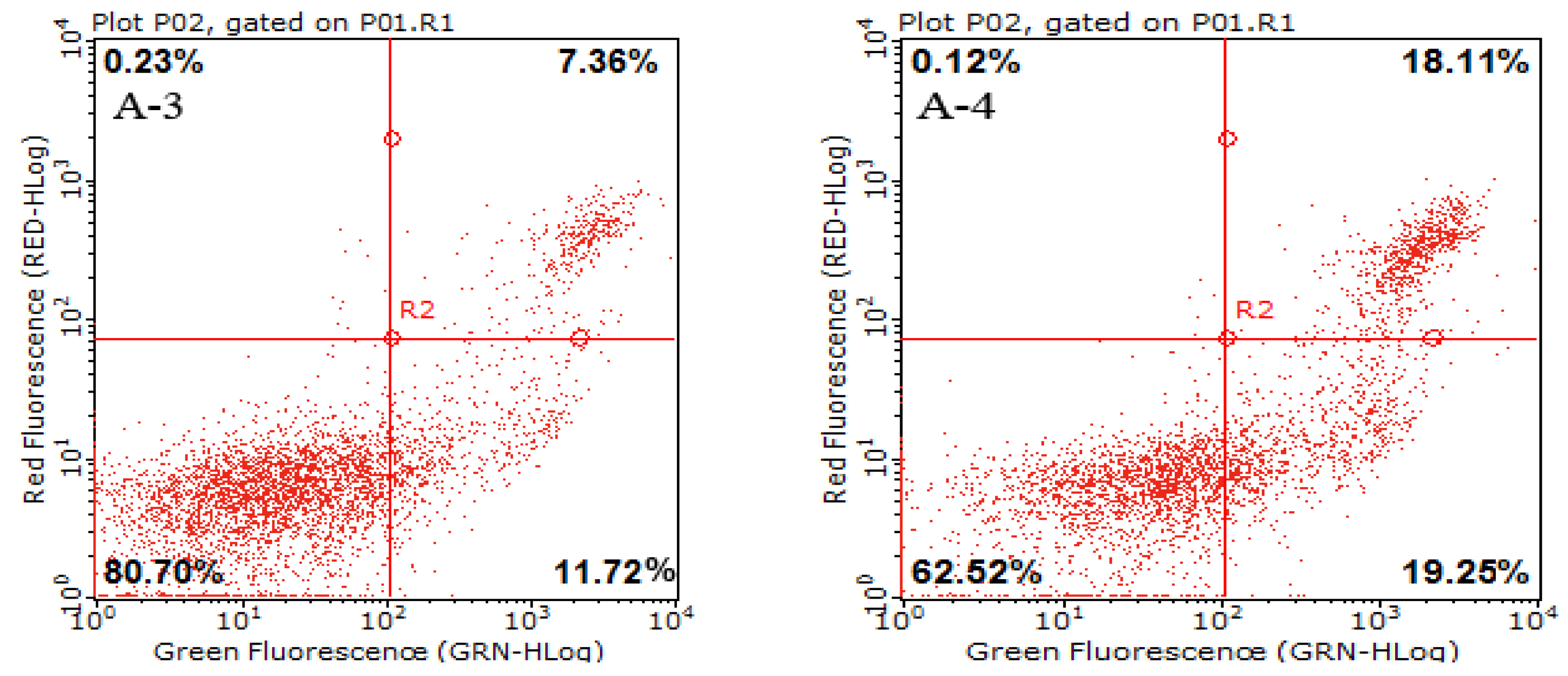
2.3. Cell Apoptotic Rate Detected by Flow Cytometry
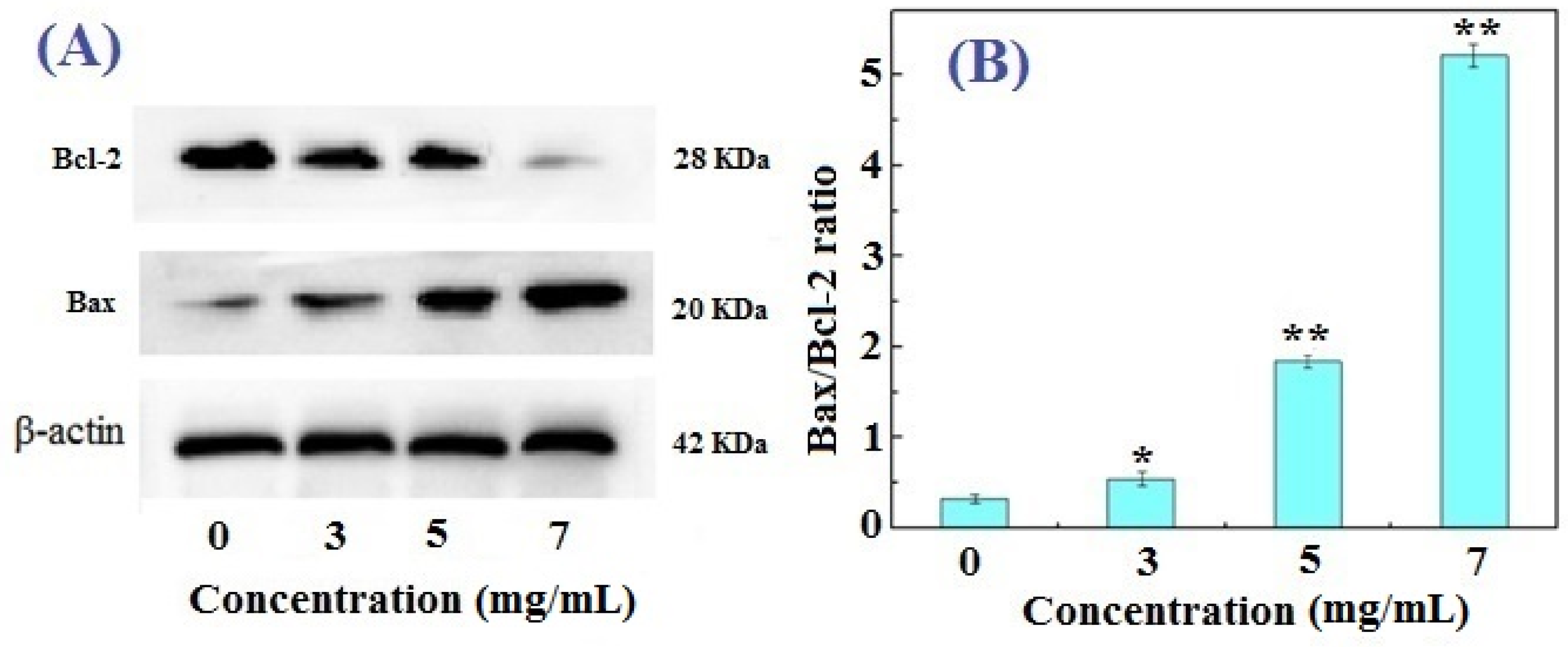
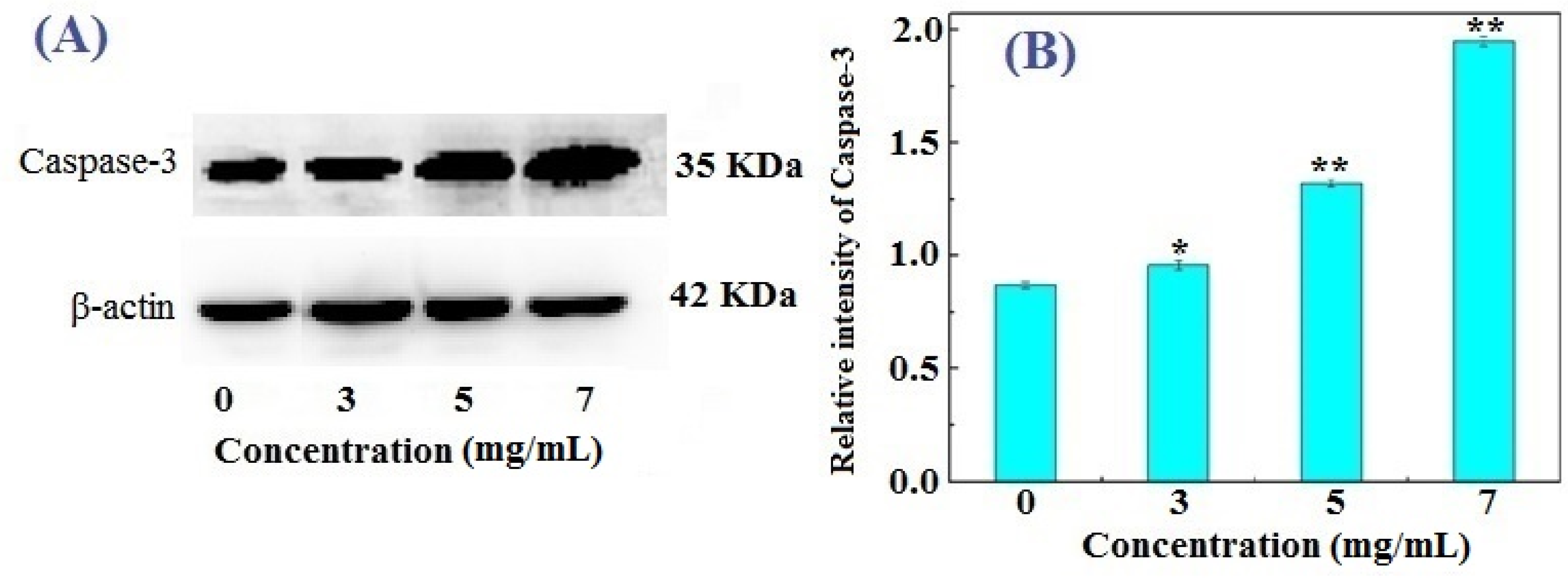
2.4. Western Blotting Results for Bcl-2, Bax, and Caspase-3 in FIMGPY-Treated HeLa Cells
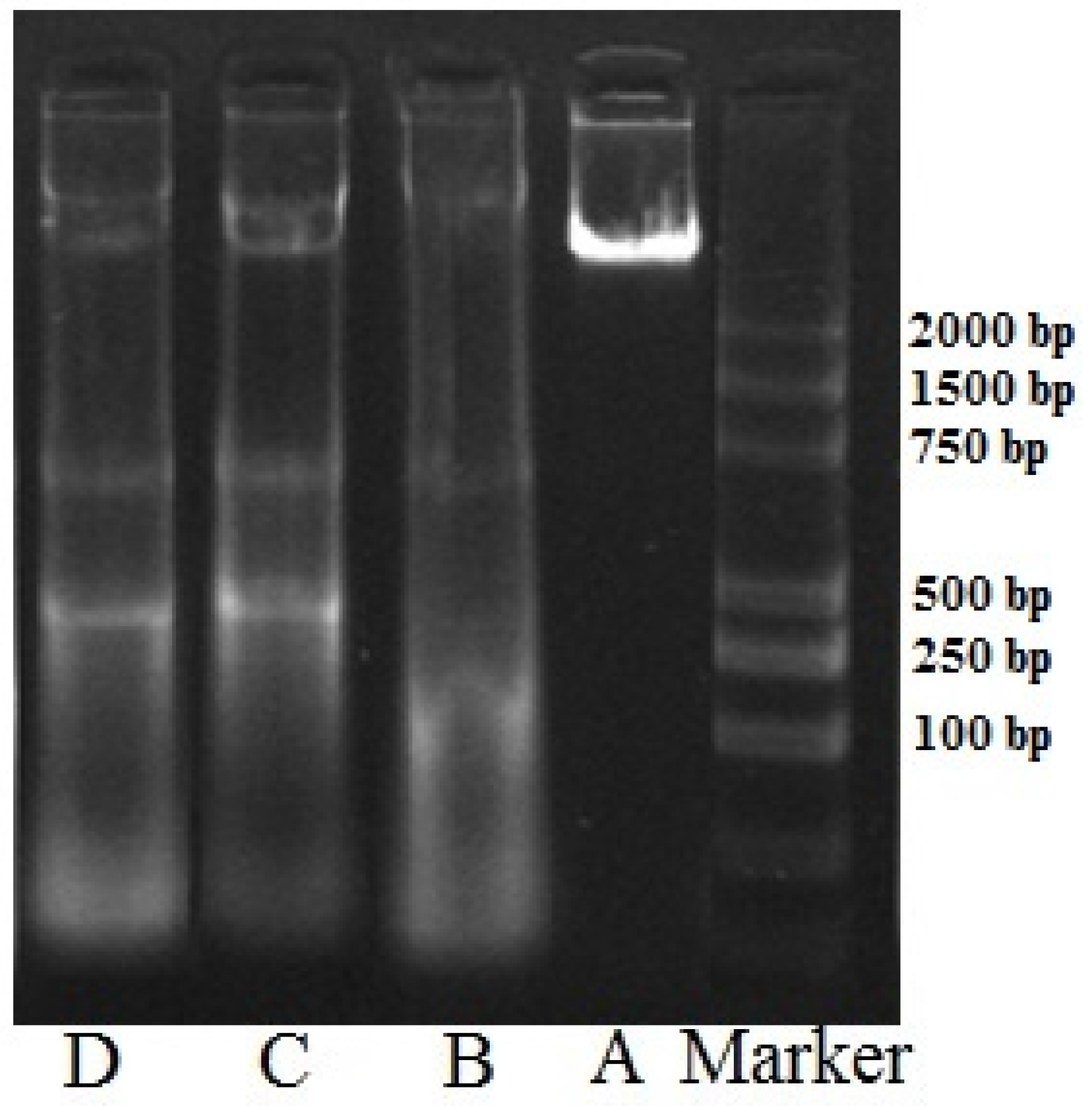
2.5. DNA Ladder Analysis
2.6. Discussion
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Preparation of Hexapeptide FIMGPY
3.3. Anti-Tumor Activity
3.3.1. Anti-Proliferative Activity
3.3.2. Morphological Study with Fluorescence Microscopy
3.3.3. Flow Cytometry Analysis
3.3.4. Western Blot Analysis
3.3.5. DNA Ladder Analysis
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Sivagami, G.; Balasubramanian, T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomed. Prev. Nutr. 2014, 4, 343–353. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Taddia, L.; D’Arca, D.; Ferrari, S.; Marraccini, C.; Severi, L.; Ponterini, G.; Assaraf, Y.G.; Marverti, G.; Costi, M.P. Inside the biochemical pathways of thymidylate synthase perturbed by anticancer drugs: Novel strategies to overcome cancer chemoresistance. Drug Resist. Update 2015, 23, 20–54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Z.; Zhang, W.G.; Zhou, G.H.; Xu, X.L.; Kang, Z.L.; Yin, Y. Isolation and identification of antioxidant peptides from Jinhua ham. J. Agric. Food Chem. 2013, 61, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- De Castro, R.J.S.; Sato, H.H. Biologically active peptides: Processes for their generation, purification and identification and applications as natural additives in the food and pharmaceutical industries. Food Res. Int. 2015, 74, 185–198. [Google Scholar] [CrossRef]
- Huang, F.; Yang, Z.; Yu, D.; Wang, J.; Li, R.; Ding, G. Sepia ink oligopeptide induces apoptosis in prostate cancer cell lines via caspase-3 activation and elevation of Bax/Bcl-2 ratio. Mar. Drugs 2012, 10, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.; Luo, H.; Yang, Z. Isolation and identification of an antiproliferative peptide derived from heated products of peptic hydrolysates of half-fin anchovy (Setipinna taty). J. Funct. Foods 2014, 10, 104–111. [Google Scholar] [CrossRef]
- Xue, Z.; Wen, H.; Zhai, L.; Yu, Y.; Li, Y.; Yu, W.; Cheng, A.; Wang, C.; Kou, X. Antioxidant activity and anti-proliferative effect of a bioactive peptide from chickpea (Cicer arietinum L.). Food Res. Int. 2015, 77, 75–81. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Memarpoor-Yazdi, M.; Asoodeh, A.; Chamani, J. A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J. Funct. Foods 2012, 4, 278–286. [Google Scholar] [CrossRef]
- Wang, B.; Gong, Y.; Li, Z.; Yu, D.; Chi, C.; Ma, J. Isolation and characterisation of five novel antioxidant peptides from ethanol-soluble proteins hydrolysate of spotless smoothhound (Mustelus griseus) muscle. J. Funct. Foods 2014, 6, 176–185. [Google Scholar] [CrossRef]
- Wang, S.; Mateos, R.; Goya, L.; Amigo-Benavent, M.; Sarriá, B.; Bravo, L. A phenolic extract from grape by-products and its main hydroxybenzoic acids protect Caco-2 cells against pro-oxidant induced toxicity. Food Chem. Toxicol. 2016, 88, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhao, Y.; Hu, F.; Wang, B. Preparation and identification of antioxidant peptides from protein hydrolysate of skate (Raja porosa) cartilage. J. Funct. Foods 2016, 25, 220–230. [Google Scholar] [CrossRef]
- Ibrahim, B.; Sowemimo, A.; Spies, L.; Koekomoer, T.; van de Venter, M.; Odukoya, O.A. Antiproliferative and apoptosis inducing activity of Markhamia tomentosa leaf extract on HeLa cells. J. Ethnopharmacol. 2013, 149, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, C.; Hansel, W. Membrane disrupting lytic peptides for cancer treatments. Curr. Pharm. Des. 2004, 10, 2299–2310. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ryu, B.; Je, J.; Kim, S. Diethylaminoethyl chitosan induces apoptosis in HeLa cells via activation of caspase-3 and p53 expression. Carbohydr. Polym. 2011, 84, 571–578. [Google Scholar] [CrossRef]
- Degterev, A.; Yuan, J. Expansion and evolution of cell death programmes. Nat. Rev. Mol. Cell Biol. 2008, 9, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huang, F.; Lin, H.; Wang, X. Isolation and purification of a peptide from Bullacta exarata and its impaction of apoptosis on prostate cancer cell. Mar. Drugs 2013, 11, 266–273. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Lecoeur, H. Nuclear apoptosis detection by flow cytometry: Influence of endogenous endonucleases. Exp. Cell Res. 2002, 277, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, Y.; Tao, X.; Zhang, M.; Sun, A. Protective effect of blueberry anthocyanins in a CCL4-induced liver cell model. LWT Food Sci. Technol. 2015, 60, 1105–1112. [Google Scholar] [CrossRef]
- Morales-Cano, D.; Calviño, E.; Rubio, V.; Herráez, A.; Sancho, P.; Tejedor, M.C.; Diez, J.C. Apoptosis induced by paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4 human leukaemia cells is not modulated by ERK inhibition. Exp. Toxicol. Pathol. 2013, 65, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Nys, K.; Agostinis, P. Bcl-2 family members: Essential players in skin cancer. Cancer Lett. 2012, 320, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Caspase function in programmed cell death. Cell Death Differ. 2007, 14, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980, 284, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Vethakanraj, H.S.; Babu, T.A.; Sudarsanan, G.B.; Duraisamy, P.K.; Kumar, S.A. Targeting ceramide metabolic pathway induces apoptosis in human breast cancer cell lines. Biochem. Biophys. Res. Commun. 2015, 464, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, Z.R.; Luo, H.Y. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna (Katsuwonus pelamis) dark muscle. Mar. Drugs 2015, 13, 2580–2601. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kim, J.Y.; Lee, K.W.; Kim, K.H.; Lee, H.J. Peptides from anchovy sauce induce apoptosis in a human lymphoma cell (U937) through the increase of caspase-3 and -8 activities. Ann. N. Y. Acad. Sci. 2003, 1010, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Lee, K.W.; Kim, J.Y.; Kim, K.H.; Lee, H.J. Induction of apoptosis in a human lymphoma cell line by hydrophobic peptide fraction separated from anchovy sauce. Biofactors 2004, 21, 63–67. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Zhao, Y.-Q.; Hu, F.-Y.; Chi, C.-F.; Wang, B. Anticancer Activity of a Hexapeptide from Skate (Raja porosa) Cartilage Protein Hydrolysate in HeLa Cells. Mar. Drugs 2016, 14, 153. https://doi.org/10.3390/md14080153
Pan X, Zhao Y-Q, Hu F-Y, Chi C-F, Wang B. Anticancer Activity of a Hexapeptide from Skate (Raja porosa) Cartilage Protein Hydrolysate in HeLa Cells. Marine Drugs. 2016; 14(8):153. https://doi.org/10.3390/md14080153
Chicago/Turabian StylePan, Xin, Yu-Qin Zhao, Fa-Yuan Hu, Chang-Feng Chi, and Bin Wang. 2016. "Anticancer Activity of a Hexapeptide from Skate (Raja porosa) Cartilage Protein Hydrolysate in HeLa Cells" Marine Drugs 14, no. 8: 153. https://doi.org/10.3390/md14080153
APA StylePan, X., Zhao, Y.-Q., Hu, F.-Y., Chi, C.-F., & Wang, B. (2016). Anticancer Activity of a Hexapeptide from Skate (Raja porosa) Cartilage Protein Hydrolysate in HeLa Cells. Marine Drugs, 14(8), 153. https://doi.org/10.3390/md14080153