Abstract
Marine micro- and macroorganisms are well known to produce metabolites with high biotechnological potential. Nearly 40 years of systematic prospecting all around the New Caledonia archipelago and several successive research programs have uncovered new chemical leads from benthic and planktonic organisms. After species identification, biological and/or pharmaceutical analyses are performed on marine organisms to assess their bioactivities. A total of 3582 genera, 1107 families and 9372 species have been surveyed and more than 350 novel molecular structures have been identified. Along with their bioactivities that hold promise for therapeutic applications, most of these molecules are also potentially useful for cosmetics and food biotechnology. This review highlights the tremendous marine diversity in New Caledonia, and offers an outline of the vast possibilities for natural products, especially in the interest of pursuing collaborative fundamental research programs and developing local biotechnology programs.
1. Introduction
Located approximately at 165° E and 21°30′ S, the New Caledonia archipelago enjoys a privileged position east of the city of Rockhampton, central province of the Great Barrier Reef, 1500 km away from the Australian Queensland coast, with surface seawater temperatures mild enough to avoid recurrent coral bleaching episodes.
This tropical subregion of Melanesia is a relic of a continental landmass that drifted away from the super-continent Gondwana at the end of the Cretaceous period (see Pelletier 2007) [1]. Successive geological events have resulted in an archipelago that comprises a main island called Grande Terre, Ile des Pins in the south, the Entrecasteaux reefs in the north, the Loyalty Islands in the east and, finally, the Chesterfield-Bellona plateau in the west, located at mid-distance to Australian coast (Figure 1).
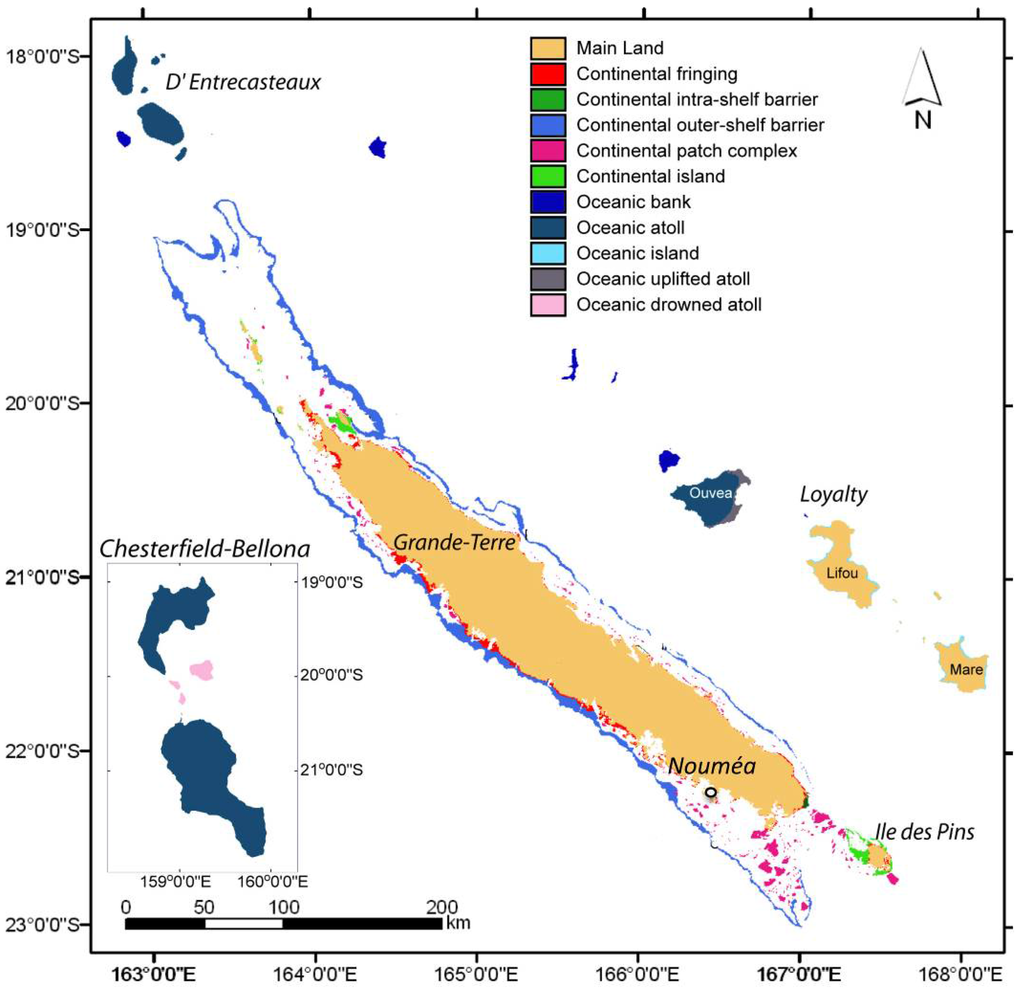
Figure 1.
The archipelago of New Caledonia and its reef complexes (adapted from Andréfouët et al. 2009 [2], with kind permission from the author).
New Caledonian waters represent an exclusive economic zone (EEZ) spanning more than 1,700,000 km2. The EEZ includes a vast and complex reef system of 4537 km2 of reef area and 31,336 km2 of non-reef area [2] and represents the second most important coral reef complex and the longest continuous barrier reef in the world, stretching over 1600 km.
The reef systems together make up a unique marine ecosystem [3] with more than 161 different reef units [4] supporting high marine species diversity and abundance [5], including so-called “living fossils” such as a stalked crinoid and a shelled cephalopod.
Only since the 19th century has attention been paid to diversity of the New Caledonian reef systems. Many expeditions have been conducted to study the oceanic life of these hitherto unexplored oceanic zones. The first oceanographic cruise, the Challenger expedition (1872–1876), led to the collection of thousands of previously unknown marine species [6]. Since the 19th century, scientific interest has shifted from the study of “exotic” specimens stored in museum collections and classified according to their morphological features, to the study and understanding of marine ecosystems. Charles Darwin’s landmark expeditions in the Eastern Pacific islands and his remarkable observations on the formation of atolls from subsiding volcanic islands sparked continued interest in ecosystems biology in oceans across the world. Research vessels are now equipped with sophisticated equipment to carry out on-site sample analysis (e.g., molecular biology) and data transmission via satellite systems. For example, the schooner Tara, specially fit out with on-board facilities, recently sailed around the world to study the impact of global warming on plankton and coral reef systems across the Pacific [7].
Pioneering scientific investigations in New Caledonian waters were carried out locally after World War II. The naturalist René Catala initiated ecological surveys and species census around Ile aux Canards [8], an island off Nouméa now directly exposed to human activities, with recent changes in reef zonation and species composition. In addition, in 1956, Catala founded the Aquarium of Nouméa (now the Aquarium des Lagons), a first-of-its-kind structure specifically designed to observe rare and fragile marine species in their environment, including homegrown corals. Catala incidentally discovered the fluorescence of living corals [9] and other invertebrates (fluorescence appears to be a photoprotective and possibly temperature-regulating mechanism). A few years later, the Singer-Polignac expedition (1960–1963) explored St. Vincent Bay and the east coast of Grande Terre [10,11,12]. Similarly, the American ecologist, Arthur Lyon Dahl, a regional adviser at the South Pacific Commission from 1974 to 1982, demonstrated the importance of surface area in ecological analysis and described several methods for conducting surveys of coral reefs [13,14].
Bioprospecting studies in New Caledonia were initiated in 1976 by Pierre Potier from the ICSN (Institute of Natural Substances Chemistry, Gif-sur-Yvette, mainland France) as part of the national research program SNOM (Substances Naturelles d’Origine Marine) and benefited from collaboration with scientific divers from IRD (Institut de Recherche pour le Développement, ex. ORSTOM, Office de la Recherche Scientifique et Technique d’Outre-Mer) in Nouméa (Figure 2). SNOM set out to explore marine biodiversity extensively with the taxonomic expertise from the National Museum of Natural History (MNHN) of Paris, to identify novel molecules and to assess their biological activities primarily for pharmaceutical purposes. Collecting efforts focused mainly on invertebrates, including octocorals, porifers, echinoderms, mollusks and ascidians. Little attention has been given to macroalgae due to the lack of taxonomic support.
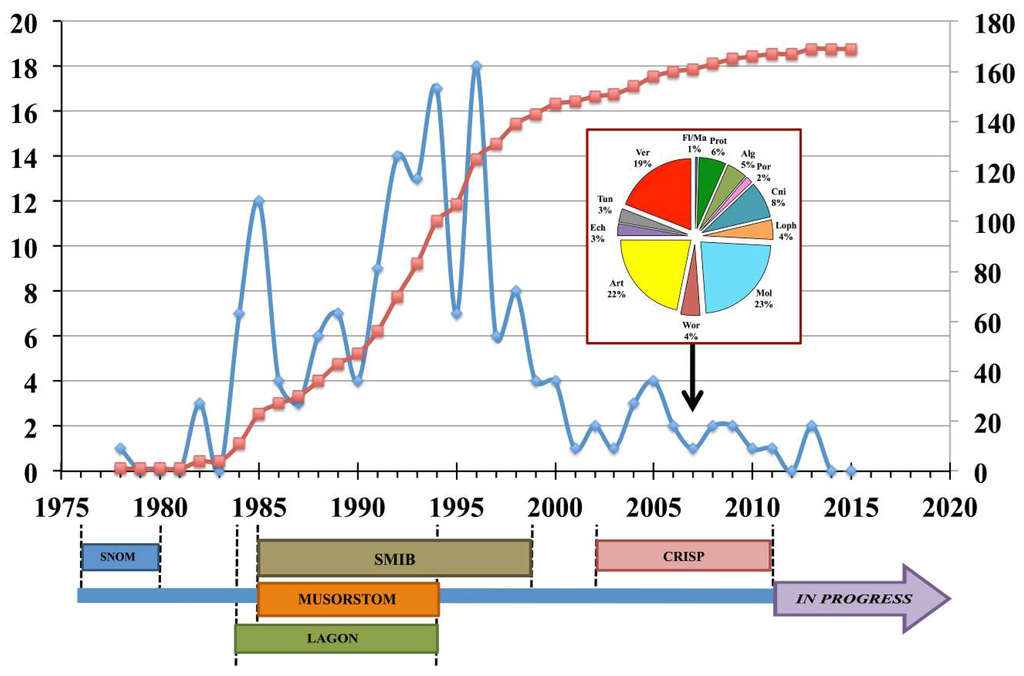
Figure 2.
Publications in scientific journals on organisms collected within various research programs. Cumulative data representing the total number of publications generated since 1976 to present (red line) and the number of publications produced per year since 1976 (blue line). The timeline on the x-axis shows the various regional marine biodiversity programs spanning the 40-year period. These statistics do not include reports, reviews and book chapters. The insert shows the relative percentages of major taxon investigated up to 2007 [5]. SNOM: Substances Naturelles d’Origine Marine; SMIB: Substances Marines d’Intérêt Biologique; LAGON; MUSORSTOM (now Tropical Deep-Sea Benthos): acronym for the joint expeditions of the National Museum of Natural History (MNHN) and the Office de la Recherche Scientifique et Technique d’Outre-Mer (ORSTOM, now IRD); CRISP: Coral Reef InitiativeS for the Pacific; in progress: various research programs including chemical and pharmacological investigations on micro- and macroalgae. Fl/Ma: Flora and Marine Angiosperms; Pro: Protozoa; Alg: Algae; Por: Porifera; Cni: Cnidaria; Loph: Lophophorates; Mol: Mollusks; Wor: Worms; Art: Arthropoda; Ech: Echinodermata; Tun: Tunicata; Ver: Vertebrata.
In 1985, the SNOM program was followed by the SMIB program (Substances Marines d’Intérêt Biologique), which was conducted jointly by ORSTOM and the Centre National de la Recherche Scientifique (CNRS), with a large collaborative network of public research institutions, e.g., the Centre d’Etudes Nucléaires de Saclay, the Institut National de la Santé et de la Recherche Médicale (INSERM), the MNHN, French and foreign universities, as well as several private companies (in particular, Pierre Fabre and Rhône-Poulenc).
The raw compounds and their fractions were extracted at the ORSTOM center in Nouméa and were originally sent for purification and structural determination to the ICSN (mainland France), and the biological screening was assigned to various mainland French laboratories. Gradually, separation and purification, as well as preliminary testing and benchtop assays, were carried out locally in New Caledonia at the ORSTOM (and later IRD) center, to avoid unnecessary duplication and to better target specific requests from collaborating partners. During this time, a number of candidate molecules were identified for their anticancer, cardiovascular or neurological interest (reviewed in [15]), prompting an extension of the research program and redefinition of the terms of scientific collaboration, now open to international experts. The purchase of a larger research vessel, the R/V Alis, in 1992, provided the opportunity to extend the bioprospecting range by dredging to 600 m deep and to access new biological resources.
The SNOM and SMIB programs led to an extensive survey of the marine biodiversity of the New Caledonian archipelago, providing many in situ observations and records of new taxon, which have been published in several papers [5] and illustrated in field guides, especially on sponges [16], echinoderms [17], gorgonians [18], ascidians [19], marine snakes [20], and fishes [21]. Among the 300 organisms studied, only 50 have been the object of chemical and therapeutic research and several original molecules have been described and tested on tumor development [22].
After the SNOM and SMIB programs, pharmacological bioprospecting in New Caledonia was integrated in the LAGON and MUSORSTOM biodiversity projects until 2000. Thereafter, research activities were extended to other countries in the Pacific region as part of the Coral Reef InitiativeS for the Pacific (CRISP), with emphasis on legal agreements and economic benefits to host countries.
In the meantime, some research activity focused on ciguatera and cyanobacteria toxicity after several severe poisoning events occurred in New Caledonia. In addition to pharmaceutical activities, natural marine compounds inspired new scientific approaches including chemotaxonomy and chemical ecology of benthic macroalgae to understand how opportunistic algae colonize living coral. These activities are conducted by the CoReUs/ENTROPIE research team at IRD (Figure 2). These projects are mentioned in Moretti et al., (1993) [22] and detailed in a comprehensive review of novel chemical structures and associated pharmacological activities described by Laurent and Pietra published in 2004 [15]. Another review emphasizes the developmental aspects of marine molecules from South Pacific zone including New Caledonia [23].
Here, we provide an updated review of 40 years of exploration of the marine micro-/macrophyte and invertebrate chemodiversity of this species-rich zone of the Southwest Pacific, with its pharmacological potential and its ecological significance. After a description of the basic operational aspects of discovering marine natural products, an overview of the work on each major taxon is presented, illustrated by case studies that have been the highlights of the abovementioned programs for the last 40 years, and carried out locally by experts in full compliance with existing regulations of biodiversity protection and the sustainability of valuable natural resources.
2. Taxonomy
SCUBA diving allows visual exploration of shallow-water marine biota, making it possible not only to collect material at depths down to 60 m, but also to take photographs and record ecological information, e.g., interactions between organisms that can be useful for selecting organisms to collect and subsequent biological tests. Upon the development of blind transect dredging with limited biomass sampling on soft bottoms, collection efforts were extended to deeper zones (down to 600 m), thus sampling entirely different organisms.
2.1. Sample Collection Sites
Collection sites were selected to cover the large diversity of habitats ranging from shallow lagoons to deep parts of the outer slopes of the barrier reefs. They include hard and sandy bottoms, sheltered and exposed areas of the mainland (Grande Terre), Loyalty Islands, as well as remote reefs and atolls (Entrecasteaux, Chesterfield) and seamounts (Figure 1).
Each site was georeferenced and described using geomorphological and biological descriptors. Historical data have been sorted, standardized and stored along with recent information in the dedicated database LagPlon [24].
2.2. Biological Material Sampling
For all the groups collected for chemical purposes, specimens from each taxon were sampled for taxonomical identification and preserved in ethanol as vouchers after labelling. Collections were duplicated, one sample was kept for the IRD reference collection to facilitate new sampling efforts and the duplicate was sent to specialists for taxonomy work. In situ macrophotographs were taken as well as additional ex situ laboratory photographs when necessary. Specimens used to describe new species (holotypes) have been deposited in various museum collections in Australia (Northern Territory Museum, Darwin and Queensland Museum of South Brisbane), New Zealand, mainland France (National Museum of Natural History, Paris), Belgium (Université Libre de Bruxelles). Paratypes were systematically filed in the IRD reference collection in Nouméa, along with photographs and field records.
3. Chemistry
3.1. Biological Material
Chemical characterization and biological assays were initially carried on samples with wet weights ranging from 300 to 3000 g. However, recent progress in analytical techniques allows molecular inventory and pharmacological tests from smaller amounts of material in compliance with international regulations on the use of biological material for research purposes.
Below, we describe the basic protocols used locally at the IRD laboratories in Nouméa. Each of the molecules that have been investigated locally or with national and international partners have been treated separately (Figure 3). It is beyond the scope of this review to detail each protocol individually; they can be found in the original research papers, or in a natural products database.
Figure 3.
Collaborative network of chemists and pharmacologists from 1976 to 2013 as compiled individually from journal publications.
3.2. Conditioning Samples for Chemistry
Freshly collected material is sorted by taxon and frozen on board at −20 °C, or at least placed in 70% ethanol in distilled water (slightly acidified to avoid oxidation of polar compounds) until it can be deep frozen and freeze-dried for subsequent use. Material used for enzymology or genome studies is snap-frozen on board (liquid nitrogen or dry ice in pure alcohol). For short collecting sessions (less than one full day), the material may be kept alive if each item is appropriately handled.
Back at the laboratory, the material must be ground/chopped/crushed then freeze-dried or ethanol-preserved and stowed away for later use, or sent abroad to partner laboratories.
3.3. Extraction
3.3.1. Routine Procedure
Unless otherwise specified, a standard protocol is used for extracting compounds and separating them into crude non-polar (organic) and polar (aqueous) fractions (Figure 4).
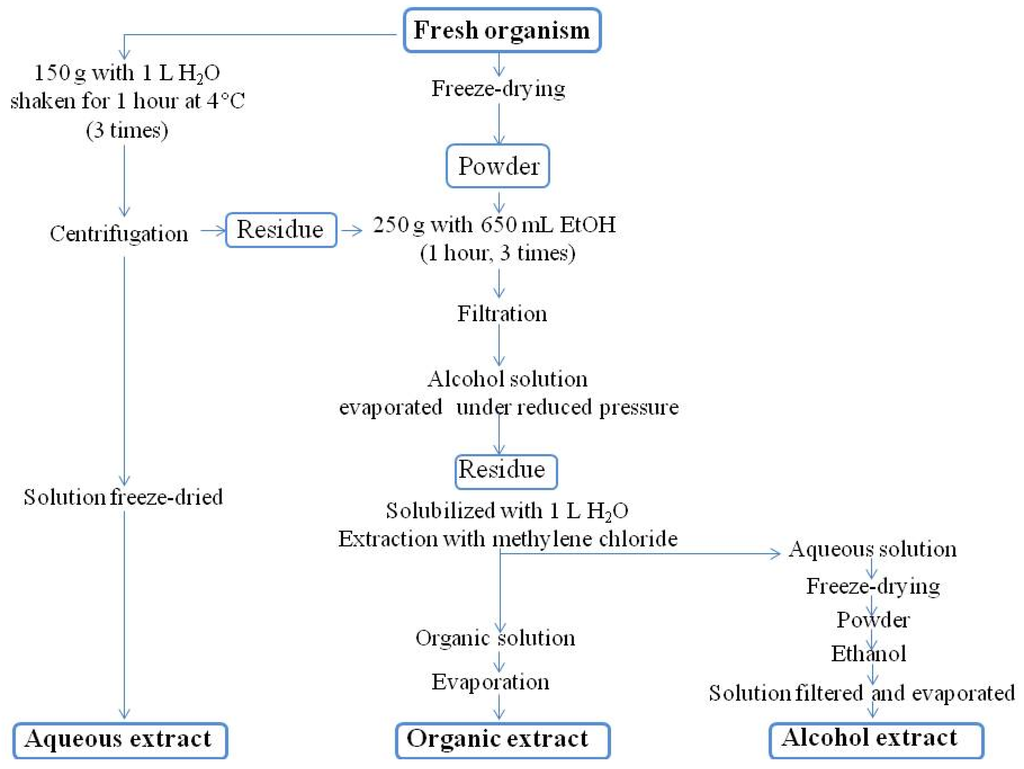
Figure 4.
Routine extraction protocol (IRD Nouméa).
Organic fractions are generally evaporated under vacuum (Rotovap) and kept in the dark at −20 °C. Water solubles may need to be desalted using size-exclusion gel chromatography (SEC) or by membrane filtration under nitrogen (Amicon®, Alsace, France) prior to separation, but for long-term storage, desalting is not necessary. Aliquoting is necessary for multiple chemical characterizations and for bioassays.
3.3.2. Peptide Protease Inhibitors
Live tissues are processed (minced or fragmented) on board and placed in an acidic solution (methanol/2 N acetic acid) to optimize the extraction of polar substances and block any potential hydrolysis by proteolytic enzymes. The cold (−20 °C) slurry is filtered twice and filtrates are reduced by evaporation and neutralized to pH 5.5 prior to freeze-drying. The total protein content of the crude extract is assayed by measuring optical densities with a UV spectrophotometer.
3.4. Separation, Purification
Classical separation and purification techniques (TLC, HPLC, SEC) were not routinely used unless requested for products presenting an interesting bioactivity profile. This data is mentioned in the original publications for each novel structure.
3.5. Structural Analysis
Basic spectroscopic (NMR, UV, IR) and spectrometric (MS) methods are used when available on site, mostly to avoid replication.
3.6. Chemical Databases
The IRD proprietary information system Cantharella [25] compiles pharmacochemical data of all organisms collected in New Caledonia and cross-Pacific oceanographic cruises for the study of their natural substances, with restricted access via Internet. The information system provides access to biological data, accounts for all chemical processes from extraction to purification, and presents the bioactivity profile detected using the complete range of tests.
4. Biological Activities
4.1. Preliminary Testing
Vacuum-dried crude extracts or non-purified fractions thereof can be used for describing general characteristics using toxicity assays on various invertebrate, vertebrate and plant models, and cultures of microbial reference strains (Table 1).

Table 1.
Field observations and crude biological tests for preliminary screening.
Preliminary tests performed on site are useful for making appropriate decisions as to whether a given sample from a newly collected organism is worth investigating further. Along with taxonomic criteria and dereplication issues, such decisions are now taken after consulting the scientific literature and specialized databases, especially the IRD databases LagPlon [24] and Cantharella [25], which, in addition to the more traditional MarinLit and other databases, considerably aid local researchers.
4.1.1. Brine Shrimp Toxicity Assay
The brine shrimp (Artemia salina) toxicity bioassay is one of the most basic and widely used tests to detect cytotoxicity. Newly hatched brine shrimp nauplii are exposed to various concentrations of soluble or solubilized test substances and mortality is recorded, leading to a median lethal dose (LD50) estimate. A typical protocol is detailed in [27].
4.1.2. Mosquito Fish Toxicity Assay
The mosquito fish Gambusia affinis is often used to evaluate the toxicity of substances to be screened for antimitotic activities, and it has been adapted from [28], a study that used this species for comparative ecotoxicological studies of soft corals. The mosquito fish toxicity test has been extended to include multiparametric behavioral observations and can provide valuable neurophysiological information as well as ecotoxicological data on crude extracts that are water soluble or solubilized [29].
4.1.3. Fertilization of Sea Urchin Eggs
The aim of this test is to observe the division pattern of eggs of the test sea urchin Echinometra mathaei fertilized in the laboratory, by adapting the method Kobayashi [30] originally designed for testing the toxicity of heavy metals on early embryonic stages. This test is highly sensitive and works at extremely low concentrations of the test substances. Cell division arrest at specific early and embryonic stages provides a preliminary indication of specific enzymatic inhibition at determined steps of the cell cycle.
4.1.4. Anti-Serpin Activity
Serine protease inhibition assays are conducted locally using bovine trypsin (protocol inspired from Green et al., 1953) [36] and porcine elastase [37], with respectively, benzoyl-arginine ethyl ester (BAEE) and succinyl (Alanyl)3 para-nitro anilide (Suc [Ala]3 pNa) as substrates. End-point titration using pH-stat benchtop equipment is used to evaluate the inhibition potential of the crude extracts on bovine trypsin. The colorimetric method is used to evaluate the anti-elastase activity of the crude extracts.
4.2. Further Biological Testing
For the identification of antiproliferative natural products, cancerous and non-cancerous mammalian cell lines are tested. The comprehensive list of cell lines that are used to characterize active molecules from sponges is given in the footnote of Table 2.

Table 2.
Natural products isolated from New Caledonian sponges and their bioactivity.
Following the preliminary anti-serpin tests (see Section 4.1.4.), other serine proteases (chymotrypsin, subtilisin), cysteine proteases (papain, viral proteinases), aspartic proteases (pepsin, renin) and metalloproteases (thermolysin, carboxypeptidase A) have been carried out by M.A. Coletti-Previero, Montpellier (mainland France).
5. Natural Products by Taxon
5.1. Porifera (Sponges)
5.1.1. General Comments
Sponges are multicellular, filter-feeding diploblastic invertebrates, i.e., without organized tissues or organs, but with a special inner layer of ciliated cells called choanocytes that generate constant water flow through numerous feeding/excretory channels. There are two major subphyla of Porifera based on their biomineralization patterns: the Calcarea or calcareous sponges and the Silicispongia or siliceous sponges, the latter group being further subdivided into Hexactinellida (glass sponges), Demospongiae, which contain a proteinaceous matrix (spongin), and Homoscleromorpha, now recognized as distinct from the latter class. Calcareous sponges are primarily found in shallow waters, and particularly abundant and diverse in tropical coral reefs. Siliceous sponges, which represent most of the living sponge species today, are found at all bathymetric levels and also in freshwater, with large populations in some rivers and lakes. Most glass sponges live in deep waters, and are difficult to access [16]. Since the 1980s, deep-water dredging on the outer reef slope of the barrier reef down to 800 m has led to the discovery of a number of non-described species. As of 2007, 149 species of Porifera have been recorded in New Caledonian waters [5].
Most shallow-water sponge species live in symbiosis with Archaea, eubacteria and cyanobacteria, forming holobiont systems that represent the main source of bioactive compounds [38,39] primarily isolated from host tissues, but occasionally isolated from cultures of associated microbiota. Sponges contain a wide range of so-called secondary metabolites, some of which afford interesting biological activities [40] (Table 2).
5.1.2. Porifera Success Stories
New Caledonian sponges have revealed more exciting chemicals than any other studied taxon, in terms of carbon skeleton, degree and patterns of unsaturation, halogenation, functional group originality, presence of unusual heteroatoms etc. Some of these features are responsible for the rather exceptional bioactivity profiles encountered. Some outstanding examples are described below:
Cymbastela Cantharella
Cymbastela cantharella (Family Axinellidae, Class Demospongiae, formerly Pseudaxinyssa cantharella, Figure 5A) was collected on the outer south reef of New Caledonia at depths ranging from 10 to 40 m (voucher #R1279, IRD Nouméa).
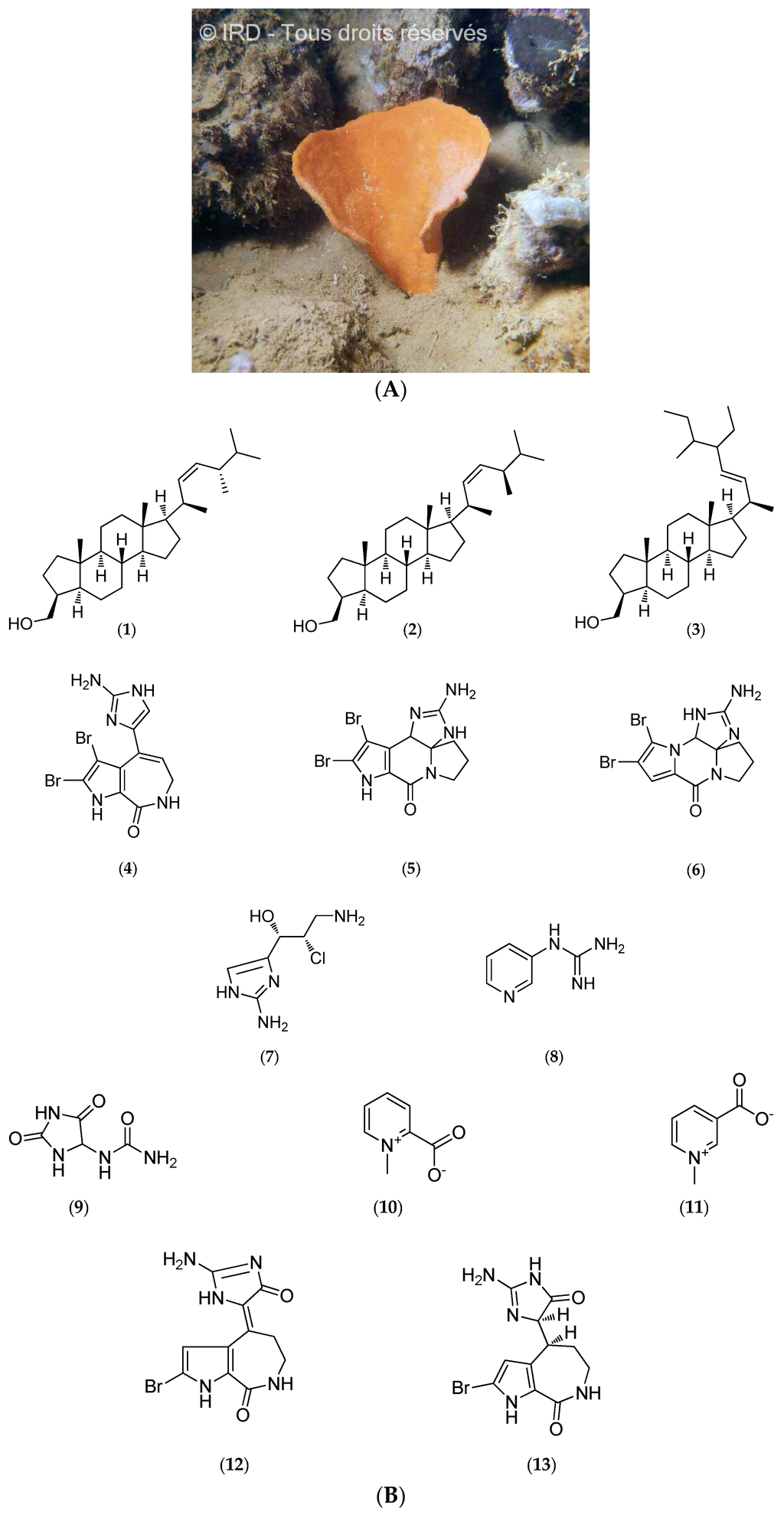
Figure 5.
(A) Cymbastela cantharella. © IRD; (B) Structure of hydroxymethyl-3β-methyl-24S-nor-A-cholest-5α-ene-22-Z (1), hydroxymethyl-3β-methyl-24R-nor-A-cholest-5α-ene-22-Z (2), hydroxymethyl-3β-ethyl-24ξ-methyl-26ξ-nor-A-cholest-5α-ene-22-E (3), odiline (4), dibromocantharelline (5), dibromophakellin (6), girolline (7), pyraxinine (8), allantoin (9), homarine (10), trigonelline (11), hymenialdisine (12) and dihydrohymenialdisine (13).
Chemical analyses led to the isolation of sterols that contain a 3β-hydroxymethyl-A-norcholestane skeleton, a typical feature of the Axinellidae (1–3, Figure 5B) [100], along with alkaloids such as odiline (4, Figure 5B), dibromocantharelline (5, Figure 5B) and dibromophakellin (6, Figure 5B) [53]. Studies performed on the crude ethanol extracts of C. cantharella led to the isolation of girolline (7, Figure 5B). Its absolute configuration has been established by X-ray diffraction [56] and total synthesis was achieved few years later [53,57,58,63,100].
Girolline (7, Figure 5B) (or girodazole) has demonstrated potent antiproliferative activity in vitro on several cell lines such as P388 murine leukemia and P388/dox, a sub-line resistant to doxorubicin, an anthracycline, or KB naso-pharyngeal and T24 bladder carcinoma human cell lines. The half maximal inhibitory concentration (IC50) values are 0.06 µM, 0.08 µM, 0.21 µM and 0.19 µM, respectively [55]. Subsequent in vivo assays have been performed on several grafted murine tumors used for preclinical evaluation including P388 and L1210 leukemia, and solid tumors such as M5076 histiocytosarcoma and MA/16C mammary adenocarcinoma cells [54,59,63]. Moreover, girodazole (7, Figure 5B) also has antitumor activity in vivo on the P388/dox cell line. This compound may inhibit the termination step of protein synthesis in vivo [60,61]. Although toxicological studies in mice and dogs do not show any major toxic effects, girodazole (7, Figure 5B) clinical development was interrupted in 1991 in phase II due to severe side effects, in particular cardiovascular toxicity [59].
Girolline (7, Figure 5B) has also shown promising results for the treatment of malaria. This compound has demonstrated potent antiplasmodial activities against four Plasmodium falciparum strains with IC50 values ranging from 77 to 215 nM, and may act synergistically with chloroquine in vitro by affecting protein synthesis [62].
Other studies have led to the characterization of pyraxinine (8, Figure 5B), a novel nitrogenous compound, along with previously identified allantoin (9, Figure 5B), homarine (10, Figure 5B) and trigonelline nitrogen compounds (11, Figure 5B) [63], hymenialdisine (previously identified; 12, Figure 5B) and new pyrrole-2-aminoimidazole alkaloids, which are dihydrohymenialdisine derivatives (13, Figure 5B) [64]. Further investigations revealed that hymenialdisine (12, Figure 5B) is a powerful inhibitor of cyclin-dependent kinases such as CDK1/cyclin B, CDK2/cyclin A and CDK2/cyclin E (IC50 values of 22 nM, 70 nM, and 40 nM, respectively), CDK5/p25 (IC50 of 28 nM), as well as against glycogen synthase kinase-3β (GSK-3β) with an IC50 of 10 nM and casein kinase 1 (CK1) with an IC50 of 35 nM [44].
Echinochalina Bargibanti
The demosponge Echinochalina bargibanti (voucher #R1858, IRD Nouméa, Figure 6A) was collected along the northeastern coast of Grande Terre between 18 and 25 m depth during the SMIB program. A bioassay-guided fractionation of its organic extracts led to the isolation of arsenicin A (14, Figure 6B), the first polyarsenic compound ever found in nature.
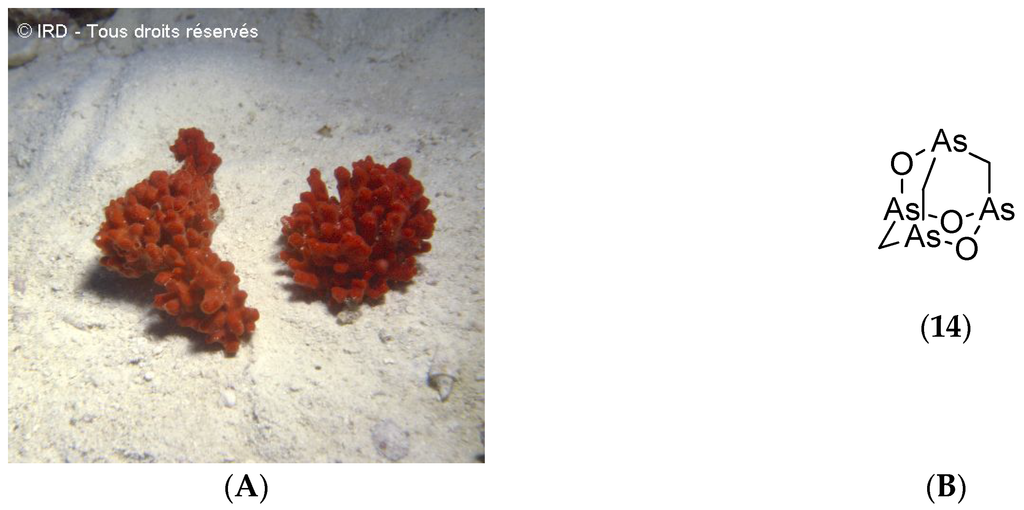
Figure 6.
(A) Echinochalina bargibanti. © IRD; (B) Structure of arsenicin A (14).
Arsenicin A (14, Figure 6B) demonstrates potent bactericidal and fungicidal activities against human pathogenic strains such as Staphylococcus aureus, Escherichia coli and Candida albicans with inhibition circles of 24, 28 and 26 mm respectively at 10 µg per disk. In contrast, gentamicin, which is the reference antibiotic, induces inhibition circles of 22, 30 and 22 mm [68]. Arsenicin A (14, Figure 6B) has been synthesized and its crystal structure determined [101]. An improvement in this synthesis has led to (±)-arsenicin A which shows potent antiproliferative activity on acute promyelocytic leukemia cell lines. This compound is more potent than arsenic trioxide (Trisenox) which is used for treating acute promyelocytic leukemia. (±)-Arsenicin A has also demonstrated antiproliferative activity against pancreatic adenocarcinomas and glioblastomas [102].
Dendrilla sp.
Dendrilla sp. (voucher #R171, IRD Nouméa) is a common shallow-water (lagoon, at approximately 20 m depth) dendroceratid sponge belonging to the Demospongiae. It features an unusual “scouring pad” appearance with its spongin fibers protruding from an elastic tissue mass that can vary in color from dark green to reddish.
R171 is cytotoxic in brine shrimp larvae bioassays [103], and its crude aqueous extract is toxic to the mosquito fish Gambusia affinis, causing erratic swimming patterns followed by 100% mortality within 12 h [29]. Reported biological activities of the crude organic extracts of Dendrilla nigra (Figure 7) include antitumor, anti-inflammatory and antimicrobial [103] properties with potential interest for shrimp aquaculture for treating Vibrio pathogens [104].

Figure 7.
Dendrilla nigra. Photo: Philippe Plailly (CNRS).
Chemically, dendrillid sponges are known to contain lamellarins A–B (15–18, Figure 8), aromatic alkaloids of probable symbiotic origin. These pyrrole derivatives are antitumoral (HIF-1 inhibitors) [105]. Dendrilla cactos contains cyclic bastadins, which are bromotyrosine-derived peptides endowed with Gram+ antibacterial and anti-inflammatory properties; in addition they inhibit topoisomerase II, dehydrofolate reductase and the endothelin A receptor [106]. Cold-water Dendrilla membranosa contains membranolides used as natural antifeedants and other microbe-derived bioactive compounds [107]. The suspicion that indwelling bacteria may be responsible for much of the reported bioactivities in sponges in the Dendrilla genus has prompted the taxonomic characterization of culturable strains in Dendrilla nigra [108].
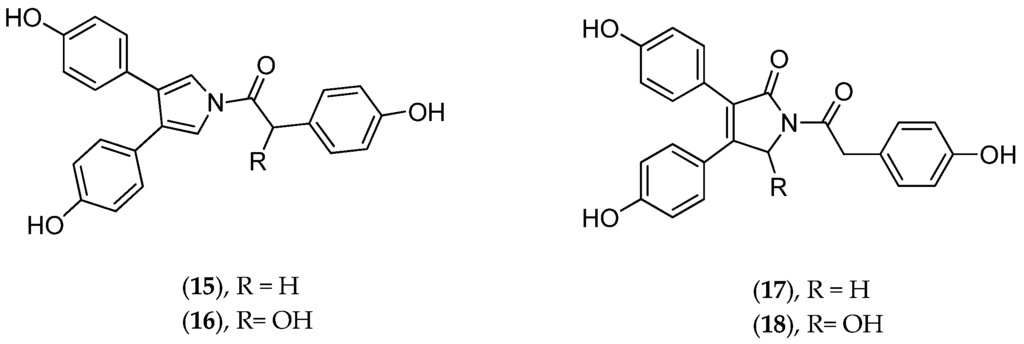
Figure 8.
Structure of neolamellarin A (15), 7-hydroxylamellarin A (16), neolamellarin B (17) and 5-hydroxylamellarin B (18).
Another interest in Dendrilla R171 stems from bioassays as serine protease inhibitors (§ 4.1.4) with remarkable anti-trypsin and anti-elastase activities in preliminary benchtop assays on crude extracts. With up to 100% inhibition on both bovine trypsin and porcine elastase activities, this sponge displays the most potent anti-serpin activity of the 133 invertebrate species tested in total. In 1988, collaborators in Montpellier isolated the active compound (a peptide) from the water-ethanol extract, bioguided by an anti-elastase assay (porcine and human) and they sequenced a 43-residue amino acid, but the structure was never published. This was long before aeruginosins, peptide anti-serine proteases first isolated from cyanobacteria in 1994. A whole family of more than 20 aeruginosins has been described since then, some from sponges of the genus Dysidea [109]. The presence of such compounds may explain the anti-trypsin activity if aeruginosins are also present in Dendrilla R171 (which has not been verified), but anti-elastase activity has never been associated with these molecules.
Thus, the identity of the trypsin and elastase inhibitor(s) from Dendrilla R171 remains unknown (or at least unpublished) to date. The fact that another tested Dendrilla (voucher #1225) does not display any inhibitory activity suggests that microbial symbionts—possibly cyanobacteria—are involved in the reported R171 activities.
Niphates sp.
A new C47 polyoxygenated acetylenic acid, nepheliosyne B (20, Figure 9B), along with the previously described nepheliosyne A (19, Figure 9B), have been isolated from Niphates sp. (Figure 9A), collected in 2008 in a southwest lagoon at 22 m depth (voucher #MHNM 1646, Natural History Museum of Marseille, mainland France). Both nepheliosyne A and B have shown cytotoxicity against K562 chronic myelogenous leukemia, U266 myeloma, SKM1 myelodysplastic syndrom and Kasumi acute myeloid leukemia human cell lines with IC50 values ranging from 150 to 200 µM [86]. Several polyhydroxylated acetylenic metabolites of marine sponges with a diacetylenic carbinol and a α-yne carboxylic group have been reported: nepheliosyne A (19, Figure 9B) from Xestospongia sp., petrosolic acid (21, Figure 9B) from Petrosia sp., osirisynes (22–27, Figure 9B) and haliclonyne (28, Figure 9B) from Haliclona sp., and fulvynes A–I (29–37, Figure 9B) from Haliclona fulva. Nepheliosyne B (20, Figure 9B) is, along with nepheliosyne A (19, Figure 9B) and petrosolic acid (21, Figure 9B), the third example of a linear acetylene with diacetylenic carbinol, α-hydroxyketone, and α-yne carboxylic groups. All these data suggest that, from a chemotaxonomic point of view, polyhydroxylated acetylenic metabolites constitute potential markers of Haplosclerida species.
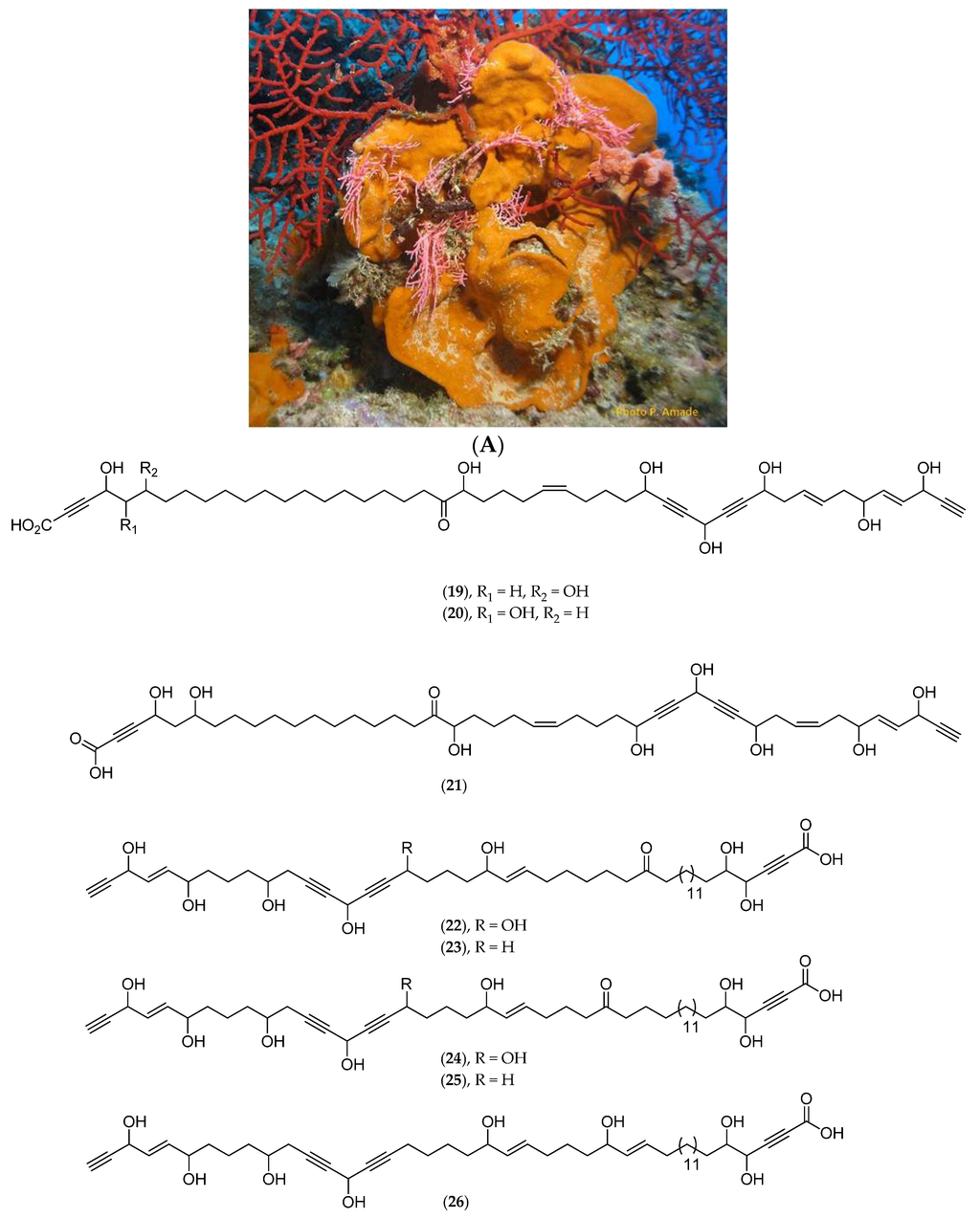
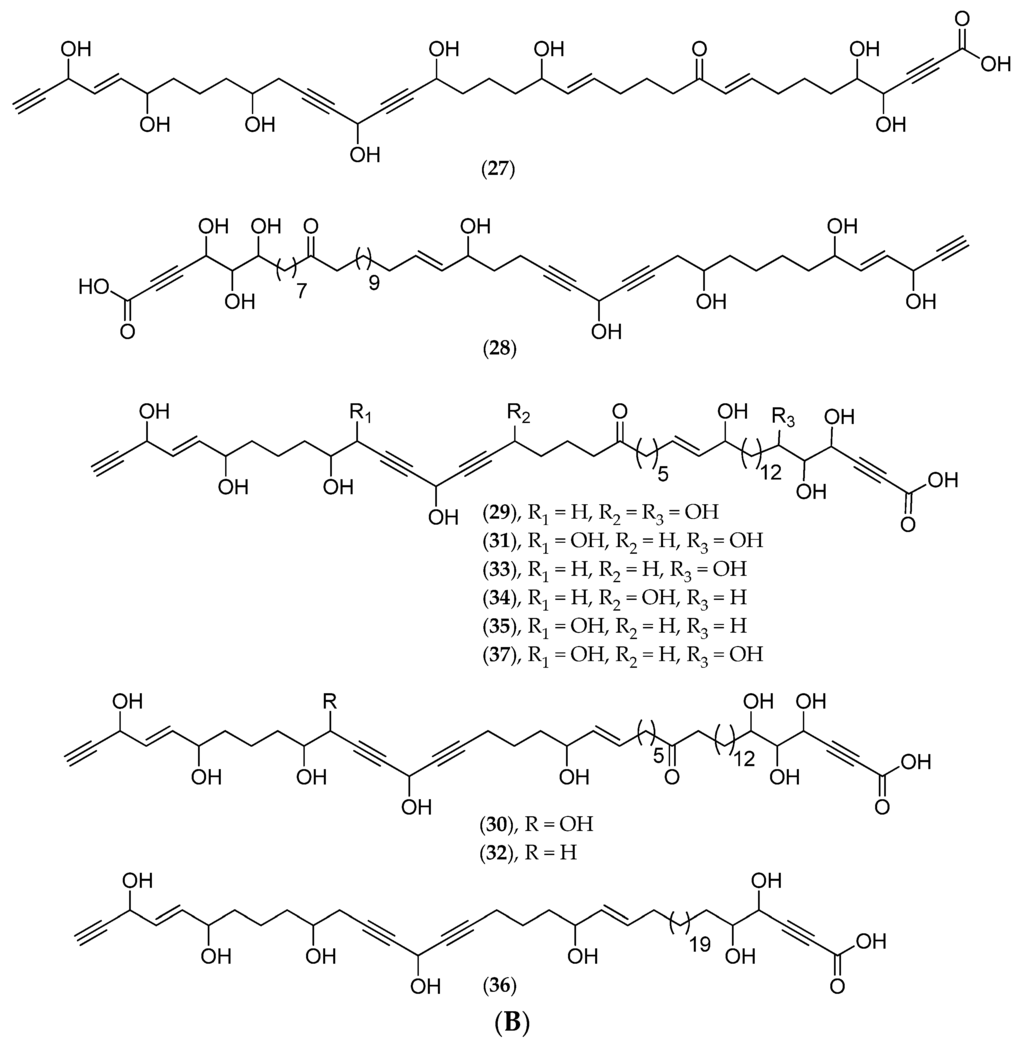
Figure 9.
(A) Niphates sp. Photo: Philippe Amade (INSERM); (B) Structure of nepheliosyne A (19), nepheliosyne B (20), petrosolic acid (21), osirisynes A–F (22–27), haliclonyne (28) and fulvynes A–I (29–37).
Corallistes sp.
This deep-sea sponge was collected by beam trawl at a depth of 350 m in the Coral Sea. The crude dichloromethanol extract shows cytotoxic activities against the KB naso-pharyngeal carcinoma cell line with an IC50 of 10 µg/mL. Subsequent chemical analyses led to the isolation of a free porphyrin called corallistin A (38, Figure 10) [50]. Its total synthesis followed a few years later [110].
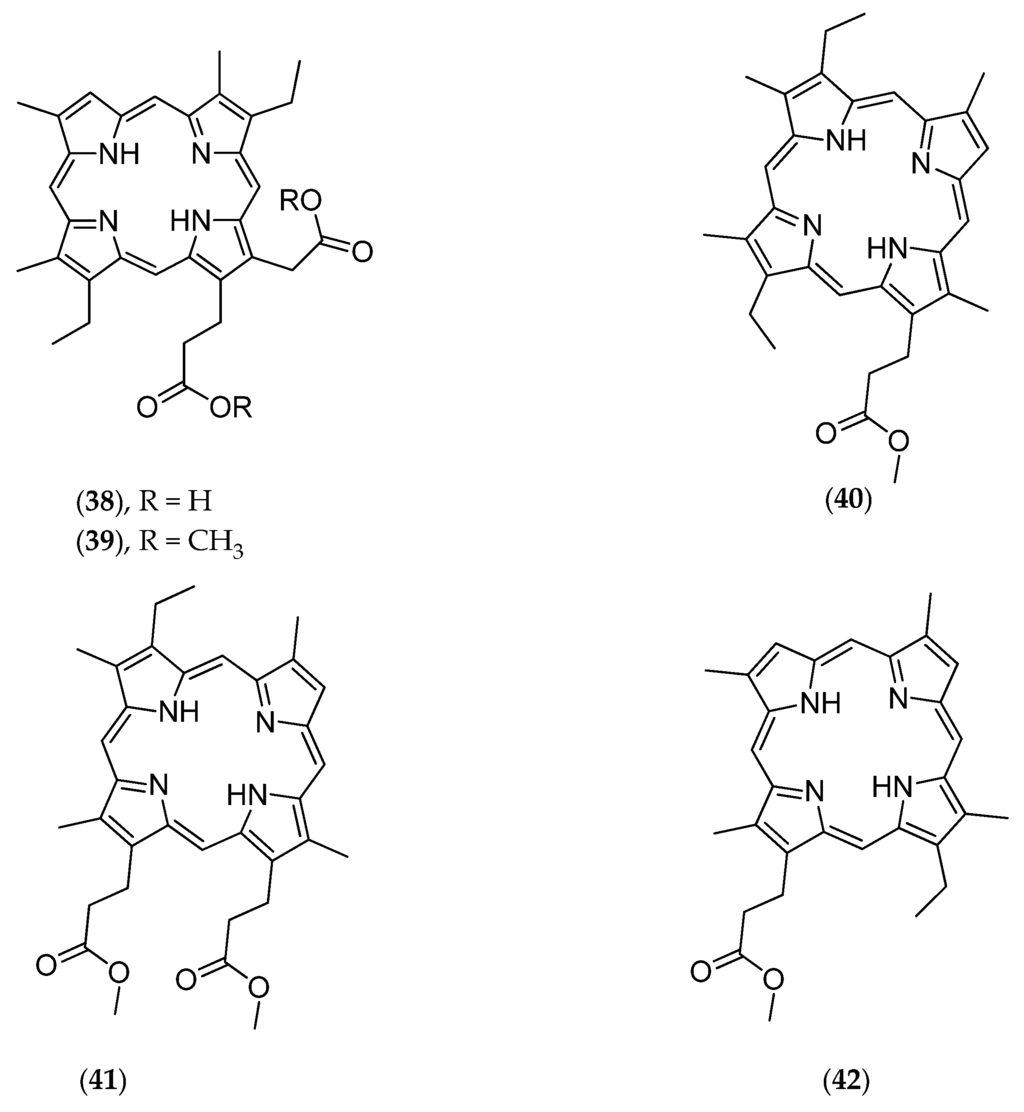
Figure 10.
Structure of corallistin methyl ester A (38), B (39), C (40), D (41), and E (42).
Further studies conducted on Corallistes sp. led to the isolation of corallistins B (39, Figure 10), C (40, Figure 10), D (41, Figure 10) and E (42, Figure 10). These porphyrins may be used as new photosensitizers in phototherapy, particularly by generating singlet oxygen toxic to cancer cells [51].
5.2. Ascidians
5.2.1. General Comments
Ascidians are invertebrate filter feeders that belong to phylum Chordata. They represent the most evolved invertebrates with a heart and a respiratory system. Living in both solitary and colonial sessile modes, ascidians can be found in all the world’s seas and at all depths. The external tunic of sea-squirts (tunicates) is made of tunicin, a cellulosic substance that is extremely rare in the animal kingdom [19]. Didemnids are soft-bodied, bag-like organisms. Like sponges, didemnids can harbor cyanobacterial photosymbionts, e.g., the photosynthetic genus Prochloron which lives in symbiosis with its host [111].
This class has a special “defense” feature: they can concentrate some toxic elements including heavy metals (notably vanadium), hydrocarbons and elementary sulfur, which may be found in Polycarpa aurata, or even sulfuric acid concentrated inside tiny vesicles of the outer tunic. Studies have also revealed high amounts of nitrogenous compounds (cyclic peptides and alkaloids) with powerful biological activities [112] (Table 3). As of 2007, 290 tunicates had been identified in New Caledonian waters [5].

Table 3.
Natural products isolated from New Caledonian ascidians and their bioactivity.
5.2.2. Lissoclinum bistratum
The didemnid Lissoclinum bistratum (voucher # UA264, IRD Nouméa, Figure 11A) was collected near the Ua islet in the southwest lagoon. Chemical studies followed when two cases of human intoxication were observed during manipulation of the lyophilized powder. The crude dichloromethanol extract of L. bistratum demonstrates acute toxicity on rats, mice and rabbits. Moreover, it has revealed cytotoxic activities with IC50 values of 45 nM, 20 nM and 22 nM on KB, P388 and normal human endothelial cells, respectively [115].

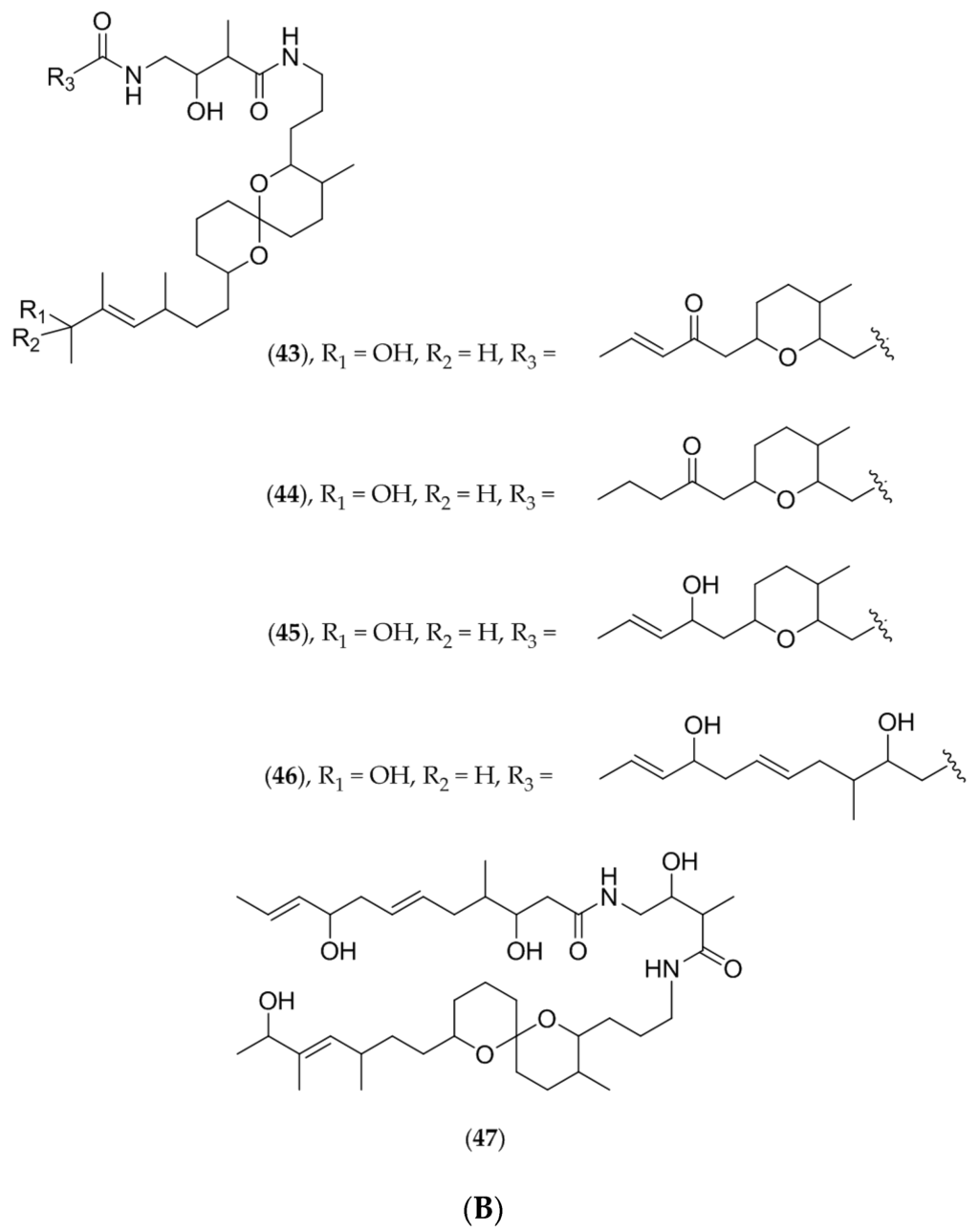
Figure 11.
(A) Lissoclinum bistratum, © IRD; (B) Structure of bistramide A (43), B (44), C (45), D (46), and K (47).
Separation and purification led to the isolation of a tetrahydropyran derivative called bistramide A (43, Figure 11B) (or bistratene). Its partial structure has been characterized using two dimensional NMR techniques [125].
Bistramide A (43, Figure 11B) appears to block the division of NSCLC-N6 (L16) cells at the G1 phase after a 24 h incubation period at a concentration of 1.42 µM. The IC50 value is 0.49 µM. Bistramide A (43, Figure 11B) may also induce polyploidy, causing unsuccessful cytokinesis [116]. It has revealed immunomodulator activities (suppressor/stimulator) on the proliferation of T and B cells [120]. Moreover, demonstrated using the voltage-clamp technique, bistramide A (43, Figure 11B) has proven to be responsible for a resting block by inhibiting sodium channels [118] and can bind to contractile proteins in competition with calcium in frog skeletal muscle fibers [119].
Later investigations performed on L. bistratum led to the isolation of new tetrahydropyran derivatives called bistramides B (44, Figure 11B), C (45, Figure 11B), D (46, Figure 11B) and K (47, Figure 11B). Bistramides A (43, Figure 11B), B (44, Figure 11B) and C (45, Figure 11B) show potent cytotoxic activities with IC50 values of less than 1 µg/mL on several cancer cell lines including KB, P388, P388 doxorubicin-resistant, B16, HT29, NSCLC-N6, MRC5CV1 fibroblasts and T24 bladder carcinoma cells. Bistramide K (47, Figure 11B), which is the least toxic compound, induces a complete blockage of NSCLC-N6 cells in the G1 phase after a 48 h incubation period with an IC50 value of 3.23 µg/mL [117,126].
5.3. Cnidaria
This phylum is divided into two subclasses, Octocorallia, which includes alcyonarians, gorgonians and pennatularians, and Hexacorallia, which includes scleratinarians. The chemical and biological properties of Cnidaria collected in New Caledonia are reported in Table 4.

Table 4.
Natural products isolated from New Caledonian Cnidaria and their bioactivity.
5.3.1. Alcyonarians
General Comments
Alcyonarians also called “soft corals” because they lack a calcium carbonate skeleton. This carbonate skeleton is replaced in most species by aragonitic sclerites, which are of major taxonomic importance for identification. They are benthic, sessile, colonial organisms often composed of many individual polyps stemming from a common sterile trunk, which is attached to the substratum by a glycoprotein “glue”. The main families are the Alcyoniidae, Nephtheidae and Xeniidae.
Alcyonarians often possess endodermal zooxanthellae which provide them with oxygen and photosynthates due to their photosynthetic activity. A total of 173 species have been reported in New Caledonian waters [5].
“Cocktails” of toxic secondary metabolites, especially diterpenes, occur in their tissues, and serve anti-predator (toxic and emetic effects) [130] and anti-competitor defense functions (contact necrosis and allelopathic growth inhibition [131], but also as anti-fouling substances and as egg protectants [132]).
Octocorals, which includes alcyonarians, gorgonians and pennatularians, are chemically characterized by terpenes, oxylipins such as prostaglandins and prostanoids, sterols and some aromatic derivatives [133]. These compounds have promising pharmacological value (Table 4).
Xenia Garciae
The “soft coral” Xenia garciae (voucher #3526, Northern Territory Museum, Darwin, Australia) was collected in shallow-water fringing reefs at a depth less than five meters. Chemical analyses isolated an original xenicane diterpene (48, Figure 12) that inhibits the growth of Ceramium codii, which is a common benthic Rhodophyta contributing to the process known as “fouling” [33]. Around the xenicane skeleton, as around the cembrane skeleton of alcyonids, are found dozens of bioactive diterpenes. The xenicane diterpenes, also found in some brown algae (Dictyotales), are particularly interesting as synthetic templates for the design of antitumor drugs [134].
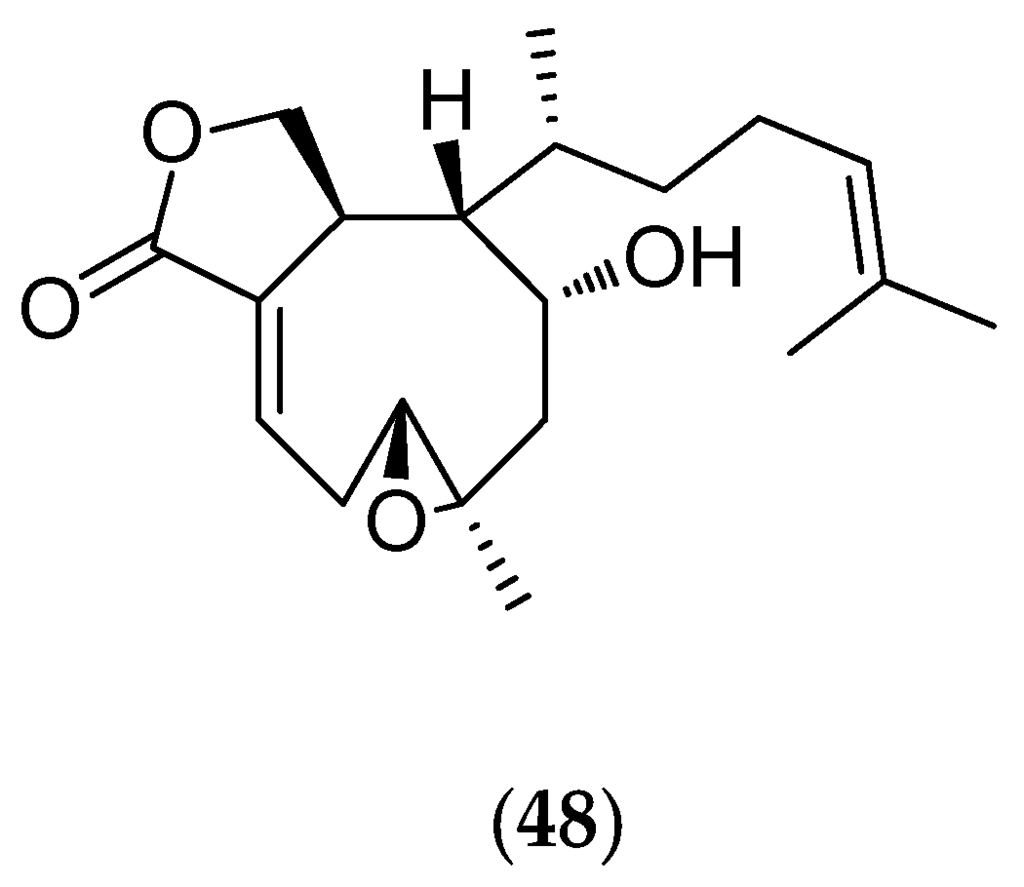
Figure 12.
Structure of xenicane (48).
5.3.2. Gorgonians
General Comments
Also known as sea whips or sea fans, gorgonians are “animal-flowers” that are supported either by a calcareous skeleton (suborder Scleraxonia) or by a flexible horny skeleton (suborder Holaxonia) made of a fibrous protein, gorgonin. Exclusively marine, these sessile invertebrates can live as solitary animals or in colonies. They are found in many places in all oceans and can occur in different growth forms such as bushes, whip-like branches and sometimes as blunt lobes or even flat crusts [18]. As of 2007, 93 species of gorgonians have been described in New Caledonian waters [5].
Melithea cf. Stormii
The sea fan Melithea cf. stormii (Figure 13A) is a large scleraxonian gorgonian commonly found on outer reef slopes. The voucher sample and the biological material used for sequencing and bioassays were taken from only one medium-sized specimen (voucher #HG163, IRD Nouméa) collected in Uitoe Pass, Southern Province at 20 m depth. Below, we summarize the main highlights of this specimen HG163; all details can be found in [37].

Figure 13.
(A) Melithea cf. stormii. Photo: Philippe Plailly (CNRS); (B) N-terminal amino acid sequence of iela melst (49). Half cysteine residues are noted in red, and correspond to half-cysteines of the non-classical Kazal-type inhibitor. The blue pair indicates P1-P1’ residues around the putative active site. One-letter codes for amino acids: A, alanine; C, cysteine; D, aspartate; E, glutamate; G, glycine; I, isoleucine; K, lysine; L, leucine; M, methionine; P, proline; S, serine; T, threonine; V, valine; X, unknown; Y, tyrosine.
From HG163, a 95% pure novel peptide elastase inhibitor was isolated using SEC and reversed-phase chromatography. This peptide of 39 residues in length was hereafter referenced as iela melst (49, Figure 13B) according to nomenclature. The amino acid sequence of the first 20 N-terminal residues of iela melst (49, Figure 13B) is homologous to that of iela anesu from the sea anemone Anemonia sulcata, a non-typical Kazal-type elastase inhibitor [135]. The Cha procedure [136] was used to monitor the inhibition kinetics of the amidolysis of the synthetic substrate [Suc(Ala)3pNA] by porcine pancreatic elastase (PPE) at different concentrations using iela melst (49, Figure 13B). This inhibitor works as a slow, tight-binding inhibitor of PPE. The equilibrium dissociation constant Ki (1.5 nM) of the complex is quite similar to that of the PPE-elafin complex, the latter being an antileuko-proteinase isolated from patients with psoriasis. The anti-elastase activity of iela melst (49, Figure 13B) is very comparable to those of most natural or synthetic PPE inhibitors. The presence of such a strong anti-elastase substance in gorgonian cortical tissues may hinder various necrotic processes caused by epibiotic fouling or extracoelenteric digestion by other coelenterates [37].
Villogorgia Rubra
Studies performed on the gorgonian Villogorgia rubra, collected near the Chesterfield Islands, led to the characterization of two new alkaloids called villogorgin A (50, Figure 14) and B (51, Figure 14), along with known compounds: caffeine (52, Figure 14), tryptamine (53, Figure 14), Nb-methyltryptamine (54, Figure 14) and 1,2,3,4-tetrahydro-β-carboline (55, Figure 14).
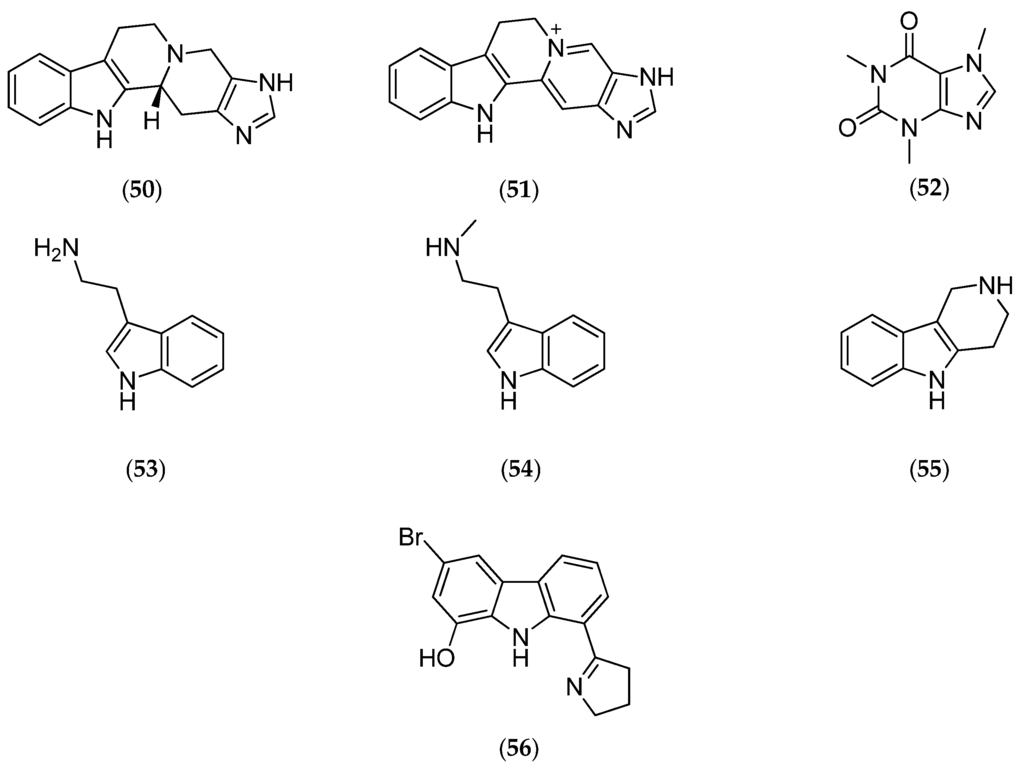
Figure 14.
Structure of villogorgin A (50), villogorgin B (51), caffeine (52), tryptamine (53), Nb-methyltryptamine (54), 1,2,3,4-tetrahydro-β-carboline (55) and eudistomidin-A (56).
Villogorgin A (50, Figure 14) inhibits (i) the contraction of the guinea pig ileum induced by acetylcholine; (ii) the aggregation of human platelets induced by thrombin and calcium ionophore A23187; this inhibition may be due to modulation of calcium/calmodulin-dependent enzymes. Villogorgin A (50, Figure 14) and B (51, Figure 14) are structurally related to the marine tunicate β-carboline alkaloid eudistomidin-A (56, Figure 14), a strong calmodulin antagonist [127].
5.3.3. Pennatularians
General Comments
Sea pens (sea feathers)—also called pennatularians—are a specialized and morphologically distinct group of octocorallian cnidarians.
Pennatularians are made of a thin tissue called the coenenchyme. These sessile animals live in colonies that are built from a single large primary polyp, the oozoid. They have been encountered in all oceans down to 6100 m depth [137].
Lituaria Australasiae
Lituaria australasiae was collected at night, inside St. Vincent Bay (west coast, Southern Province). From this octocoral, a novel class of macrocyclic lactones has been identified: lituarines A (57, Figure 15), B (58, Figure 15) and C (59, Figure 15). Lituarines have demonstrated antifungal activities on Fusarium oxysporum, Helminthosporium turscicum, Penicillium italicum and Phytophtora parasitica, as well as cytotoxic activities on KB cells with IC50 values of 3.7–5.0 ng/mL for lituarine A (57, Figure 15), 1–2 ng/mL for lituarine B (58, Figure 15) and ranging from 5–6 ng/mL for lituarine C (59, Figure 15) [128].
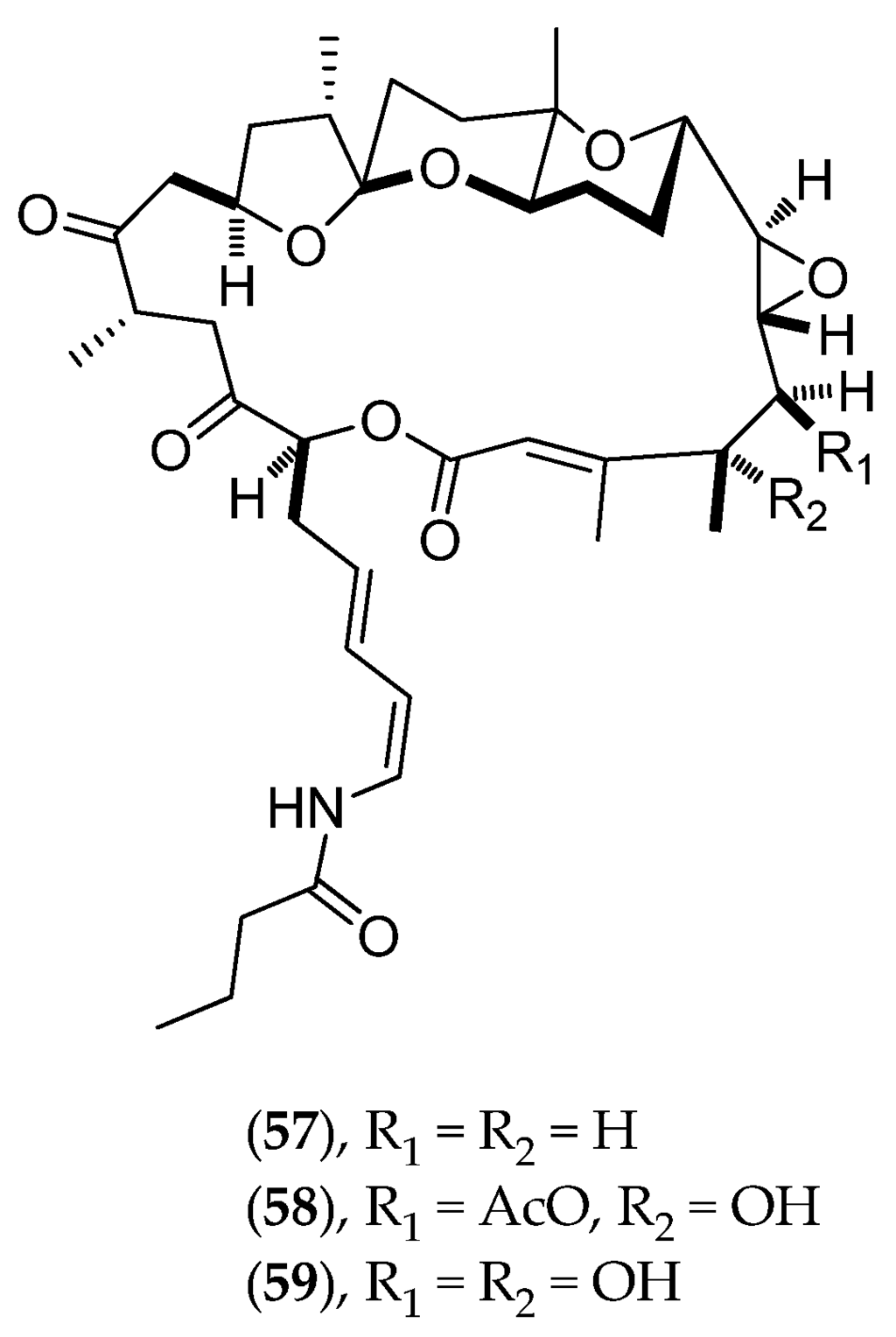
Figure 15.
Structure of lituarine A (57), lituarine B (58) and lituarine C (59).
5.3.4. Scleractinians
Scleractinians are also called “stony corals” or “hard corals”. Scleractinian colonies act as biological photosystems during sunlit hours and feed on plankton at night. Closely related to sea anemones, they are also armed with cnidocytes that can be used for predation as well as for territorial defense [138].
“Stony corals” have a large range of colonial growth forms, from crustose to massive, but mostly branching, corymbose, tabular or lamellate, with a few solitary and free-living forms, e.g., mushroom corals [14]. All corals originate from a minute swimming planula that settles and metamorphoses into a single solitary polyp, which itself undergoes successive budding to form a colony that encases itself in an aragonitic (calcium/magnesium carbonate) shell. Growth rates can vary from a few centimeters per year for fast growing branching acroporids that are shorter-lived and rarely very large, to a few millimeters per year at most for large poritid corals that can grow up to 10 m in diameter and live for many centuries.
Shallow-water corals contain Symbiodinium (symbiotic unicellular microalgae known as zooxanthellae) within their endodermal cells. These microalgae give the coral its color, which thus can vary in hue depending on the symbiont species. Scleractinians are usually photosymbiotes.
As of 2007, 310 species of scleractinians have been reported in New Caledonian waters [5]. Furthermore, the anthozoan Hexacorallia class may produce toxins (e.g., palytoxin) and other secondary metabolites such as venoms, similar to those of sea anemones, or photoprotection pigments (e.g., mycosporins, zoanthoxanthins) [139].
5.4. Echinoderms
5.4.1. General Comments
Phylum Echinodermata is divided into two subphyla, the Pelmatozoa including the class of Crinoidea (crinoids, sea lilies and feather stars), and the Eleutherozoa which comprises Asteroidea (starfish, sea stars), Echinoidea (sea urchins), Holothuroidea (sea cucumbers) and Ophiuroidea (brittle stars) [17].
Echinoderms are exclusively marine invertebrates that have an endoskeleton consisting of magnesium calcite. In all, 257 species of echinoderms have been reported in New Caledonian waters as of 2007 [5].
The therapeutic potential of these marine organisms is outlined in Table 5. Secondary metabolites produced by Crinoidea are sulfated anthraquinonic pigments, whereas Eleutherozoa produce quinonic pigments, naphthoquinones or sulfated saponins (e.g., asterosaponins, holothurins) [140].

Table 5.
Natural products isolated from New Caledonian Echinodermata and their bioactivity.
5.4.2. Actinopyga Flammea
The sea cucumber Actinopyga flammea (voucher #EH 025, IRD Nouméa) was collected at depths ranging from 35 to 50 m on the outer reef slope on the New Caledonian west coast. Highly ichthyotoxic, this species is used by fishermen in the Philippines to kill fish in coral holes. A specimen similar to that shown in Figure 16A reportedly caused the death of all fish overnight after a caretaker accidentally placed it in a fish tank at the Aquarium of Nouméa.
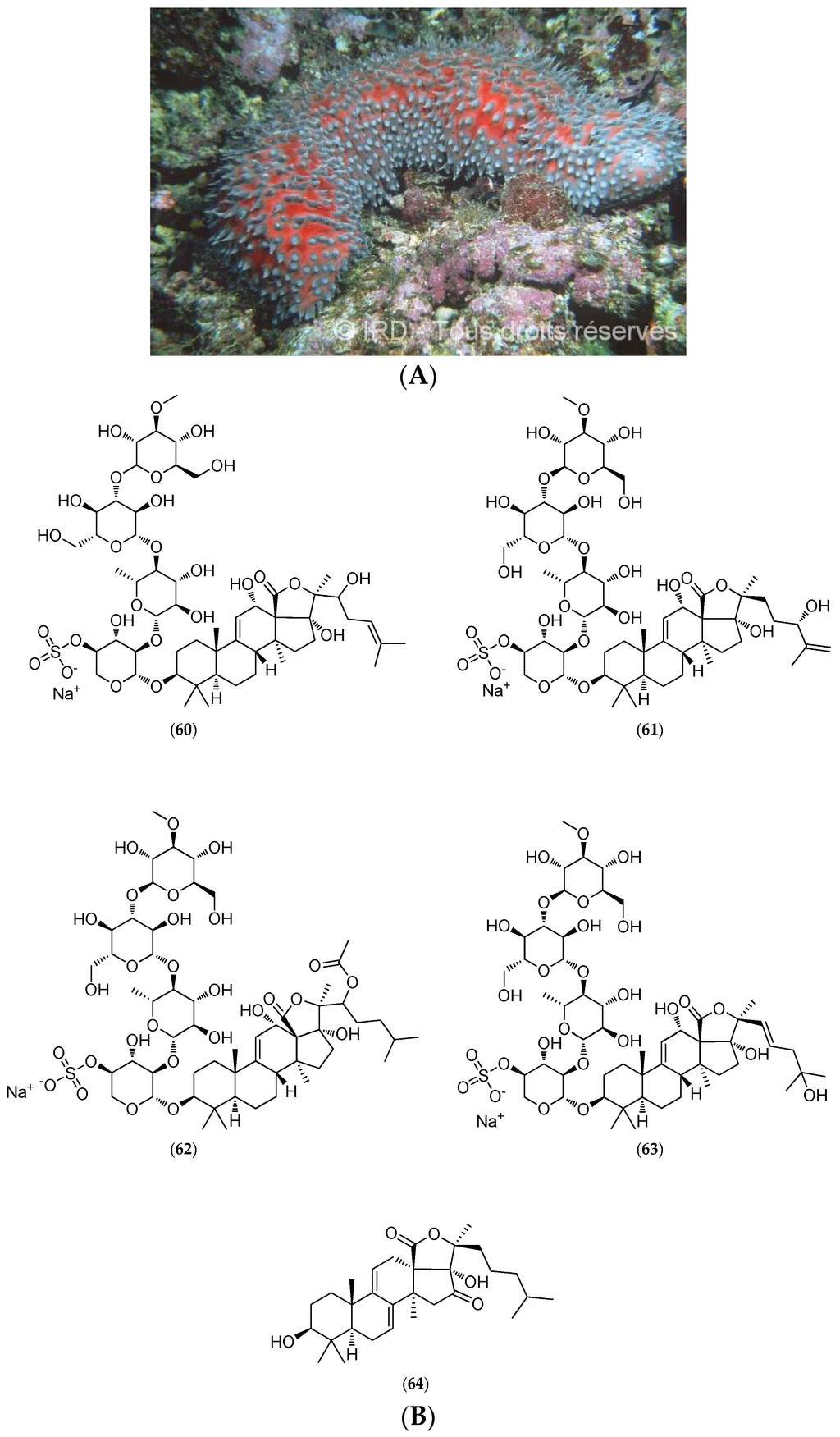
Figure 16.
(A) Actinopyga flammea. © IRD; (B) Structure of 24(S)-hydroxy-25-dehydro-echinoside A (60), 22-hydroxy-24-dehydro-echinoside A (61), 22-acetoxy-echinoside A (62), 25-hydroxy-dehydroechinoside A (63) and 16-keto-holothurinogenin (64).
Chemical studies led to the purification and characterization of triterpenoid saponins comprising the novel 24(S)-hydroxy-25-dehydro-echinoside A (60, Figure 16B), 22 ξ-hydroxy-24-dehydro-echinoside A (61, Figure 16B), 22 ξ-acetoxy-echinoside A (62, Figure 16B) and 25-hydroxy-dehydroechinoside A (63, Figure 16B) which were minor compounds. A new sapogenin, 16-keto-holothurinogenin (64, Figure 16B), was also obtained by acid hydrolysis of crude saponins [145].
5.4.3. Gymnochrinus Richeri
The “living fossil” Gymnochrinus richeri (voucher MNHN, Paris, mainland France, Figure 17A) was collected in 1987 at a depth of 520 m on Stylaster Bank, Norfolk Ridge. Chemical studies isolated gymnochromes A–D (65–68, Figure 17B), isogymnochrome D (69, Figure 17B), which constitute a new group of brominated phenanthroperylenequinones with an hypericin core [141], and also of some sterols such as cholest-4-en-3-one (70, Figure 17B) and cholesta-1,4-dien-3-one (71, Figure 17B) [146].
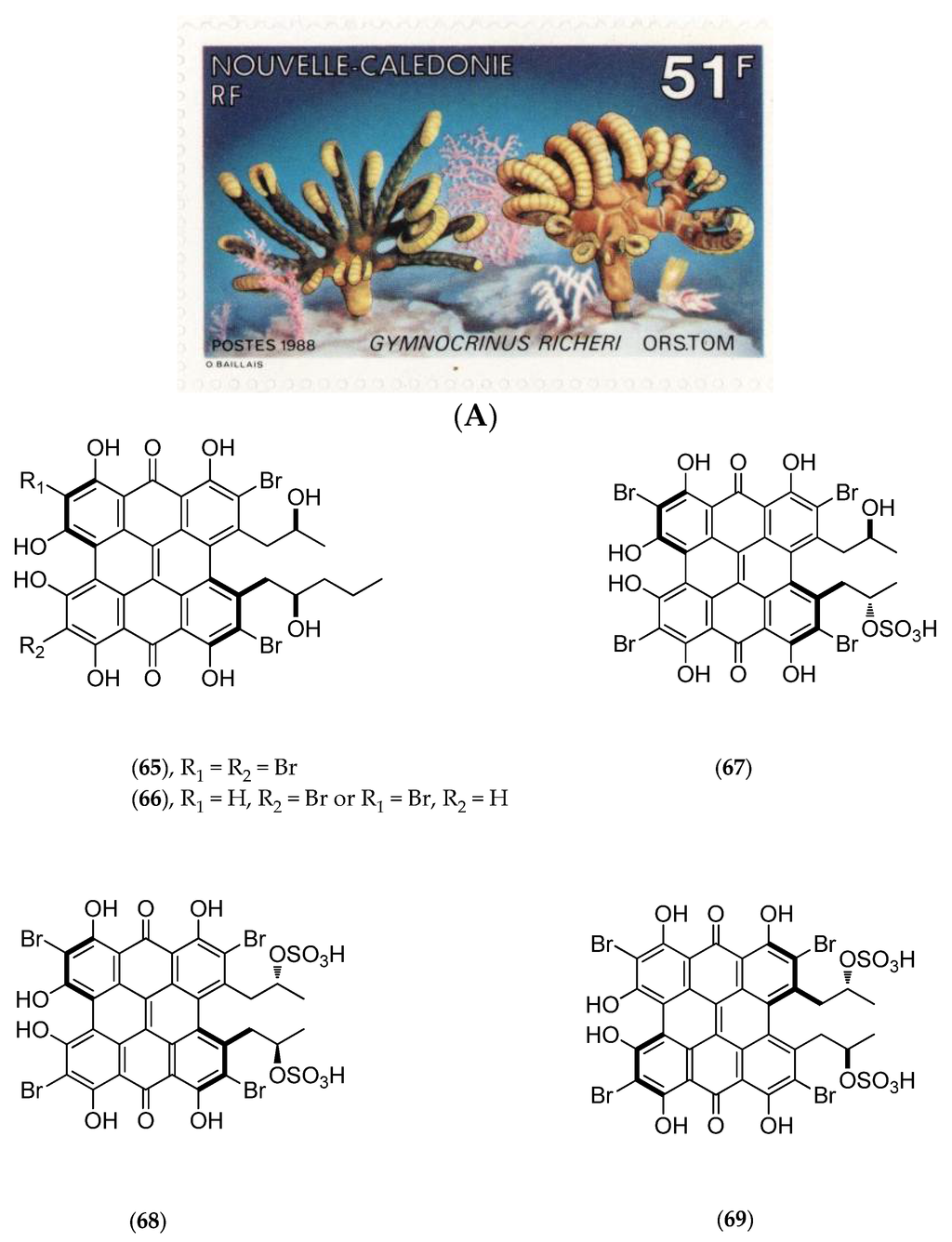
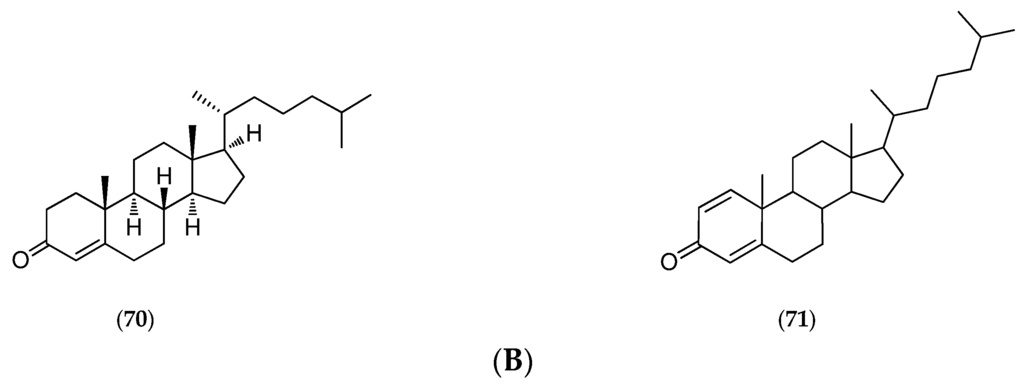
Figure 17.
(A) Gymnochrinus richeri. Postage stamp issued by the Office des Postes et Télécommunications (1988) (courtesy of a private collection); (B) Structure of gymnochrome A (65), B (66), C (67), D (68), isogymnochrome D (69), cholest-4-en-3-one (70) and cholesta-1,4-dien-3-one (71).
Gymnochromes B (66, Figure 17B), D (68, Figure 17B) and isogymnochrome D (69, Figure 17B) possess antiviral activities in vitro against the human immunodeficiency virus (A. Bousseau, unpublished results) and the dengue virus with a 50% viral titer reduction factor (RF50%) of less than 1 µg/mL [142].
Further studies have revealed that photoexcitation of gymnochrome A (65, Figure 17B) may induce electrophysiological effects such as the blockage of background K+ current in the atrial region of the frog heart muscle [147]. Moreover, gymnochrome B (66, Figure 17B) has demonstrated powerful virucidal and antiviral photoactivity with median effective dose (ED50) of 0.042 nM/mL and 0.029 nM/mL, respectively. This photoactivity may be partly due to its brominated hypericin core [148].
5.4.4. Thromidia Catalai
Thromidia catalai (voucher #EA065, IRD Nouméa, Figure 18A) is one of the largest known starfish species, discovered by René Catala in the inner lagoon close to Dumbea Pass in the barrier reef of the Southern Province, around 25 m in depth. This massive five-armed species reaches up to 60 cm in diameter and weighs several kilograms.
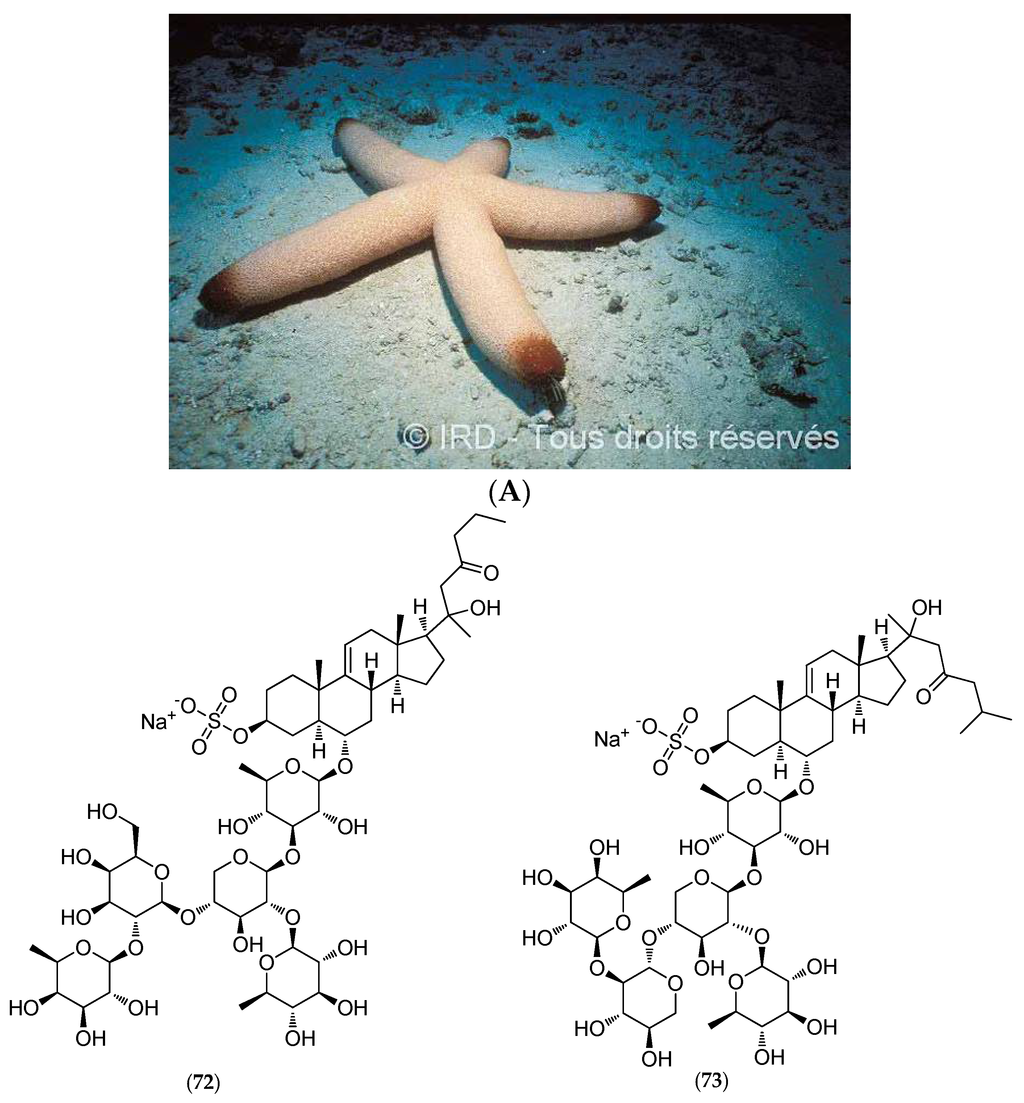
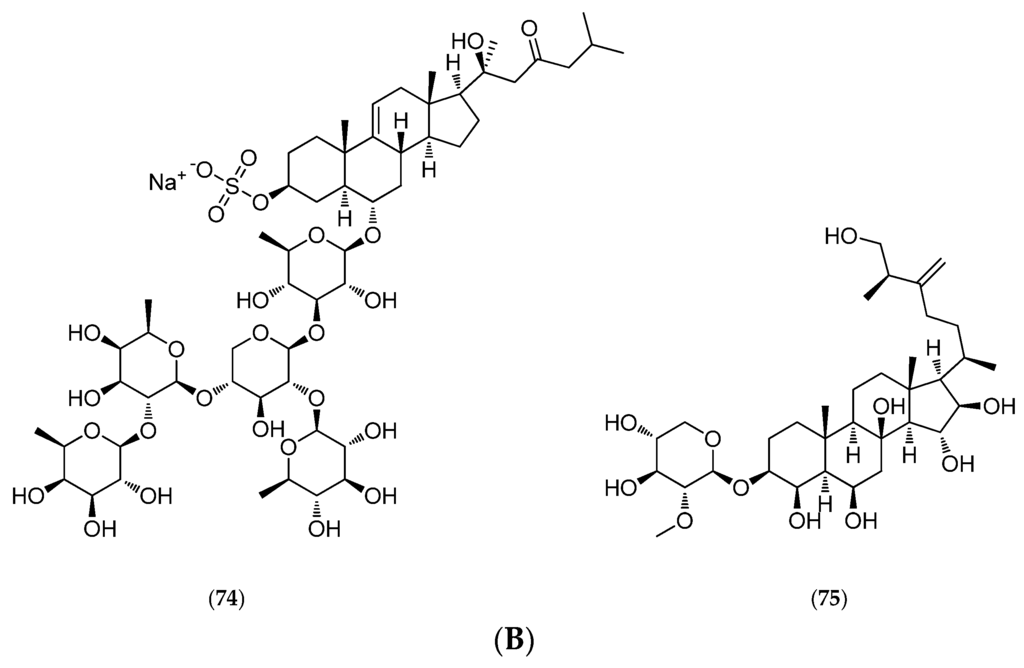
Figure 18.
(A) Thromidia catalai. © IRD; (B) Structure of thornasteroside A (72), ophidianoside F (73), regularoside B (74) and thromidioside (75).
In addition to three previously described sulfated asterosaponins (thornasteroside A (72, Figure 18B), ophidianoside F (73, Figure 18B) and regularoside B (74, Figure 18B)), which belong to a family of strong surfactants with ichthyotoxic properties, polar T. catalai extracts revealed a new steroid monoglycoside called thromidioside (75, Figure 18B) [149].
5.5. Macroalgae
Macroalgae belong to three different divisions: red algae (Rhodophyta), brown algae (Heterokontophyta, also known as the Ochrophyta, class Phaeophyceae) and green algae (Chlorophyta). All three evolutionary lineages diverged early on according to their respective plastid configurations, reflecting their endosymbiotic history [150].
Algae have a relatively unspecialized vegetative apparatus called a thallus. Important differences are noted in many ultrastructural and biochemical features including photosynthetic pigments, storage compounds, composition of cell walls, presence/absence of flagella, ultrastructure of mitosis, connections between adjacent cells, and the fine structure of chloroplasts. They vary from small, single-celled forms to complex multicellular forms, such as the giant kelp Macrocystis pyrifera that can grow up to 49 m in length in New Zealand and holds the record of the fastest growing organism (0.6 m per day).
Marine macroalgae generally live attached to rocks or other hard substrata in coastal areas but can be also found in sandy areas such as the Udoteaceae in tropical areas. They occur in all of the world’s oceans where they occupy the seabed (phytobenthos), although a few species float freely on the surface (e.g., the pelagic algae of the Sargasso Sea). In New Caledonia [151], the large brown algae are mainly Fucales (Sargassum, Turbinaria) and Dictyotales and form large stands on rocky lagoon bottoms, while several Chlorophyta species from the genera Halimeda and Caulerpa colonize sandy bottoms [152,153,154,155]. The large fleshy Rhodophyta are mainly restricted to outer slopes or the cooler waters of the southern lagoon. As of 2007, 454 species of algae and marine angiosperms had been reported in New Caledonian waters [5].
Despite their capacity to produce large amounts of natural products, macroalgae from New Caledonia have been little documented in this respect. Caulerpa species were investigated for caulerpicin (76–80, Figure 19) activity in the 1980s for cosmetic purposes, but the highly publicized invasion of Caulerpa taxifolia in Mediterranean Sea brought the project to an immediate halt [156]. The genus Lobophora (Phaeophyta) has been investigated in biological interaction studies between macroalgae and several corals as part of a PhD thesis. Three new compounds have been isolated and described [34], and their bioactivity experimentally tested on corals results in severe coral bleaching.
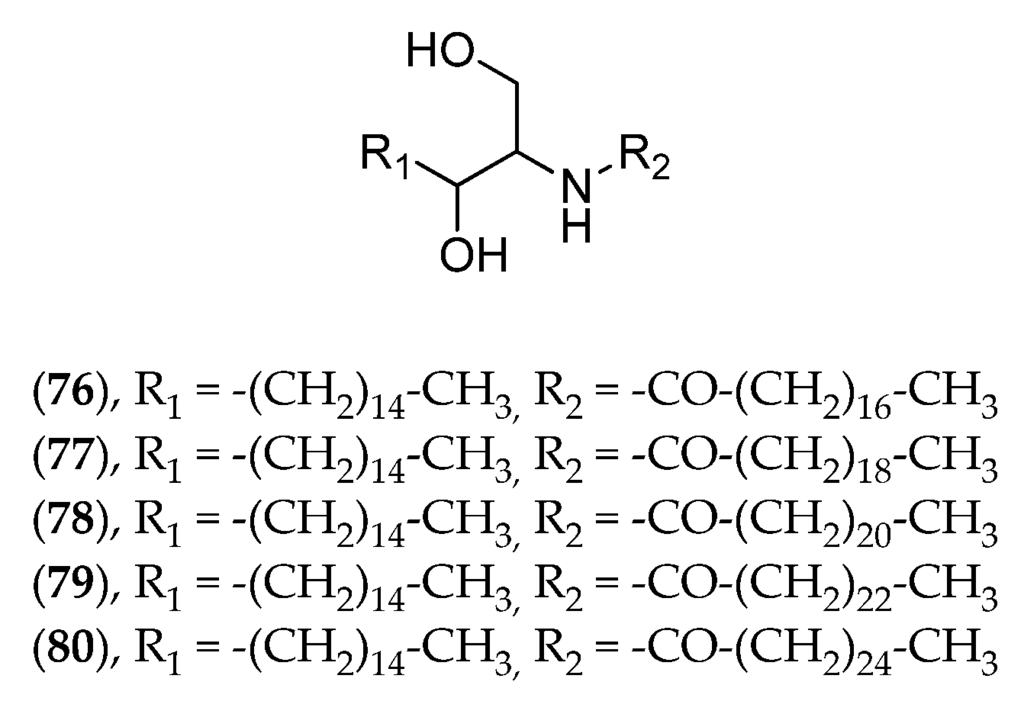
Figure 19.
Structure of caulerpicin C18 (76), C20 (77), C22 (78), C24 (79) and C26 (80).
5.6. Microalgae and Cyanobacteria
Less than 1% of described marine phytoplanktonic species are known to produce potent toxins [157]. Microalgal benthic dinoflagellates species are involved, including Gambierdiscus toxicus that produces maitotoxin (81, Figure 20). In vivo toxicity in mice indicates that intraperitoneal injection of 0.13 µg/kg is lethal [158]. A culture of ca. 4000 L of dinoflagellate cells is needed for the purification of 20 mg of MTX, enough to kill about four million mice. MTX activates both voltage-sensitive and receptor-operated calcium channels in the plasma membrane, thus inducing calpain protease that rapidly leads to cell death. MTX has since been considered as a useful tool for investigating the physiological and pathophysiological roles of calpain in neuronal cells [159].
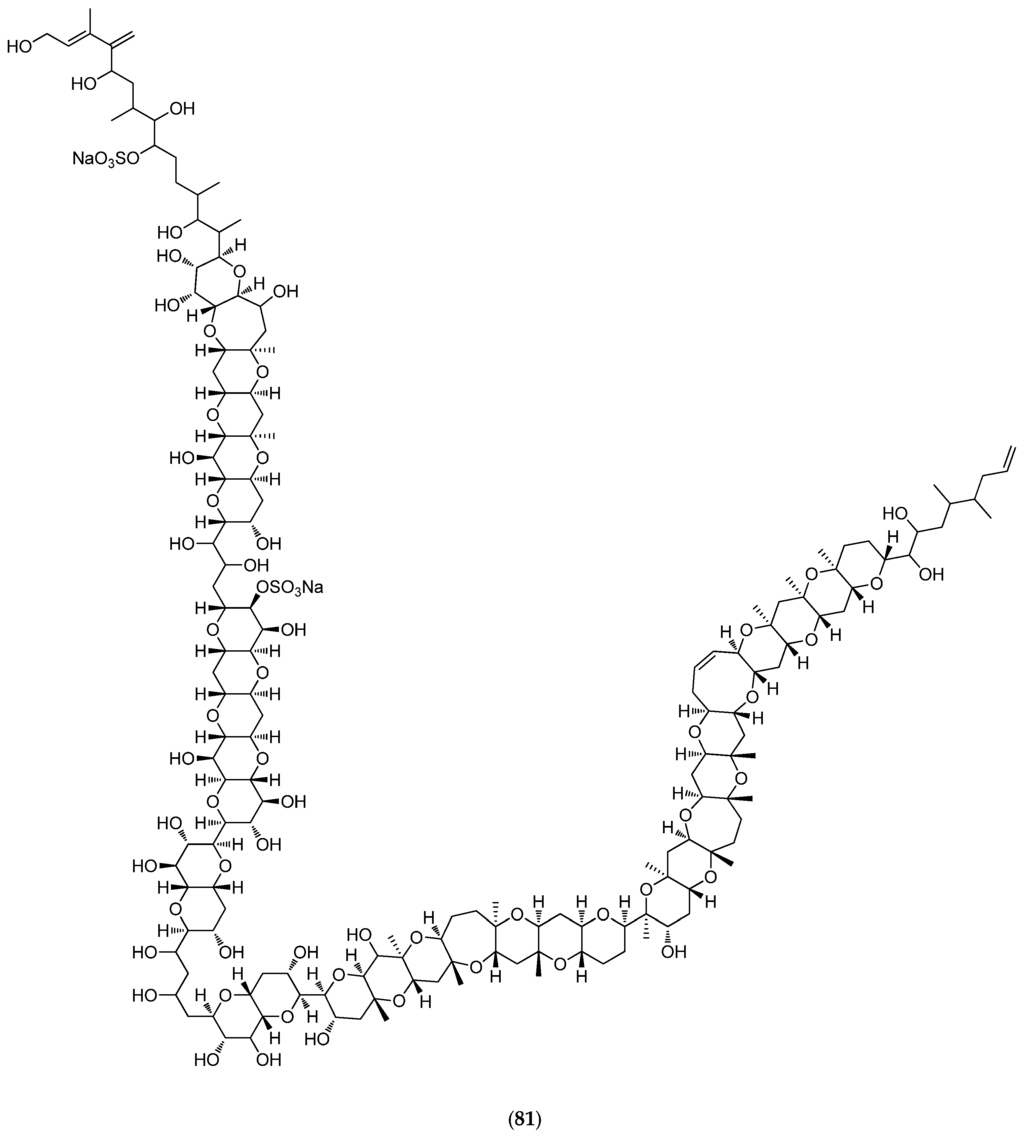
Figure 20.
Structure of maitotoxin (81).
Species belonging to the genus Gambierdiscus have also been shown to produce ciguatoxin analogues (CTX analogs) causing ciguatera fish poisoning (CFP). New Caledonia is a CFP-endemic area. CFP constitutes a global health problem with more than 50,000 people worldwide affected by this disease annually. CTXs arise from biotransformation/metabolization of CTX analogs through the food chain: from herbivorous fish that accumulate dinoflagellate toxins by eating dead coral and marine algae to carnivorous fish [157]. In Pacific areas, the principal and most potent CTX is Pacific ciguatoxin-1 (82, Figure 21). CTXs are potent activators of voltage-sensitive sodium channels and cause an increase in neuronal excitability and neurotransmitter release [160]. In New Caledonia, traditional remedies are commonly employed in the treatment of CFP: 90 plant species have been catalogued as useful CFP remedies, including the leaves of Heliotropium foertherianum used to prepare the most popular herbal remedy [161].
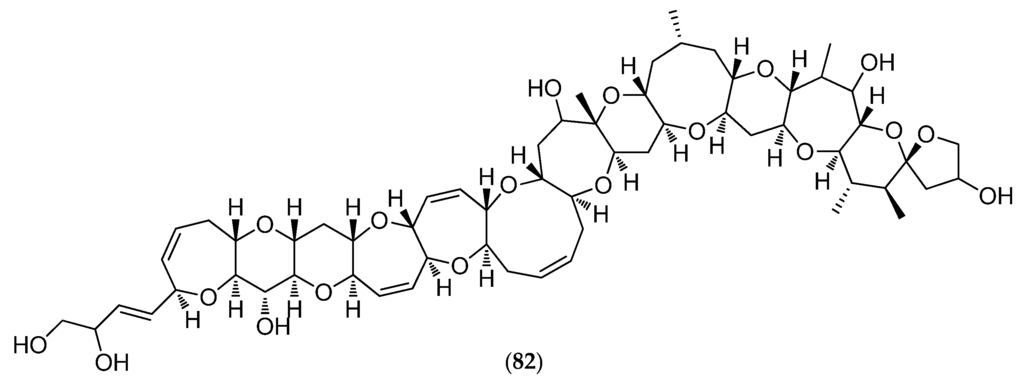
Figure 21.
Structure of Pacific ciguatoxin-1 (P-CTX-1) (82).
A study conducted by D. Laurent et al. following the observation of many cases of seafood poisoning on Lifou Island between 2001 and 2005 (Loyalty Islands, New Caledonia, Figure 1), indicates that bloom-forming cyanobacteria, such as the filamentous Trichodesmium erythraeum, can also produce CTX-like compounds [160].
In line with programs exploring the biodiversity of planktonic species such as OCEANOMICs (see [162] for details), the project AMICAL (Aquaculture of MIcroalgae in New CALedonia, [163]) instigated by IFREMER-New Caledonia, the Physiology and Biotechnology of Algae (PBA) laboratory (Nantes, mainland France) and ADECAL (Caledonian Economic Development Agency-Nouméa, [164]), aims to develop industrial microalgal production based on indigenous microalgae species isolated along the mainland coasts (Figure 1). Partners of this program will transfer the cultivation and extraction techniques to selected algal species for industrial programs in New Caledonia targeting the animal nutrition market and high added-value compounds (cosmetics, health food, etc.).
5.7. Other Biological Sources
Statistically “minor” phyla in terms of the number of studies on local species include prokaryotes, several invertebrate phyla, and snakes as the sole vertebrate representatives.
5.7.1. Prokaryotes and Fungi
Micrococcus Luteus
Micrococcus luteus is a Gram+ bacterium that was isolated from Xestospongia exigua sponges collected off Nouméa in the Southwest Pacific. Chemical studies reveal that this bacterium can produce lutoside (83, Figure 22), an acyl-1-(acyl-6′-mannobiosyl)-3-glycerol, and also triclosan (84, Figure 22) (2,4,4′-trichloro-2′-hydroxydiphenylether), with both molecules presenting antibacterial activities [165,166].
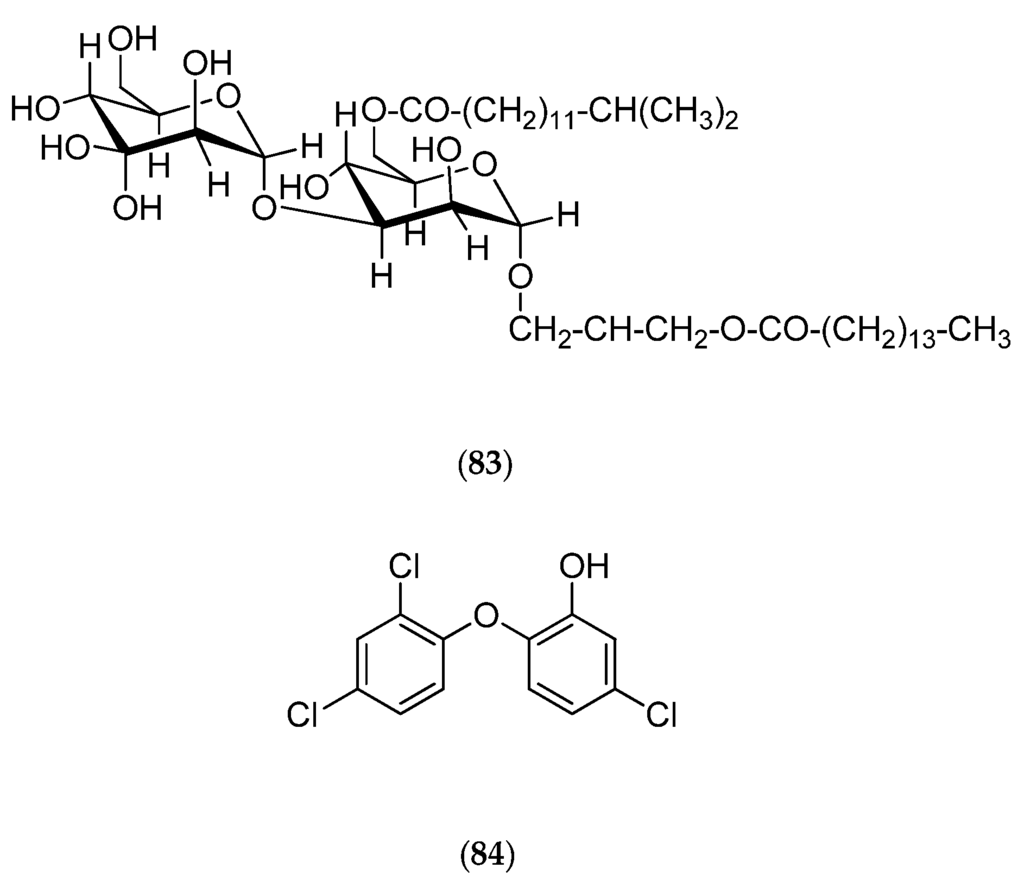
Figure 22.
Structure of lutoside (83) and triclosan (84).
Bacteria from Pseudoalteromonas and Vibrio Genus
Along the west coast of New Caledonia, 205 environmental samples were collected on a variety of surfaces (sediments, intertidal rocks, invertebrates, plants, fish and biofilms found on organic substrates). This sampling led to the isolation of 493 marine bacteria [167,168]. Studies were at first performed to assess their ability to produce exopolysaccharides (EPSs). Among them, the new strain Vibrio neocaledonicus sp. nov. (NC470), isolated from a biofilm found on Holothuroidea in St. Vincent Bay, has demonstrated the production of EPSs that have a high N-acetyl-hexosamine and uronic acid content with a low amount of neutral sugars. Preliminary experiments conducted on these EPSs have shown high metal-binding capacity [167]. Further studies have shown that four other strains (NC282, NC412, NC272 and NC120), which belong to the genus Pseudoalteromonas, have antibacterial potential against reference and multidrug-resistant pathogen strains such as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Enterococcus faecalis [168].
Acremonium Neocaledoniae
The culture of the marine fungus Acremonium neocaledoniae (moniliaceae), collected on driftwood in the southwestern lagoon of New Caledonia, led to the production of verrol 4-acetate (85, Figure 23) [169], a new cytotoxic metabolite sesquiterpene trichothecene, along with known trichothecenic mycotoxins (e.g., verrucarine A (86, Figure 23), isororidine A (87, Figure 23) and some previously described styrylpyrones, such as kawain (88, Figure 23), 7,8-dihydrokawain (89, Figure 23) and 5,6-dehydrokawain (90, Figure 23) [170].
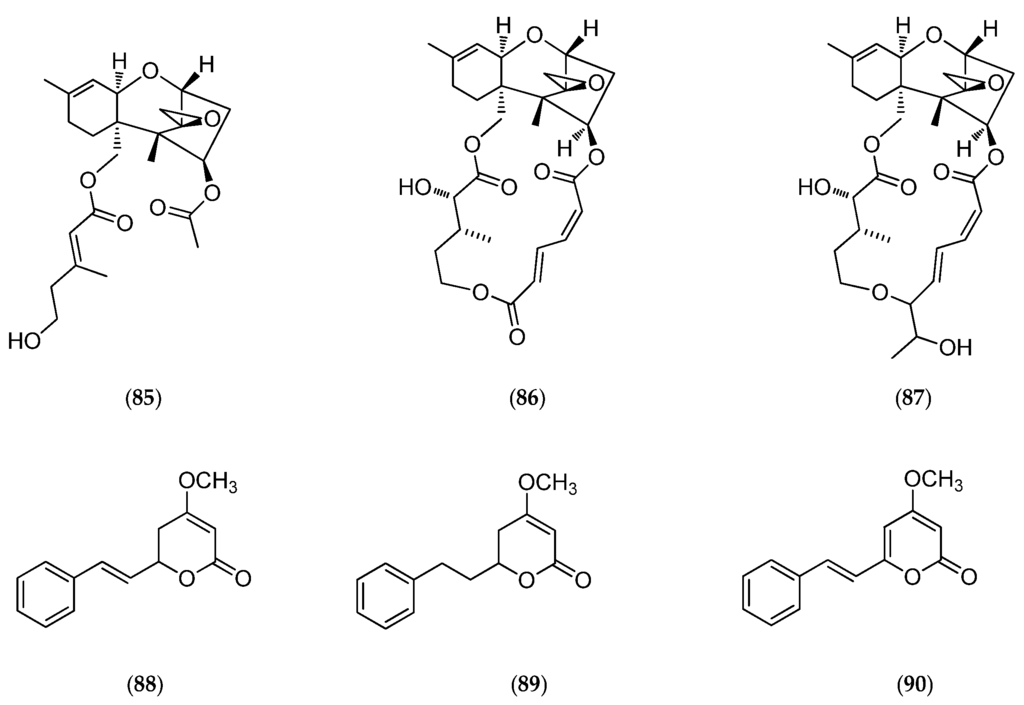
Figure 23.
Structure of verrol 4-acetate (85), verrucarine A (86), isororidine A (87), kawain (88), 7,8-dihydrokawain (89) and 5,6-dehydrokawain (90).
5.7.2. Venomous Cone Snails
Venomous marine cone snails (Conoidea) are neogastropod mollusks that predate on marine worms and small benthic invertebrates (thick-shelled snails, e.g., Conus textile) or that actively “hunt” fish (thin-shelled snails, e.g., Conus geographus) primarily by inflicting cocktails of peptide neurotoxins produced by venom glands via their modified harpoon-like radula (see review in Kaas and Craik, 2014 [171]). Fish-hunting cone snails may switch from prey-stimulated to defensive envenomation strategies as shown by the use of different and more potent “high threat” neurotoxins if threatened, the latter being regarded as a specialization in response to predation pressure from fish and cephalopods [172]. In addition, C. geographus (common in New Caledonia) diffuses a “cloud” of fish-like insulin that literally "tetanizes" the fish by eliciting hypoglycemic shock prior to envenomation [173], a possible metabolic cost-saving feature. Pharmacological applications of cone snail venoms have stemmed from the work of Olivera’s group on Conus magus, which inspired the biosynthesis of the most potent pain-killer on the market (ziconotide, Prialt®, Dublin, Ireland), claimed to be 1000 times more potent than morphine for the sedation of terminally ill cancer patients. The CONCO cone snail genome project for health funded by the European Commission was launched in 2005 to study the toxins composing the venom of the cone snail Conus consors (Figure 24) from the Chesterfield Islands using genomic, transcriptomic and proteomic approaches. The mitochondrial genome of C. consors has now been sequenced and gene annotated. The authors report the presence of a novel 700 bp control region absent from the hitherto known mitochondrial genomes of cone shells [174]. The species richness of venomous cone mollusks in New Caledonia is exceptional and prompts further investigations in the molecular biology, pharmacology and ecology of this fascinating group.

Figure 24.
Conus consors. Photo Jan Delsing [175].
5.8. Vertebrates: Venomous Marine Snakes
Globally, snake venom studies are of primordial importance for the design of novel antivenoms, for research tools in neurophysiology, and for a better understanding of the adaptive history of reptiles.
Like Australia, New Caledonia has no terrestrial venomous snakes, and Gail and Rageau established the first census of venomous marine snakes from the Elapidae family in 1955 [176]. Of the marine snakes that have been investigated by toxicologists, the most common ones are Laticauda laticaudata and its congener Laticauda colubrina, both highly venomous snakes that essentially hunt small fish underwater at night and rest on land during the day (Figure 25A).

Figure 25.
(A) Laticauda colubrina and Laticauda laticaudata. Postage stamps issued by the Office des Postes et Télécommunications (1983) (courtesy of a private collection); (B) Amino-acid sequence of erabutoxin b (91). Half cysteine residues are noted in red. One-letter codes for amino acids: C, cysteine; D, aspartate; E, glutamate; F, phenylalanine; G, glycine; H, histidine; I, isoleucine; K, lysine; L, leucine; N, asparagine; P, proline; Q, glutamine; R, arginine; S, serine; T, threonine; V, valine; W, tryptophan; Y, tyrosine. Adapted from [179].
L. laticaudata and L. colubrina rank, respectively, 9th and 46th among the 163 most venomous snakes (terrestrial and marine species of all continents). Being related to well-known Asian cobras and African mambas (Elapidae), but being much easier to handle (the mouth is very small and the hooks are adapted to bite prey during the swallowing process), laticaudas are an excellent model for toxicologists. A major component, erabutoxin b (91, Figure 25B) from Laticauda semifasciata binds with high affinity to muscular nicotinic acetylcholine receptors (nAChRs), but with low affinity to neuronal alpha-7 nAChRs and inhibits acetylcholine from binding to the receptor, thereby impairing neuromuscular transmission [177]. As a result, it produces peripheral paralysis by blocking neuromuscular transmission at the postsynaptic site. The first cDNA studies undertaken as part of a collaboration between the Centre d'Etudes Nucléaires de Saclay and Tokyo University [178] later included New Caledonian snakes in a review of the evolution of Elapid snake venoms [179].
Aipisurus laevis is a fully pelagic species, collected in surface waters of the lagoon in the Southern Province. A. laevis ranks 10 out of the 163 known most “globally dangerous” snakes, and 30 out of 163 in terms of toxicity (LD50 by injection in mice). Two cDNAs of short-chain neurotoxins were cloned and sequenced by Ducancel et al., (1990) [180], and compared with similar work on erabutoxin b (91, Figure 25B) from Laticauda snakes.
6. Recent Advances on Selected New Caledonian Marine Natural Products
Following their discovery in the course of ongoing New Caledonian scientific programs, and after promising results were revealed by preliminary biological screening and spectral characterization, further biological and chemical investigations on several molecules have been pursued to carry out (i) total synthesis; (ii) synthesis of chemical derivatives selected on the basis of structure-activity relationship (SAR) studies; (iii) determination of the mechanism of action; (iv) determination of additional bioactivities including in vivo and receptor studies. These studies and relevant updates are listed in Table 6.

Table 6.
Subsequent and ongoing development of selected natural products isolated from New Caledonian marine organisms.
Despite recent advances in developing therapeutic strategies, cancer is still one of the leading causes of death worldwide. Cancer is a group of diseases characterized by the uncontrolled growth and spread of abnormal cells. Drug-induced programmed cell death holds promise for cancer therapeutic approaches. Apoptosis is one of the well-known pathways used by cells to die. It is a morphological event characterized by chromosomal DNA fragmentation, nuclear disintegration, cell shrinkage, translocation of phosphatidyl serine moieties to the outer membrane leaflet, and membrane blebbing [209]. Numerous anticancer drugs, both on the market and in development, have apoptosis-modulating properties (e.g., Yondelis®, Madrid, Spain, has been recently approved by the American Food and Drug Administration (FDA) to treat patients with advanced soft tissue sarcoma [210]). Apoptogens are agents that can induce rapid death by modulating the apoptosis pathway. Numerous marine natural products reported in Table 6 are apoptogenic compounds associated with antitumor activity.
Agelastatins, members of the chemically diverse pyrrole-imidazole alkaloids (PIA), are among the best examples of this class of molecules. Since the discovery of agelastatin A (92, Figure 26) in 1993 by Pietra et al., in the New Caledonian coral sea sponge Agelas dendromorpha, more than ten different research groups have reported innovative solutions to synthesize it. As reported in Han et al., 2013, recent development of a concise, stereo-controlled, and biosynthetically inspired strategy to synthesize agelastatin alkaloids and a new synthetic methodology for azaheterocycle synthesis has given rise to many agelastatin derivatives. These developments also facilitate the first side-by-side testing of all known agelastatin alkaloids for their ability to induce cell death in various cancer cells [181]: U-937 (lymphoma), HeLa (cervical carcinoma), A549 (non-small-cell lung carcinoma), BT549 (breast carcinoma), and IMR90 (immortalized lung fibroblasts) human cell lines. Agelastatins A (92, Figure 26) and D (95, Figure 26) both induce dose-dependent apoptosis (in particular cell membrane permeabilization, activation of procaspase-3 to active caspase-3 and proteolytic cleavage of poly(ADP-ribose) polymerase (PARP)) and exhibit dose-dependent G2/M cell cycle arrest in synchronized U-937 cells without affecting tubulin dynamics within cells [181]. In addition, the potency of all agelastatins (92–97, Figure 26) has been evaluated in five human blood cancer cell lines. Interestingly, agelastatin C (94, Figure 26) is only weakly active and agelastatin F (97, Figure 26) is inactive against the tested cell lines. Agelastatin A (92, Figure 26) is remarkably active, particularly against CEM from acute lymphoblastic leukemia and against Daudi from Burkitt’s lymphoma (values reported in Table 6) and specifically target these white blood cell lines over normal red blood cells: it is 16,650 times more active on cancer cells than on normal cells! These results are in line with a previous study showing the inhibition of osteopontin-mediated neoplastic transformation (notably by β-catenin inhibition) and metastasis by agelastatin A (92, Figure 26) in MDA-MB-231 and MDA-MB-435 human breast cancer cell lines [211]. Therefore, agelastatins exhibit considerable potential as antitumor drugs that can simultaneously inhibit cancer cell growth as well as act as a potent anti-metastatic drug [211]. However, the mechanism through which agelastatin A (92, Figure 26) causes this cellular effect, particularly cycle arrest, still needs to be elucidated. Identification of potential intracellular targets of agelastatins can be performed by affinity chromatography on immobilized agelastatin. This approach has previously provided valuable insights into the cellular targets of various protein kinases inhibitors including hymenialdisine (12, Figure 5) [212].
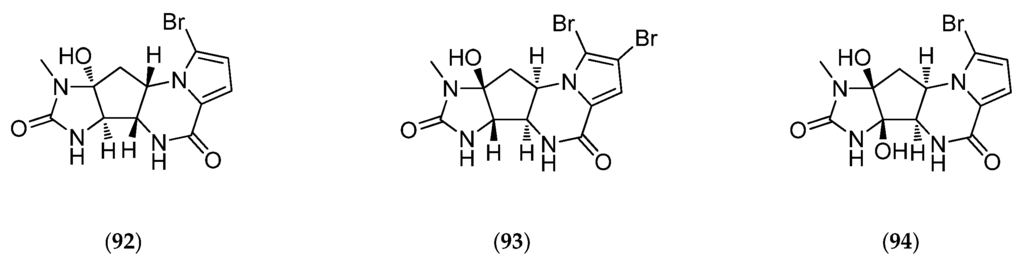
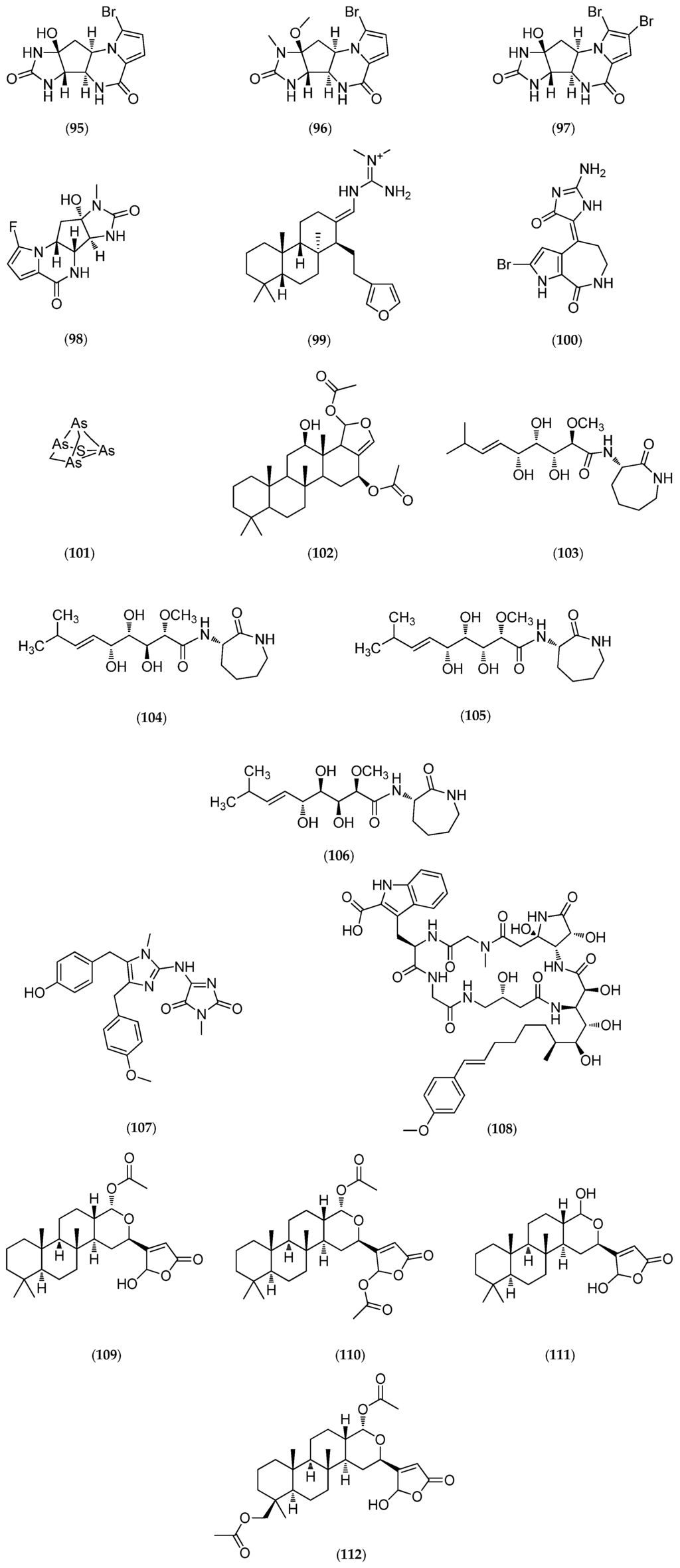
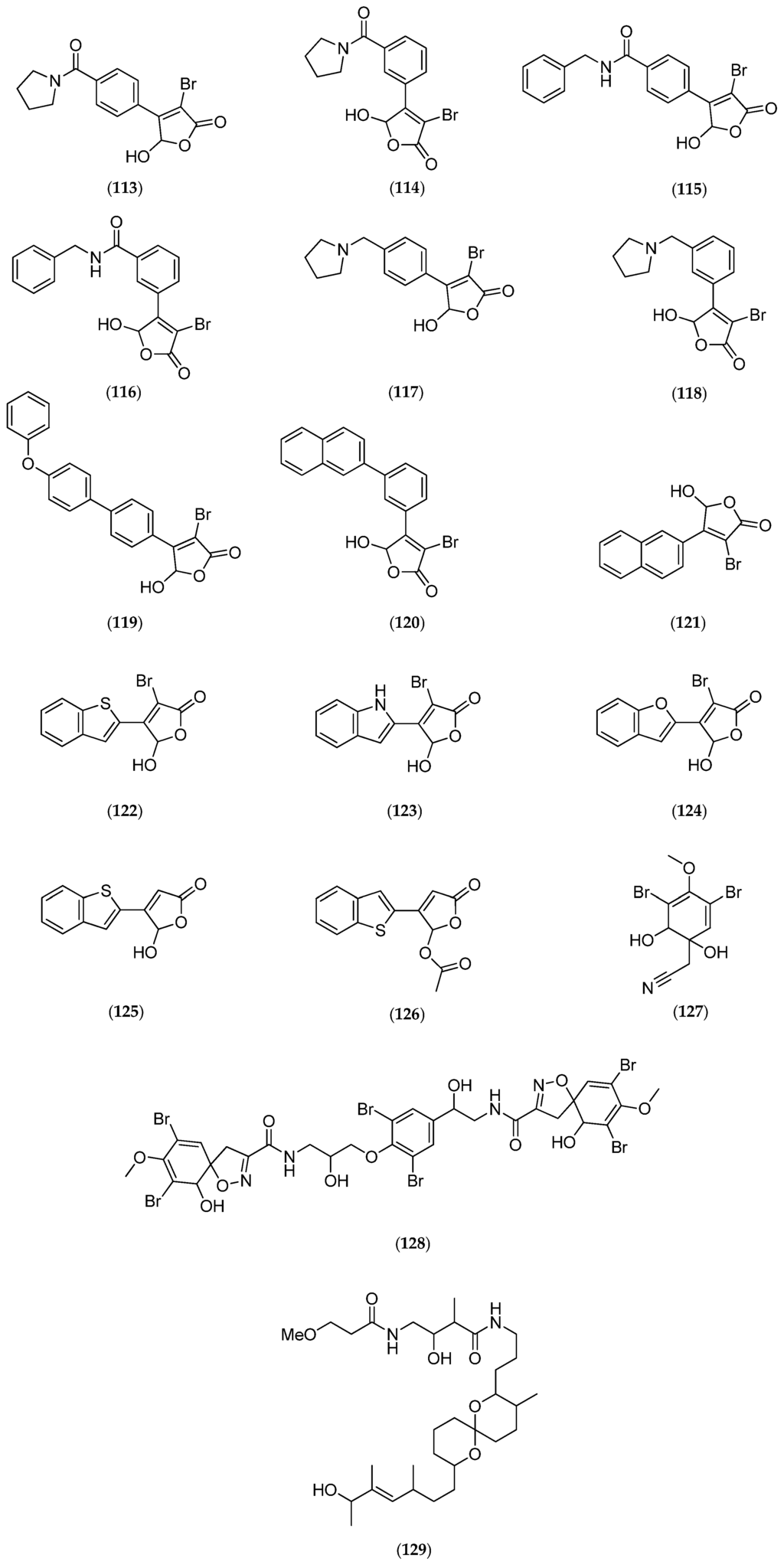
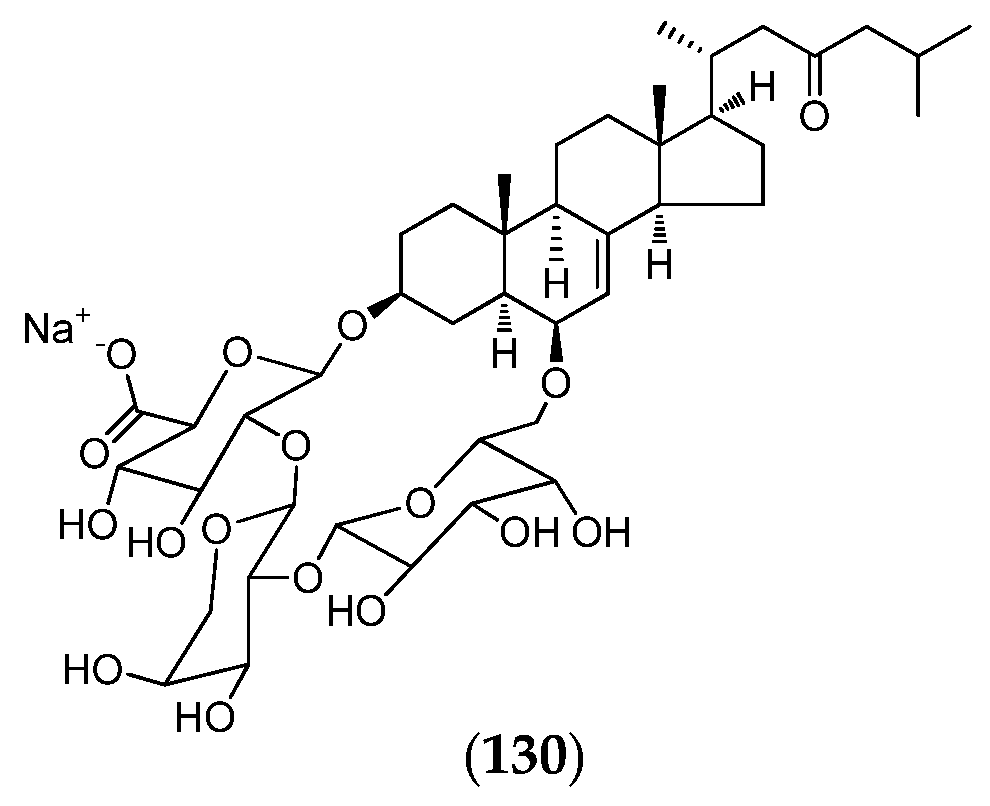
Figure 26.
Structure of agelastatin A–F (92–97), 13-debromo-13-trifluoromethyl agelastatin A (98), suvanine (99), isohymenialdisine (100), monosulfide arsenicin A (101), heteronemin (102), bengamide E (103) and analogs: 2,3-bis-epi-bengamide E (104), 2-epi-bengamide E (105) and 3,4-bis-epi-bengamide E (106), naamidine A (107), microsclerodermin A (108), petrosaspongiolide M (109), natural analogs of petrosaspongiolide M (110–112), first generation of synthetic petrosaspongiolide M analogs (113–120), second generation of synthetic petrosaspongiolide M analogs (121–126), aeroplysinin-1 (127), fistularin-3 (128), analogs of bistramide A (129), adapted from [206] and luzonicoside A (130).
Among the molecules reported in Table 6, heteronemin (102, Figure 26), a marine sesterterpene, has been shown to induce apoptosis in A-498 human renal carcinoma cells by downregulating apoptosis inhibitors Bcl-2 and Bcl-xL and upregulating the death agonist Bax, leading to the disruption of the mitochondrial membrane potential and the release of cytochrome c from mitochondria [192]. As also reported for agelastatins, these effects are associated with the activation of caspases (3/8 and 9), followed by PARP cleavage. Interestingly, the same study showed that heteronemin (102, Figure 26) also induces autophagy in A-498 cells, but the inhibition of autophagy enhances the anticancer effect of heteronemin (102, Figure 26) in A-498 cells. This result suggests that the combination of heteronemin (102, Figure 26) with autophagy inhibitors further enhances its therapeutic effects for cancer treatment [192]. Therefore, marine molecules can represent chemical tools for the exploration of novel combined therapeutic strategies. Petrosaspongiolide M (109, Figure 26) may also represent an equally valuable chemical tool for its capacity to modulate intracellular proteolysis through dual inhibition of the immunoproteasome and autophagy [202].
Synergistic effects (e.g., treatment with heteronemin (102, Figure 26) and inhibitors of autophagy such as chloroquine) of marine products and therapeutic drugs on disease-related phenotypes highlight the concept of combination therapy. The cytotoxicity of new marine molecules should be tested alone or in combination with known anticancer compounds to overcome drug resistance, as reported in Elmallah and Micheau (2015) for the resistance of cancer cells to TNF-related apoptosis inducing ligand (TRAIL)-induced cell death [209]. TRAIL is a molecule that selectively kills—via apoptosis—transformed and cancer cells, but not most normal cells [213]. Manzamine A, an alkaloid originally isolated from an Okinawan sponge Haliclona sp., may restore TRAIL-induced apoptotic cell death in the TRAIL-resistance pancreatic AsPC-1 cell line via inhibition of glycogen synthase kinase-3β (GSK-3β) and subsequent inhibition of the survival factor NF-κB [209]. It will thus be very interesting to evaluate the TRAIL-sensitizing activity of agelastatin A (92, Figure 26); which is known to inhibit GSK-3β with an IC50 of 12 µM [44] (manzamine A has an IC50 for GSK-3β inhibition of 10 µM [214]). All new marine natural products, including those from biodiversity hotspots such as the New Caledonia archipelago, represent putative new hope for cancer treatments, in particular for overcoming defects in apoptosis signaling.
As highlighted above, targeting apoptotic pathways may have a direct role in inducing tumor cell death, as confirmed by recent advances on marine molecules found in New Caledonia. In addition to agelastatins and heteronemin (102, Figure 26), naamidine A (107, Figure 26), microsclerodermin A (108, Figure 26), aeroplysinin-1 (127, Figure 26), fistularin-3 (128, Figure 26) have been shown to induce apoptosis (Table 6). Cancer is the principal target of the marine molecules released on the market today. Of the seven marine compounds currently on the market, only three (Prialt®, Dublin, Ireland; Yondelis®, Madrid, Spain and Lovaza®, London, UK) were not chemically modified to become drugs [215,216]. The four other molecules underwent lead optimization during the various different stages of their development. Developments in structural chemistry must be followed by the characterization of biological targets to optimize therapeutic applications.
7. Conclusions
Though far from being exhaustive, this review outlines 40 years of exciting research on the chemodiversity of marine organisms, ranging from microbes and invertebrates to vertebrates, from microalgae to macroalgae and halophytes, belonging to very different biota in association with the complex coral reef systems of New Caledonia.
Traditionally “interesting” lead groups such as sponges, cnidarians and ascidians have been intensely investigated because they not only provide the most interesting array of original chemical structures, but they also show the most potent anticancer, anti-inflammatory and antibiotic properties. Other groups have occasionally led to original and stimulating research: echinoderms, mollusks, etc. This review focused on novel products although several studies have been performed on New Caledonian marine species leading to the rediscovery of some secondary metabolites (e.g., Acremonium neocaledoniae, p. 41).
The fact that “minor” groups have been largely left aside does not reflect their lack of intrinsic interest for chemical exploration, but rather the technical difficulties to collect or cultivate them in New Caledonia. Only about 2% of marine bacteria can be cultivated using classical growth media. Some small invertebrates require time-consuming field and laboratory work to get enough molecular material to work on (e.g., bryozoans), are haphazardly encountered (e.g., many “naked” nudibranch and opistobranch mollusks) or are on an endangered species list, etc. Noteworthy are independent investigations on different models: e.g., biomaterials from scleractinian corals for maxillofacial replacement of bone tissue [217] or from oyster nacre as an osteoinductor in odontology [218]. Another example is that of the endemic Nautilus macromphallus cephalopod excretory metabolism. This metabolism relies on a unique and seemingly very stable symbiosis with specific bacterial symbionts to produce molecular nitrogen in the chambered shell and regulate the buoyancy [219].
Figure 2 indicates that the “golden age” of traditional screening for novel molecules and bioactivity has ceased after several periods of intense investigation. This reflects the successive explorations of new “territories”, e.g., sea mounts, outer reef slopes and deep benthos in general. Based on what has been achieved thus far, taking into account the exceptional richness of the local biota and the growing need to develop local economy in a balanced and sustainable way, it appears necessary to develop a permanent, reliable and collaborative research and development pipeline linking field exploration to:
- -drug development, including (i) isolation of active principles; (ii) high-throughput bioactivity screening; (iii) structure-activity investigations and structural elucidation; (iv) cultivation technologies or bioinspired synthesis; (v) clinical trials and beyond;
- -aquaculture focusing on the treatment of locally grown species of prawns and oyster varieties that are sensitive to seasonal blooms of toxigenic bacteria and microalgae.
Molecular approaches have now come of age, making new biological models more attractive and promising. New Caledonia represents a living laboratory with its unique source of marine organisms that have not been investigated to date. There is now a need to better understand the relationships between host organisms and their microbial flora. This requires on-site operations and specialized equipment (aquaria, cultivation facilities for microorganisms).
Finally, in the general context of “blue growth”, New Caledonia is ideally located for investigating the production of third-generation biofuels and of high value-added products (e.g., cosmetics, nutraceuticals) via the biomass of microalgal primary producers.
New Caledonia is a treasure chest for scientists to explore, but is also fragile. With the perspective of climate change due to global warming and emerging anthropogenic forcings, the sustainability of the sources of molecules, including resident bacteria, must take precedence. New Caledonian coral reefs—of which some portions were added to UNESCO’s World Heritage list in 2008—must be protected for the livelihood of resident populations, as well as for biological inspiration for the sciences and the arts. Extensive studies of their chemodiversity should contribute to their protection.
Acknowledgments
This review is dedicated in memoriam to the late Pierre Potier (CNRS) and to the late André Ménez (MNHN) who made the important decisions and provided essential support at crucial times. Major chemistry/pharmacology contributors historically involved in program management include (in alphabetical order): Ali Al-Mourabit, Philippe Amade, Dominique Bourret, Cécile Debitus, Stéphane La Barre, Dominique Laurent, Jacques Pusset and Thierry Sévenet. Major contributors with respect to the biology, ecology and history of New Caledonia include (in alphabetical order): Georges Bargibant, Pierre Laboute, Claude Lévy, Jean-Louis Menou, Claude Payri, Bertrand Richer de Forges and Philippe Tirard. Chemists and biologists from Italian Universities (Trente and Napoli) who have significantly contributed are gratefully acknowledged. Georges de Noni is warmly acknowledged for his continuing support. Serge Andréfouët is acknowledged for providing maps. The authors thank the Managing Editor for the publication fee waiver kindly offered to S. La Barre. S.-E. Motuhi is the recipient of a thesis grant from the Government of New Caledonia (Gov. NC). Authors warmly thank the Gov. NC for continuing support for research on marine natural products. S. Bach is supported by Biogenouest, Cancéropôle Grand-Ouest (axis: Natural sea products in cancer treatment), ANR/Investissements d’Avenir program via the OCEANOMICs project (grant #ANR-11-BTBR-0008) and INCa (“NECROTRAIL” Program).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pelletier, B. Geology of the New Caledonia Region and its Implications for the Study of the New Caledonian Biodiversity. In Compendium of Marine Species from New Caledonia, 2nd ed.; Doc. Sci. Tech. II7; Payri, C.E., de Forges, B.R., Eds.; IRD Nouméa: Nouvelle-Calédonie, France, 2007; pp. 19–32. [Google Scholar]
- Andréfouët, S.; Cabioch, G.; Flamand, B.; Pelletier, B. A reappraisal of the diversity of geomorphological and genetic processes of New Caledonian coral reefs: A synthesis from optical remote sensing, coring and acoustic multibeam observations. Coral Reefs 2009, 28, 691–707. [Google Scholar] [CrossRef]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.; Calado, R. Trends in the discovery of new marine natural products from invertebrates over the last two decades—Where and what are we bioprospecting. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Andréfouët, S.; Cabioch, G.; Flamand, B.; Pelletier, B. The diversity of New Caledonia Coral Reef Geomorphology and Genetic Processes: A Synthesis from Optical Remote Sensing, Coring and Acoustic Multi-beam Observations. In Compendium of Marine Species from New Caledonia, 2nd ed.; Payri, C.E., de Forges, B.R., Eds.; IRD Editions: Nouméa, Nouvelle-Calédonie, 2007; pp. 33–49. [Google Scholar]
- Payri, C.; de Forges Richer, B. Compendium of Marine Species of New Caledonia, 2nd ed.; IRD Editions: Nouméa, Nouvelle-Calédonie, 2007. [Google Scholar]
- Natural History Museum. Available online: http://www.nhm.ac.uk/nature-online/science-of-natural-history/expeditions-collecting/hms-challenger-expedition/ (accessed on 21 May 2015).
- Armbrust, E.V.; Palumbi, S.R. Uncovering hidden worlds of ocean biodiversity. Science 2015, 348, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Catala, R. Contribution à l’étude écologique des îlots coralliens du Pacifique Sud. Premiers éléments d’écologie terrestre et marine des îlots voisins du littoral de la Nouvelle-Calédonie. Bull. Biol. Fr. Belg. 1950, 84, 234–310. (In French) [Google Scholar] [PubMed]
- Catala-Stucki, R. Fluorescence effects from corals irradiated with ultra-violet rays. Nature 1959, 183, 949. [Google Scholar] [CrossRef]
- Salvat, B. Prospections faunistiques en Nouvelle-Calédonie dans le cadre de la mission d’Etudes des Récifs Coralliens. Cahtay. Pac. 1964, 6, 77–119. (In French) [Google Scholar]
- Plessis, Y. Un voyage en Nouvelle-Calédonie dans le cadre de la mission Singer-Polignac. Cahtay. Pac. 1962, 4, 81–83. [Google Scholar]
- Foundation Singer-Polignac. Expédition Française sur les récifs Coralliens de la Nouvelle-Calédonie, Organisée Sous l'égide de la Fondation Singer-Polignac, 1960–1962; Editions de la Fondation Singer-Polignac: Paris, France, 1961. (In French) [Google Scholar]
- Dahl, A.L. Surface area in ecological analysis: Quantification of benthic coral reef algae. Mar. Biol. 1973, 23, 239–249. [Google Scholar] [CrossRef]
- Dahl, A.L. Coral Reef Monitoring Handbook; South Pacific Commission: Noumea, New Caledonia, 1981. [Google Scholar]
- Laurent, D.; Pietra, F. Natural-Product Diversity of the New Caledonian Marine Ecosystem Compared to Other Ecosystems: A Pharmacologically Oriented View. Chem. Biodivers. 2004, 1, 539–594. [Google Scholar] [CrossRef] [PubMed]
- Lévi, C.; Laboute, P.; Bargibant, G.; Menou, J.L. Sponges of the New Caledonian Lagoon; ORSTOM Editions (IRD): Nouméa, Nouvelle-Calédonie, 1998. [Google Scholar]
- Guille, A.; Laboute, P.; Menou, J.L. Guide des étoiles de mer, Oursins et Autres échinodermes du lagon de Nouvelle-Calédonie; ORSTOM Editions (IRD): Nouméa, Nouvelle-Calédonie, 1986. (In French) [Google Scholar]
- Grasshoff, M.; Bargibant, G. Coral Reef Gorgonians of New Caledonia; IRD Editions: Nouméa, Nouvelle-Calédonie, 2001. [Google Scholar]
- Monniot, C.; Monniot, F.; Laboute, P. Coral Reef Ascidians of New Caledonia; IRD Editions: Nouméa, Nouvelle-Calédonie, 1991. [Google Scholar]
- Ineich, Y.; Laboute, P. Sea Snakes of New Caledonia; IRD Editions/Muséum National d’Histoire Naturelle: Paris, France, 2002. [Google Scholar]
- Laboute, P.; Grandperrin, R. Poissons de Nouvelle-Calédonie; Editions Catherine Ledru: Nouméa, Nouvelle-Calédonie, 2000. (In French) [Google Scholar]
- Moretti, C.; Debitus, C.; Fournet, A.; Sauvain, M.; Bourdy, G.; Laurent, D. Diversité biologique tropicale et innovation thérapeutique. Les recherches menées par l’ORSTOM. Ann. Soc. Belg. Med. Trop. 1993, 73, 169–178. (In French) [Google Scholar] [PubMed]
- Debitus, C.; Guézennec, J. Pharmacologie des organismes marins des milieux récifaux. In Valorisation et économie des Ressources Marines; Monaco, A., Prouzet, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 113–148. (In French) [Google Scholar]
- LagPlon Database. Available online: http://lagplon.ird.nc/index.xhtml (accessed on 18 June 2015).
- Cantharella Database. Available online: http://cantharella.ird.nc (accessed on 19 June 2015).
- Kerr, T.J.; McHale, B.B. Applications in General Microbiology: A Laboratory Manual, 6th ed.; Hunter Textbooks: Winston-Salem, NC, USA, 2001; pp. 202–203. [Google Scholar]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A microwell cytotoxicity assay using. Artemia Salina Plant Med. 1993, 59, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.C.; La Barre, S.; Sammarco, P.W.; Williams, W.T.; Bakus, G.J. Chemical defenses in soft corals (Coelenterata: Octocorallia) of the Great Barrier Reef. Part I: A study of comparative toxicities. Mar. Ecol. Prog. Ser. 1982, 8, 271–278. [Google Scholar] [CrossRef]
- La Barre, S.; Laurent, D.; Sammarco, P.; Williams, W.T.; Coll, J.C. Comparative ichthyotoxicity of shallow and deep-water sponges of New Caledonia. In Proceedings of the 6th International Symposium on Coral Reefs, Townsville, Australia, 8–12 August 1988; Volume 3, pp. 55–59.
- Kobayashi, N. Comparative sensitivity of various developmental stages of sea urchins to some chemicals. Mar. Biol. 1980, 58, 163–171. [Google Scholar] [CrossRef]
- Brun, L.-O.; Urbain, R.; Wacapo, E.; Debitus, C. A Method for the Evaluation of Marine extracts Toxicity for the Coffee Berry Borer: Hypothenemus Hampei. In Proceeding of the Third Pacific-Asia Symposium on Biologically Active Natural Products, Noumea, New Caledonia, 26–30 August 1991; p. 31.
- Stone, B.F.; Haydock, K.P. Method derived from Stone and Haydock. A method for measuring the acaricide-susceptibility of the cattle tick Boophilus microplus (Can.). Bull. Entomol. Res. 1962, 53, 563–578. [Google Scholar] [CrossRef]
- König, G.M.; Coll, J.C.; Bowden, B.F.; Gulbis, J.M.; MacKay, M.F.; La Barre, S.C.; Laurent, D. The structure determination of a Xenicane diterpene from Xenia garciae. J. Nat. Prod. 1989, 52, 294–299. [Google Scholar] [CrossRef]
- Vieira, C.; Thomas, O.P.; Culioli, G.; Genta-Jouve, G.; Houlbreque, F.; Gaubert, J.; De Clerk, O.; Payri, C.E. Allelopathic interactions between the brown algal genus Lobophora (Dictyotales, Phaeophyceae) and scleractinian corals. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- La Barre, S.; (CNRS, Nouméa, New Caledonia). Personal communication, 1989.
- Green, N.M.; Work, E. Pancreatic trypsin inhibitor. 2. Reaction with trypsin. Biochem. J. 1953, 54, 347–352. [Google Scholar] [CrossRef] [PubMed]
- La Barre, S.; Longeon, A.; Barthélémy, M.; Guyot, M.; Le Caer, J.P.; Bargibant, G. Characterization of a novel elastase inhibitor from a fan coral. C. R. Acad. Sci. III 1996, 319, 365–370. [Google Scholar] [PubMed]
- Faulkner, D.J.; Unson, M.D.; Bewley, C.A. The chemistry of some sponges and their symbionts. Pure Appl. Chem. 1994, 66, 1983–1990. [Google Scholar] [CrossRef]
- Kobayashi, J.; Ishibashi, M. Bioactive metabolites of symbiotic marine microorganisms. Chem. Rev. 1993, 93, 1753–1769. [Google Scholar] [CrossRef]
- Kornprobst, J.M. Porifera (Sponges). In Encyclopedia of Marine Natural Products, Greatly Enlarged Edition, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; Volume 2, pp. 607–1086. [Google Scholar]
- D’Ambrosio, M.; Guerriero, A.; Debitus, C.; Ribes, O.; Pusset, J.; Leroy, S.; Pietra, F. Agelastatin A, a new skeleton cytotoxic alkaloid of the oroidin family. Isolation from the axinellid sponge Agelas dendromorpha of the Coral Sea. J. Chem. Soc. Chem. Commun. 1993, 16, 1305–1306. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Guerriero, A.; Chiasera, G.; Pietra, F. Conformational preferences and absolute configuration of agelastatin A, a cytotoxic alkaloid of the axinellid sponge Agelas dendromorpha from the Coral Sea, via combined molecular modelling, NMR, and exciton splitting for diamide and hydroxyamide derivatives. Helv. Chim. Acta 1994, 77, 1895–1902. [Google Scholar]
- D’Ambrosio, M.; Guerriero, A.; Ripamonti, M.; Debitus, C.; Waikedre, J.; Pietra, F. The active centres of agelastatin A, a strongly cytotoxic alkaloid of the Coral Sea axinellid sponge Agelas dendromorpha, as determined by comparative bioassays with semisynthetic derivatives. Helv. Chim. Acta 1996, 79, 727–735. [Google Scholar] [CrossRef]
- Meijer, L.; Thunnissen, A.M.; White, A.W.; Garnier, M.; Nikolic, M.; Tsai, L.H.; Walter, J.; Cleverley, K.E.; Salinas, P.C.; Wu, Y.Z.; et al. Inhibition of cyclin-dependent kinases, GSK-3β and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 2000, 7, 51–63. [Google Scholar] [CrossRef]
- Vassas, A.; Bourdy, G.; Paillard, J.J.; Lavayre, J.; Pais, M.; Quirion, J.C.; Debitus, C. Naturally occurring somatostatin and vasoactive intestinal peptide inhibitors. Isolation of alkaloids from two marine sponges. Plant. Med. 1996, 62, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Zampella, A.; D’Auria, M.V.; Paloma, L.G.; Casapullo, A.; Minale, L.; Debitus, C.; Henin, Y. Callipeltin A. an anti-HIV cyclic depsipeptide from the New Caledonian Lithistida sponge Callipelta sp. J. Am. Chem. Soc. 1996, 118, 6202–6209. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Zampella, A.; Paloma, L.G.; Minale, L.; Debitus, C.; Roussakis, C.; Le Bert, V. Callipeltins B and C; bioactive peptides from a marine Lithistida sponge Callipelta sp. Tetrahedron 1996, 52, 9589–9596. [Google Scholar]
- Zampella, A.; D’Auria, M.V.; Minale, L.; Debitus, C.; Roussakis, C. Callipeltoside A: A cytotoxic aminodeoxy sugar-containing macrolide of a new type from the marine Lithistida sponge Callipelta sp. J. Am. Chem. Soc. 1996, 118, 11085–11088. [Google Scholar] [CrossRef]
- Zampella, A.; D’Auria, M.V.; Minale, L.; Debitus, C. Callipeltosides B and C, two novel cytotoxic glycoside macrolides from a marine lithistida sponge Callipelta sp. Tetrahedron 1997, 53, 3243–3248. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Guerriero, A.; Debitus, C.; Ribes, O.; Richer de Forges, B.; Pietra, F. Corallistin A, a second example of a free porphyrin from a living organism. Isolation from the demosponge Corallistes sp. of the Coral Sea and inhibition of abnormal cells. Helv. Chim. Acta 1989, 72, 1451–1454. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Guerriero, A.; Debitus, C.; Ribes, O.; Pietra, F. On the Novel Free Porphyrins Corallistin B, C, D, and E: Isolation from the demosponge Corallistes sp. of the Coral Sea and Reactivity of Their Nickel 55 (II) Complexes toward Formylating Reagents. Helv. Chim. Acta 1993, 76, 1489–1496. [Google Scholar] [CrossRef]
- Loukaci, A.; Le Saout, I.; Samadi, M.; Leclerc, S.; Damiens, E.; Meijer, L.; Debitus, C.; Guyot, M. Coscinosulfate, a CDC25 phosphatase inhibitor from the sponge Coscinoderma mathewsi. Bioorg. Med. Chem. 2001, 9, 3049–3054. [Google Scholar] [CrossRef]
- De Nanteuil, G.; Ahond, A.; Guilhem, J.; Poupat, C.; Tran Huu Dau, E.; Potier, P.; Pusset, M.; Pusset, J.; Laboute, P. Invertébrés marins du lagon néo-calédonien-V. Isolement et identification des métabolites d’une nouvelle espèce de spongiaire, Pseudaxinyssa cantharella. Tetrahedron 1985, 41, 6019–6033. (In French) [Google Scholar] [CrossRef]
- Ahond, A.; Laboute, P.; Laurent, D.; Potier, P.; Poupat, C.; Pusset, J.; Pusset, M.; Thoison, O. New Biologically Active Substance Called Girolline, Extracted from the Sponge Pseudaxinyssa cantharella Process for its Preparation and Pharmaceutical Compositions Containing It. U.S. Patent 4,801,602, 31 January 1989. [Google Scholar]
- Ahond, A.; Bedoya Zurita, M.; Colin, M.; Fizames, C.; Laboute, P.; Lavelle, F.; Laurent, D.; Poupat, C.; Pusset, J.; Pusset, M.; et al. La girolline, nouvelle substance antitumorale extraite de l’éponge, Pseudaxinyssa cantharella n. sp. (Axinellidae). C. R. Acad. Sci. Ser. 1988, 307, 145–148. (In French) [Google Scholar]
- Chiaroni, A.; Riche, C.; Ahond, A.; Poupat, C.; Pusset, M.; Potier, P. Structure cristalline et configuration absolue de la girolline. C. R. Acad. Sci. Ser. 1991, 312, 49–53. [Google Scholar]
- Bedoya Zurita, M.; Ahond, A.; Poupat, C.; Potier, P. Première synthèse totale de la girolline. Tetrahedron 1989, 45, 6713–6720. (In French) [Google Scholar] [CrossRef]
- Marchais, S.; Al Mourabit, A.; Ahond, A.; Poupat, C.; Potier, P. A short synthesis of the marine bioactive metabolite (+/−) girolline. Tetrahedron Lett. 1998, 39, 8085–8088. [Google Scholar] [CrossRef]
- Colson, G.; Rabault, B.; Lavelle, F.; Zerial, A. Mode of action of the antitumor compound girodazole (RP 49532A, NSC 627434). Biochem. Pharmacol. 1992, 43, 1717–1723. [Google Scholar] [CrossRef]
- Catimel, G.; Coquard, R.; Guastalla, J.P.; Merrouche, Y.; Le Bail, N.; Alakl, M.K.; Dumortier, A.; Foy, M.; Clavel, M. Phase I study of RP 49532A, a new protein-synthesis inhibitor, in patients with advanced refractory solid tumors. Cancer Chemother. Pharm. 1995, 35, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Diop, D.; Chauvin, C.; Salhi, S.; Poupat, C.; Ahond, A.; Jean-Jean, O. Girolline interferes with cell-cycle progression, but not with translation. C. R. Biol. 2007, 330, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Benoit-Vical, F.; Saléry, M.; Soh, P.N.; Ahond, A.; Poupat, C. Girolline: A potential lead structure for antiplasmodial drug research. PlantMed 2008, 74, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Al Mourabit, A.; Pusset, M.; Chtourou, M.; Gaigne, C.; Ahond, A.; Poupat, C.; Potier, P. Pyraxinine, a novel nitrogenous compound from the marine sponge Cymbastela cantharella. J. Nat. Prod. 1997, 60, 290–291. [Google Scholar] [CrossRef]
- Sauleau, P.; Retailleau, P.; Nogues, S.; Carletti, I.; Marcourt, L.; Raux, R.; Al Mourabit, A.; Debitus, C. Dihydrohymenialdisines, new pyrrole-2-aminoimidazole alkaloids from the marine sponge Cymbastela cantharella. Tetrahedron Lett. 2011, 52, 2676–2678. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Guerriero, A.; Debitus, C.; Waikedre, J.; Pietra, F. Relative contributions to antitumoral activity of lipophilic vs. polar reactive moieties in marine terpenoids. Tetrahedron Lett. 1997, 38, 6285–6288. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Guerriero, A.; Deharo, E.; Debitus, C.; Munoz, V.; Pietra, F. New Types of Potentially Antimalarial Agents: Epidioxy-substituted norditerpene and norsesterpenes from the marine sponge Diacarnus Levii. Helv. Chim. Acta 1998, 81, 1285–1292. [Google Scholar] [CrossRef]
- Mancini, I.; Guella, G.; Debitus, C.; Waikedre, J.; Pietra, F. From inactive nortopsentin D, a novel bis (indole) alkaloid isolated from the axinellid sponge Dragmacidon sp. from deep waters south of New Caledonia, to a strongly cytotoxic derivative. Helv. Chim. Acta 1996, 79, 2075–2082. [Google Scholar] [CrossRef]
- Mancini, I.; Guella, G.; Frostin, M.; Hnawia, E.; Laurent, D.; Debitus, C.; Pietra, F. On the first polyarsenic organic compound from nature: Arsenicin A from the New Caledonian marine sponge Echinochalina bargibanti. Chem. Eur. J. 2006, 12, 8989–8994. [Google Scholar] [CrossRef] [PubMed]
- Dopeso, J.; Quiñoá, E.; Riguera, R.; Debitus, C.; Bergquist, P.R. Euryspongiols: Ten new highly hydroxylated 9, 11-secosteroids with antihistaminic activity from the sponge Euryspongia sp. Stereochemistry and reduction. Tetrahedron 1994, 50, 3813–3828. [Google Scholar] [CrossRef]
- Zampella, A.; Sepe, V.; Luciano, P.; Bellotta, F.; Monti, M.C.; D’Auria, M.V.; Jepsen, T.; Petek, S.; Adeline, M.T.; Laprévôte, O.; et al. Homophymine A, an anti-HIV cyclodepsipeptide from the sponge Homophymia sp. J. Org. Chem. 2008, 73, 5319–5327. [Google Scholar] [CrossRef] [PubMed]
- Zampella, A.; Sepe, V.; Bellotta, F.; Luciano, P.; D’Auria, M.V.; Cresteil, T.; Debitus, C.; Petek, S.; Poupat, C.; Ahond, A. Homophymines B–E and A1–E1, a family of bioactive cyclodepsipeptides from the sponge Homophymia sp. Org. Biomol. Chem. 2009, 7, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Bourguet-Kondracki, M.L.; Martin, M.T.; Debitus, C.; Guyot, M. 12-epi-heteronemin: New sesterterpene from the marine sponge Hyrtios erecta. Tetrahedron Lett. 1994, 35, 109–110. [Google Scholar] [CrossRef]
- Ledroit, V.; Debitus, C.; Ausseil, F.; Raux, R.; Menou, J.L.; Hill, B. Heteronemin as a protein farnesyl transferase inhibitor. Pharm. Biol. 2004, 42, 454–456. [Google Scholar] [CrossRef]
- Bourguet-Kondracki, M.L.; Debitus, C.; Guyot, M. Biologically active sesterterpenes from a New Caledonian marine sponge Hyrtios sp. J. Chem. Res. 1996, 192–193. [Google Scholar]
- Bourguet-Kondracki, M.L.; Debitus, C.; Guyot, M. Dipuupehedione, a cytotoxic new red dimer from a New Caledonian marine sponge Hyrtios sp. Tetrahedron Lett. 1996, 37, 3861–3864. [Google Scholar] [CrossRef]
- Bourguet-Kondracki, M.L.; Lacombe, F.; Guyot, M. Methanol adduct of puupehenone, a biologically active derivative from the marine sponge Hyrtios species. J. Nat. Prod. 1999, 62, 1304–1305. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, G.; Bruno, I.; Minale, L.; Riccio, R.; Debitus, C.; Bourdy, G.; Vassas, A.; Lavayre, J. Bioactive prenylhydroquinone sulfates and a novel C31 furanoterpene alcohol sulfate from the marine sponge, Ircinia sp. J. Nat. Prod. 1995, 58, 1444–1449. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Guerriero, A.; Debitus, C.; Pietra, F. Leucascandrolide A, a new type of macrolide: The first powerfully bioactive metabolite of calcareous sponges (Leucascandra caveolata, a new genus from the Coral Sea). Helv. Chim. Acta 1996, 79, 51–60. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Tatò, M.; Pocsfalvi, G.; Debitus, C.; Pietra, F. Leucascandrolide B, a New 16-Membered, Extensively Methyl-Branched Polyoxygenated Macrolide from the Calcareous Sponge Leucascandra caveolata from Northeastern Waters of New Caledonia. Helv. Chim. Acta 1999, 82, 347–353. [Google Scholar] [CrossRef]
- Bewley, C.A.; Debitus, C.; Faulkner, D.J. Microsclerodermins A and B. Antifungal cyclic peptides from the Lithistid sponge Microscleroderma sp. J. Am. Chem. Soc. 1994, 116, 7631–7636. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Paloma, L.G.; Minale, L.; Zampella, A.; Verbist, J.F.; Roussakis, C.; Debitus, C. Three new potent cytotoxic macrolides closely related to sphinxolide from the New Caledonian sponge Neosiphonia superstes. Tetrahedron 1993, 49, 8657–8664. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Paloma, L.G.; Minale, L.; Zampella, A.; Debitus, C.; Perez, J. Neosiphoniamolide A, a novel cyclodepsipeptide, with antifungal activity from the marine sponge Neosiphonia superstes. J. Nat. Prod. 1995, 58, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Carbonelli, S.; Zampella, A.; Randazzo, A.; Debitus, C.; Gomez-Paloma, L. Sphinxolides E–G and reidispongiolide C: Four new cytotoxic macrolides from the New Caledonian lithistida sponges N. superstes and R. coerulea. Tetrahedron 1999, 55, 14665–14674. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Debitus, C.; Paloma, L.G.; Minale, L.; Zampella, A. Superstolide A: A potent cytotoxic macrolide of a new type from the New Caledonian deep water marine sponge Neosiphonia superstes. J. Am. Chem. Soc. 1994, 116, 6658–6663. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Paloma, L.G.; Minale, L.; Zampella, A.; Debitus, C. A novel cytotoxic macrolide, superstolide B, related to superstolide A, from the New Caledonian marine sponge Neosiphonia superstes. J. Nat. Prod. 1994, 57, 1595–1597. [Google Scholar] [CrossRef] [PubMed]
- Legrave, N.; Hamrouni-Buonomo, S.; Dufies, M.; Guérineau, V.; Vacelet, J.; Auberger, P.; Amade, P.; Mehiri, M. Nepheliosyne B, a new polyacetylenic acid from the New Caledonian marine sponge Niphates sp. Mar. Drugs 2013, 11, 2282–2292. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, G.; Bruno, I.; Minale, L.; Riccio, R.; Calignano, A.; Debitus, C. (±)-Gelliusines A and B, two diastereomeric brominated tris-indole alkaloids from a deep water New Caledonian marine sponge (Gellius or Orina sp.). J. Nat. Prod. 1994, 57, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, G.; Bruno, I.; Riccio, R.; Lavayre, J.; Bourdy, G. Further brominated bis- and tris-indole alkaloids from the deep-water New Caledonian marine sponge Orina sp. J. Nat. Prod. 1995, 58, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Paloma, L.G.; Randazzo, A.; Minale, L.; Debitus, C.; Roussakis, C. New cytotoxic sesterterpenes from the New Caledonian marine sponge Petrosaspongia nigra (Bergquist). Tetrahedron 1997, 53, 10451–10458. [Google Scholar] [CrossRef]
- Randazzo, A.; Debitus, C.; Minale, L.; Garcia Pastor, P.; Alcaraz, M.J.; Payá, M.; Gomez-Paloma, L. Petrosaspongiolides M–R: New Potent and Selective Phospholipase A2 Inhibitors from the New Caledonian Marine Sponge Petrosaspongia nigra. J. Nat. Prod. 1998, 61, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Kourany-Lefoll, E.; Pais, M.; Sévenet, T.; Guittet, E.; Montagnac, A.; Fontaine, C.; Guénard, D.; Adeline, M.T.; Debitus, C. Phloeodictines A and B: New antibacterial and cytotoxic bicyclic amidinium salts from the New Caledonian sponge, Phloeodictyon sp. J. Org. Chem. 1992, 57, 3832–3835. [Google Scholar] [CrossRef]
- Kourany-Lefoll, E.; Laprévote, O.; Sévenet, T.; Montagnac, A.; Pais, M.; Debitus, C. Phloeodictines A1–A7 and C1–C2, antibiotic and cytotoxic guanidine alkaloids from the New Caledonian sponge, Phloeodictyon sp. Tetrahedron 1994, 50, 3415–3426. [Google Scholar] [CrossRef]
- Chevallier, C.; Laprévote, O.; Bignon, J.; Debitus, C.; Guénard, D.; Sévenet, T. Isolation of cytotoxic chondropsins, macrolide lactams from the New Caledonian marine sponge Psammoclemma sp. and electrospray ion trap multiple stage MS study of these macrolides. Nat. Prod. Res. 2004, 18, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Rubnov, S.; Chevallier, C.; Thoison, O.; Debitus, C.; Laprevote, O.; Guénard, D.; Sévenet, T. Echinosulfonic acid D: An ESI MSn evaluation of a new cytotoxic alkaloid from the New Caledonian sponge Psammoclemma sp. Nat. Prod. Res. 2005, 19, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.S.; Carroll, A.R.; Addepalli, R.; Avery, V.M.; Hooper, J.N.; Quinn, R.J. Psammaplysenes C and D, cytotoxic alkaloids from Psammoclemma sp. J. Nat. Prod. 2007, 70, 1827–1829. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, M.V.; Paloma, L.G.; Minale, L.; Zampella, A.; Verbist, J.F.; Roussakis, C.; Debitus, C.; Patissou, J. Reidispongiolide A and B, two new potent cytotoxic macrolides from the New Caledonian sponge Reidispongia coerulea. Tetrahedron 1994, 50, 4829–4834. [Google Scholar] [CrossRef]
- Bourguet-Kondracki, M.L.; Longeon, A.; Debitus, C.; Guyot, M. New cytotoxic isomalabaricane-type sesterterpenes from the New Caledonian marine sponge Rhabdastrella globostellata. Tetrahedron Lett. 2000, 41, 3087–3090. [Google Scholar] [CrossRef]
- Debitus, C.; Guella, G.; Mancini, I.; Waikedre, J.; Guemas, J.P.; Nicolas, J.L.; Pietra, F. Quinolones from a bacterium and tyrosine metabolites from its host sponge, Suberea creba from the Coral Sea. J. Mar. Biotechnol. 1998, 6, 136–141. [Google Scholar] [PubMed]
- Quirion, J.C.; Sévenet, T.; Husson, H.P.; Weniger, B.; Debitus, C. Two new alkaloids from Xestospongia sp., a New Caledonian sponge. J. Nat. Prod. 1992, 55, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- De Nanteuil, G.; Ahond, A.; Poupat, C.; Potier, P.; Pusset, M.; Pusset, J.; Laboute, P. Invertebres marins du lagon neo-caledonien—VI : Isolement et identification de onze sterols de type hydroxymethyl-3β nor-A cholestane du spongiaire, Pseudaxinyssa cantharella. Tetrahedron 1985, 41, 6035–6039. [Google Scholar] [CrossRef]
- Lu, D.; Rae, A.D.; Salem, G.; Weir, M.L.; Willis, A.C.; Wild, S.B. Arsenicin A, a natural polyarsenical: Synthesis and crystal structure. Organometallics 2010, 29, 32–33. [Google Scholar] [CrossRef]
- Lu, D.; Coote, M.L.; Ho, J.; Kilah, N.L.; Lin, C.Y.; Salem, G.; Weir, M.L.; Willis, A.C.; Wild, S.B. Resolution and Improved Synthesis of (±)-Arsenicin A: A Natural Adamantane-Type Tetraarsenical Possessing Strong Anti-Acute Promelocytic Leukemia Cell Line Activity. Organometallics 2012, 31, 1808–1816. [Google Scholar] [CrossRef]
- Valentin, B.B.; Vinod, V.; Beulah, M.C. Biopotential of secondary metabolites isolated from marine sponge Dendrilla nigra. Asian Pac. J. Trop. Dis. 2011, 1, 299–303. [Google Scholar] [CrossRef]
- Selvin, J.; Lipton, A.P. Dendrilla nigra, a marine sponge, as potential source of antibacterial substances for managing shrimp diseases. Aquaculture 2004, 236, 277–283. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Zhou, Y.D.; Nagle, D.G. Molecular-targeted antitumor agents. 15. Neolamellarins from the marine sponge Dendrilla nigra inhibit hypoxia-inducible factor-1 activation and secreted vascular endothelial growth factor production in breast tumor cells. J. Nat. Prod. 2007, 70, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.V.; Ravinder, K.; Narasimhulu, M.; Sridevi, A.; Satyanarayana, N.; Kondapi, A.K.; Venkateswarlu, Y. New anticancer bastadin alkaloids from the sponge Dendrilla cactos. Bioorg. Med. Chem. 2006, 14, 4452–4457. [Google Scholar] [CrossRef] [PubMed]
- Witowski, C.G. Investigation of Bioactive Metabolites from the Antarctic Sponge Dendrilla Membranosa and Marine Microorganisms. Ph.D. thesis, University of South Florida, Tampa, FL, USA, 10 April 2015. [Google Scholar]
- Selvin, J.; Gandhimathi, R.; Kiran, G.S.; Priya, S.S.; Ravji, T.R.; Hema, T.A. Culturable heterotrophic bacteria from the marine sponge Dendrilla nigra: Isolation and phylogenetic diversity of actinobacteria. Helgol. Mar. Res. 2009, 63, 239–247. [Google Scholar] [CrossRef]
- Ersmark, K.; Del Valle, J.R.; Hanessian, S. Chemistry and biology of the aeruginosin family of serine protease inhibitors. Angew. Chem. Int. Ed. 2008, 47, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Yon-Hin, P.; Scott, A.I. Total synthesis of corallistin A. Tetrahedron Lett. 1991, 32, 4231–4234. [Google Scholar] [CrossRef]
- Donia, M.S.; Fricke, W.F.; Partensky, F.; Cox, J.; Elshahawi, S.I.; White, J.R.; Phillippy, A.M.; Schatz, M.C.; Piel, J.; Haygoood, M.G.; et al. Complex microbiome underlying secondary and primary metabolism in the tunicate-Prochloron symbiosis. Proc. Natl. Acad. Sci. USA 2011, 108, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Kornprobst, J.M. Ascidians (Tunicates). In Encyclopedia of Marine Natural Products, Greatly Enlarged Edition, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; Volume 3, pp. 1609–1731. [Google Scholar]
- Adesanya, S.A.; Chbani, M.; Pais, M.; Debitus, C. Brominated β-carbolines from the marine tunicate Eudistoma album. J. Nat. Prod. 1992, 55, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Debitus, C.; Laurent, D.; Pais, M. Alcaloides d’une ascidie néocalédonienne, Eudistoma fragum. J. Nat. Prod. 1988, 51, 799–801. (In French) [Google Scholar] [CrossRef]
- Gouiffes, D.; Juge, M.; Grimaud, N.; Welin, L.; Sauviat, M.P.; Barbin, Y.; Laurent, D.; Roussakis, C.; Hénichart, J.P.; Verbist, J.F. Bistramide A, a new toxin from the Urochordata Lissoclinum bistratum Sluiter: Isolation and preliminary characterization. Toxicon 1988, 26, 1129–1136. [Google Scholar] [CrossRef]
- Roussakis, C.; Robillard, N.; Riou, D.; Biard, J.F.; Pradal, G.; Piloquet, P.; Debitus, C.; Verbist, J.F. Effects of bistramide A on a non-small-cell bronchial carcinoma line. Cancer Chemother. Pharmacol. 1991, 28, 283–292. [Google Scholar] [PubMed]
- Biard, J.F.; Roussakis, C.; Kornprobst, J.M.; Gouiffes-Barbin, D.; Verbist, J.F.; Cotelle, P.; Foster, M.P.; Ireland, C.M.; Debitus, C. Bistramides A, B, C, D, and K: A new class of bioactive cyclic polyethers from Lissoclinum bistratum. J. Nat. Prod. 1994, 57, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Sauviat, M.P.; Gouiffes-Barbin, D.; Ecault, E.; Verbist, J.F. Blockade of sodium channels by bistramide A in voltage-clamped frog skeletal muscle fibres. Biochim. BBA-Biomembr. 1992, 1103, 109–114. [Google Scholar] [CrossRef]
- Sauviat, M.P.; Chesnais, J.M.; Choukri, N.; Diacono, J.; Biard, J.F.; Verbist, J.F. The polyether bistramide A affects the calcium sensitivity of the contractile proteins in frog atrial heart muscle. Cell Calcium 1993, 14, 301–309. [Google Scholar] [CrossRef]
- Pusset, J.; Maillere, B.; Debitus, C. Evidence that bistramide A, from the ascidian Lissoclinum bistratum Sluiter, has immunomodulating properties in vitro. J. Nat. Toxins 1996, 5, 1–6. [Google Scholar]
- Malochet-Grivois, C.; Cotelle, P.; Biard, J.F.; Hénichart, J.P.; Debitus, C.; Roussakis, C.; Verbist, J.F. Dichlorolissoclimide, a new cytotoxic labdane derivative from Lissoclinum voeltzkowi Michaelson (Urochordata). Tetrahedron Lett. 1991, 32, 6701–6702. [Google Scholar] [CrossRef]
- Malochet-Grivois, C.; Roussakis, C.; Robillard, N.; Biard, J.F.; Riou, D.; Debitus, C.; Verbist, J.F. Effects in vitro of two marine substances, chlorolissoclimide and dichlorolissoclimide, on a non-small-cell bronchopulmonary carcinoma line (NSCLC-N6). Anticancer Drug Des. 1992, 7, 493–502. [Google Scholar] [PubMed]
- Biard, J.F.; Malochet-Grivois, C.; Roussakis, C.; Cotelle, P.; Hénichart, J.P.; Debitus, C.; Verbist, J.F. Lissoclimides, cytotoxic diterpenes from Lissoclinum voeltzkowi Michaelsen. Nat. Prod. Lett. 1994, 4, 43–50. [Google Scholar] [CrossRef]
- Chbani, M.; Pais, M.; Delauneux, J.M.; Debitus, C. Brominated indole alkaloids from the marine tunicate Pseudodistoma arborescens. J. Nat. Prod. 1993, 56, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Gouiffes, D.; Moreau, S.; Helbecque, N.; Bernier, J.L.; Hénichart, J.P.; Barbin, Y.; Laurent, D.; Verbist, J.F. Proton nuclear magnetic study of bistramide A, a new cytotoxic drug isolated from Lissoclinum bistratum Sluiter. Tetrahedron 1988, 44, 451–459. [Google Scholar] [CrossRef]
- Foster, M.P.; Mayne, C.L.; Dunkel, R.; Pugmire, R.J.; Grant, D.M.; Kornprobst, J.M.; Verbist, J.F.; Biard, J.F.; Ireland, C.M. Revised structure of bistramide A (bistratene A): Application of a new program for the automated analysis of 2D INADEQUATE spectra. J. Am. Chem. Soc. 1992, 114, 1110–1111. [Google Scholar] [CrossRef]
- Espada, A.; Jiménez, C.; Debitus, C.; Riguera, R. Villagorgin A and B. New type of indole alkaloids with acetylcholine antagonist activity from the gorgonian Villagorgia rubra. Tetrahedron Lett. 1993, 34, 7773–7776. [Google Scholar] [CrossRef]
- Vidal, J.P.; Escale, R.; Girard, J.P.; Rossi, J.C.; Chantraine, J.M.; Aumelas, A. Lituarines A, B, and C: A new class of macrocyclic lactones from the New Caledonian sea pen Lituaria australasiae. J. Org. Chem. 1992, 57, 5857–5860. [Google Scholar] [CrossRef]
- Clastres, A.; Ahond, A.; Poupat, C.; Potier, P.; Kan, S.K. Invertébrés Marins Du Lagon Néo-Calédonien, II. Étude Structurale De Trois Nouveaux Diterpènes Isolés Du Pennatulaire Pteroides laboutei. J. Nat. Prod. 1984, 47, 155–161. (In French) [Google Scholar] [CrossRef]
- La Barre, S.; Coll, J.C.; Sammarco, P.W. Defensive strategies of soft corals (Coelenterata: Octocorallia) of the Great Barrier Reef. II—The relationship between toxicity and feeding deterrence. Biol. Bull. 1986, 171, 565–576. [Google Scholar] [CrossRef]
- La Barre, S.; Coll, J.C.; Sammarco, P.W. Competitive strategies of soft corals (Coelenterata: Octocorallia): III. Spacing and aggressive interactions between alcyonaceans. Mar. Ecol. Prog. Ser. 1986, 28, 147–156. [Google Scholar] [CrossRef]
- Coll, J.C.; Bowden, B.F.; Heaton, A.; Scheuer, P.J.; Li, M.K.W.; Clardy, J.; Schulte, G.K.; Finer-Moore, J.S. Structures and possible functions of epoxypu-kalide and pukalide: Diterpenes associated with eggs of sinularian soft corals (Cnidaria, Anthozoa, Octocorallia, Alcyonacea, Alcyoniidae). J. Chem. Ecol. 1989, 15, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Kornprobst, J.M. Cnidaria and Ctenophora. In Encyclopedia of Marine Natural Products, Greatly Enlarged Edition, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; Volume 3, pp. 1087–1286. [Google Scholar]
- Kornprobst, J.M. Phaeophyceae (Brown Algae). In Encyclopedia of Marine Natural Products, Greatly Enlarged Edition, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; Volume 1, pp. 435–436. [Google Scholar]
- Tschesche, H; Kolkenbrock, H.; Bode, W. The covalent structure of the elastase inhibitor from Anemonia sulcata—A “non classical” Kazal-type protein. Biol. Chem. Hoppe-Seyler 1989, 368, 1297–1304. [Google Scholar]
- Cha, S. Tight-binding inhibitors-kinetic behaviour. Biochem. Pharmacol. 1975, 24, 2177–2185. [Google Scholar] [CrossRef]
- Williams, G.C. Living genera of sea pens (Coelenterata: Octocorallia: Pennatulacea): Illustrated key and synopses. Zool. J. Linn. Soc. 1995, 113, 93–140. [Google Scholar] [CrossRef]
- Lang, J.C. Interspecific aggression by scleractinian corals. II. Why the race is not only to the swift. Bull. Mar. Sci. 1973, 23, 261–279. [Google Scholar]
- Salih, A.; Larkum, A.; Cox, G.; Kühl, M.; Hoegh-Guldberg, O. Fluorescent pigments in corals are photoprotective. Nature 2000, 408, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Kornprobst, J.M. Echinodermata. In Encyclopedia of Marine Natural Products, Greatly Enlarged Edition, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; Volume 3, pp. 1499–1599. [Google Scholar]
- De Riccardis, F.; Iorizzi, M.; Minale, L.; Riccio, R.; de Forges, B. R.; Debitus, C. The gymnochromes: Novel marine brominated phenanthroperylenequinone pigments from the stalked crinoid Gymnocrinus richeri. J. Org. Chem. 1991, 56, 6781–6787. [Google Scholar] [CrossRef]
- Laille, M.; Gerald, F.; Debitus, C. In vitro antiviral activity on dengue virus of marine natural products. Cell. Mol. Life Sci. 1998, 54, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Palagiano, E.; De Marino, S.; Minale, L.; Riccio, R.; Zollo, F.; Iorizzi, M.; Carré, J.B.; Debitus, C.; Lucarain, L.; Provost, J. Ptilomycalin A, crambescidin 800 and related new highly cytotoxic guanidine alkaloids from the starfishes Fromia monilis and Celerina heffernani. Tetrahedron 1995, 51, 3675–3682. [Google Scholar] [CrossRef]
- Bruno, I.; Minale, L.; Riccio, R.; La Barre, S.; Laurent, D. Isolation and structure of new polyhydroxylated sterols from a deep-water starfish of the genus Rosaster. Gazz. Chim. Ital. 1990, 120, 449–451. [Google Scholar]
- Bhatnagar, S.; Dudouet, B.; Ahond, A.; Poupat, C.; Thoison, O.; Clastres, A.; Laurent, D.; Potier, P. Invertébrés marins du lagon néocalédonien. IV: Saponines et sapogénines d’une holothurie, Actinopyga flammea. Bull. Soc. Chim. Fr. 1985, 1, 124–129. (In French) [Google Scholar]
- De Riccardis, F.; Giovannitti, B.; Iorizzi, M.; Minale, L.; Riccio, R.; Debitus, C.; Richer de Forges, B. Sterol composition of the “living fossil” crinoid Gymnocrinus richeri. Comp. Biochem. Physiol. B Comp. Biochem. 1991, 100, 647–651. [Google Scholar] [CrossRef]
- Sauviat, M.P.; Benoit, A.G.; Debitus, C.; Pouny, I.; Laurent, D. Alterations of transmembrane currents in frog atrial heart muscle induced by photoexcited gymnochrome A purified from the crinoid, Gymnochrinus richeri. Photochem. Photobiol. 2001, 74, 115–119. [Google Scholar] [CrossRef]
- Laurent, D.; Baumann, F.; Benoit, A.G.; Mortelecqe, A.; Nitatpattana, N.; Desvignes, I.; Debitus, C.; Laille, M.; Gonzalez, J.P.; Chungue, E. Structure-activity relationships of dengue antiviral polycyclic quinones. South. Asian J. Trop. Med. Public Health 2005, 36, 901–905. [Google Scholar]
- Riccio, R.; Greco, O.S.; Minale, L.; La Barre, S.; Laurent, D. Starfish Saponins, Part 36. Steroidal Oligoglycosides from the Pacific Starfish Thromidia catalai. J. Nat. Prod. 1988, 51, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Kornprobst, J.M. Photosynthetic eukaryotes. In Encyclopedia of Marine Natural Products, Greatly Enlarged Edition, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; Volume 1, pp. 285–504. [Google Scholar]
- Garrigue, C.; Tsuda, R.T. Catalog of marine benthic algae from New Caledonia. Micronesica 1988, 21, 53–70. [Google Scholar]
- Garrigue, C. Biomass and production of two Halimeda species in the Southwest New Caledonian lagoon. Oceano. Acta 1991, 14, 581–588. [Google Scholar]
- Laurent, D.; Garrigue, C.; Bargibant, G.; Menou, J.L.; Tirard, P. Répartition bathymétrique des Caulerpes (Chlorophyces) et corrélation avec la présence de caulerpine. In Proceedings of the 5th International Coral Reef Congress, Tahiti, French Polynesia, 27 May–1 June 1985.
- Mattio, L.; Dirberg, G.; Payri, C.E.; Andréfouët, S. Diversity, biomass and distribution pattern of Sargassum beds in the South West lagoon of New Caledonia (South Pacific). In Proceedings Nineteenth International Seaweed Symposium, Kobe, Japan, 26–31 March 2007; Springer: Dordrecht, The Netherlands, 2007; pp. 361–373. [Google Scholar]
- Mattio, L.; Payri, C.E.; Verlaque, M. Taxonomic revision and geographic distribution of the subgenus Sargassum (Fucales, Phaeophyceae) in the Western and central pacific islands based on morphological and molecular analyses. J. Phycol. 2009, 45, 1213–1227. [Google Scholar] [CrossRef]
- Vidal, J.P.; Laurent, D.; Kabore, S.A.; Rechencq, E.; Boucard, M.; Girard, J.P.; Escale, R.; Rossi, J.C. Caulerpin, caulerpicin, Caulerpa scalpelliformis: Comparative acute toxicity study. Bot. Mar. 1984, 27, 533–538. [Google Scholar] [CrossRef]
- Amade, P.; Mehiri, M.; Lewis, R.J. Outstanding Marine Biotoxins: Stx, Ttx, and Ctx; Wiley-Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Yokoyama, A.; Murata, M.; Oshima, Y.; Iwashita, T.; Yasumoto, T. Some chemical properties of maitotoxin, a putative calcium channel agonist isolated from a marine dinoflagellate. J. Biochem. 1988, 104, 184–187. [Google Scholar] [PubMed]
- Wang, K.K.; Nath, R.; Raser, K.J.; Hajimohammadreza, I. Maitotoxin induces calpain activation in sh-sy5y neuroblastoma cells and cerebrocortical cultures. Arch. Biochem. Biophys. 1996, 331, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Roué, M.; Gugger, M.; Golubic, S.; Amzil, Z.; Aráoz, R.; Turquet, J.; Chinain, M.; Laurent, D. Marine cyanotoxins potentially harmul to human health. Chapter 1. In Outstanding Marine Molecules—Chemistry, Biology, Analysis, 1st ed.; La Barre, S., Kornprobst, J.M., Eds.; Wiley-VCH Verlag GmbH & Co KgaA: Weinheim, Germany, 2014; pp. 3–22. [Google Scholar]
- Kumar-Roine, S.; Taiana Darius, H.; Matsui, M.; Fabre, N.; Haddad, M.; Chinain, M.; Pauillac, S.; Laurent, D. A review of traditional remedies of ciguatera fish poisoning in the pacific. Phytother. Res. 2011, 25, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Abida, H.; Ruchaud, S.; Rios, L.; Humeau, A.; Probert, I.; De Vargas, C.; Bach, S.; Bowler, C. Bioprospecting marine plankton. Mar. Drugs 2013, 11, 4594–4611. [Google Scholar] [CrossRef] [PubMed]
- Physiology and Biotechnology of Algae laboratory. AMICAL. Available online: http://wwz.ifremer.fr/pba_eng/Projects/AMICAL (accessed on 26 May 2015).
- ADECAL. Available online: http://www.adecal.nc/en/2013-10-25-03-02-53/adecal (accessed on 26 May 2015).
- Bultel-Poncé, V.; Debitus, C.; Blond, A.; Cerceau, C.; Guyot, M. Lutoside: An acyl-1-(acyl-6′-mannobiosyl)-3-glycerol isolated from the sponge-associated bacterium Micrococcus luteus. Tetrahedron Lett. 1997, 38, 5805–5808. [Google Scholar] [CrossRef]
- Bultel-Poncé, V.; Berge, J.P.; Debitus, C.; Nicolas, J.L.; Guyot, M. Metabolites from the sponge-associated bacterium Pseudomonas species. Mar. Biotechnol. 1999, 1, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadakis, E.; Dufourcq, R.; Schmitt, S.; Brandily, C.; Kervarec, N.; Coatanea, D.; Amir, H.; Loubersac, L.; Chanteau, S.; Guezennec, J.; et al. Partial characterization of an exopolysaccharide secreted by a marine bacterium, Vibrio neocaledonicus sp. nov, from New Caledonia. J. Appl. Microbiol. 2013, 114, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Dufourcq, R.; Chalkiadakis, E.; Fauchon, M.; Deslandes, E.; Kerjean, V.; Chanteau, S.; Petit, E.; Guezennec, J.; Dupont-Rouzeyrol, M. Isolation and partial characterization of bacteria (Pseudoalteromonas sp.) with potential antibacterial activity from a marine costal environment from New Caledonia. Lett. Appl. Microbiol. 2014, 58, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Bettucci, L.; Pietra, F.; Laurent, D.; Roquebert, M.F. Acremonium neocaledoniae, a new species from wood in the Southwestern Lagoon of New Caledonia. Mycotaxon 2000, 75, 349–356. [Google Scholar]
- Laurent, D.; Guella, G.; Roquebert, M.F.; Farinole, F.; Mancini, I.; Pietra, F. Cytotoxins, mycotoxins and drugs from a new deuteromycete, Acremonium neocaledoniae, from the southwestern lagoon of New Caledonia. Plant. Med. 2000, 66, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Kaas, Q.; Craik, J. Conotoxins and Other Conopeptides. Chapter 14. In Outstanding Marine Molecules—Chemistry, Biology, Analysis, 1st ed.; La Barre, S., Kornprobst, J.M., Eds.; Wiley-VCH Verlag GmbH & Co KgaA: Weinheim, Germany, 2014; pp. 319–332. [Google Scholar]
- Dutertre, S.; Jin, A.H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J.; et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Hemami, H.; Gajewiak, J.; Karanth, S.; Robinson, S.D.; Ueberheide, B.; Douglass, A.D.; Schlegel, A.; Imperial, J.S.; Watkins, M.; Bandyopadhyay, P.K.; et al. Specialized insulin is used for chemical warfare by fish-hunting cone snails. Proc. Natl. Acad. Sci. USA 2015, 112, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Brauer, A.; Kurz, A.; Sockwell, T.; Baden-Tillson, H.; Heidler, J.; Wittig, I.; Kauferstein, S.; Mebs, D.; Stöcklin, R.; Remm, M. The mitochondrial genome of the venomous cone snail Conus consors. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Conus consors. Available online: http://www.biolib.cz/en/taxonimages/id582835/?type=1 (accessed on 28 March 2015).
- Gail, R.; Rageau, J. First findings on a venomous marine fish from New Caledonia, the stonefish Synanceia verrucos. Bull. Soc. Path. Exot. Film. 1956, 49, 846–854. [Google Scholar]
- Servent, D.; Winckler-Dietrich, V.; Hu, H.-Y.; Kessler, P.; Drevet, P.; Bertrand, D.; Ménez, A. Only snake curaremimetic toxins with a fifth disulfide bond have high affinity for the neuronal α7 nicotinic receptor. J. Biol. Chem. 1997, 272, 24279–24286. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Fuse, N.; Tsuchiya, T.; Nonomura, Y.; Ménez, A.; Tamiya, T. Sequence analysis of a cDNA encoding erabutoxin b from the sea-snake Laticauda semifasciata. Nucl. Acids Res. 1989, 17, 10490. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, N.; Yagi, T. Studies on sea snake venom. Proc Jpn. Acad. Ser. B. 2011, 87, 41–52. [Google Scholar] [CrossRef]
- Ducancel, F.; Guignery-Frelat, G.; Boulain, J.C.; Ménez, A. Nucleotide sequencing and structure analysis of cDNAs encoding short-chain neurotoxins from venom glands of a sea snake (Aipisurus laevis). Toxicon 1990, 28, 119–123. [Google Scholar] [CrossRef]
- Han, S.; Siegel, D.S.; Morrison, K.C.; Hergenrother, P.J.; Movassaghi, M. Synthesis and anticancer activity of all known (−)-Agelastatin alkaloids. J. Org. Chem. 2013, 78, 11970–11984. [Google Scholar] [CrossRef] [PubMed]
- Stout, E.P.; Choi, M.Y.; Castro, J.E.; Molinski, T.F. Potent Fluorinated Agelastatin Analogues for Chronic Lymphocytic Leukemia: Design, Synthesis, and Pharmacokinetic Studies. J. Med. Chem. 2014, 57, 5085–5093. [Google Scholar] [CrossRef] [PubMed]
- Cassiano, C.; Monti, M.C.; Festa, C.; Zampella, A.; Riccio, R.; Casapullo, A. Chemical proteomics reveals heat shock protein 60 to be the main cellular target of the marine bioactive sesterterpene suvanine. ChemBioChem 2012, 13, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, F.S.; Festa, C.; D’Amore, C.; De Marino, S.; Renga, B.; D’Auria, M.V.; Novellino, E.; Limongelli, V.; Zampella, A.; Fiorucci, S. Binding mechanism of the farnesoid X receptor marine antagonist suvanine reveals a strategy to forestall drug modulation on nuclear receptors. Design, synthesis, and biological evaluation of novel ligands. J. Med. Chem. 2013, 56, 4701–4717. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Salam, K.A.; Hermawan, I.; Akimitsu, N.; Tanaka, J.; Tani, H.; Yamashita, A.; Moriishi, K.; Nakakoshi, M.; Tsubuki, M.; et al. Identification and biochemical characterization of halisulfate 3 and suvanine as novel inhibitors of hepatitis C virus NS3 helicase from a marine sponge. Mar. Drugs 2014, 12, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Karthikeyan, C.; Hari Narayana Moorthy, N.S.; Kumar Waiker, D.; Kumar Jain, A.; Trivedi, P. Human CDC2-like kinase 1 (CLK1): A novel target for Alzheimer’s disease. Curr. Drug Targets 2014, 15, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Shin, U.; Williams, D.E.; Kozakov, D.; Hall, D.R.; Beglov, D.; Vajda, S.; Andersen, R.J.; Pelletier, J. Stimulators of translation identified during a small molecule screening campaign. Anal. Biochem. 2014, 447, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Saleem, R.S.Z.; Tepe, J.J. A concise total synthesis of hymenialdisine. Tetrahedron Lett. 2015, 56, 3011–3013. [Google Scholar] [CrossRef]
- Lu, D.; Arulmozhiraja, S.; Coote, M.L.; Rae, A.D.; Salem, G.; Willis, A.C.; Wild, S.B.; Benhenda, S.; Lallemand Breitenbach, V.; de Thé, H.; Zhai, X.; et al. Sulfur Derivatives of the Natural Polyarsenical Arsenicin A: Biologically Active, Organometallic Arsenic–Sulfur Cages Related to the Minerals Realgar and Uzonite. Organometallics 2015, 34, 829–840. [Google Scholar] [CrossRef]
- Kopf, S.; Viola, K.; Atanasov, A.G.; Jarukamjorn, K.; Rarova, L.; Kretschy, N.; Teichmann, M.; Vonach, C.; Saiko, P.; Giessrigl, B.; et al. In vitro characterisation of the anti-intravasative properties of the marine product heteronemin. Arch. Toxicol. 2013, 87, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Cassiano, C.; Esposito, R.; Tosco, A.; Zampella, A.; D’Auria, M.V.; Riccio, R.; Casapullo, A.; Monti, M.C. Heteronemin, a marine sponge terpenoid, targets TDP-43, a key factor in several neurodegenerative disorders. Chem. Commun. 2014, 50, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Sung, P.J.; Chang, Y.L.; Pan, S.L.; Teng, C.M. Heteronemin, a Spongean Sesterterpene, Induces Cell Apoptosis and Autophagy in Human Renal Carcinoma Cells. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.Y.; Zhang, R.T.; Ma, Y.M.; Gu, M.; Liu, G.; Li, J.; Nan, F.J. Design, Synthesis, and Biological Evaluation of Ring-Opened Bengamide Analogues. ChemMedChem 2011, 6, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lu, J.P.; Ye, Q.Z. Structural analysis of bengamide derivatives as inhibitors of methionine aminopeptidases. J. Med. Chem. 2012, 55, 8021–8027. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.P.; Yuan, X.H.; Ye, Q.Z. Structural analysis of inhibition of Mycobacterium tuberculosis methionine aminopeptidase by bengamide derivatives. Eur. J. Med. Chem. 2012, 47, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gálvez, F.; García-Ruiz, C.; Sánchez-Ruiz, A.; Valeriote, F.A.; Sarabia, F. An array of bengamide E analogues modified at the terminal olefinic position: Synthesis and antitumor properties. ChemMedChem 2013, 8, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Sarabia, F.; Martin-Galvez, F.; García-Ruiz, C.; Sánchez-Ruiz, A.; Vivar-García, C. Epi-, epoxy-, and C2-modified bengamides: Synthesis and biological evaluation. J. Org. Chem. 2013, 78, 5239–5253. [Google Scholar] [CrossRef] [PubMed]
- LaBarbera, D.V.; Modzelewska, K.; Glazar, A.I.; Gray, P.D.; Kaur, M.; Liu, T.; Grossman, D.; Harper, M.K.; Kuwada, S.K.; Moghal, N.; et al. The marine alkaloid naamidine A promotes caspase-dependent apoptosis in tumor cells. Anticancer Drugs 2009, 20, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Copp, B.R.; Fairchild, C.R.; Cornell, L.; Casazza, A.M.; Robinson, S.; Ireland, C.M. Naamidine A is an antagonist of the epidermal growth factor receptor and an in vivo active antitumor agent. J. Med. Chem. 1998, 41, 3909–3911. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.A.; Maers, K.; Roberts, J.; Kemami-Wangun, H.V.; Harmody, D.; Wright, A.E. The marine natural product microsclerodermin A is a novel inhibitor of the nuclear factor kappa B and induces apoptosis in pancreatic cancer cells. Investig. New Drugs 2015, 33, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Andrés, R.M.; Montesinos, M.C.; Navalón, P.; Payá, M.; Terencio, M.C. NF-κB and STAT3 inhibition as a therapeutic strategy in psoriasis: In vitro and in vivo effects of BTH. J. Investig. Dermatol. 2013, 133, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Margarucci, L.; Riccio, R.; Bonfili, L.; Mozzicafreddo, M.; Eleuteri, A.M.; Casapullo, A. Mechanistic insights on petrosaspongiolide M inhibitory effects on immunoproteasome and autophagy. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2014, 1844, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Poveda, B.; Rodríguez-Nieto, S.; García-Caballero, M.; Medina, M.Á.; Quesada, A.R. The antiangiogenic compound aeroplysinin-1 induces apoptosis in endothelial cells by activating the mitochondrial pathway. Mar. Drugs 2012, 10, 2033–2046. [Google Scholar] [CrossRef] [PubMed]
- Stuhldreier, F.; Kassel, S.; Schumacher, L.; Wesselborg, S.; Proksch, P.; Fritz, G. Pleiotropic effects of spongean alkaloids on mechanisms of cell death, cell cycle progression and DNA damage response (DDR) of acute myeloid leukemia (AML) cells. Cancer Lett. 2015, 361, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Mijares, M.R.; Ochoa, M.; Barroeta, A.; Martinez, G.P.; Suarez, A.I.; Compagnone, R.S.; Chirinos, P.; Avila, R.; De Sanctis, J.B. Cytotoxic effects of Fisturalin-3 and 11-Deoxyfisturalin-3 on Jurkat and U937 cell lines. Biomed. Pap. 2013, 157, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.; Liu, S.; Chen, Z.; Skau, C.; Pytynia, M.; Kovar, D.R.; Chmura, S.J.; Kozmin, S.A. Rationally simplified bistramide analog reversibly targets actin polymerization and inhibits cancer progression in vitro and in vivo. J. Am. Chem. Soc. 2010, 132, 7288–7290. [Google Scholar] [CrossRef] [PubMed]
- Herkommer, D.; Dreisigacker, S.; Sergeev, G.; Sasse, F.; Gohlke, H.; Menche, D. Design, Synthesis, and Biological Evaluation of Simplified Side Chain Hybrids of the Potent Actin Binding Polyketides Rhizopodin and Bistramide. ChemMedChem 2015, 10, 470–489. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Kalinovsky, A.I.; Malyarenko, T.V.; Ivanchina, N.V.; Dmitrenok, P.S.; Menchinskaya, E.S.; Yurchenko, E.A.; Pislyagin, E.A.; Aminin, D.L.; Huong, T.T.T.; et al. Cyclic Steroid Glycosides from the Starfish Echinaster luzonicus: Structures and Immunomodulatory Activities. J. Nat. Prod. 2015, 78, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Elmallah, M.I.; Micheau, O. Marine drugs regulating apoptosis induced by tumor necrosis factor-related apoptosis-inducing ligand (trail). Mar. Drugs 2015, 13, 6884–6909. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves New Therapy for Certain Types of Advanced Soft Tissue Sarcoma. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm468832.htm (accessed on 23 October 2015).
- Mason, C.K.; McFarlane, S.; Johnston, P.G.; Crowe, P.; Erwin, P.J.; Domostoj, M.M.; Campbell, F.C.; Manaviazar, S.; Hale, K.J.; El-Tanani, M. Agelastatin A: A novel inhibitor of osteopontin-mediated adhesion, invasion, and colony formation. Mol. Cancer Ther. 2008, 7, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Hur, W.; Cho, C.Y.; Liu, Y.; Adrian, F.J.; Lozach, O.; Bach, S.; Mayer, T.; Fabbro, D.; Meijer, L. Synthesis and target identification of hymenialdisine analogs. Chem. Biol. 2004, 11, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.; Alonso, D.; Martin-Aparicio, E.; Fuertes, A.; Perez-Puerto, M.J.; Castro, A.; Morales, S.; Navarro, M.L.; del Monte-Millan, M.; Medina, M. Glycogen synthase kinase-3 (GSK-3) inhibitory activity and structure-activity relationship (sar) studies of the manzamine alkaloids. Potential for alzheimer’s disease. J. Nat. Prod. 2007, 70, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Marine Pharmaceuticals: The Clinical Pipeline. Available online: http://marinepharmacology.midwestern.edu/clinPipeline.htm (accessed on 21 February 2016).
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Kwon, H.J.; Jung, H.S. Osteogenic potency of nacre on human mesenchymal stem cells. Mol. Cells 2015, 38, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.; Vidal, B.; Berland, S.; Camprasse, S.; Camprasse, G.; Silve, C. Demonstration of the capacity of nacre to induce bone formation by human osteoblasts maintained in vitro. Tissue Cell 1992, 24, 667–679. [Google Scholar] [CrossRef]
- Pernice, M; Wetzel, S.; Gros, O.; Boucher-Rodoni, N.; Dubilier, N. Enigmatic dual symbiosis in the excretory organ of Nautilus macromphalus (Cephalopoda: Nautiloidea). Proc. R. Soc. B 2007, 274, 1143–1152. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).