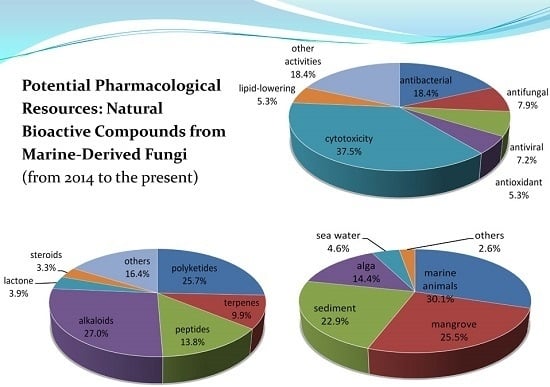
Potential Pharmacological Resources: Natural Bioactive Compounds from Marine-Derived Fungi
Abstract
:1. Introduction
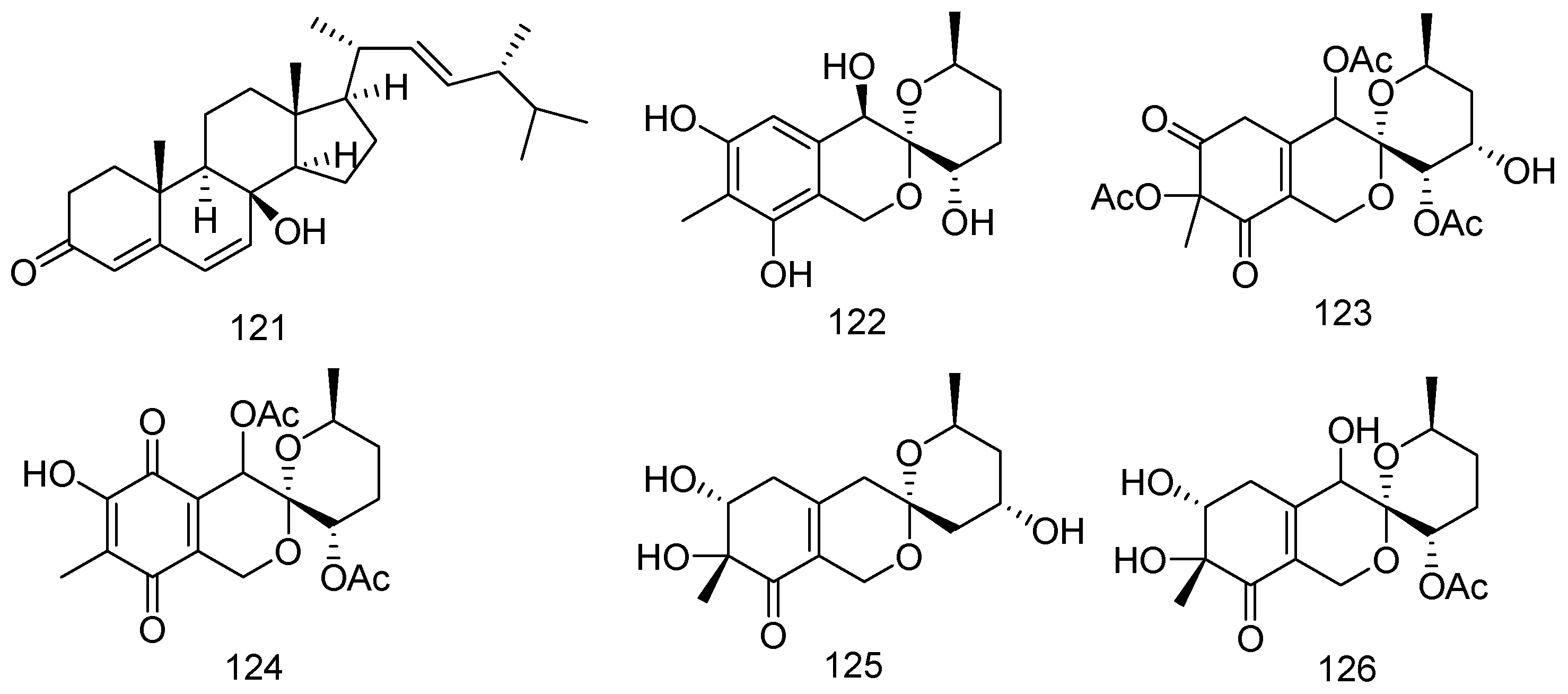
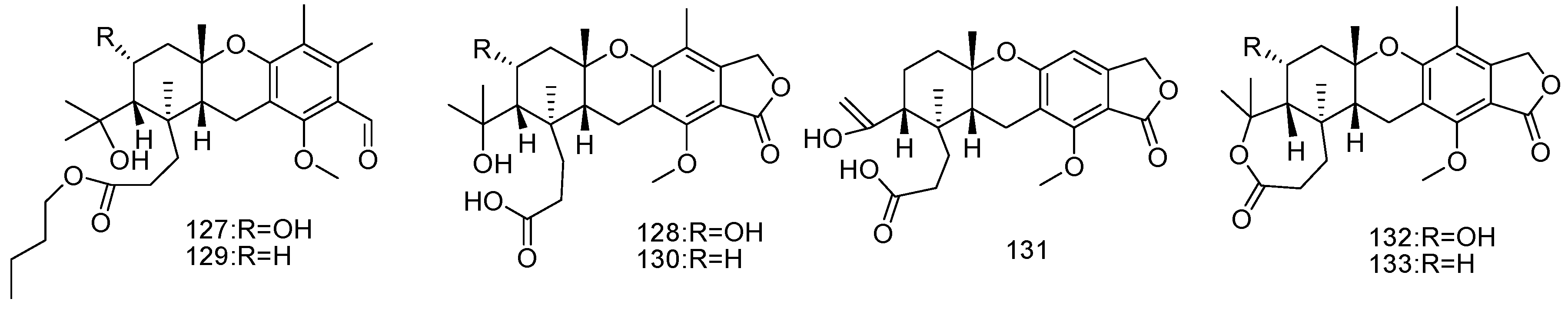
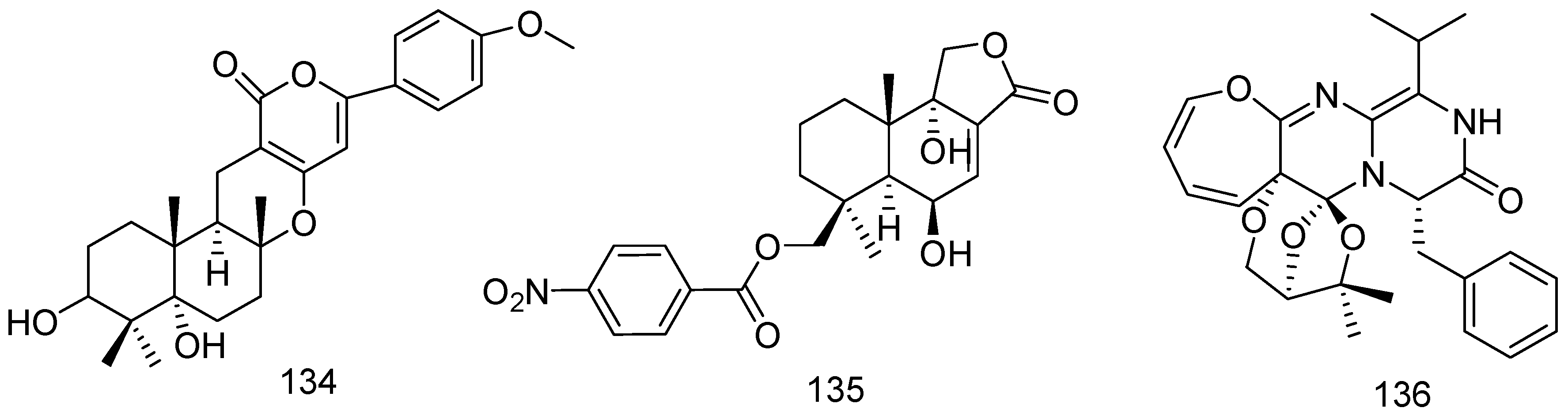
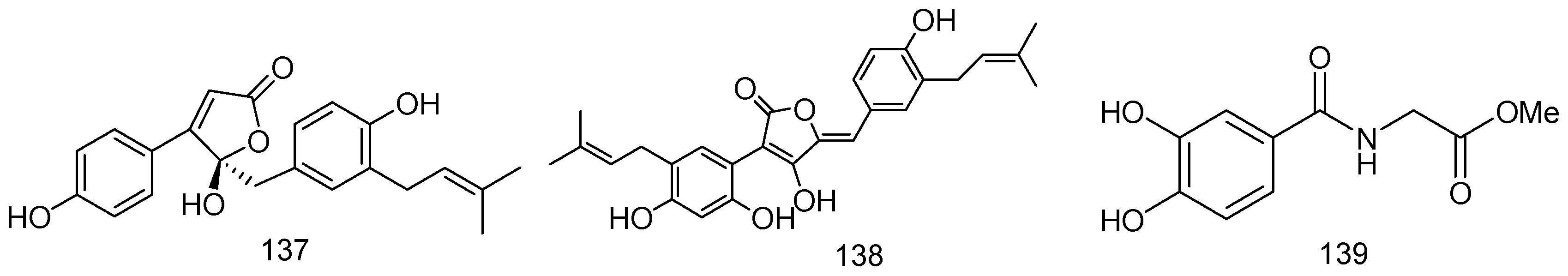
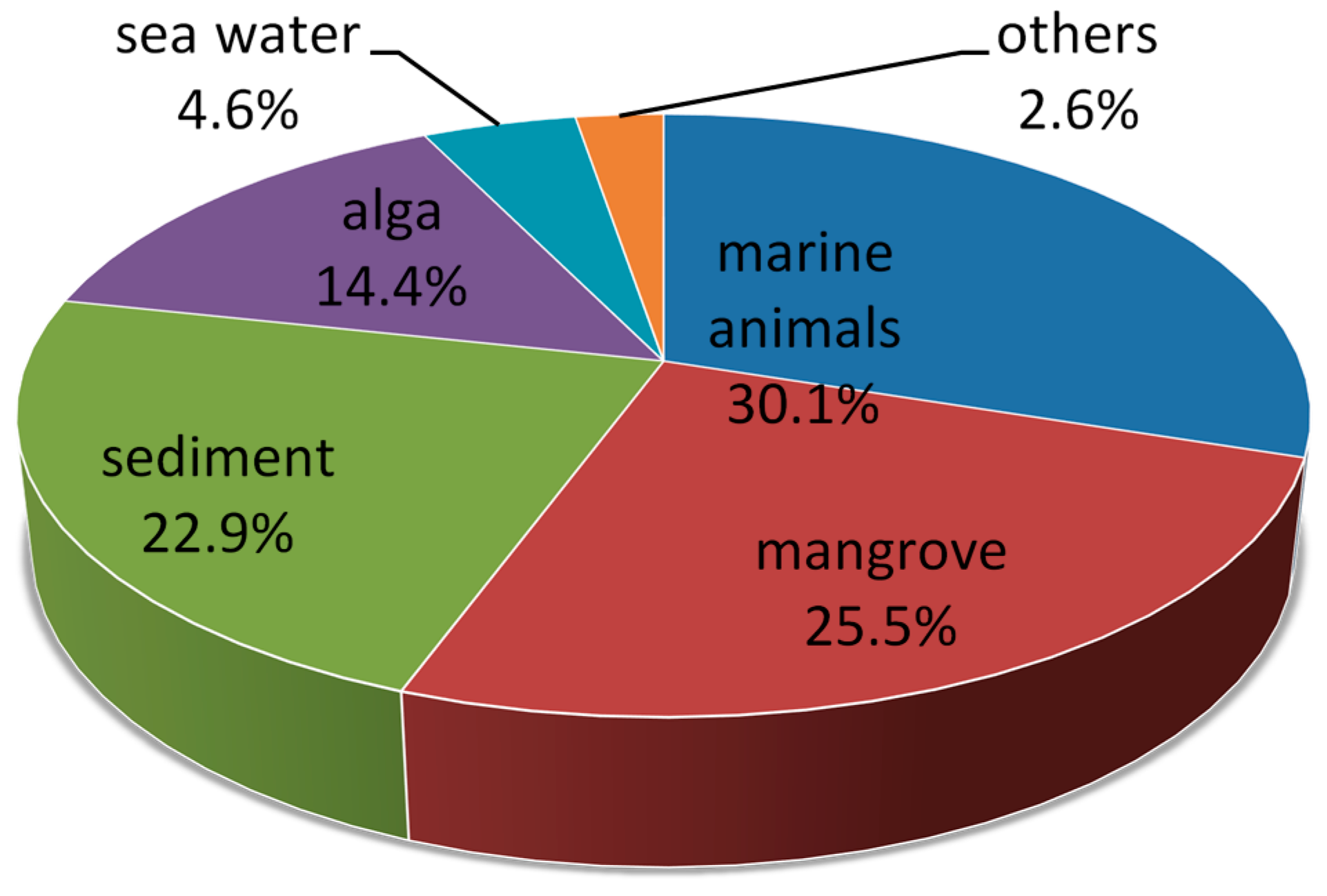
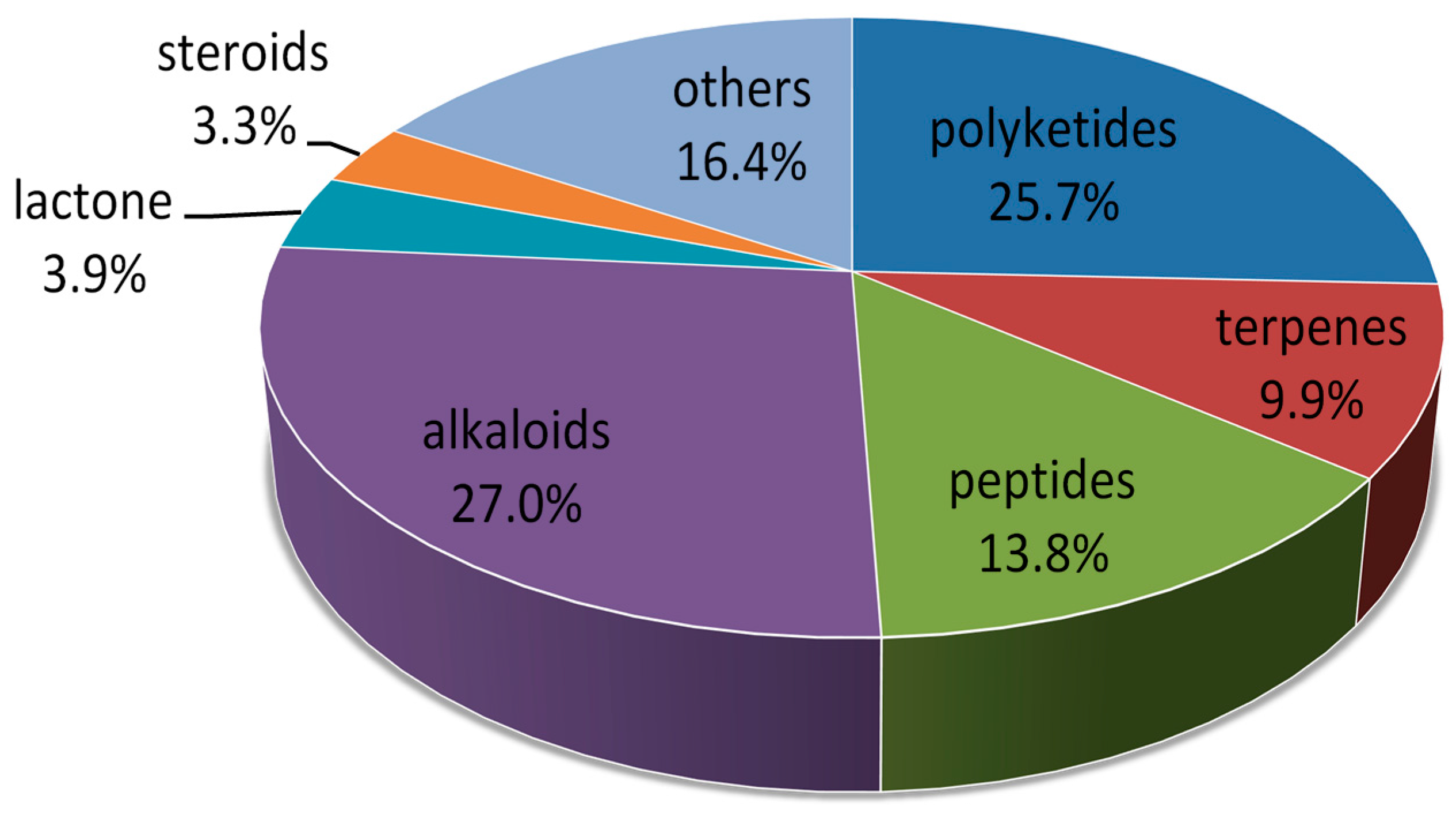
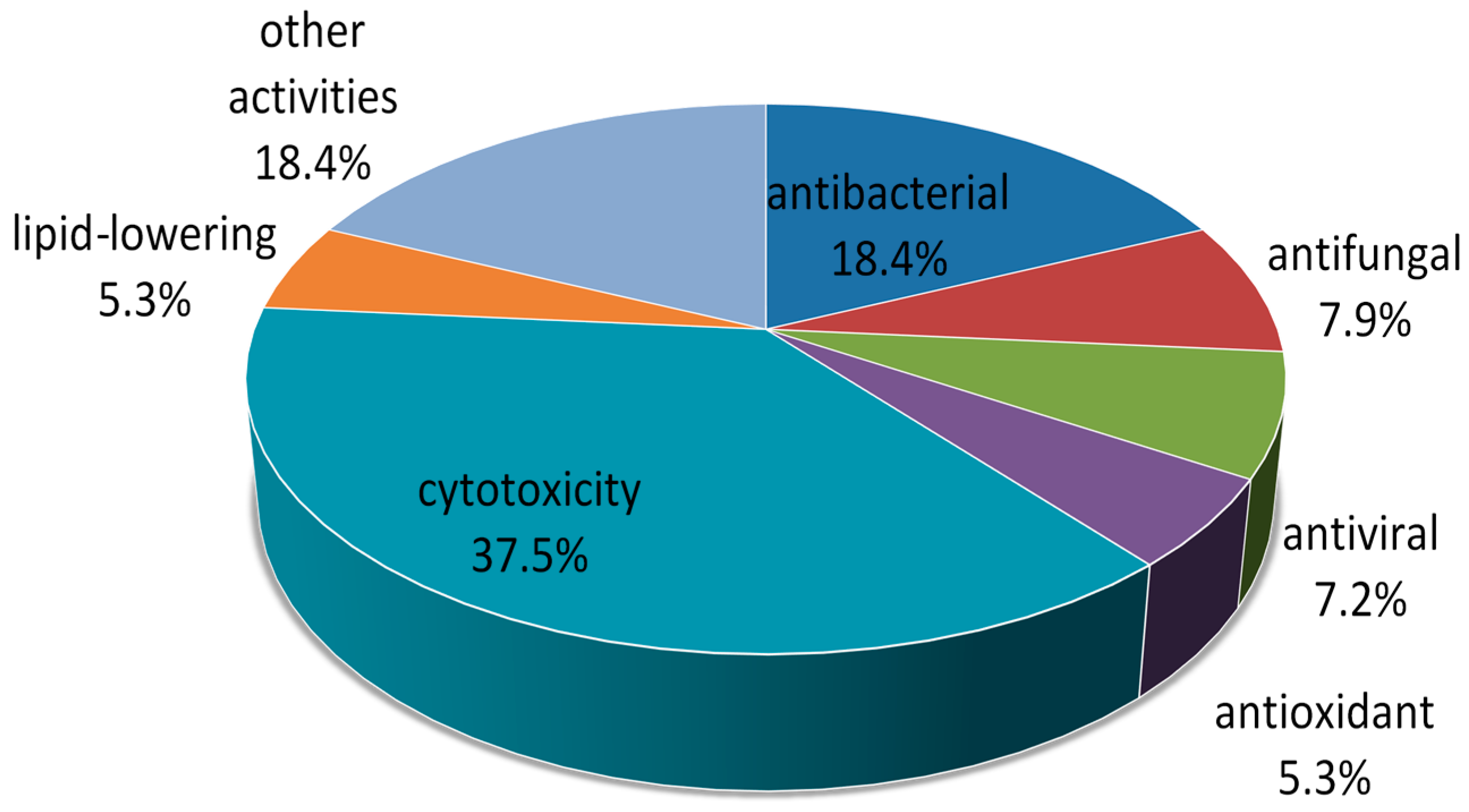
2. Metabolites from Marine-Derived Fungi
2.1. Marine Animals
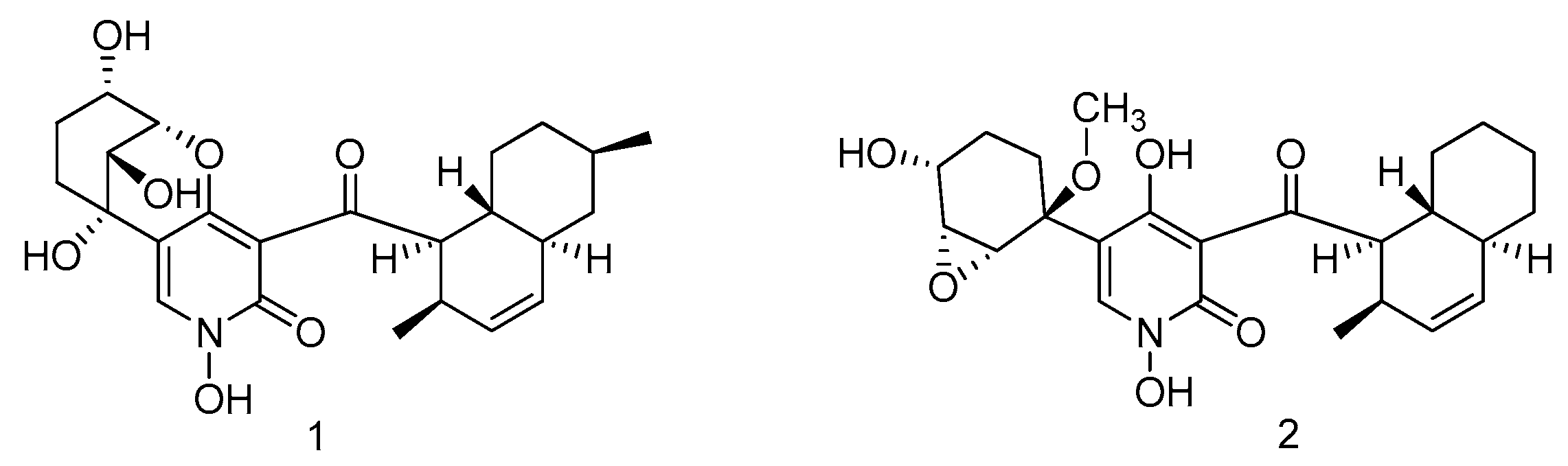
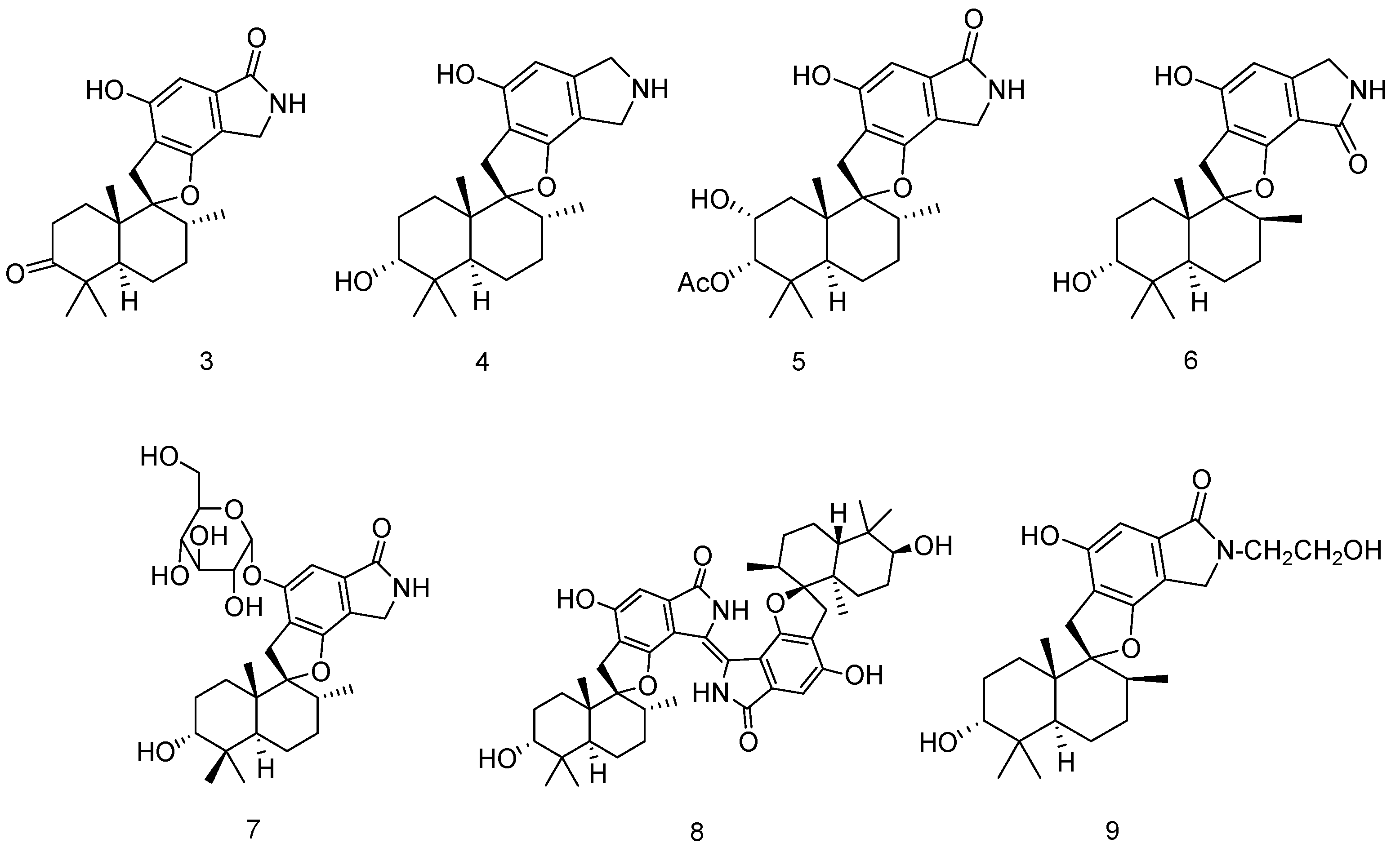
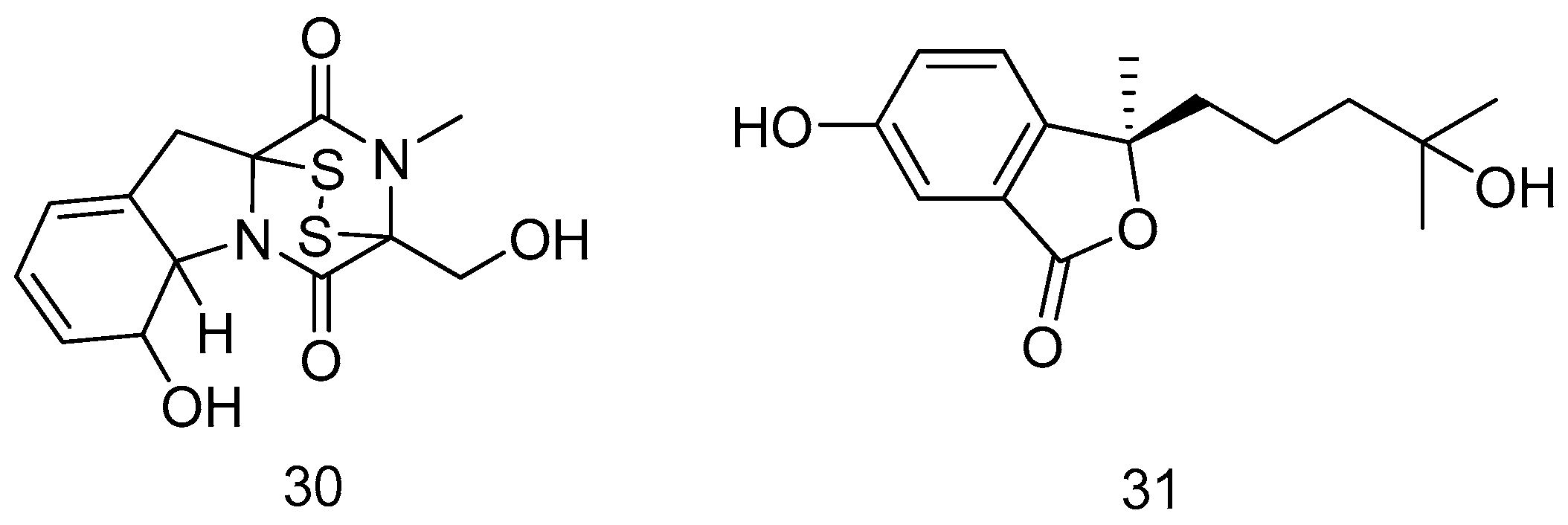
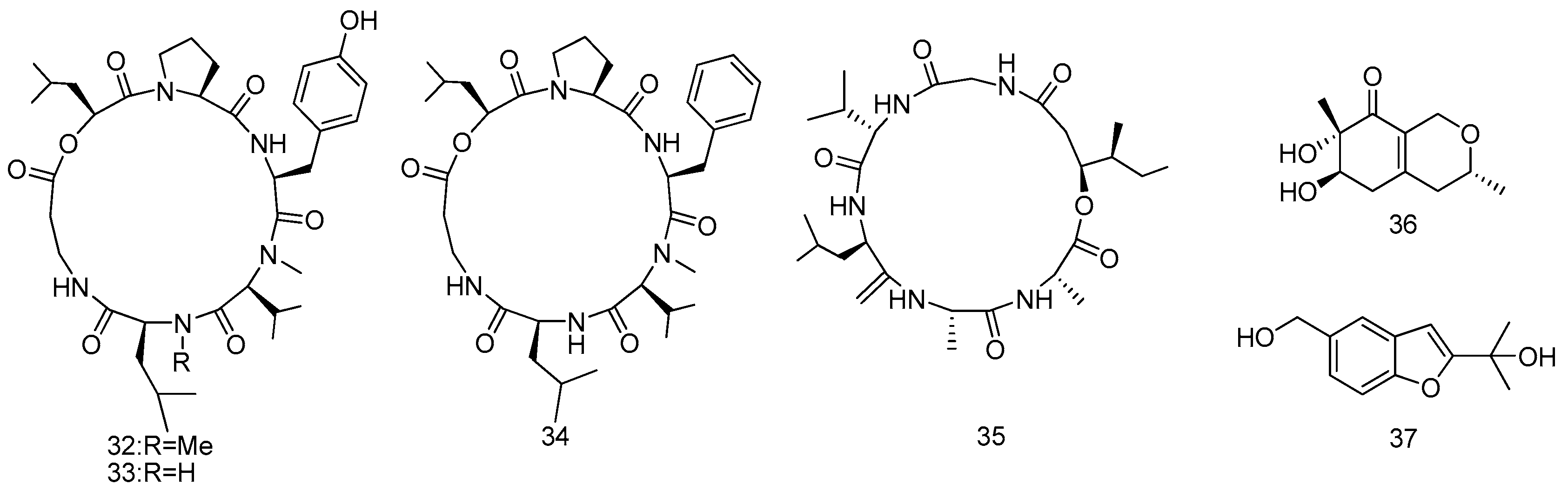
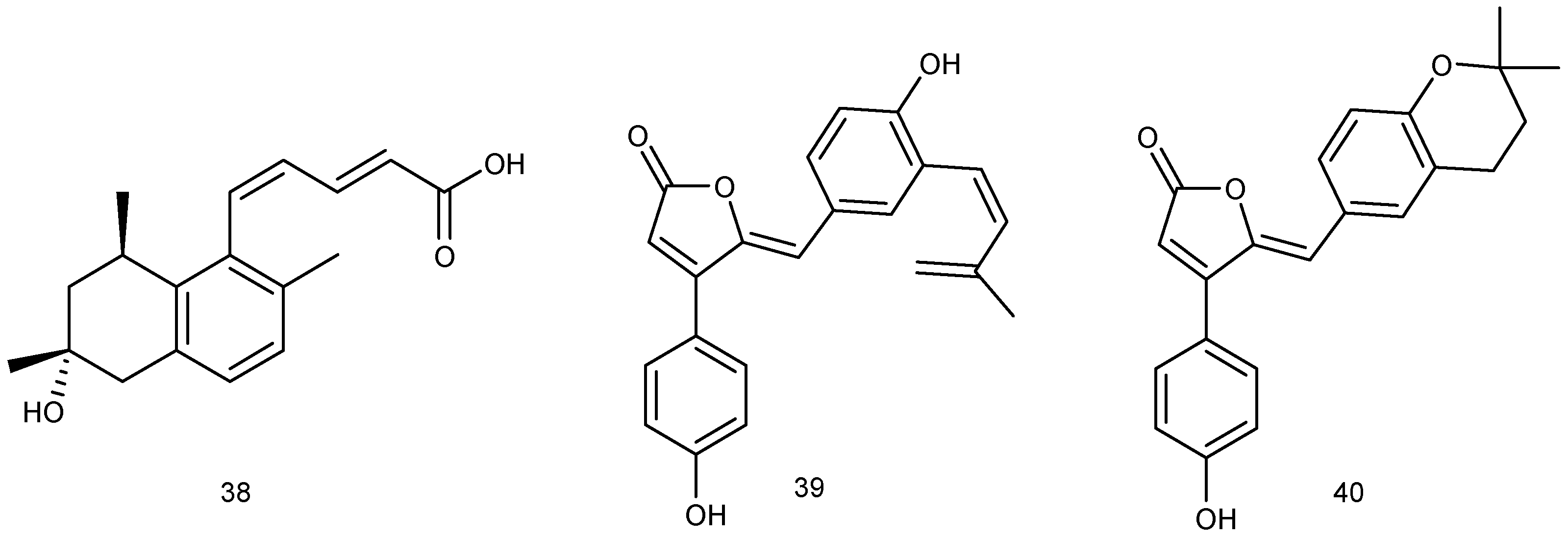
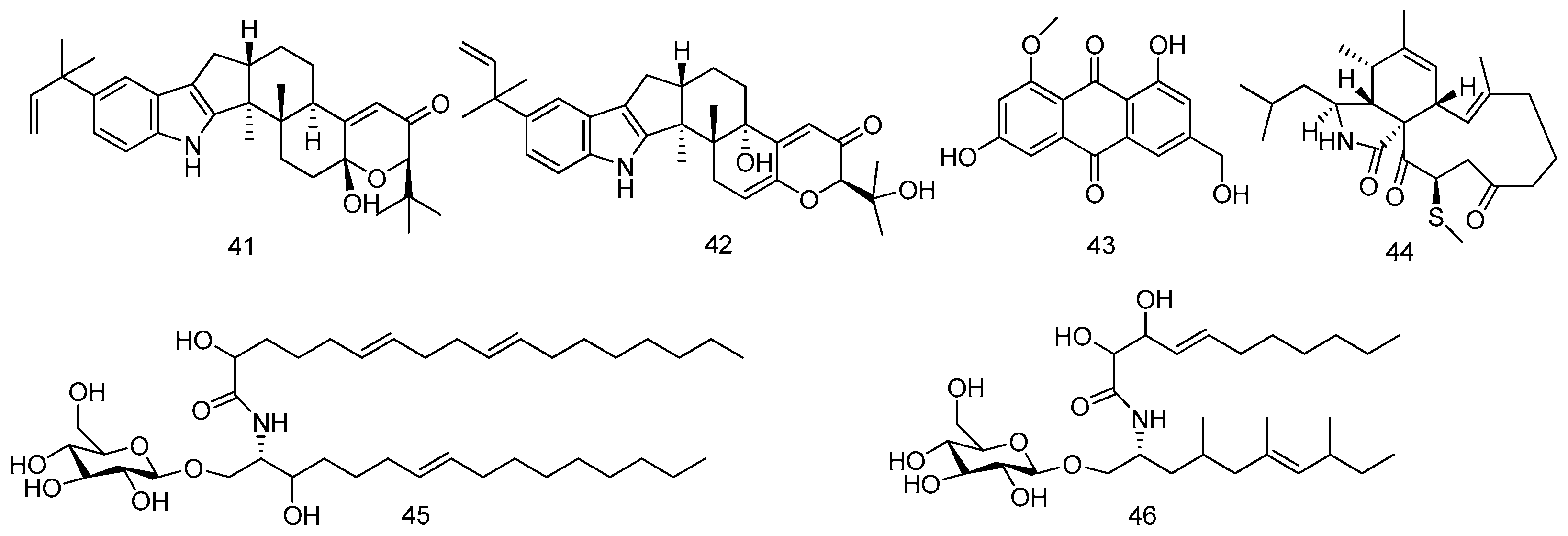
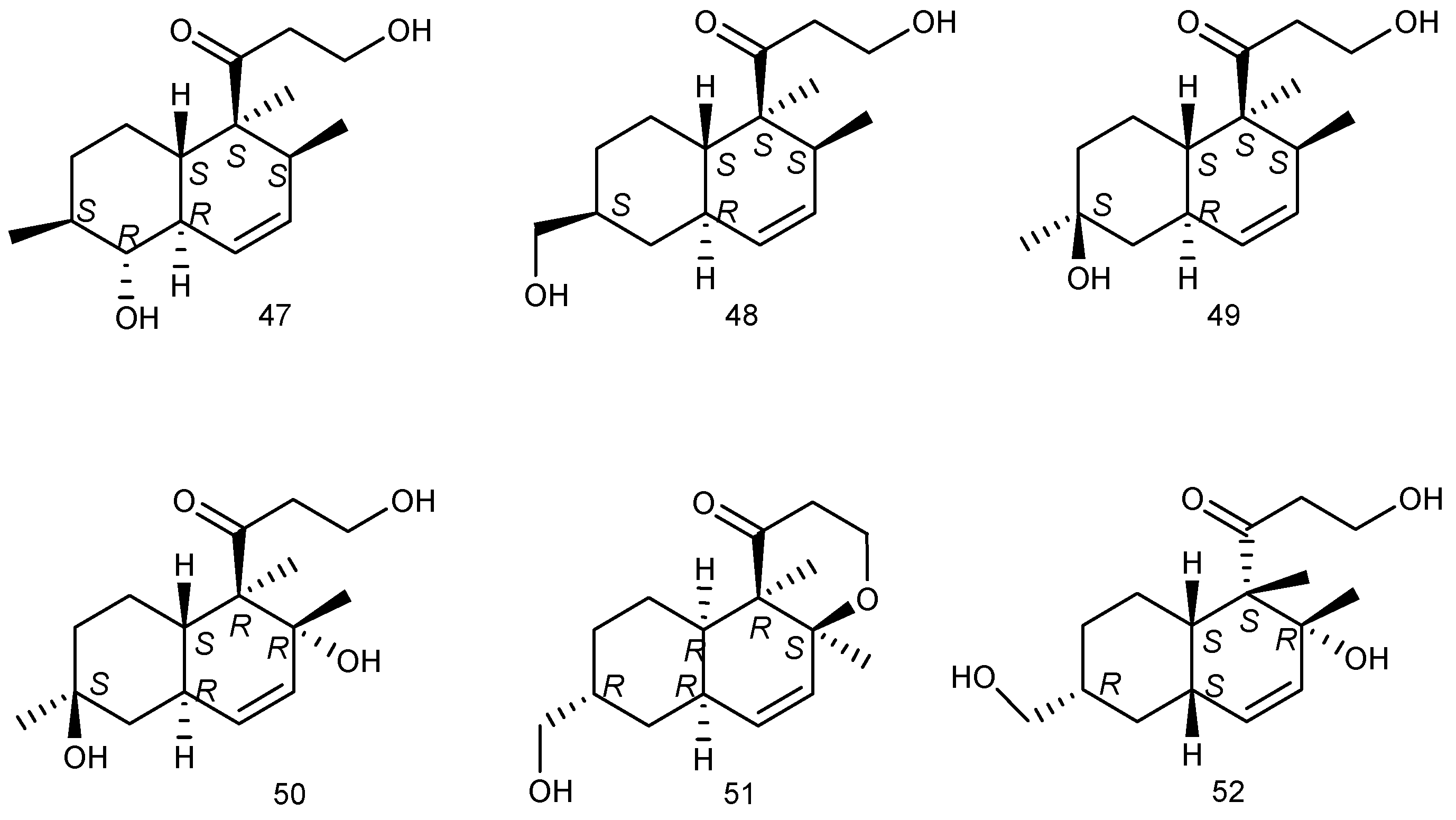
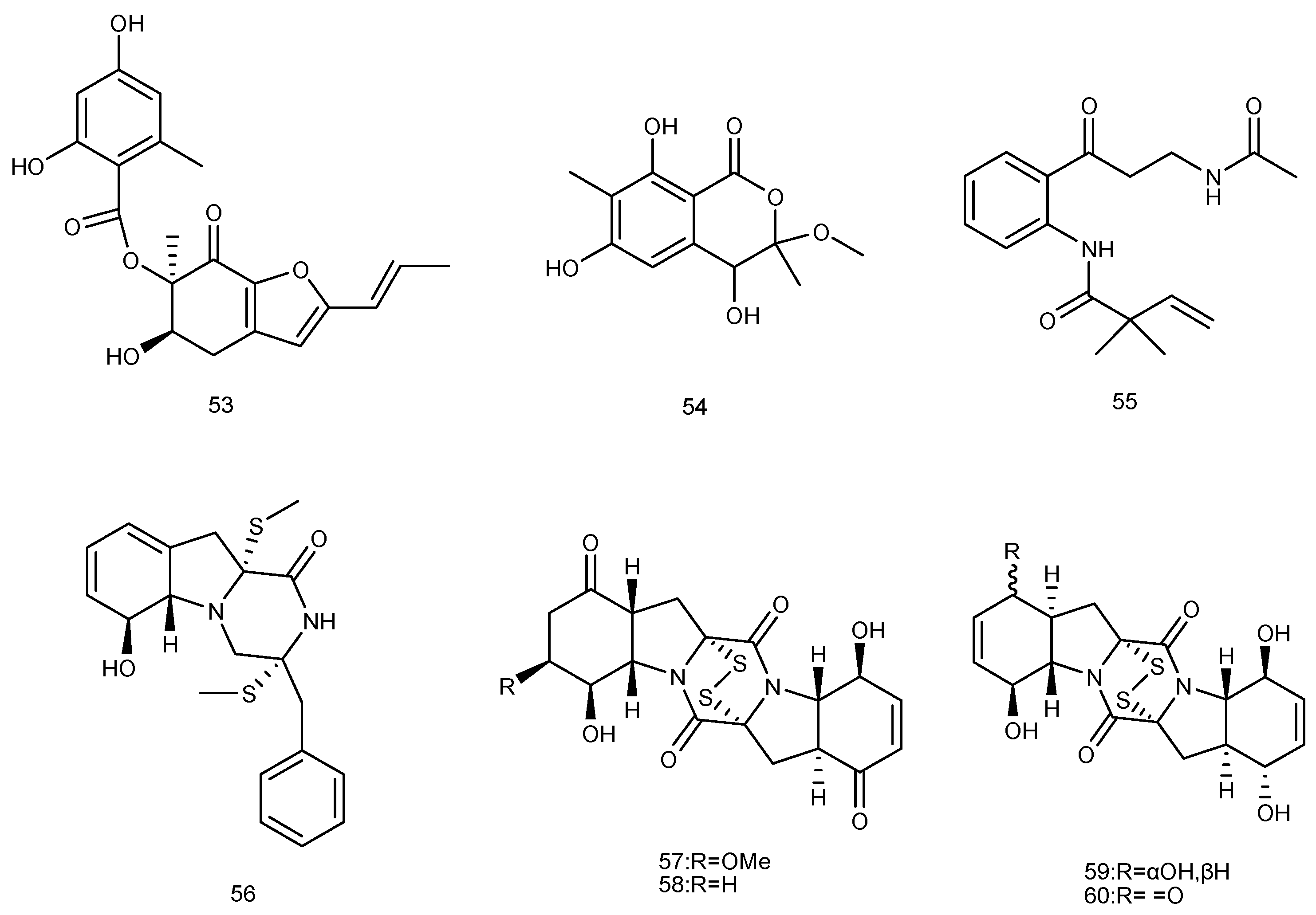
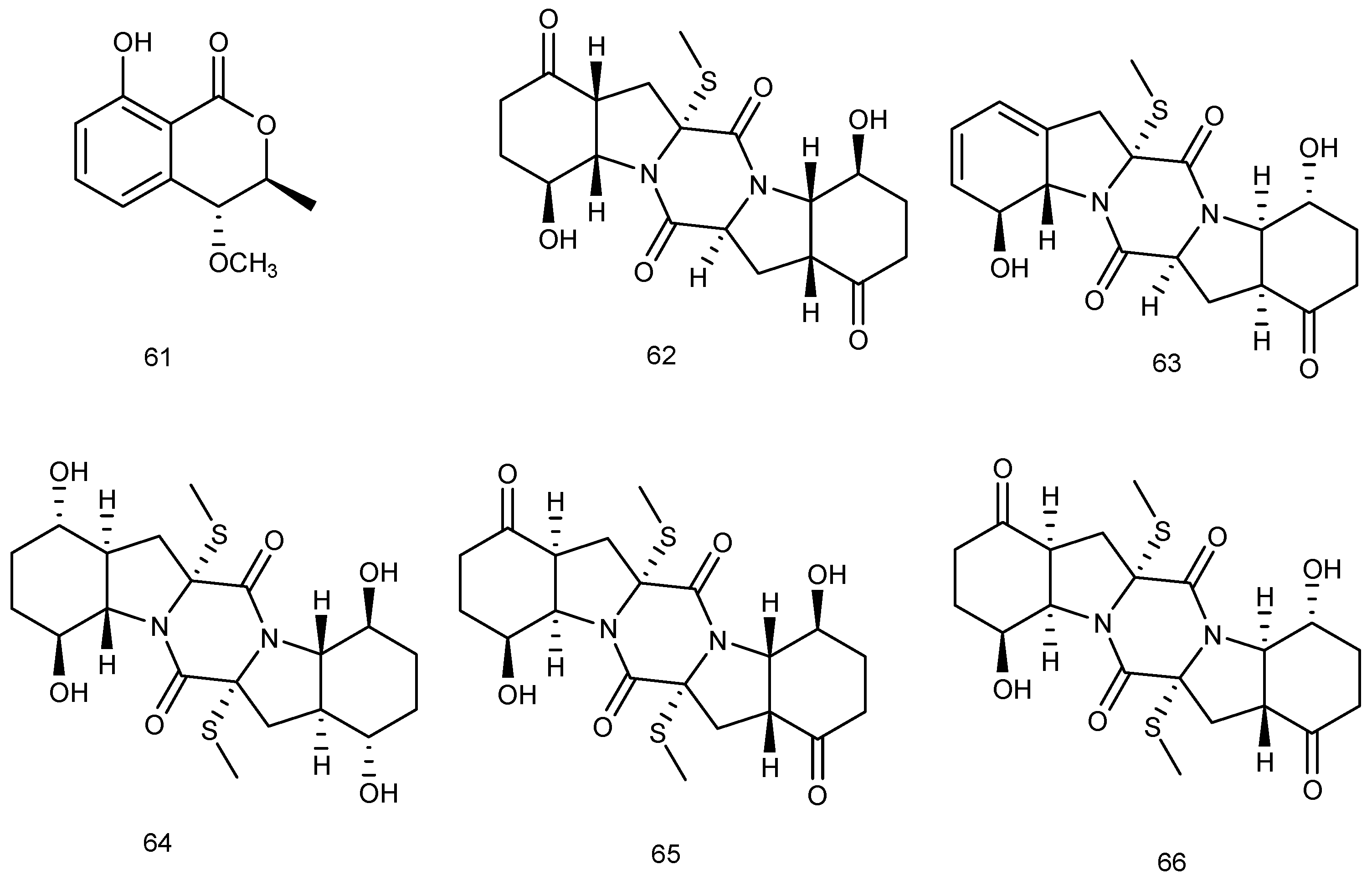
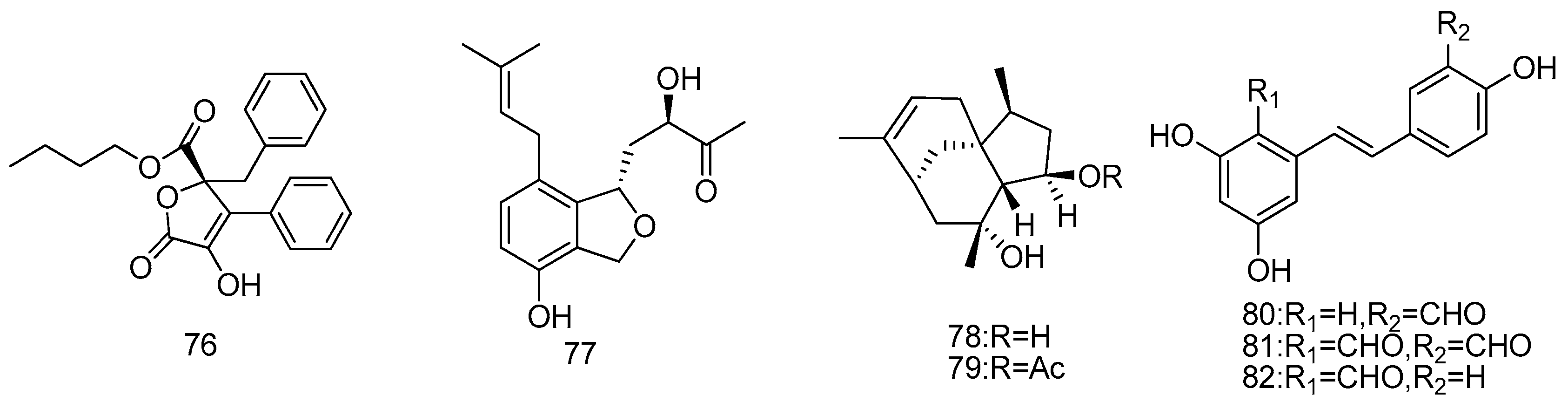
2.1.1. Sponge
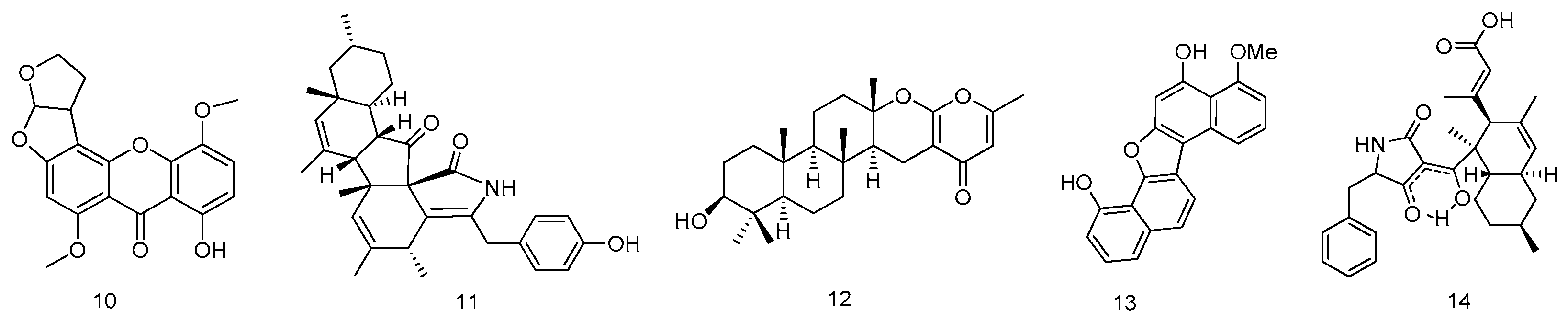
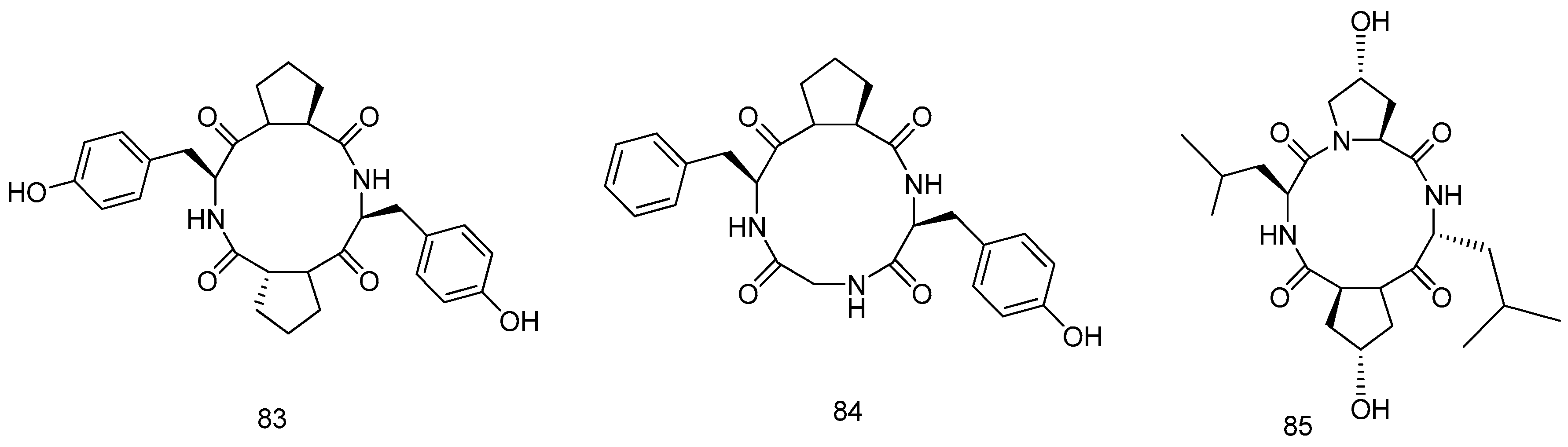
2.1.2. Coral
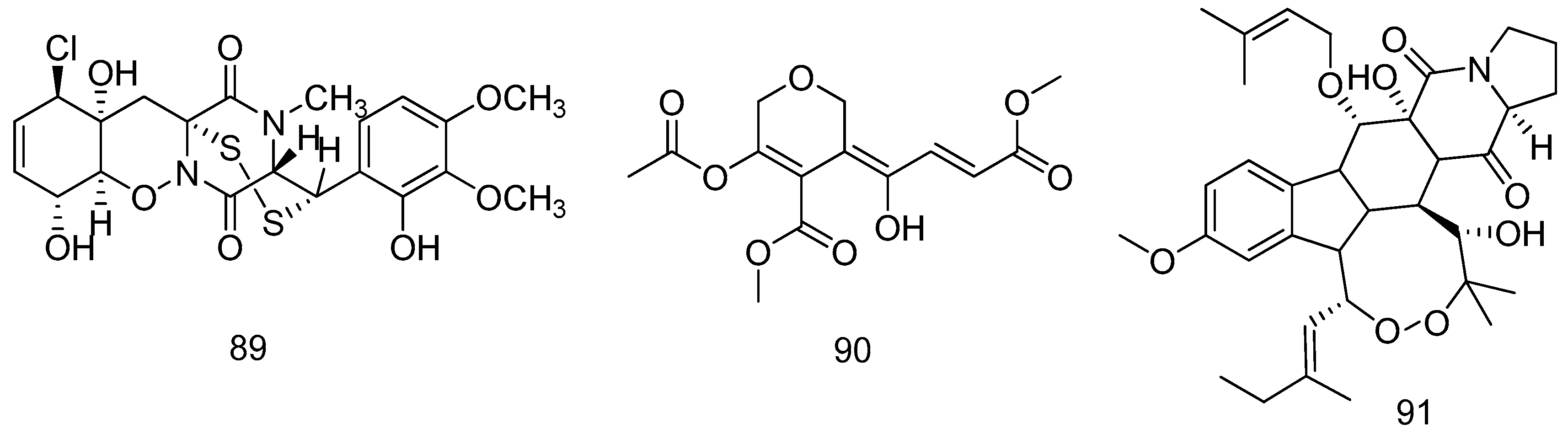
2.1.3. Starfish
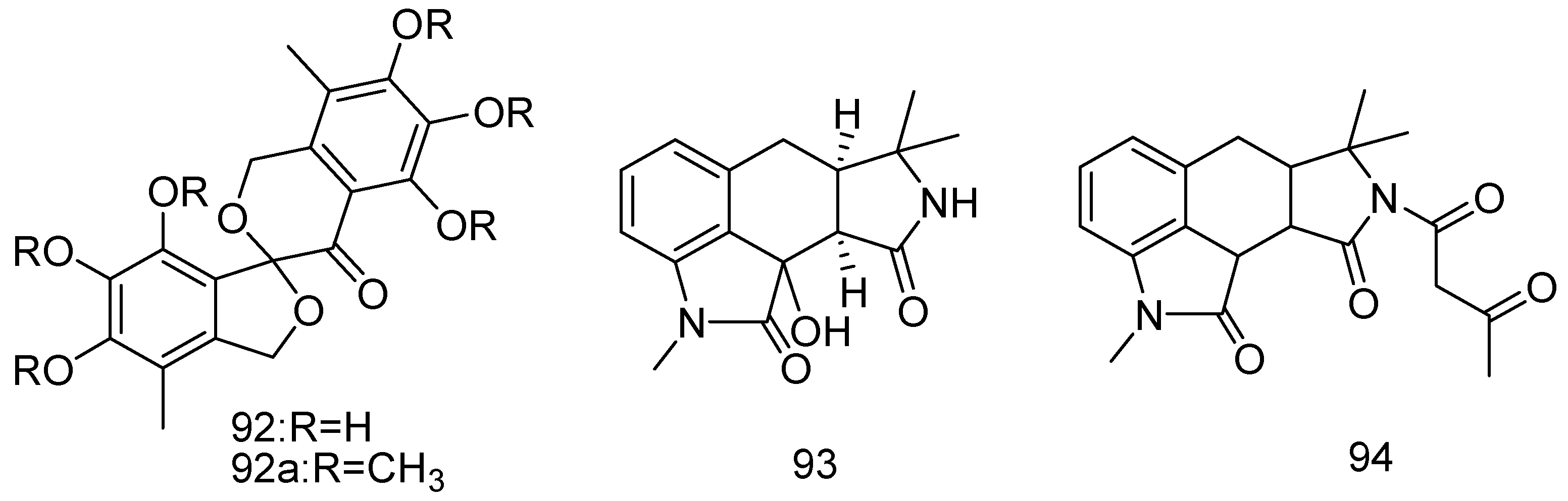
2.1.4. Bryozoan
2.1.5. Sea Urchin
2.1.6. Fish
2.1.7. Prawn
2.1.8. Others
2.2. Mangrove
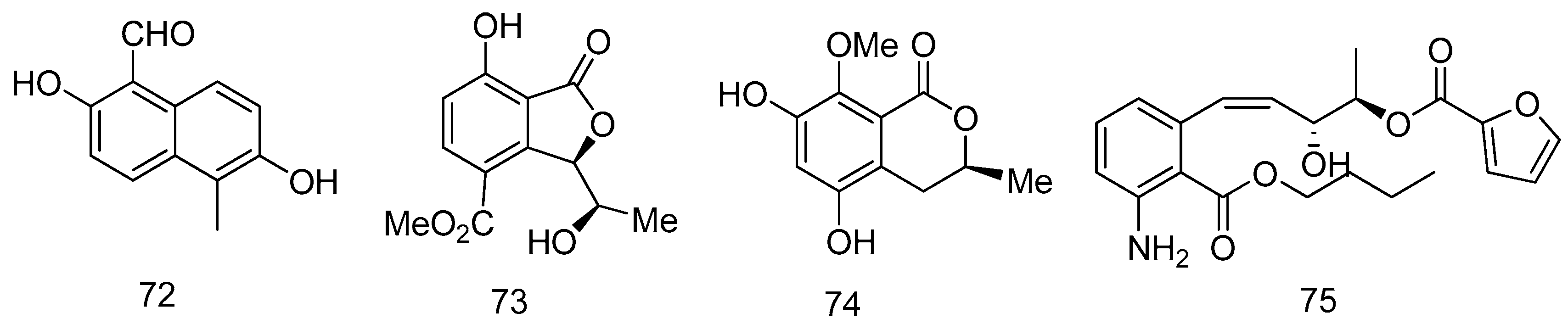
2.3. Sediment
2.4. Alga
2.5. Sea Water
2.6. Others
3. Future Perspectives and Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Miller, J.M.T. Development of the semi-synthetic penicillins and cephalosporins. Int. J. Antimicrob. Agents 2008, 31, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeyer, J.; Kohlmeyer, E. Marine Mycology: The Higher Fungi; Academic Press: New York, NY, USA, 1979; pp. 704–705. [Google Scholar]
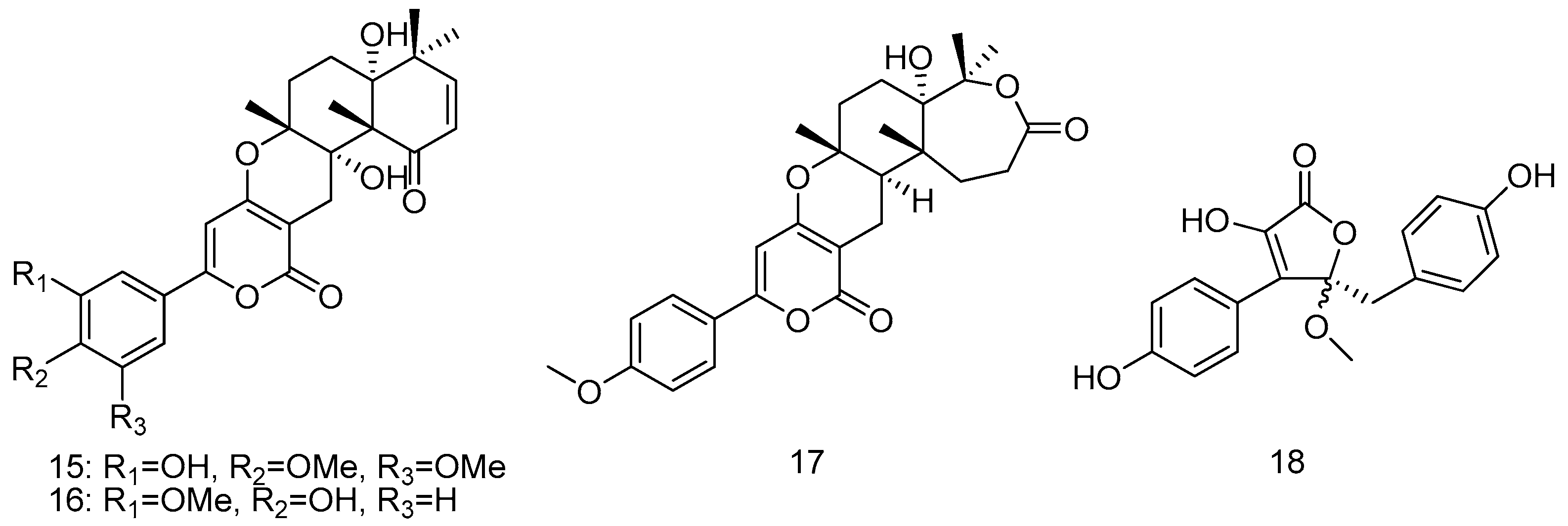
- Wang, J.; Wei, X.; Qin, X.; Lin, X.; Zhou, X.; Liao, S.; Yang, B.; Liu, J.; Tu, C.; Liu, H. Arthpyrones A–C, pyridone alkaloids from a sponge-derived fungus Arthrinium arundinis ZSDS1-F3. Org. Lett. 2015, 17, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, C.; Liu, D.; Proksch, P.; Guo, P.; Lin, W.H. Chartarlactams A–P, phenylspirodrimanes from the sponge-associated fungus Stachybotrys chartarum with antihyperlipidemic activities. J. Nat. Prod. 2014, 77, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Ren, B.; Chen, C.; Yu, K.; Liu, X.; Zhang, Y.; Yang, N.; He, H.; Liu, X.; Dai, H.; Zhang, L. Three new sterigmatocystin analogues from marine-derived fungus Aspergillus versicolor MF359. Appl. Microbiol. Biotechnol. 2014, 98, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. An antibacterial cytochalasin derivative from the marine-derived fungus Diaporthaceae sp. PSU-SP2/4. Phytochem. Lett. 2014, 10, 5–9. [Google Scholar] [CrossRef]
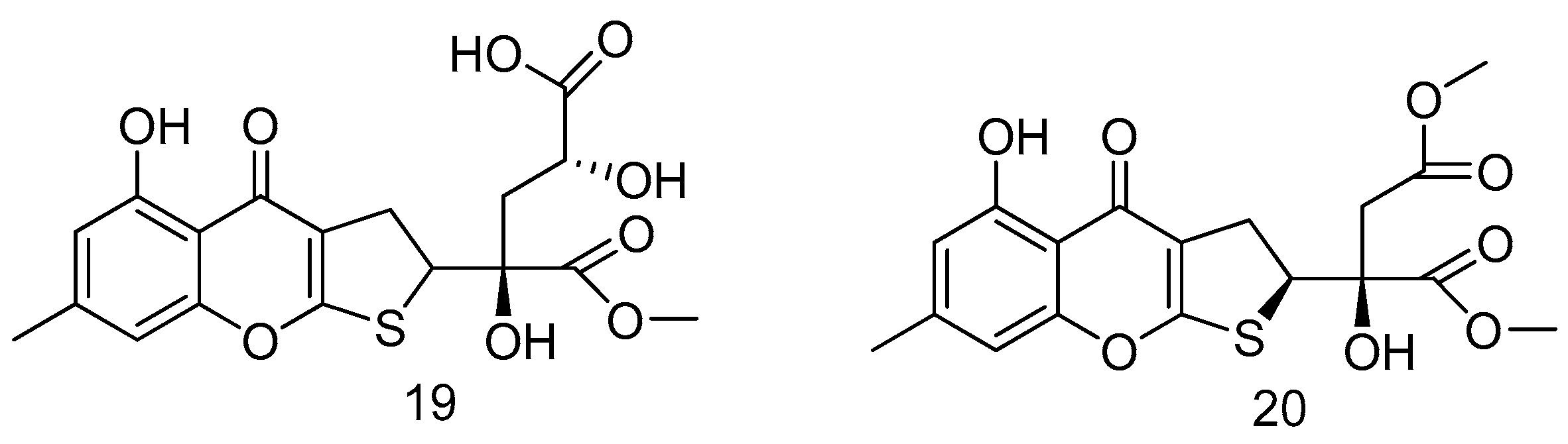
- Prompanya, C.; Dethoup, T.; Bessa, L.J.; Pinto, M.M.M.; Gales, L.; Costa, P.M.; Silva, A.M.S.; Kijjoa, A. New isocoumarin derivatives and meroterpenoids from the marine sponge-associated fungus Aspergillus similanensis sp. Nov. KUFA 0013. Mar. Drugs 2014, 12, 5160–5173. [Google Scholar] [CrossRef] [PubMed]
- Kotoku, N.; Higashimoto, K.; Kurioka, M.; Arai, M.; Fukuda, A.; Sumii, Y.; Sowa, Y.; Sakai, T.; Kobayashi, M. Xylarianaphthol-1, a novel dinaphthofuran derivative, activates p21 promoter in a p53-independent manner. Bioorg. Med. Chem. Lett. 2014, 24, 3389–3391. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wiese, J.; Labes, A.; Kramer, A.; Schmaljohann, R.; Imhoff, J.F. Lindgomycin, an unusual antibiotic polyketide from a marine fungus of the Lindgomycetaceae. Mar. Drugs 2015, 13, 4617–4632. [Google Scholar] [CrossRef] [PubMed]
- Nong, X.; Wang, Y.; Zhang, X.; Zhou, M.; Xu, X.; Qi, S. Territrem and butyrolactone derivatives from a marine-derived fungus Aspergillus terreus. Mar. Drugs 2014, 12, 6113–6124. [Google Scholar] [CrossRef] [PubMed]
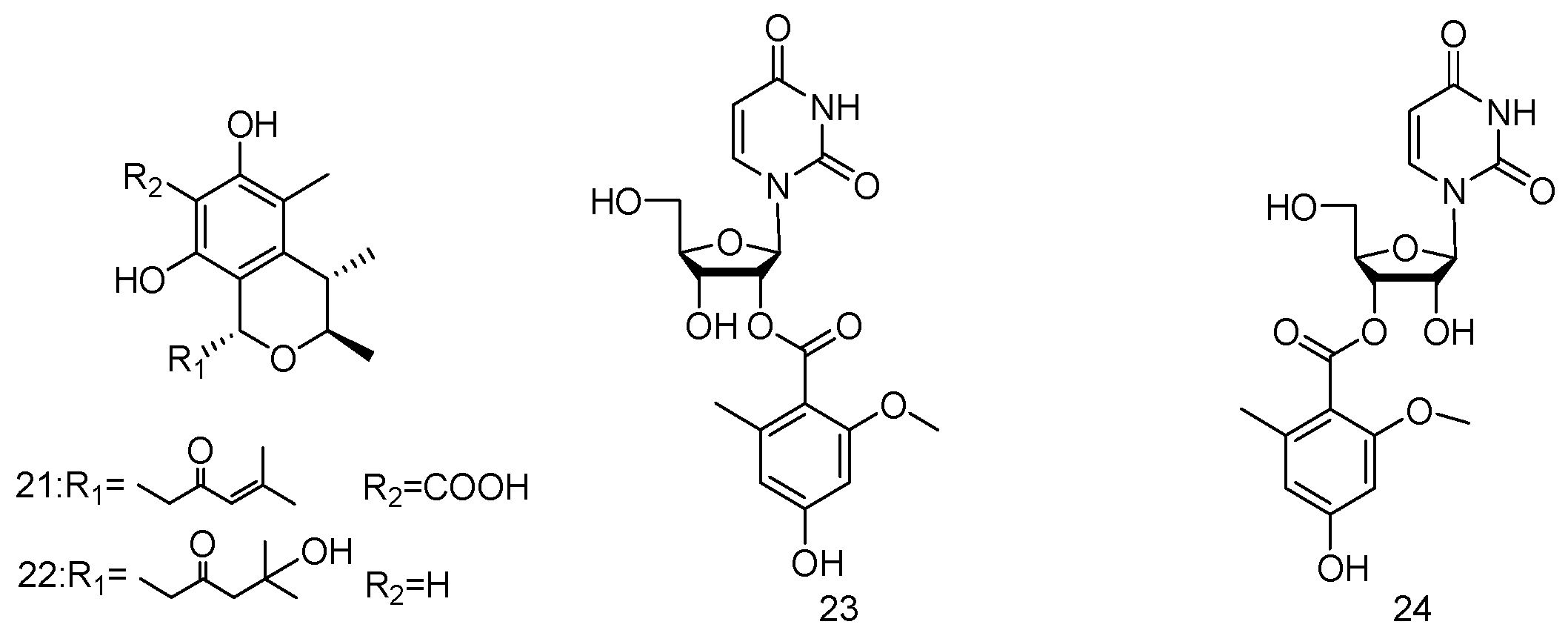
- Bao, J.; Luo, J.; Qin, X.; Xu, X.; Zhang, X.; Tu, Z.; Qi, S. Dihydrothiophene-condensed chromones from a marine-derived fungus Penicillium oxalicum and their structure-bioactivity relationship. Bioorg. Med. Chem. Lett. 2014, 24, 2433–2436. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Bao, J.; Zhang, X.; Xu, X.; Nong, X.; Qi, S. Alkaloids and citrinins from marine-derived fungus Nigrospora oryzae SCSGAF 0111. Tetrahedron Lett. 2014, 55, 2749–2753. [Google Scholar] [CrossRef]
- Chen, M.; Fu, X.; Kong, C.; Wang, C. Nucleoside derivatives from the marine-derived fungus Aspergillus versicolor. Nat. Prod. Res. 2014, 28, 895–900. [Google Scholar] [CrossRef] [PubMed]
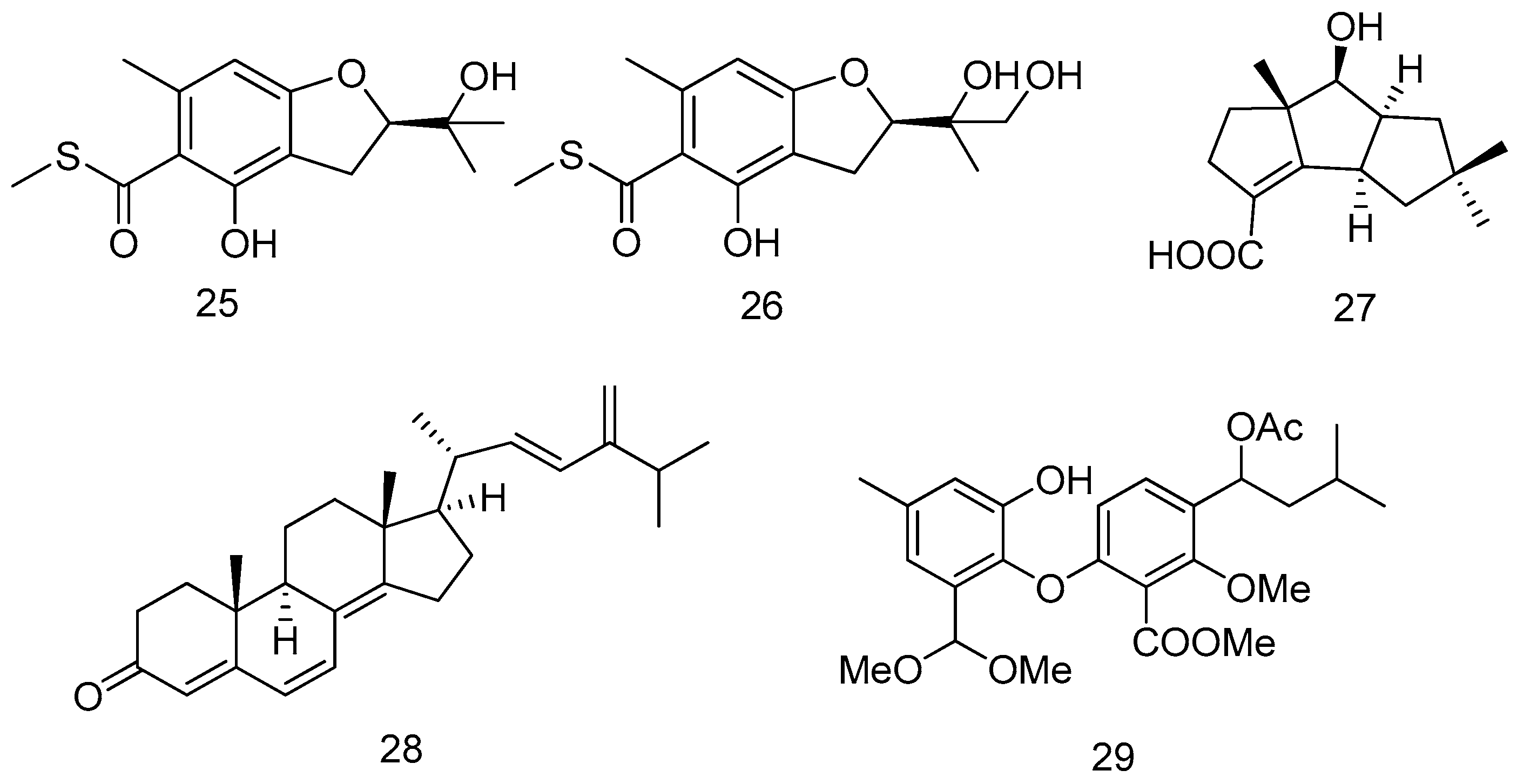
- Liu, Z.; Xia, G.; Chen, S.; Liu, Y.; Li, H.; She, Z. Eurothiocin A and B, sulfur-containing benzofurans from a soft coral-derived fungus Eurotium rubrum SH-823. Mar. Drugs 2014, 12, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, W.; Liang, W.; Huang, J.; Mo, Y.; Ding, Y.; Lam, C.; Qian, X.; Zhu, X.; Lan, W. Induced marine fungus Chondrostereum sp. as a means of producing new sesquiterpenoids chondrosterins I and J by using glycerol as the carbon source. Mar. Drugs 2014, 12, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, K.; Liu, M.; She, Z.; Wang, C. Bioactive steroid derivatives and butyrolactone derivatives from a gorgonian-derived Aspergillus sp. fungus. Chem. Biodivers. 2015, 12, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Han, L.; Shao, C.; She, Z.; Wang, C. Bioactive diphenyl ether derivatives from a gorgonian-derived fungus Talaromyces sp. Chem. Biodivers. 2015, 12, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Le, X.; Li, H.; Yang, X.; Chen, J.; Xu, J.; Liu, H.; Wang, L.; Wang, K.; Hu, K.; et al. Exploring the chemodiversity and biological activities of the secondary metabolites from the marine fungus Neosartorya Pseudofischeri. Mar. Drugs 2014, 12, 5657–5676. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Liu, W.; Liang, W.; Xu, Z.; Le, X.; Xu, J.; Lam, C.K.; Yang, D.; Li, H.; Wang, L. Pseudaboydins A and B: Novel isobenzofuranone derivatives from marine fungus Pseudallescheria boydii associated with starfish Acanthaster planci. Mar. Drugs 2014, 12, 4188–4199. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhang, P.; Li, X.; Li, C.; Cui, C.; Wang, B. Cyclohexadepsipeptides of the isaridin class from the marine-derived fungus Beauveria felina EN-135. J. Nat. Prod. 2014, 77, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Li, X.; Zhang, P.; Li, C.; Wang, B. Cyclodepsipeptides and other o-containing heterocyclic metabolites from Beauveria felina EN-135, a marine-derived entomopathogenic fungus. Mar. Drugs 2014, 12, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Ngan, T.T.N.; Ko, W.; Kim, D.C.; Yoon, S.C.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Tanzawaic acid derivatives from a marine isolate of Penicillium sp. (SF-6013) with anti-inflammatory and PTP1B inhibitory activities. Bioorg. Med. Chem. Lett. 2014, 24, 5787–5791. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, Z.; Liu, P.; Wang, Y.; Xin, Z.; Zhu, W. New rubrolides from the marine-derived fungus Aspergillus Terreus OUCMDZ-1925. J. Antibiot. 2014, 67, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, Y.; Guo, L.; Wang, Y.; Liu, P.; Zhu, W. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs 2014, 12, 3970–3981. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kang, M.; Li, Y.; Kim, E.A.; Kang, S.; Jeon, Y.J. Anti-inflammatory activity of questinol isolated from marine-derived fungus Eurotium amstelodami in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Microbiol. Biotechnol. 2014, 24, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, S.; Ding, W.; Wang, P.; Yang, X.; Xu, J. Methylthio-aspochalasins from a marine-derived fungus Aspergillus sp. Mar. Drugs 2014, 12, 5124–5131. [Google Scholar] [CrossRef] [PubMed]
- Murshid, S.S.A.; Badr, J.M.; Youssef, D.T.A. Penicillosides A and B: New cerebrosides from the marine-derived fungus Penicillium species. Rev. Bras. Farmacogn. 2016, 26, 29–33. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Huang, M.; Liu, L.; Wang, J.; Lin, Y. Six new polyketide decalin compounds from mangrove endophytic fungus Penicillium aurantiogriseum 328#. Mar. Drugs 2015, 13, 6306–6318. [Google Scholar] [PubMed]
- Liu, Y.; Chen, S.; Liu, Z.; Lu, Y.; Xia, G.; Liu, H.; He, L.; She, Z. Bioactive metabolites from mangrove endophytic fungus Aspergillus sp. 16–5B. Mar. Drugs 2015, 13, 3091–3102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, L.; Mandi, A.; Kurtan, T.; Li, X.; Liu, Y.; Li, X.; Li, C.; Wang, B. Brocaeloids A–C, 4-oxoquinoline and indole alkaloids with C-2 reversed prenylation from the mangrove-derived endophytic fungus Penicillium brocae. Eur. J. Org. Chem. 2014, 19, 4029–4036. [Google Scholar] [CrossRef]
- Kong, F.; Wang, Y.; Liu, P.; Dong, T.; Zhu, W. Thiodiketopiperazines from the marine-derived fungus Phoma sp. OUCMDZ-1847. J. Nat. Prod. 2014, 77, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, X.; Lu, C.; Huang, C.; Wang, B. Brocazines A–F, cytotoxic bisthiodiketopiperazine derivatives from Penicillium brocae MA-231, an endo-phytic fungus derived from the marine mangrove plant Avicennia marina. J. Nat. Prod. 2014, 77, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qiu, S.; She, Z.; Lin, Y. A new isochroman derivative from the marine fungus Phomopsis sp. (No.Gx-4). Chem. Nat. Compd. 2014, 50, 424–426. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, P.; Li, X.; Wang, B. Penicibrocazines A–E, five new sulfide diketopiperazines from the marine-derived endophytic fungus Penicillium brocae. Mar. Drugs 2015, 13, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, J.; Liu, Z.; Lin, S.; Xia, G.; Xia, X.; Ding, B.; He, L.; Lu, Y.; She, Z. Peniphenones A–D from the mangrove fungus Penicillium dipodomyicola HN4-3A as inhibitors of Mycobacterium tuberculosis Phosphatase MptpB. J. Nat. Prod. 2014, 77, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Li, X.; Li, C.; Wang, B. Diphenyl ether and benzophenone derivatives from the marine mangrove-derived fungus Penicillium sp. MA-37. Phytochem. Lett. 2014, 9, 22–25. [Google Scholar] [CrossRef]
- Wang, J.; Wei, X.; Lu, X.; Xu, F.; Wan, J.; Lin, X.; Zhou, X.; Liao, S.; Yang, B.; Tu, Z.; Liu, Y. Eight new polyketide metabolites from the fungus Pestalotiopsis vaccinii endogenous with the mangrove plant Kandelia candel (L.) Druce. Tetrahedron 2014, 70, 9695–9701. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, X.; Ma, W.; Fang, W.; Chen, Z.; Yang, B.; Liu, Y. A new aromatic amine from fungus Pestalotiopsis vaccinii. Phytochem. Lett. 2014, 7, 35–37. [Google Scholar] [CrossRef]
- Kornsakulkarn, J.; Saepua, S.; Komwijit, S.; Rachtawee, P.; Thongpanchang, C. Bioactive polyketides from the fungus Astrocystis sp. BCC 22166. Tetrahedron 2014, 70, 2129–2133. [Google Scholar] [CrossRef]
- Bai, Z.; Lin, X.; Wang, Y.; Wang, J.; Zhou, X.; Yang, B.; Liu, J.; Yang, X.; Wang, Y.; Liu, Y. New phenyl derivatives from endophytic fungus Aspergillus flavipes AIL8 derived of mangrove plant Acanthus ilicifolius. Fitoterapia 2014, 95, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, X.; Qin, X.; Chen, P.; Lin, X.; Zhang, T.; Yang, X.; Liao, S.; Yang, B.; Liu, J.; Zhou, X.; Tu, Z.; Liu, Y. Two new prenylated phenols from endogenous fungus Pestalotiopsis vaccinii of mangrove plant Kandelia candel (L.) Druce. Phytochem. Lett. 2015, 12, 59–62. [Google Scholar] [CrossRef]
- Meng, L.; Li, X.; Liu, Y.; Wang, B. Penicibilaenes A and B, sesquiterpenes with a tricyclo[6.3.1.0(1,5)]dodecane skeleton from the marine isolate of Penicillium bilaiae MA-267. Org. Lett. 2014, 16, 6052–6055. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cox, D.G.; Ding, W.; Huang, G.; Lin, Y.; Li, C. Three new resveratrol derivatives from the mangrove endophytic fungus Alternaria sp. Mar. Drugs 2014, 12, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ding, W.; Li, C.; Cox, D.G. Two new cyclopeptides from the co-culture broth of two marine mangrove fungi and their antifungal activity. Pharmacogn. Mag. 2014, 10, 410–414. [Google Scholar] [PubMed]
- Li, C.; Wang, J.; Luo, C.; Ding, W.; Cox, D.G. A new cyclopeptide with antifungal activity from the co-culture broth of two marine mangrove fungi. Nat. Prod. Res. 2014, 28, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, X.; Du, L.; Wang, W.; Zhu, T.; Cu, Q.; Li, D. Sorbicatechols A and B, antiviral sorbicillinoids from the marine-derived fungus Penicillium chrysogenum PJX-17. J. Nat. Prod. 2014, 77, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Lee, J.H.; You, M.J.; Choi, T.J.; Park, W.; Lee, S.K.; Oh, D.C.; Oh, K.B.; Shin, J. Penicillipyrones A and B, meroterpenoids from a marine-derived Penicillium sp. fungus. J. Nat. Prod. 2014, 77, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Orfali, R.S.; Aly, A.H.; Ebrahim, W.; Abdel-Aziz, M.S.; Müller, W.E.G.; Lin, W.H.; Daletos, G.; Proksch, P. Pretrichodermamide C and N-methylpretrichodermamide B, two new cytotoxic epidithiodiketopiperazines from hyper saline lake derived Penicillium sp. Phytochem. Lett. 2015, 3, 168–172. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Kalinovsky, A.I.; Pushilin, M.A.; Glazunov, V.P.; Khudyakova, Y.V.; Kirichuk, N.N.; Ermakova, S.P.; Dyshlovoy, S.A.; Yurchenko, E.A.; et al. Oxirapentyns F–K from the marine-sediment-derived fungus Isaria felina KMM 4639. J. Nat. Prod. 2014, 77, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Li, X.; Li, C.; Xu, G.; Wang, B. Prenylated indolediketopiperazine peroxides and related homologues from the marine sediment-derived fungus Penicillium brefeldianum SD-273. Mar. Drugs 2014, 12, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Wei, H.; Zhang, Z.; Che, Q.; Liu, Y.; Zhu, T.; Mandi, A.; Kurtan, T.; Gu, Q.; Li, D. Eleganketal A, a highly oxygenated dibenzospiroketal from the marine-derived fungus Spicaria elegans KLA03. J. Nat. Prod. 2014, 77, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xia, Q.; Zhao, Y.; Zheng, Q.; Liu, Q.; Chen, L.; Zhang, Q. Speradines F–H, three new oxindole alkaloids from the marine-derived fungus Aspergillus oryzae. Chem. Pharm. Bull. 2014, 62, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, H.; Zhang, X.; Wang, S.; Wu, C.; Gu, Q.; Guo, P.; Zhu, T.; Li, D. Psychrophilins E–H and versicotide C, cyclic peptides from the marine-derived fungus Aspergillus versicolor ZLN-60. J. Nat. Prod. 2014, 77, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; An, C.; Wang, B. Prenylated indole alkaloid derivatives from marine sediment-derived fungus Penicillium paneum SD-44. Helvetica. Chim. Acta 2014, 97, 1440–1444. [Google Scholar] [CrossRef]
- Peng, J.; Gao, H.; Li, J.; Ai, J.; Geng, M.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Prenylated indole diketopiperazines from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2014, 79, 7895–7904. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wu, C.; Li, C.; Cui, C. A practical strategy to discover new antitumor compounds by activating silent metabolite production in fungi by diethyl sulphate mutagenesis. Mar. Drugs 2014, 12, 1788–1814. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Cui, C.; Li, C.; Wu, C. Three new and eleven known unusual C25 steroids: Activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulphate. Mar. Drugs 2014, 12, 1545–1568. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, C.; Cui, C. Seven new and two known lipopeptides as well as five known polyketides: The activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulphate. Mar. Drugs 2014, 12, 1815–1838. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, D.; Li, Y.; Hua, H.; Ma, E.; Li, Z. Caryophyllene sesquiterpenes from the marine-derived fungus Ascotricha sp. ZJ-M-5 by the one strain-many compounds strategy. J. Nat. Prod. 2014, 77, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhang, Y.; Ding, L.; He, S.; Wu, B.; Dong, J.; Zhu, P.; Chen, J.; Zhang, J.; Yan, X. Preparative separation of sulfur-containing diketopiperazines from marine fungus Cladosporium sp. using high-speed counter-current chromatography in stepwise elution mode. Mar. Drugs 2015, 13, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fang, P.; Tang, J.; Wu, Z.; Li, X.; Li, S.; Wang, Y.; Liu, G.; He, Z.; Gou, D.; et al. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, J.; Wang, Y.; Zhang, X.; Nong, X.; Chen, M.; Xu, X.; Qi, S. Cytotoxic and antiviral tetramic acid derivatives from the deep-sea-derived fungus Trichobotrys effus DFFSCS021. Tetrahedron 2015, 71, 9328–9332. [Google Scholar] [CrossRef]
- Liu, X.; Miao, F.; Liang, X.; Ji, N. Ergosteroid derivatives from an algicolous strain of Aspergillus ustus. Nat. Prod. Res. 2014, 28, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Sobolevskaya, M.P.; Afiyatullov, S.S.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Yurchenko, E.A.; Dyshlovoy, S.A. Sargassopenillines A–G, 6,6-spiroketals from the alga-derived fungi Penicillium thomii and Penicillium lividum. Mar. Drugs 2014, 12, 5930–5943. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Sobolevskaya, M.P.; Leshchenko, E.V.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Zakharenko, A.M.; Kim, N.Y.; Afiyatullov, S.S. Meroterpenoids from the alga-derived fungi Penicillium thomii maire and Penicillium lividum westling. J. Nat. Prod. 2014, 77, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Miao, F.; Liang, X.; Ji, N. Meroterpenes from an algicolous strain of Penicillium echinulatum. Magn. Reson. Chem. 2014, 52, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Lin, X.; Zhou, X.; Wan, J.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.; Tu, Z.; Liu, Y. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus Aspergillus ochraceus Jcma1F17. Med. Chem. Commun. 2014, 5, 701–705. [Google Scholar] [CrossRef]
- Zhang, P.; Mandi, A.; Li, X.; Du, F.; Wang, J.; Li, X.; Kurtan, T.; Wang, B. Varioxepine A, a 3H-oxepine-containing alkaloid with a new oxa-cage from the marine algal-derived endophytic fungus Paecilomyces variotii. Org. Lett. 2014, 16, 4834–4837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, X.; Wang, J.; Li, X.; Wang, B. New butenolide derivatives from the marine-derived fungus Paecilomyces variotii with DPPH radical scavenging activity. Phytochem. Lett. 2015, 11, 85–88. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Xu, G.; Li, C.; Wang, B. Antioxidant metabolites from marine alga-derived fungus Aspergillus wentii EN-48. Phytochem. Lett. 2014, 7, 120–123. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Wang, J.; Wang, B. Oxepine-containing diketopiperazine alkaloids from the algal-derived endophytic fungus Paecilomyces variotii EN-291. Helv. Chim. Acta 2015, 98, 800–804. [Google Scholar] [CrossRef]
- Alarif, W.M.; Al-Footy, K.O.; Zubair, M.S.; Halid Ph, M.; Ghandourah, M.A.; Basaif, S.A.; Al-Lihaibi, S.S.; Ayyad, S.N.; Badria, F.A. The role of new eudesmane-type sesquiterpenoid and known eudesmane derivatives from the red alga Laurencia obtusa as potential antifungal-antitumour agents. Nat. Prod. Res. 2015, 20, 1–6. [Google Scholar]
- Wu, B.; Oesker, V.; Wiese, J.; Schmaljohann, R.; Imhoff, J.F. Two new antibiotic pyridones produced by a marine fungus, Trichoderma sp. strain MF106. Mar. Drugs 2014, 12, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Lin, Y.; Yuan, J.; Lu, Y.; Zhu, X.; Li, J.; Li, M.; Lin, Y.; He, J.; Liu, L. Meroterpenes and azaphilones from marine mangrove endophytic fungus Penicillium 303#. Fitoterapia 2014, 97, 241–246. [Google Scholar] [PubMed]
- Wu, B.; Oesker, V.; Wiese, J.; Malien, S.; Schmaljohann, R.; Imhoff, J.F. Spirocyclic drimanes from the marine fungus Stachybotrys sp. strain MF347. Mar. Drugs 2014, 12, 1924–1938. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ji, Y.; Hu, D.; Zhang, S.; Yan, H.; Liu, X.; Luo, H.; Zhu, H. Structure and absolute configuration of penicilliumine, a new alkaloid from Penicillium commune 366606. Tetrahedron Lett. 2014, 55, 2684–2686. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Men, L.; Zhang, Y.; Liu, Z.; Sun, T.; Geng, Y.; Yu, Z. Secondary metabolites of the marine fungus Penicillium chrysogenum. Chem. Nat. Compd. 2014, 50, 405–407. [Google Scholar] [CrossRef]
- Xiao, Z.; Lin, S.; Tan, C.; Lu, Y.; He, L.; Huang, X.; She, Z. Asperlones A and B, dinaphthalenone derivatives from a mangrove endophytic fungus Aspergillus sp. 16–5C. Mar. Drugs 2015, 13, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Root, N.; Jabeen, F.; Al-Harrasi, A.; Ahmad, M.; Mabood, F.; Hassan, Z.; Shah, A.; Green, I.R.; Schulz, B.; et al. Microsphaerol and seimatorone: Two new compounds isolated from the endophytic fungi, Microsphaeropsis sp. and Seimatosporium sp. Chem. Biodivers. 2015, 12, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F. Natural products from marine fungi—Still an underrepresented resource. Mar. Drugs 2016, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B.; Henrikson, J.C.; Hoover, A.R.; Lee, A.E.; Cichewicz, R.H. Epigenetic remodeling of the fungal secondary metabolome. Org. Biomol. Chem. 2008, 6, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Wei, C.; Chuang, D.; El-Shazly, M.; Hsieh, C.T.; Asai, T.; Oshima, Y.; Hsieh, T.J.; Hwang, T.L.; Wu, Y.; et al. An epigenetic modifier enhances the production of anti-diabetic and anti-inflammatory sesquiterpenoids from Aspergillus sydowii. Bioorg. Med. Chem. 2013, 21, 3866–3872. [Google Scholar] [CrossRef] [PubMed]
- Beau, J.; Mahid, N.; Burda, W.N.; Harrington, L.; Shaw, L.N.; Mutka, T.; Kyle, D.E.; Barisic, B.; Olphen, A.; Baker, B.J. Epigenetic tailoring for the production of anti- infective cytosporones from the marine fungus Leucostoma persoonii. Mar. Drugs 2012, 10, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, I.; Kim, S.K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef] [PubMed]
- Duarte, K.; Rocha-Santos, T.A.P.; Freitas, A.C.; Duarte, A.C. Analytical techniques for discovery of bioactive compounds from marine fungi. Trends Anal. Chem. 2012, 34, 97–110. [Google Scholar] [CrossRef]


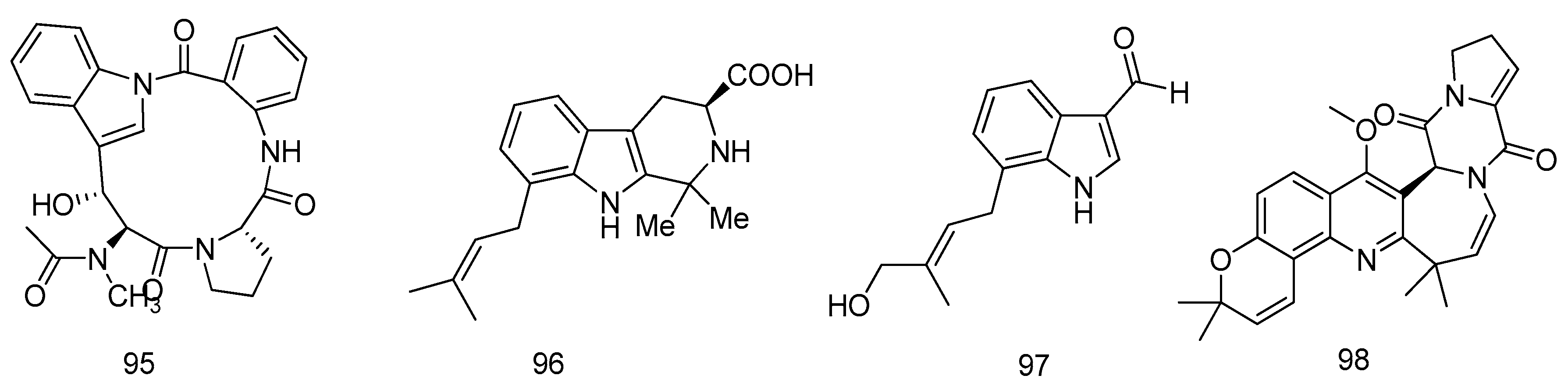
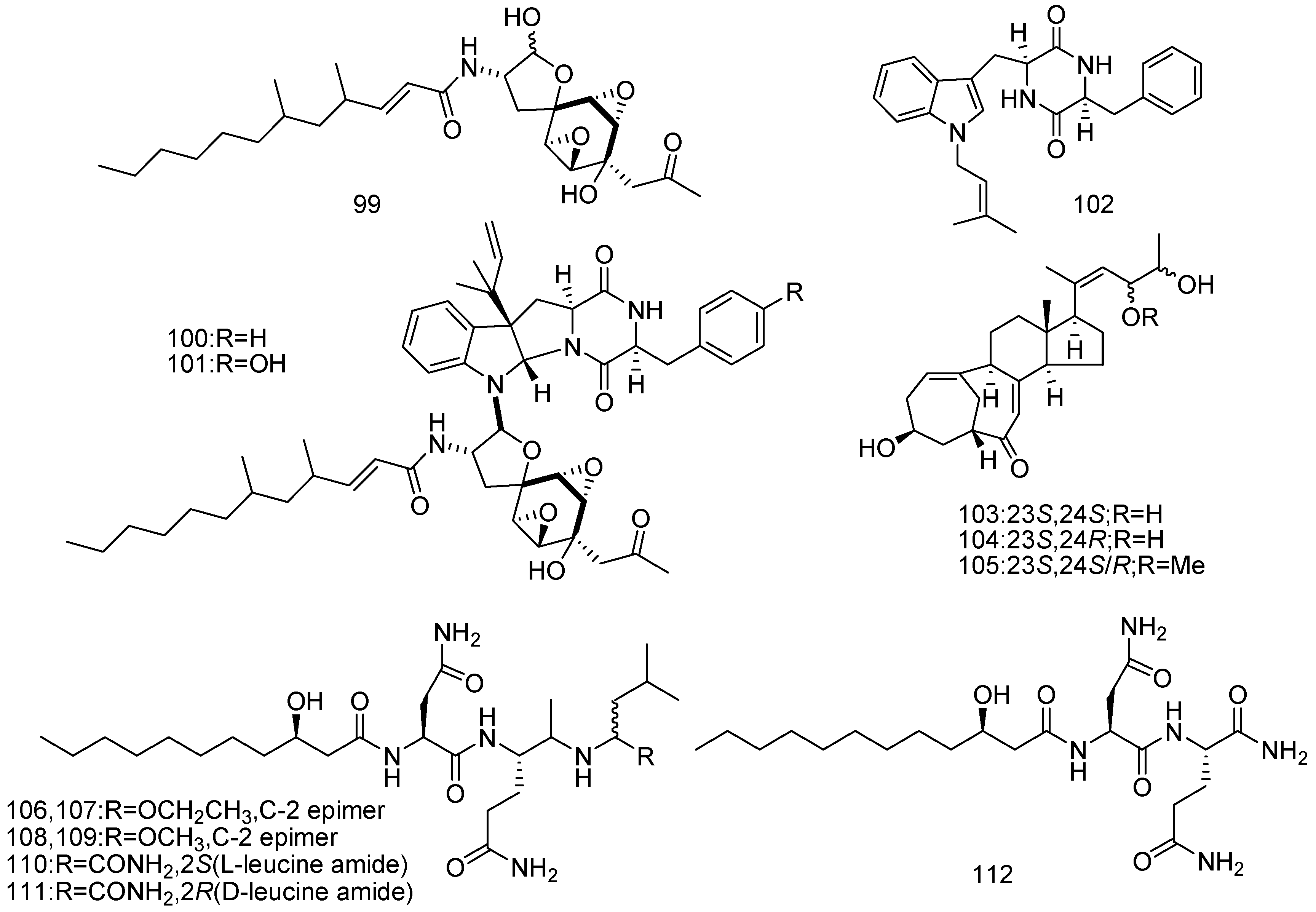
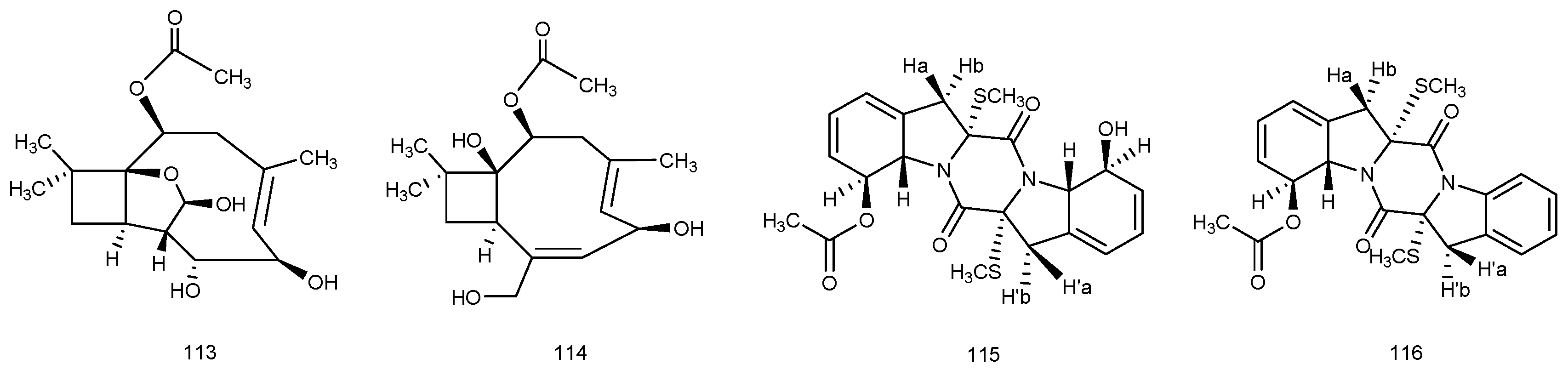
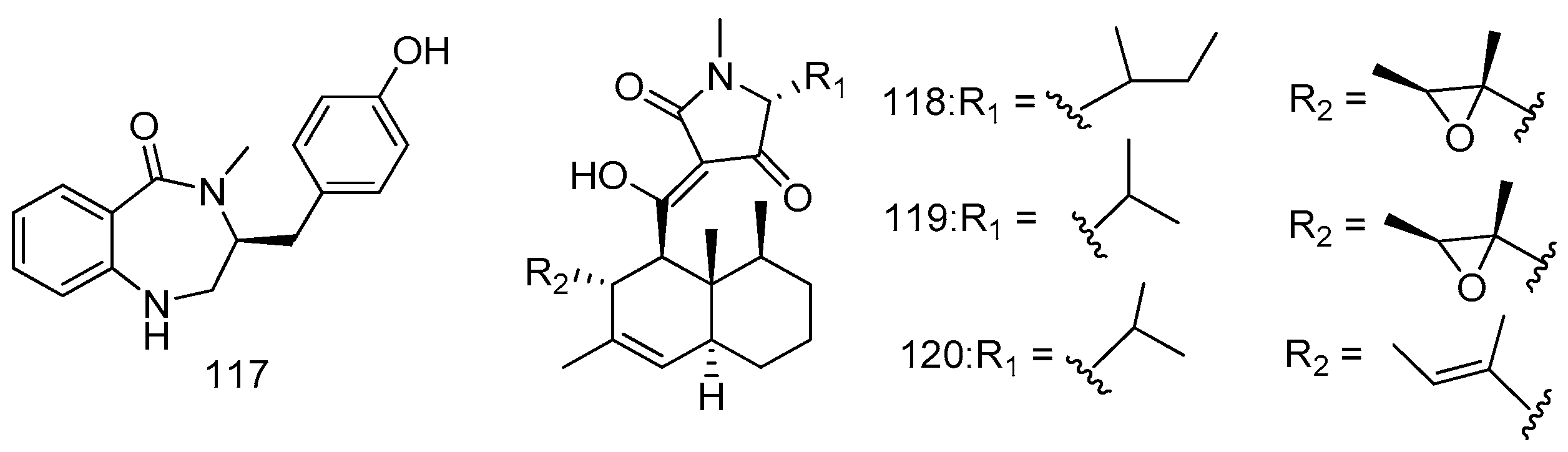
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential Pharmacological Resources: Natural Bioactive Compounds from Marine-Derived Fungi. Mar. Drugs 2016, 14, 76. https://doi.org/10.3390/md14040076
Jin L, Quan C, Hou X, Fan S. Potential Pharmacological Resources: Natural Bioactive Compounds from Marine-Derived Fungi. Marine Drugs. 2016; 14(4):76. https://doi.org/10.3390/md14040076
Chicago/Turabian StyleJin, Liming, Chunshan Quan, Xiyan Hou, and Shengdi Fan. 2016. "Potential Pharmacological Resources: Natural Bioactive Compounds from Marine-Derived Fungi" Marine Drugs 14, no. 4: 76. https://doi.org/10.3390/md14040076
APA StyleJin, L., Quan, C., Hou, X., & Fan, S. (2016). Potential Pharmacological Resources: Natural Bioactive Compounds from Marine-Derived Fungi. Marine Drugs, 14(4), 76. https://doi.org/10.3390/md14040076