Tiny Microbes with a Big Impact: The Role of Cyanobacteria and Their Metabolites in Shaping Our Future
Abstract
:1. Introduction
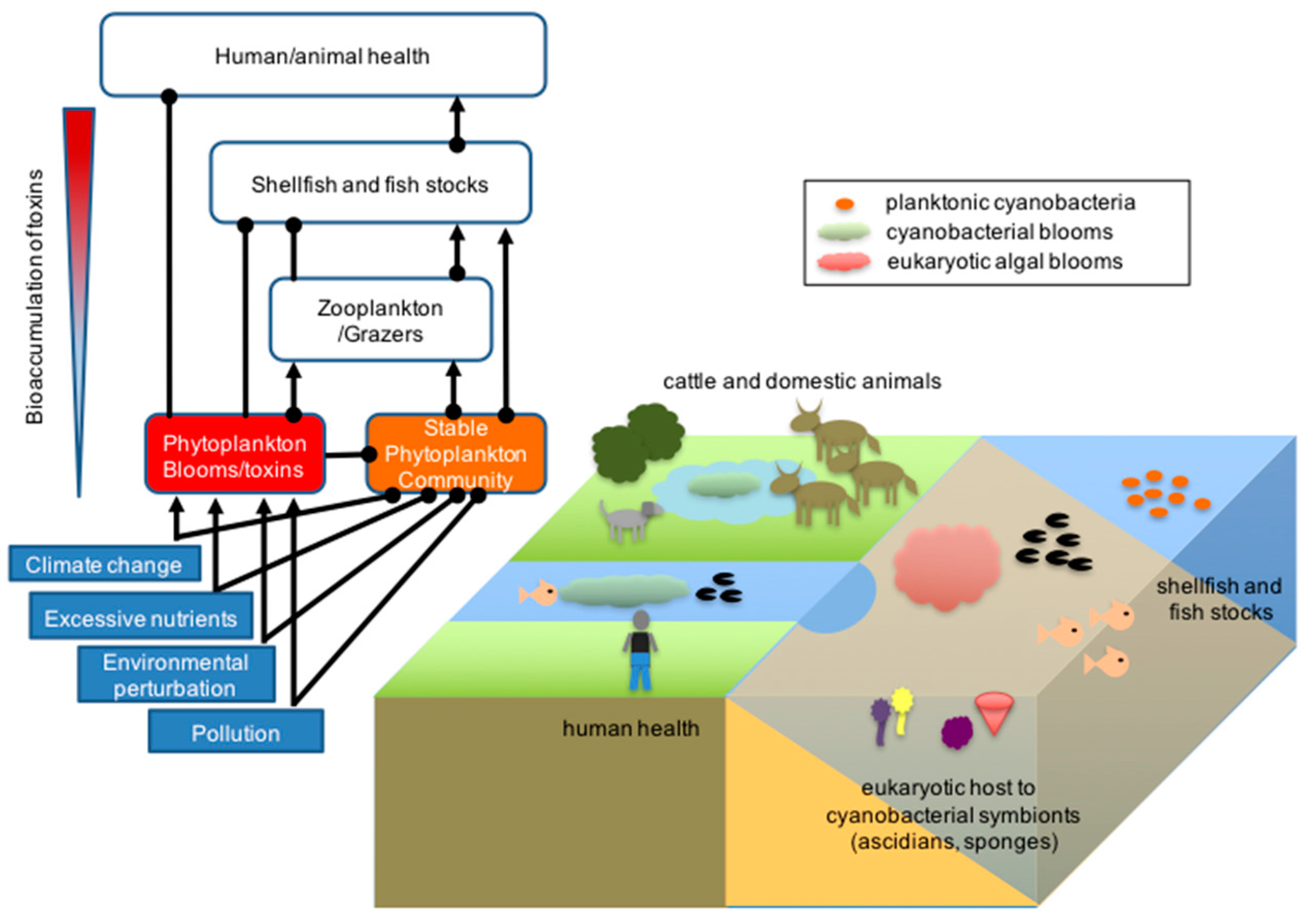
2. Environmental Impact of Marine Cyanobacterial Secondary Metabolites
3. Ecological Role of Marine Cyanobacterial Secondary Metabolites
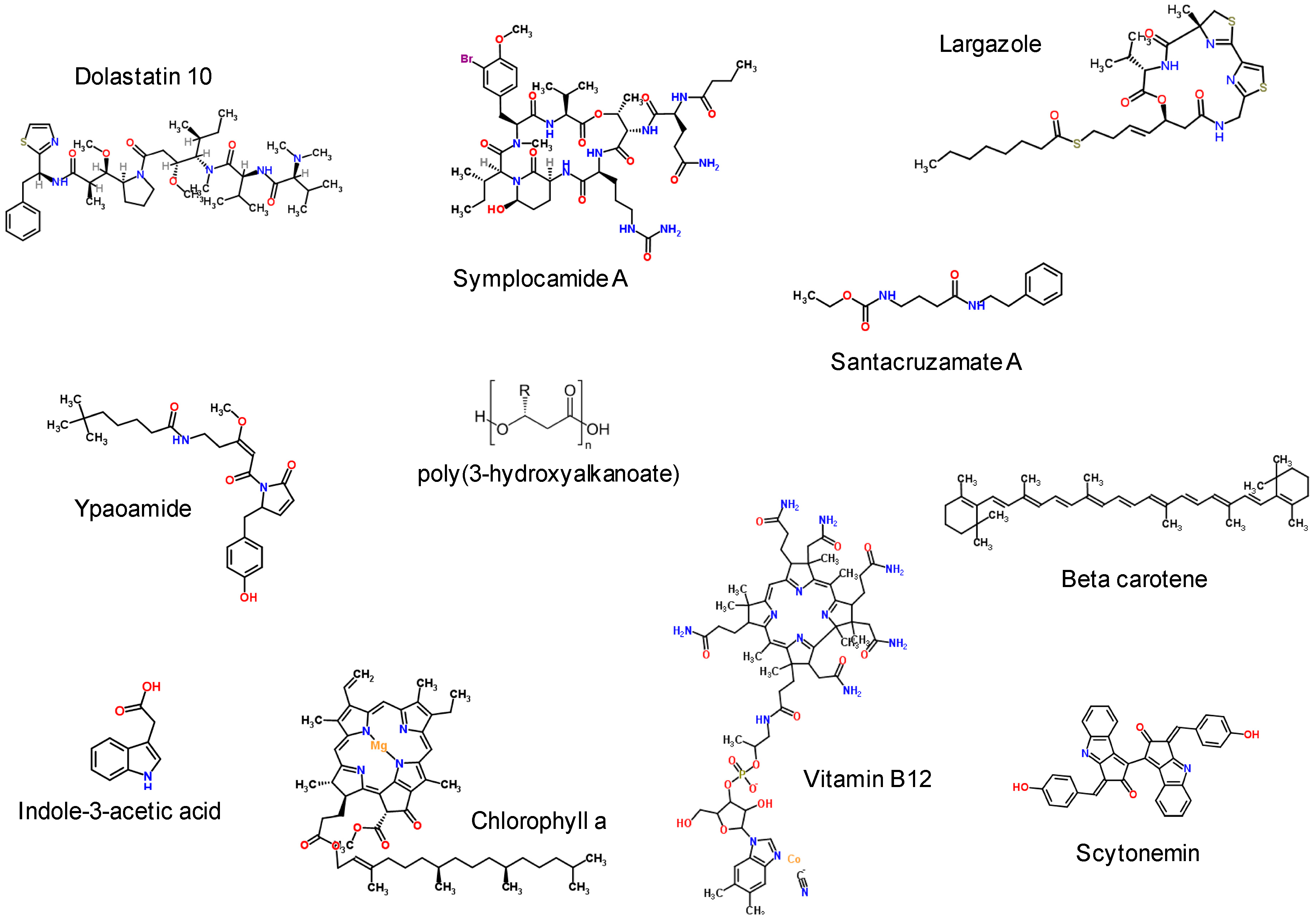
4. Biotechnological Applications for Marine Cyanobacterial Secondary Metabolites
4.1. Inhibitory Bioactive Metabolites
4.2. Nutritional Supplements, Pigments and Chromophores
4.3. Biofuels, Industrial Processes and Engineering
4.4. Frontier Technologies
4.4.1. Food Supplements
4.4.2. High Value Products
4.4.3. Biofuels
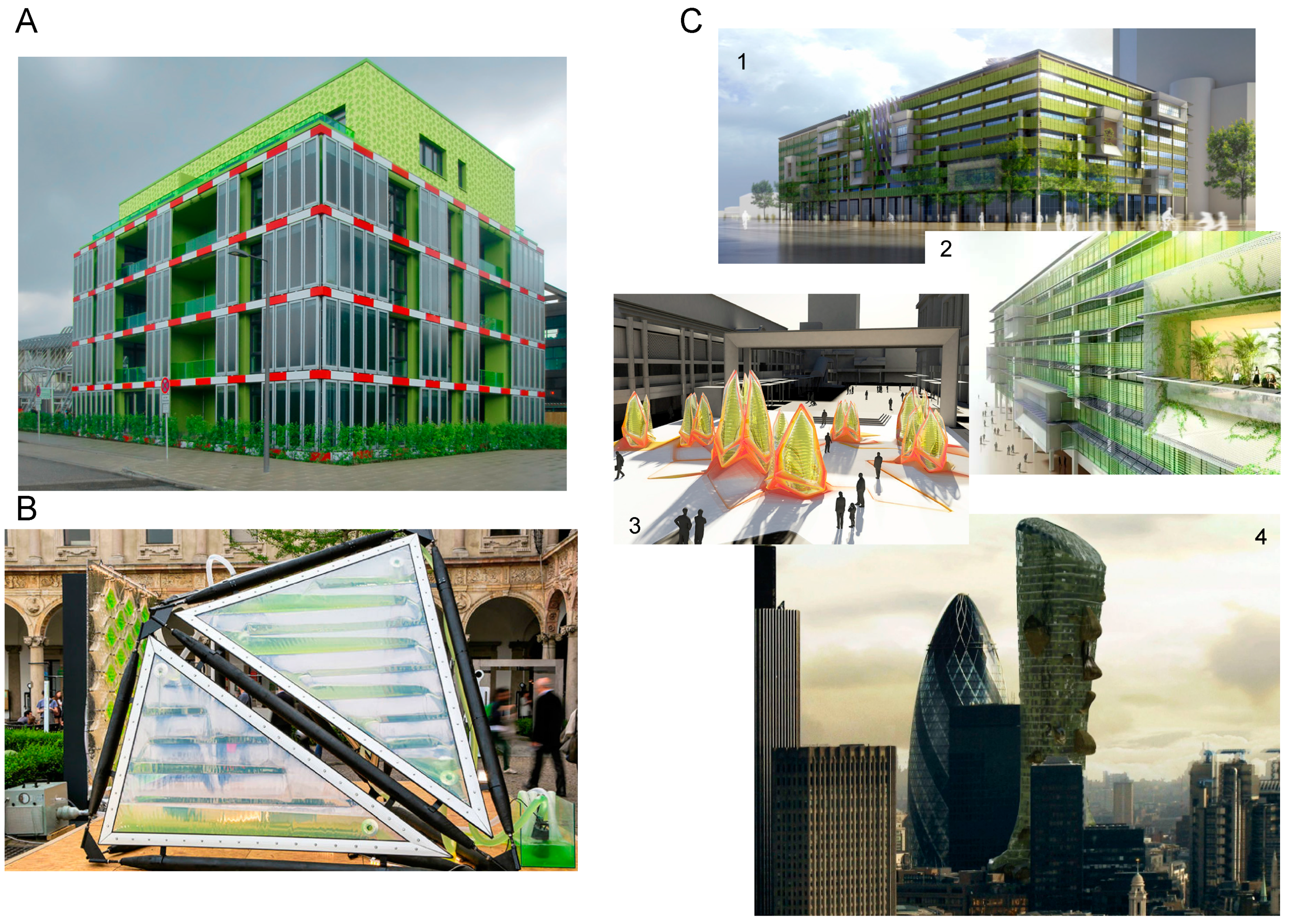
4.4.4. Energy-Efficient Green Buildings
4.4.5. Genetics and Synthetic Biology
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| CO2 | carbon dioxide |
| FMSA | financial market service authority |
| GSA | general services administration |
| HIV | human immunodeficiency virus |
| HOK | formerly Hellmuth, Obata + Kassabaum, design firm |
| IBA | International Building Exhibition |
| N | nitrogen |
| P | phosphorus |
| R&D | research and development |
| U.S. | United States (of America) |
| UV | ultra-violet |
References
- Schopf, J.W.; Packer, B.M. Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science 1987, 237, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W. Microfossils of the early Archean apex chert: New evidence of the antiquity of life. Science 1993, 260, 640–646. [Google Scholar]
- Bekker, A.; Holland, H.D.; Wang, P.L.; Rumble, D., 3rd; Stein, H.J.; Hannah, J.L.; Coetzee, L.L.; Beukes, N.J. Dating the rise of atmospheric oxygen. Nature 2004, 427, 117–120. [Google Scholar] [PubMed]
- Foster, R.A.; Kuypers, M.M.; Vagner, T.; Paerl, R.W.; Musat, N.; Zehr, J.P. Nitrogen fixation and transfer in open ocean diatom-cyanobacterial symbioses. ISME J. 2011, 5, 1484–1493. [Google Scholar]
- Freeman, C.J.; Thacker, R.W. Complex interactions between marine sponges and their symbiotic microbial communities. Limnol. Oceanogr. 2011, 56, 1577–1586. [Google Scholar]
- Steunou, A.S.; Bhaya, D.; Bateson, M.M.; Melendrez, M.C.; Ward, D.M.; Brecht, E.; Peters, J.W.; Kuhl, M.; Grossman, A.R. In situ analysis of nitrogen fixation and metabolic switching in unicellular thermophilic cyanobacteria inhabiting hot spring microbial mats. Proc. Natl. Acad. Sci. USA 2006, 103, 2398–2403. [Google Scholar] [PubMed]
- Comte, K.; Sabacka, M.; Carre-Mlouka, A.; Elster, J.; Komarek, J. Relationships between the Arctic and the Antarctic cyanobacteria; three Phormidium-like strains evaluated by a polyphasic approach. FEMS Microbiol. Ecol. 2007, 59, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Waterbury, J.; Cohen-Bazire, G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 1974, 100, 419–436. [Google Scholar]
- Saw, J.H.W.; Schatz, M.; Brown, M.V.; Kunkel, D.D.; Foster, J.S.; Shick, H.; Christensen, S.; Hou, S.; Wan, X.; Donachie, S.P. Cultivation and complete genome sequencing of Gloeobacter kilaueensis sp. nov., from a lava cave in Kīlauea caldera, Hawaii. PLoS ONE 2013, 8, e76376. [Google Scholar]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [PubMed]
- Ducat, D.C.; Way, J.C.; Silver, P.A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 2011, 29, 95–103. [Google Scholar] [PubMed]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar]
- Tan, L.T. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar] [PubMed]
- Niedermeyer, T.H.J. Anti-infective Natural Products from Cyanobacteria. Planta Med. 2015, 81, 1309–1325. [Google Scholar]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [PubMed]
- Carmichael, W.W. Health Effects of Toxin-Producing Cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1393–1407. [Google Scholar]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar]
- Carmichael, W. A world overview—One-hundred-twenty-seven years of research on toxic cyanobacteria—Where do we go from here? In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 105–125. [Google Scholar]
- Devlin, J.P.; Edwards, O.E.; Gorham, P.R.; Hunter, N.R.; Pike, R.K.; Stavric, B. Anatoxin-a, a toxic alkaloid from Anabaena flos-aquae NRC-44 h. Can. J. Chem. 1977, 55, 1367–1371. [Google Scholar]
- Carmichael, W.W.; Gorham, P.R. Anatoxins from clones of Anabaena flos-aquae isolated from lakes of western Canada. Mitt. Int. Ver. Theor. Angew. Limnol. 1978, 21, 285–295. [Google Scholar]
- Dawson, R.M. The toxicology of microcystins. Toxicon 1998, 36, 953–962. [Google Scholar] [PubMed]
- Engene, N.; Rottacker, E.C.; Kaštovský, J.; Byrum, T.; Choi, H.; Ellisman, M.H.; Komárek, J.; Gerwick, W.H. Moorea producens gen. nov., sp. nov. and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evolut. Microbiol. 2012, 62, 1171–1178. [Google Scholar]
- Turner, J.T.; Tester, P.A. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol. Oceanogr. 1997, 42, 1203–1214. [Google Scholar]
- Hudnell, H.K. The state of US freshwater harmful algal blooms assessments, policy and legislation. Toxicon 2010, 55, 1024–1034. [Google Scholar]
- Watkinson, A.J.; O’Neil, J.M.; Dennison, W.C. Ecophysiology of the marine cyanobacterium, Lyngbya majuscula (Oscillatoriaceae) in Moreton Bay, Australia. Harmful Algae 2005, 4, 697–715. [Google Scholar]
- Edwards, D.J.; Gerwick, W.H. Lyngbyatoxin biosynthesis: Sequence of biosynthetic gene cluster and identification of a novel aromatic prenyltransferase. J. Am. Chem. Soc. 2004, 126, 11432–11433. [Google Scholar] [PubMed]
- Kudela, R.M.; Berdalet, E.; Bernard, S.; Burford, M.; Fernand, L.; Lu, S.; Roy, S.; Tester, P.; Usup, G.; Magnien, R.; et al. Harmful Algal Blooms: A Scientific Summary for Policy Makers; IOC/UNESCO: Paris, France, 2015. [Google Scholar]
- Turgeon, D.D.; Sellner, K.G.; Scavia, D.; Anderson, D. Status of U.S. harmful algal blooms: Progress towards a National Program; NOAA, National Ocean Service, Centers for Coastal Ocean Science, Center for Monitoring and Assessment; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 1998; p. 22.
- Bushaw-Newton, K.L.; Sellner, K.G. Harmful Algal Blooms. In NOAA’s State of the Coast Report; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 1999. [Google Scholar]
- Atech Group Pty Ltd. Cost of algal blooms. In Land and Water Resources Research and Development Corporation; Land & Water Australia Legacy: Canberra, Australia, 2000. [Google Scholar]
- Steffensen, D.A. Economic cost of cyanobacterial blooms. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 855–865. [Google Scholar]
- Francis, G. Poisonous Australian Lake. Nature 1878, 18, 11–12. [Google Scholar] [CrossRef]
- McGregor, G.B.; Stewart, I.; Sendall, B.C.; Sadler, R.; Reardon, K.; Carter, S.; Wruck, D.; Wickramasinghe, W. First report of a toxic Nodularia spumigena (Nostocales/Cyanobacteria) bloom in sub-tropical Australia. I. Phycological and public health investigations. Int. J. Environ. Res. Public Health 2012, 9, 2396–2411. [Google Scholar]
- Jones, A.C.; Monroe, E.A.; Podell, S.; Hess, W.R.; Klages, S.; Esquenazi, E.; Niessen, S.; Hoover, H.; Rothmann, M.; Lasken, R.S.; et al. Genomic insights into the physiology and ecology of the marine filamentous cyanobacterium Lyngbya majuscula. Proc. Natl. Acad. Sci. USA 2011, 108, 8815–8820. [Google Scholar]
- Leao, P.N.; Engene, N.; Antunes, A.; Gerwick, W.H.; Vasconcelos, V. The chemical ecology of cyanobacteria. Nat. Prod. Rep. 2012, 29, 372–391. [Google Scholar] [PubMed]
- Simmons, T.L.; Coates, R.C.; Clark, B.R.; Engene, N.; Gonzalez, D.; Esquenazi, E.; Dorrestein, P.C.; Gerwick, W.H. Biosynthetic origin of natural products isolated from marine microorganism–invertebrate assemblages. Proc. Natl. Acad. Sci. USA 2008, 105, 4587–4594. [Google Scholar]
- Penesyan, A.; Marshall-Jones, Z.; Holmstrom, C.; Kjelleberg, S.; Egan, S. Antimicrobial activity observed among cultured marine epiphytic bacteria reflects their potential as a source of new drugs. FEMS Microbiol. Ecol. 2009, 69, 113–124. [Google Scholar] [CrossRef]
- Penesyan, A.; Kjelleberg, S.; Egan, S. Development of novel drugs from marine surface associated microorganisms. Mar. Drugs 2010, 8, 438–459. [Google Scholar] [PubMed]
- Luesch, H.; Harrigan, G.G.; Goetz, G.; Horgen, F.D. The cyanobacterial origin of potent anticancer agents originally isolated from sea hares. Curr. Med. Chem. 2002, 9, 1791–1806. [Google Scholar]
- König, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Müller, D. Natural products from marine organisms and their associated microbes. ChemBioChem 2006, 7, 229–238. [Google Scholar] [PubMed]
- Salvador-Reyes, L.A.; Luesch, H. Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep. 2015, 32, 478–503. [Google Scholar]
- Ainslie, R.D.; Barchi, J.J., Jr.; Kuniyoshi, M.; Moore, R.E.; Mynderse, J.S. Structure of malyngamide C. J. Org. Chem. 1985, 50, 2859–2862. [Google Scholar]
- Dobretsov, S.; Teplitski, M.; Alagely, A.; Gunasekera, S.P.; Paul, V.J. Malyngolide from the cyanobacterium Lyngbya majuscula interferes with quorum sensing circuitry. Environ. Microbiol. Rep. 2010, 2, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Teplitski, M.; Gunasekera, S.P.; Paul, V.J.; Luesch, H. Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.C.; Meickle, T.; Ladwa, D.; Teplitski, M.; Paul, V.; Luesch, H. Lyngbyoic acid, a “tagged” fatty acid from a marine cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginosa. Mol. BioSyst. 2011, 7, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Teplitski, M.; Bayer, M.; Gunasekera, S.; Proksch, P.; Paul, V.J. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling 2011, 27, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Paz-Yepes, J.; Brahamsha, B.; Palenik, B. Role of a Microcin-C-like biosynthetic gene cluster in allelopathic interactions in marine Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 12030–12035. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.; Costa, M.; Ramos, V.; Leão, P.N.; Barreiro, A.; Vasconcelos, V.; Martins, R. Picocyanobacteria from a clade of marine cyanobium revealed bioactive potential against microalgae, bacteria, and marine invertebrates. J. Toxicol. Environ. Health A 2015, 78, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Paul, V.J. Chemical defense of a marine cyanobacterial bloom. J. Exp. Mar. Biol. Ecol. 1998, 225, 29–38. [Google Scholar] [CrossRef]
- Cruz-Rivera, E.; Paul, V.J. Chemical deterrence of a cyanobacterial metabolite against generalized and specialized grazers. J. Chem. Ecol. 2007, 33, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Camacho, F.T.; Paul, V.J. Dietary preferences of the opisthobranch mollusc Stylocheilus longicauda for secondary metabolites produced by the tropical cyanobacterium Lyngbya majuscula. Mar. Biol. 1998, 132, 267–273. [Google Scholar] [CrossRef]
- Cole, J. Interactions Between Bacteria and Algae in Aquatic Ecosystems. Ann. Rev. Ecol. Syst. 1982, 131, 191–314. [Google Scholar] [CrossRef]
- Wilkinson, C.R. Nutrient translocation from symbiotic cyanobacteria to coral reef sponges. In Biologie des Spongiaires; Levi, C., Boury-Esnault, N., Eds.; CNRS: Paris, France, 1979; pp. 373–380. [Google Scholar]
- Thacker, R.W. Impacts of shading on sponge-cyanobacteria symbioses: A comparison between host-specific and generalist associations. Integr. Comp. Biol. 2005, 45, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, E.; Liaimer, A.; Bergman, B. Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 2002, 215, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Hamayun, M.; Shah, S.T. Root colonization and phytostimulation by phytohormones producing entophytic Nostoc sp. AH-12. Curr. Microbiol. 2013, 67, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Shah, S.T.; Rahman, H.; Irshad, M.; Iqbal, A. Effect of IAA on in vitro growth and colonization of Nostoc in plant roots. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, R.N.; Babu, S.V.; Ashokkumar, B.; Malliga, P.; Varalakshmi, P. Antioxidant property of fresh and marine water cyanobacterial extracts in Swiss mice. J. Biopestic. 2012, 5, 250–254. [Google Scholar]
- Guedes, A.; Gião, M.S.; Seabra, R.; Ferreira, A.C.; Tamagnini, P.; Moradas-Ferreira, P.; Malcata, F.X. Evaluation of the antioxidant activity of cell extracts from microalgae. Mar. Drugs 2013, 11, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Eckes, M.J.; Siebeck, U.E.; Dove, S.; Grutter, A.S. Ultraviolet sunscreens in reef fish mucus. Mar. Ecol. Prog. Ser. 2008, 353, 203–211. [Google Scholar] [CrossRef]
- Kicklighter, C.E.; Kamio, M.; Nguyen, L.; Germann, M.W.; Derby, C.D. Mycosporine-like amino acids are multifunctional molecules in sea hares and their marine community. Proc. Natl. Acad. Sci. USA 2011, 108, 11494–11499. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Häder, D.P. UV-protectants in cyanobacteria. Plant Sci. 2008, 174, 278–289. [Google Scholar] [CrossRef]
- Plavsic, M.; Terzic, S.; Ahel, M.; Van Den Berg, C.M.G. Folic acid in coastal waters of the Adriatic Sea. Mar. Freshw. Res. 2004, 53, 1245–1252. [Google Scholar] [CrossRef]
- Bonnet, S.; Webb, E.A.; Panzeca, C.; Karl, D.M.; Capone, D.G.; Wilhelmy, S.A.S. Vitamin B12 excretion by cultures of the marine cyanobacteria Crocosphaera and Synechococcus. Limnol. Oceanogr. 2010, 55, 1959–1964. [Google Scholar] [CrossRef]
- Bertrand, E.M.; Allen, A.E. Influence of vitamin B auxotrophy on nitrogen metabolism in eukaryotic phytoplankton. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, S.J.; Tripp, H.J.; Givan, S.; Podar, M.; Vergin, K.L.; Baptista, D.; Bibbs, L.; Eads, J.; Richardson, T.H.; Noordewier, M.; et al. Genome streamlining in a cosmopolitan oceanic bacterium. Science 2005, 309, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Tripp, H.J.; Kitner, J.B.; Schwalbach, M.S.; Dacey, J.W.; Wilhelm, L.J.; Giovannoni, S.J. SAR11 marine bacteria require exogenous reduced sulphur for growth. Nature 2008, 452, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, I.; Kim, S.K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef] [PubMed]
- Calteau, A.; Fewer, D.P.; Latifi, A.; Coursin, T.; Laurent, T.; Jokela, J.; Kerfeld, C.A.; Sivonen, K.; Piel, J.; Gugger, M. Phylum-wide comparative genomics unravel the diversity of secondary metabolism in Cyanobacteria. BMC Genom. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Uzair, B.; Tabassum, S.; Rasheed, M.; Rehman, S.F. Exploring marine cyanobacteria for lead compounds of pharmaceutical importance. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.; Hemaiswarya, S.; Ganesan, V.; Carvalho, I.S. Recent developments in therapeutic applications of Cyanobacteria. Crit. Rev. Microbiol. 2016, 42, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.; Cahoon, N.; Trakhtenberg, E.M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S.S. Use of ichip for high-throughput in situ cultivation of “uncultivable” microbial species. Appl. Environ. Microbiol. 2010, 76, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fewer, D.P.; Sivonen, K. Genome mining demonstrates the widespread occurrence of gene clusters encoding bacteriocins in cyanobacteria. PLoS ONE 2011, 6, e22384. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sher, D.; Kelly, L.; Shi, Y.; Huang, K.; Knerr, P.J.; Joewono, I.; Rusch, D.; Chisholm, S.W.; van der Donk, W.A. Catalytic promiscuity in thebiosynthesis of cyclic peptide secondary metabolites in planktonic marine cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 10430–10435. [Google Scholar] [CrossRef] [PubMed]
- Bhadury, P.; Wright, P.C. Exploitation of marine algae: Biogenic compounds for potential antifouling applications. Planta 2004, 219, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. New apratoxins of marine cyanobacterial origin from Guam and Palau. Bioorg. Med. Chem. 2002, 10, 1973–1978. [Google Scholar] [CrossRef]
- Simmons, T.L.; Andrianasolo, E.; McPhail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [Google Scholar] [PubMed]
- Tan, L.T. Filamentous tropical marine cyanobacteria: A rich source of natural products for anticancer drug discovery. J. Appl. Phycol. 2010, 22, 659–676. [Google Scholar] [CrossRef]
- Do Rosário Martins, M.; Costa, M. Marine cyanobacteria compounds with anticancer properties: Implication of apoptosis. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 621–647. [Google Scholar]
- Linington, R.G.; Clark, B.R.; Trimble, E.E.; Almanza, A.; Ureña, L.D.; Kyle, D.E.; Gerwick, W.H. Antimalarial peptides from marine cyanobacteria: Isolation and structural elucidation of gallinamide A. J. Nat. Prod. 2009, 72, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K.; Leikoski, N.; Fewer, D.P.; Jokela, J. Cyanobactins-ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 2010, 86, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Linington, R.G.; Tidgewell, K.; Fenner, A.M.; Ureña, L.D.; Togna, G.D.; Kyle, D.E.; Gerwick, W.H. Dragonamide E, a modified linear lipo-peptide from Lyngbya majuscula with antileishmanial activity. J. Nat. Prod. 2010, 73, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.V.; Flatt, P.M.; Edwards, D.J.; Simmons, T.L.; Han, B.; Gerwick, W.H. The secondary metabolites and biosynthetic gene clusters of marine cyanobacteria. Applications in biotechnology. In Frontiers in Marine Biotechnology; Proksch, P., Müller, W.E., Eds.; Horizon Bioscience: Norfolk, UK, 2006; pp. 175–224. [Google Scholar]
- Matthew, S.; Ross, C.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatin 4, a dolastatin 13 analogue with elastase and chymotrypsin inhibitory activity from the marine cyanobacterium Lyngbya confervoides. J. Nat. Prod. 2007, 70, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Matthew, S.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatins 5–7, potent elastase inhibitors from Floridian marine cyanobacteria, Lyngbya sp. J. Nat. Prod. 2007, 70, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Linington, R.G.; Edwards, D.J.; Shuman, C.F.; McPhail, K.L.; Matainaho, T.; Gerwick, W.H. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2007, 71, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Costa-Rodrigues, J.; Fernandes, M.H.; Barros, P.; Vasconcelos, V.; Martins, R. Marine cyanobacteria compounds with anticancer properties: A review on the implication of apoptosis. Mar. Drugs 2012, 10, 2181–2207. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Paul, V.J.; Luesch, H. Tiglicamides A–C, cyclodepsipeptides from the marine cyanobacterium Lyngbya confervoides. Phytochemistry 2009, 70, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.T.; Workman, J.L. Histone deacetylase inhibitors: Anticancer compounds. Int. J. Biochem. Cell B 2009, 41, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, C.M.; Wong, C.Y.; Ononye, S.; Lopez, D.D.; Engene, N.; McPhail, K.L.; Gerwick, W.H.; Balunas, M.J. Santacruzamate A, a potent and selective histone deacetylase inhibitor from the Panamanian marine cyanobacterium cf. Symploca sp. J. Nat. Prod. 2013, 76, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Bowers, A.; West, N.; Taunton, J.; Schreiber, S.L.; Bradner, J.E.; Williams, R.M. Total synthesis and biological mode of action of largazole: A potent class I histone deacetylase inhibitor. J. Am. Chem. Soc. 2008, 130, 11219–11222. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Paul, V.J.; Luesch, H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008, 130, 1806–1807. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Liu, Y.; Byeon, S.R.; Kim, H.; Luesch, H.; Hong, J. Synthesis and activity of largazole analogues with linker and macrocycle modification. Org. Lett. 2008, 10, 4021–4024. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Luesch, H. Largazole: From discovery to broad-spectrum therapy. Nat. Prod. Rep. 2012, 29, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.S. Biosynthesis of marine natural products: Microorganisms (Part A). Nat. Prod. Rep. 2005, 22, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Trentacoste, E.M.; Martinez, A.M.; Zenk, T. The place of algae in agriculture: Policies for algal biomass production. Photosynth. Res. 2014, 123, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, V.; Perretti, A.; Palomba, I.; Verde, A.; Cuomo, A. Utlization of Ulva rigida biomass in the Venice Lagoon (Italy): Biotransformation in compost. J. Appl. Phycol. 1995, 7, 479–485. [Google Scholar] [CrossRef]
- Angus, S.; Dargie, T. The UK Machair habitat action plan: Progress and problems. Bot. J. Scotl. 2002, 54, 63–74. [Google Scholar] [CrossRef]
- Tung, H.F.; Shen, T.C. Studies of the Azolla pinnata—Anabaena azollae symbiosis: Concurrent growth of Azolla with rice. Aquat. Bot. 1985, 22, 145–152. [Google Scholar] [CrossRef]
- Peng, J.; Shen, X.; El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Bobzin, S.; Huesing, J.; Camp, R.; et al. Marine natural products as prototype agrochemical agents. J. Agric. Food Chem. 2003, 51, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Batard, P.; Szollosi, J.; Luescher, I.; Cerottini, J.C.; MacDonald, R.; Romero, P. Use of phycoerythrin and allophycocyanin for fluorescence resonance energy transfer analyzed by flow cytometry: Advantages and limitations. Cytometry 2002, 48, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bracmort, K. Algae’s Potential as a Transportation Biofuel; R42122; Congressional Research Service Report for Congress: Washington, DC, USA, 2013. [Google Scholar]
- Tyner, W.E. Policy update: The US renewable fuel standard up against the wall. Biofuels 2013, 4, 475–477. [Google Scholar] [CrossRef]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)—Prospects and challenges. Algal Res. 2013, 2, 79–97. [Google Scholar] [CrossRef]
- Algenol. Available online: www.algenol.com (accessed on 8 Feburary 2016).
- Joule Unlimited. Available online: www.jouleunlimited.com (accessed on 8 April 2016).
- Audi and Joule Unlimited Partnership. Available online: www.businesswire.com/news/home/201209 17005123/en/Joule-Partners-AUDI-Accelerate-Development-Commercialization-Sustainable (accessed on 8 April 2016).
- Red Rock Biofuels and Joule Unlimited Merging Intent. Available online: www.jouleunlimited.com/ joule-and-red-rock-biofuels-announce-intent-merge-creating-industry-leading-carbon-neutral-fuel (accessed on 8 April 2016).
- Möllers, K.B.; Cannella, D.; Jørgensen, H.; Frigaard, N.U. Cyanobacterial biomass as carbohydrate and nutrient feedstock for bioethanol production by yeast fermentation. Biotechnol. Biofuels 2014, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Stöckel, J.; Min, H.; Sherman, L.A.; Pakrasi, H.B. High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat. Commun. 2010, 1, 139. [Google Scholar] [CrossRef] [PubMed]
- Melnicki, M.R.; Pinchuk, G.E.; Hill, E.A.; Kucek, L.A.; Fredrickson, J.K.; Konopka, A.; Beliaev, A.S. Sustained H2 production driven by photosynthetic water splitting in a unicellular cyanobacterium. mBio. 2012, 3, e00197-12. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.M.; Köster, J. The direct role of aerobic heterotrophic bacteria associated with cyanobacteria in the degradation of oil compounds. Int. Biodeterior. Biodegrad. 2005, 55, 29–37. [Google Scholar] [CrossRef]
- Paniagua-Michel, J.J.; Olmos-Soto, J.; Morales-Guerrero, E.R. Algal and microbial exo- polysaccharides: New insights as biosurfactants and bioemulsifiers. Adv. Food Nutr. Res. 2013, 73, 221–257. [Google Scholar]
- Marti, M.E.; Colonna, W.J.; Patra, P.; Zhang, H.; Green, C.; Reznik, G.; Pynn, M.; Jarrell, K.; Nyman, J.A.; Somasundaran, P.; et al. Production and characterization of microbial biosurfactants for potential use in oil-spill remediation. Enzyme Microb. Technol. 2014, 55, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Fattom, A.; Shilo, M. Phormidium J-1 bioflocculant: Production and activity. Arch. Microbiol. 1984, 139, 421–426. [Google Scholar] [CrossRef]
- Ennesys, Environmental Energy Systems. Available online: www.ennesys.com/technologie/ (accessed on 8 Feburaey 2016).
- Bravo-Fritz, C.P.; Sáez-Navarrete, C.A.; Zeppelin, L.A.H.; Cea, R.G. Site selection for microalgae farming on an industrial scale in Chile. Algal Res. 2015, 11, 343–349. [Google Scholar] [CrossRef]
- Scanlan, D.J.; Ostrowski, M.; Mazard, S.; Dufresne, A.; Garczarek, L.; Hess, W.R.; Post, A.F.; Hagemann, M.; Paulsen, I.; Partensky, F. Ecological genomics of marine picocyanobacteria. Microbiol. Mol. Biol. Rev. 2009, 73, 249–299. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods—Possibilities and Challenges. Comp. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe; Vigani, M., Parisi, C., Rodríguez Cerezo, E., Eds.; JRC Scientific and Policy Reports, EU publications: Luxembourg, 2014. [Google Scholar]
- Vigani, M.; Parisi, C.; Rodríguez-Cerezo, E.; Barbosa, M.J.; Sijtsma, L.; Ploeg, M.; Enzing, C. Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends Food Sci. Technol. 2015, 42, 81–92. [Google Scholar] [CrossRef]
- Spirulina Source: Resource Center for Spirulina, Algae and Green Superfoods. Available online: www.spirulinasource.com (accessed on 8 February 2016).
- Freitas, A.C.; Rodrigues, D.; Rocha-Santos, T.A.; Gomes, A.M.; Duarte, A.C. Marine biotechnology advances towards applications in new functional foods. Biotechnol. Adv. 2012, 30, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- ESA: MELiSSA Project (Micro-Ecological Life Support System Alternative). Available online: www.esa.int/Our_Activities/Space_Engineering_Technology/Melissa/Targets_Scientific_domains (accessed on 8 February 2016).
- Hays, S.G.; Ducat, D.C. Engineering cyanobacteria as photosynthetic feedstock factories. Photosynth. Res. 2014, 123, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Driver, T.; Bajhaiya, A.; Pittman, J.K. Potential of bioenergy production from microalgae. Curr. Sustain. Renew. Energy Rep. 2014, 1, 94–103. [Google Scholar] [CrossRef]
- Originclear, Live Algae Oil Extraction. Available online: www.originclear.com/company-news/originoil-announces-breakthrough-process-for-live-algae-oil-extraction (accessed on 8 Febuary 2016).
- US EPA Approval for the Helioculture Process from Joule Unlimited. Available online: epa.gov/sites/production/files/2016-04/documents/joule-deter-ltr-2016-03-29.pdf (accessed on 29 April 2016).
- Henrikson, R.; Edwards, M. Algae Microfarms: For Home, School, Community and Urban Gardens, Rooftop, Mobile and Vertical Farms and Living Buildings; Create Space Independent Publishing Platform: North Charleston, SC, USA, 2013. [Google Scholar]
- Henrikson, R.; Edwards, M. Imagine Our Algae Future: Visionary Algae Architecture and Landscape Design; Create Space Independent Publishing Platform: North Charleston, SC, USA, 2012. [Google Scholar]
- Cervera-Sardá, R.; Gómez-Pioz, J.; Ruiz-de-Elvira, A. Architecture as an Energy Factory: Pushing the Envelope. In Construction and Building Research; Llinares-Millán, C., Fernandez-Plazaola, I., Hidalgo-Delgado, F., Martínez-Valenzuela, M.M., Medina-Ramon, F.J., Oliver-Faubel, I., Rodriguez-Abad, I., Salandin, A., Sanchez-Grandia, R., Tort-Ausina, I., Eds.; Springer: Amsterdam, The Netherlands, 2014; pp. 209–217. [Google Scholar]
- Bio Intelligent Quotient (BIQ) House in Hamburg. Available online: www.iba-hamburg.de/en/themes-projects/the-building-exhibition-within-the-building-exhibition/smart-material-houses/biq/projekt/biq.html (accessed on 8 Febuary 2016).
- Origin Oil Receives Firm Order for its Algae Appliance in Urban Test Program at Paris La Défense Complex. Available online: www.originclear.com/company-news/originoil-receives-firm-order-for-its-algae-appliance-in-urban-test-program-at-paris-la-defense-complex (accessed on 8 Febuary 2016).
- Brooks, R. World’s First Urban Algae Canopy Produces the Oxygen Equivalent of Four Hectares of Woodland Every Day. Inhabitat.com. 2015. Available online: http://inhabitat.com/incredible-urban-algae-canopy-produces-the-oxygen-equivalent-of-four-hectares-of-woodland-every-day/ (accessed on 8 Febuary 2016).
- Cesare Griffa Architecture Lab and projects. Available online: cesaregriffa.com/bioskin-microalgae-facades/cesaregriffa.com/waterlilly (accessed on 10 April 2016).
- Wijffels, R.H.; Kruse, O.; Hellingwerf, K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013, 24, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Ivanova, N.N.; Jose, N.; Garcia-Pichel, F.; Kyrpides, N.C.; Gugger, M.; Northen, T.R. Functional genomics of novel secondary metabolites from diverse cyanobacteria using untargeted metabolomics. Mar. Drugs 2013, 11, 3617–3631. [Google Scholar] [CrossRef] [PubMed]
- Ufarté, L.; Potocki-Veronese, G.; Laville, É. Discovery of new protein families and functions: New challenges in functional metagenomics for biotechnologies and microbial ecology. Front. Microbiol. 2015, 6, 563. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Hathaway, B.J.; Sudek, S.; Haygood, M.G.; Rosovitz, M.J.; Ravel, J.; Schmidt, E.W. Natural combinatorial peptide libraries in cyanobacterial symbionts of marine ascidians. Nat. Chem. Biol. 2006, 2, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, T.; Tatsuke, T.; Tsuruno, K.; Hirokawa, Y.; Atsumi, S.; Liao, J.C.; Hanai, T. Engineering a synthetic pathway in cyanobacteria for isopropanol production directly from carbon dioxide and light. Metab. Eng. 2013, 20, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Llerena, M.E.; Burja, A.M.; Wright, P.C. Genetic analysis of polyketide synthase and peptide synthetase genes in cyanobacteria as a mining tool for secondary metabolites. J. Ind. Microbiol. Biot. 2007, 34, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Gu, L.; Sorrels, C.M.; Sherman, D.H.; Gerwick, W.H. New tricks from ancient algae: Natural products biosynthesis in marine cyanobacteria. Curr. Opin. Chem. Biol. 2009, 13, 216–223. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny Microbes with a Big Impact: The Role of Cyanobacteria and Their Metabolites in Shaping Our Future. Mar. Drugs 2016, 14, 97. https://doi.org/10.3390/md14050097
Mazard S, Penesyan A, Ostrowski M, Paulsen IT, Egan S. Tiny Microbes with a Big Impact: The Role of Cyanobacteria and Their Metabolites in Shaping Our Future. Marine Drugs. 2016; 14(5):97. https://doi.org/10.3390/md14050097
Chicago/Turabian StyleMazard, Sophie, Anahit Penesyan, Martin Ostrowski, Ian T. Paulsen, and Suhelen Egan. 2016. "Tiny Microbes with a Big Impact: The Role of Cyanobacteria and Their Metabolites in Shaping Our Future" Marine Drugs 14, no. 5: 97. https://doi.org/10.3390/md14050097
APA StyleMazard, S., Penesyan, A., Ostrowski, M., Paulsen, I. T., & Egan, S. (2016). Tiny Microbes with a Big Impact: The Role of Cyanobacteria and Their Metabolites in Shaping Our Future. Marine Drugs, 14(5), 97. https://doi.org/10.3390/md14050097






