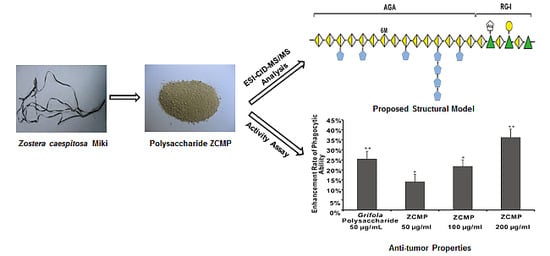
Extraction, Isolation, Structural Characterization and Anti-Tumor Properties of an Apigalacturonan-Rich Polysaccharide from the Sea Grass Zostera caespitosa Miki
Abstract
:1. Introduction
2. Results and Discussion
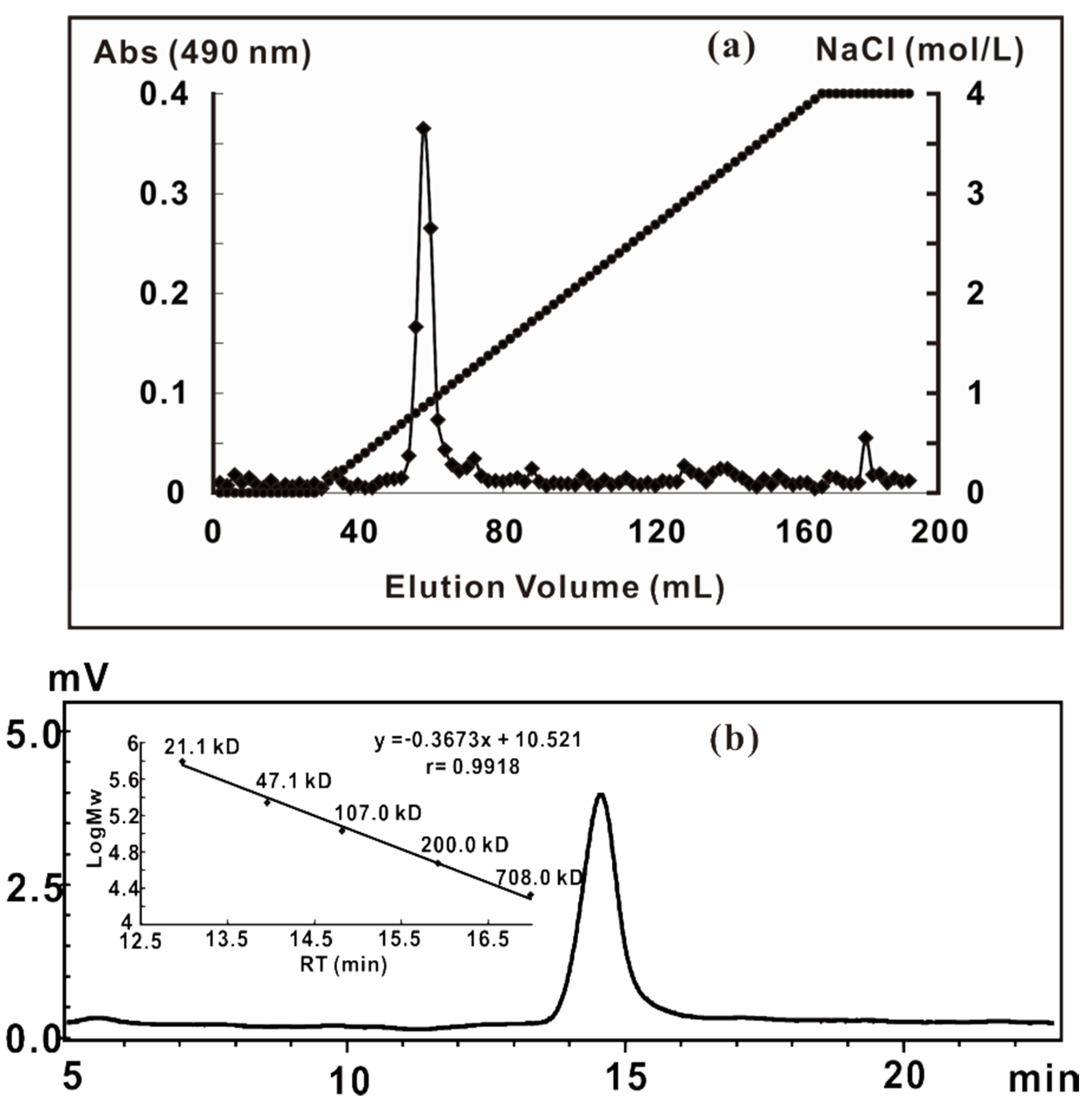
2.1. Extraction, Purification and General Analysis of ZCMP
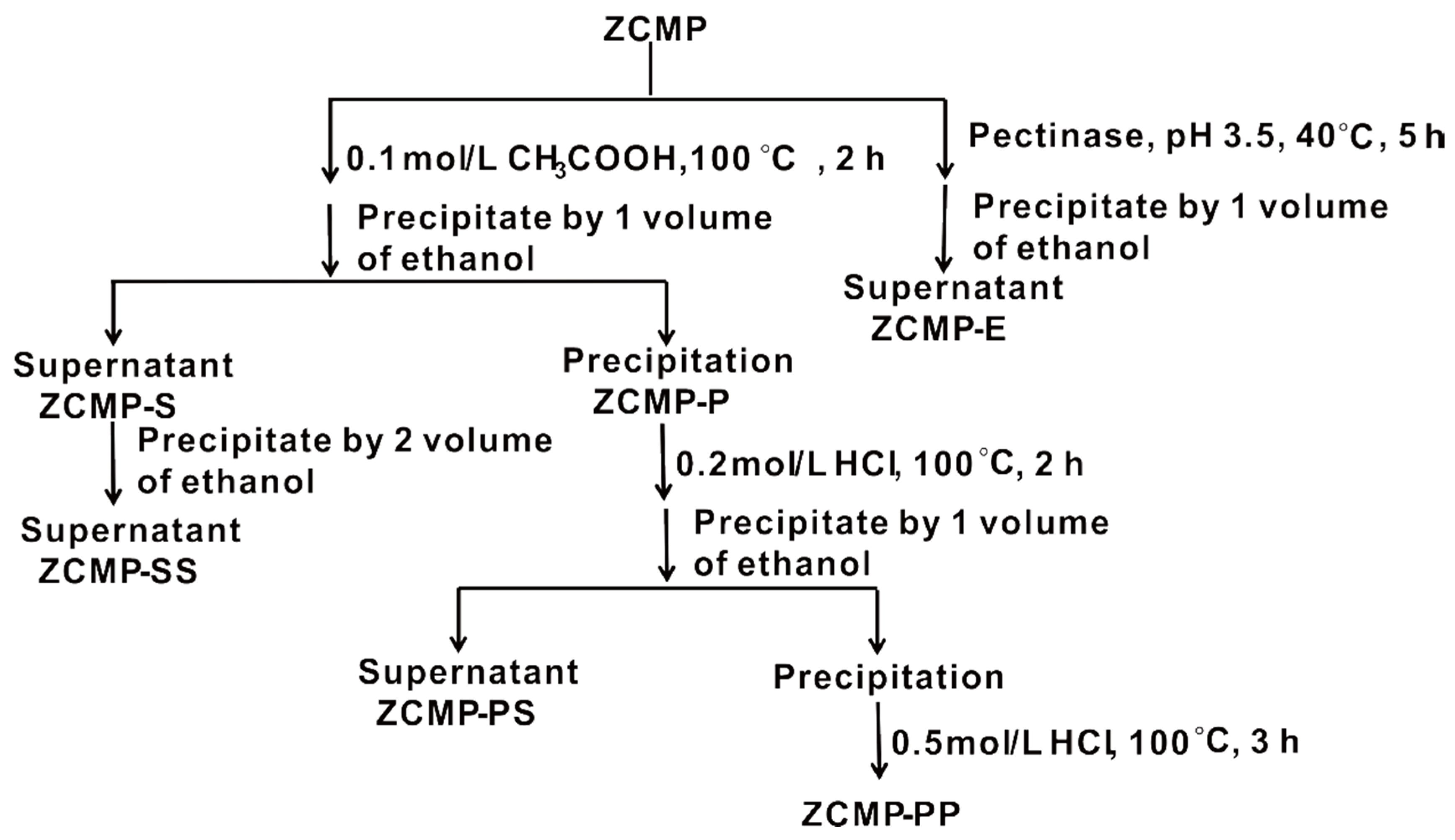
2.2. Preparation of ZCMP-Derived Oligosaccharides
2.2.1. Degradation of ZCMP
| Molecular Weight (kD) | Monosaccharides (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | GlcA | GalA | Api | Gal | Xyl | Ara | ||
| ZCMP | 77.2 | 4.2 | 11.8 | - | 51.4 | 15.5 | 6.0 | 4.4 | 4.3 |
| ZCMP-SS | - | 2.3 | 6.7 | 3.9 | 5.7 | 52.1 | 10.7 | 5.8 | 12.2 |
| ZCMP-PS | - | 3.0 | 27.0 | 5.0 | 39.9 | 2.5 | 10.1 | 4.7 | 7.8 |
| ZCMP-P | 23.4 | 3.9 | 5.8 | 0.9 | 79.9 | 2.9 | 3.1 | 1.2 | 2.2 |
| ZCMP-PP | - | 3.4 | 3.0 | 0.4 | 93.2 | - | - | - | - |
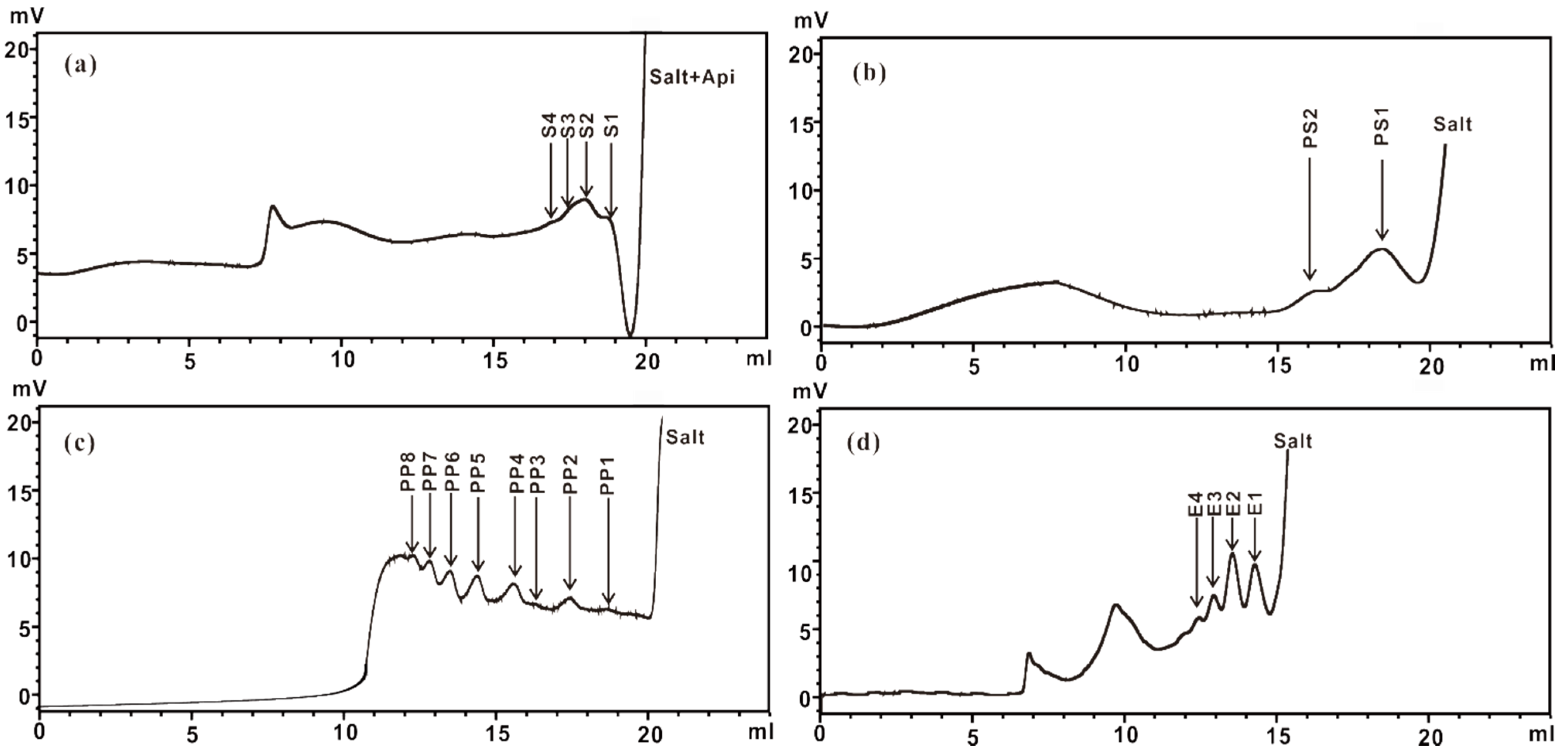
2.2.2. Purification of ZCMP-Derived Oligosaccharides
| Fraction | Ions | Mw (H Form) | Dp | Composition |
|---|---|---|---|---|
| E1 | 272.05 (z = 2) | 546.10 | 3 | GalA3 |
| E2 | 448.08 (z = 2) | 898.16 | 5 | GalA5 |
| E3 | 536.09 (z = 2) | 1074.18 | 6 | GalA6 |
| E4 | 415.74 (z = 3) | 1250.22 | 7 | GalA7 |
| S1 | 149.05 (z = 1) | 150.05 | 1 | Api |
| S2 | 281.10 (z = 1) | 282.10 | 2 | Api2 |
| S3 | 413.14 (z = 1) | 414.14 | 3 | Api3 |
| S4 | 545.18 (z = 1) | 546.18 | 4 | Api4 |
| PS1 | 339.09 (z = 1) | 340.09 | 2 | GalARha |
| PS2 | 661.17 (z = 1) | 662.17 | 4 | GalA2Rha2 |
| PP1 | 193.08 (z = 1) | 194.08 | 1 | GalA |
| PP2 | 369.10 (z = 1) | 370.10 | 2 | GalA2 |
| PP3 | 545.10 (z = 1) | 546.10 | 3 | GalA3 |
| PP4 | 721.12 (z = 1) | 722.12 | 4 | GalA4 |
| PP5 | 448.08 (z = 2) | 898.16 | 5 | GalA5 |
| PP6 | 536.09 (z = 2) | 1074.18 | 6 | GalA6 |
| PP7 | 624.10 (z = 2) | 1250.20 | 7 | GalA7 |
| PP8 | 474.41 (z = 3) | 1426.24 | 8 | GalA8 |
2.3. ESI-CID-MS2 Analysis of the Oligosaccharides Derived from ZCMP
2.3.1. ESI-CID-MS2 Analysis of the Sequences of Oligosaccharides from ZCMP-S
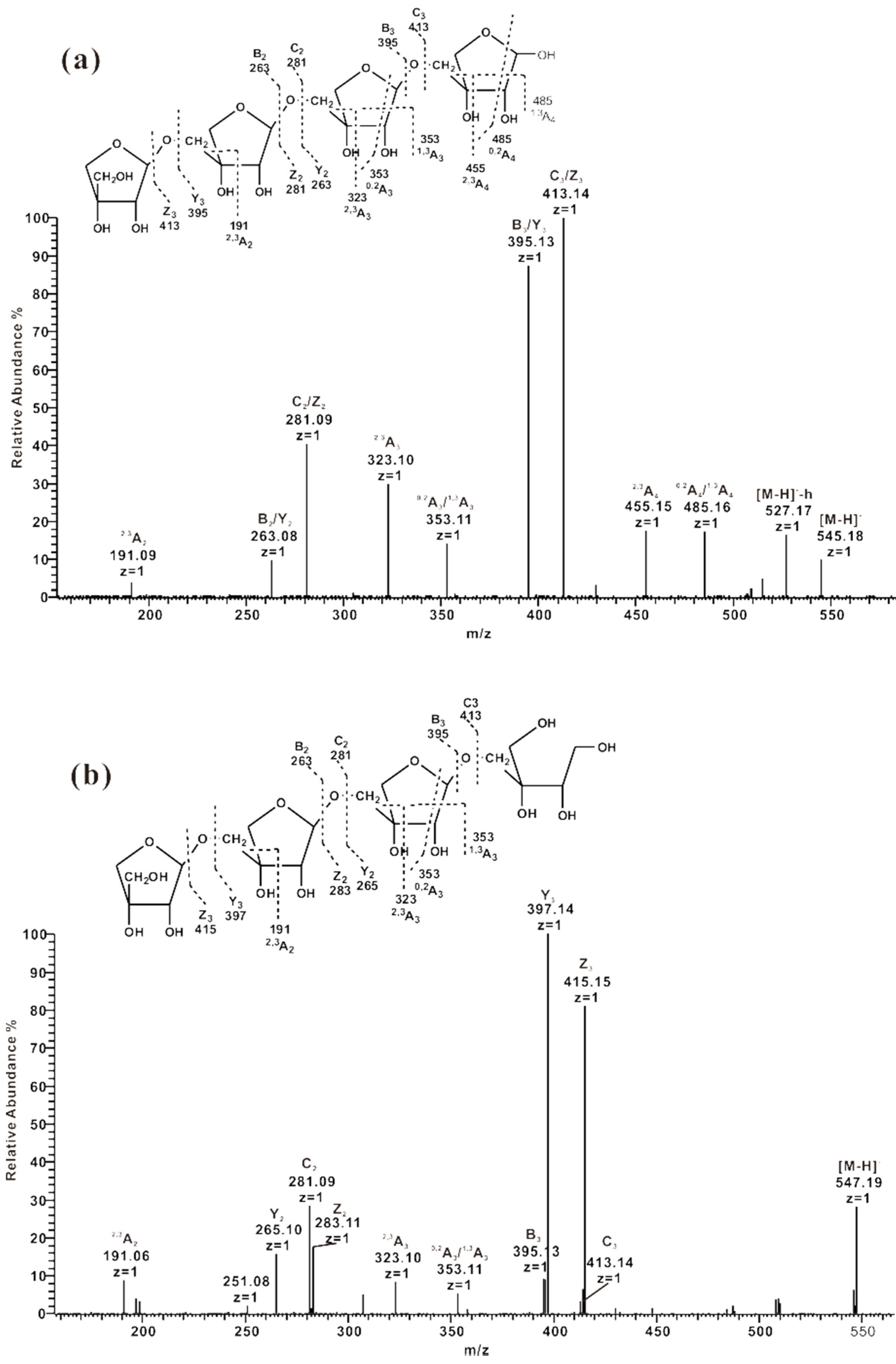
2.3.2. ESI-CID-MS2 Analysis of Oligosaccharides from ZCMP-PS
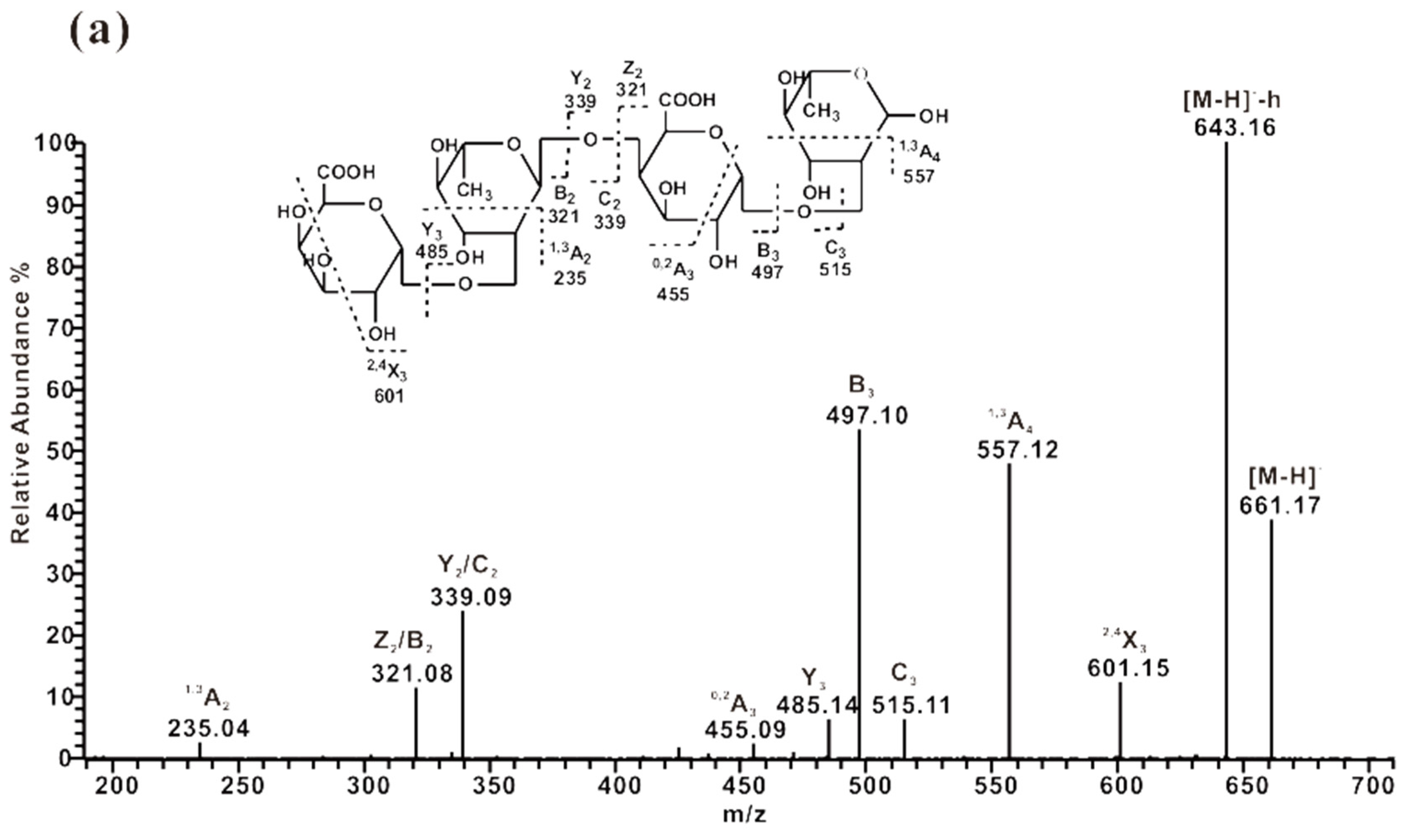
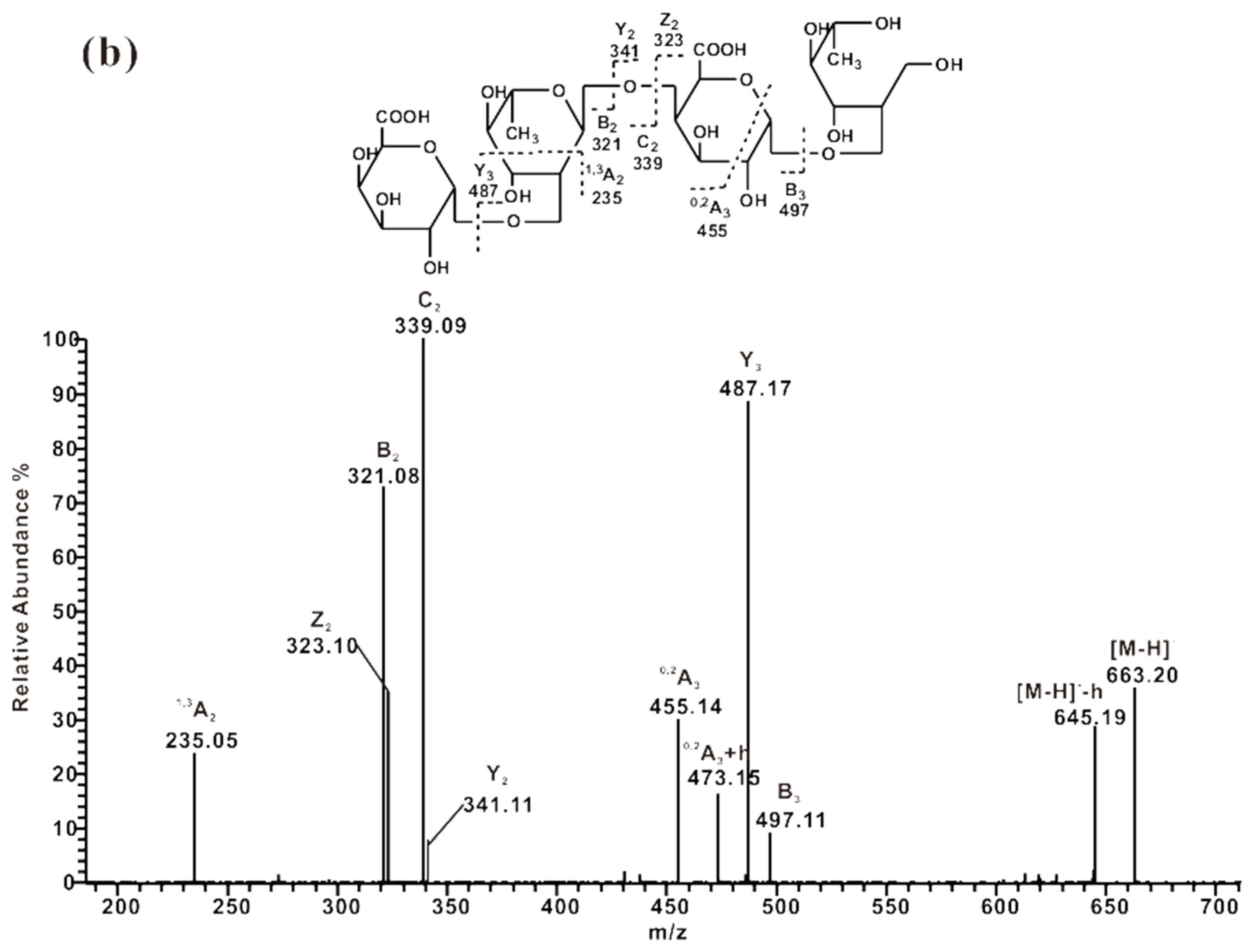
2.3.3. ESI-CID-MS2 Analysis of Oligosaccharides from ZCMP-PP
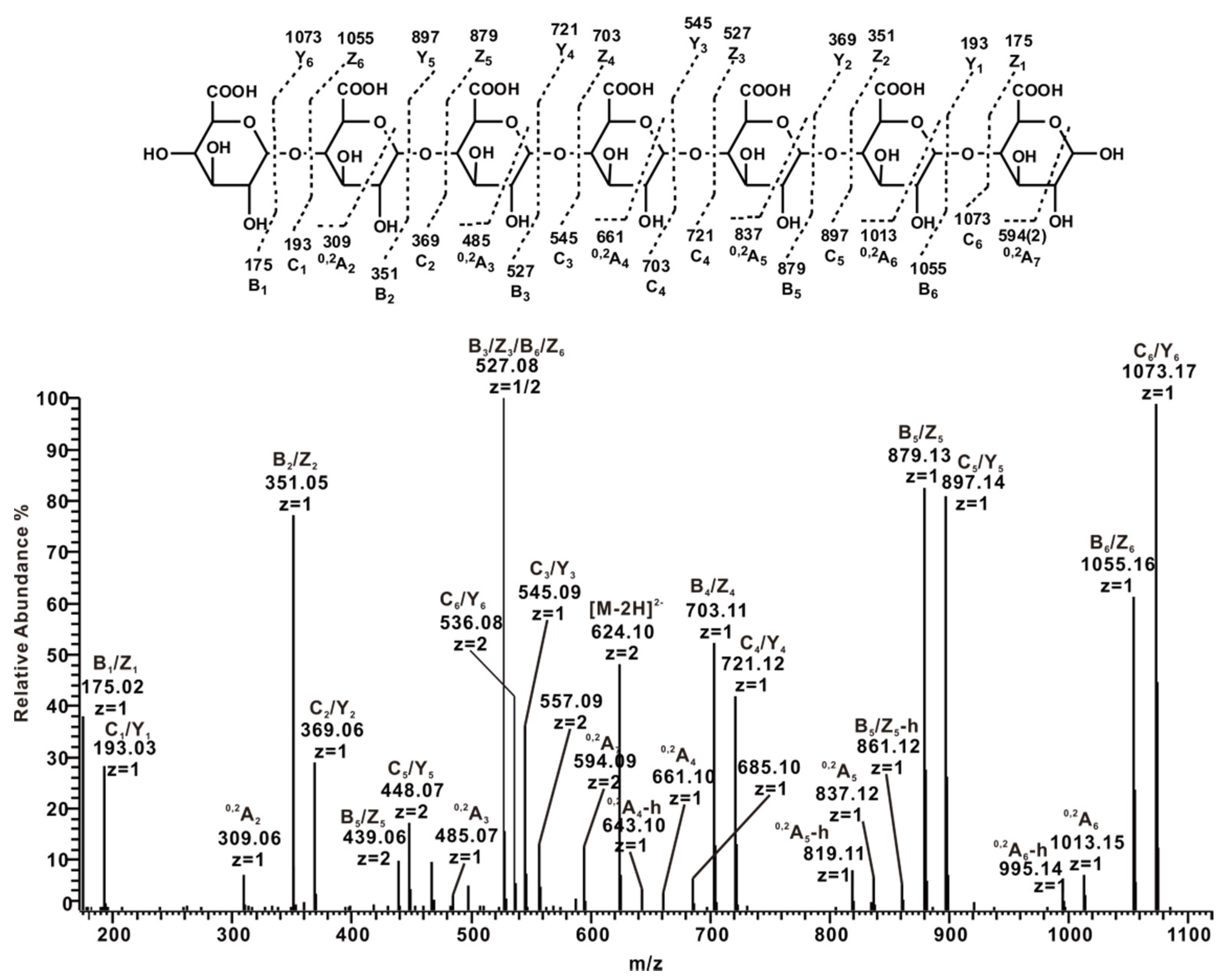
2.3.4. ESI-CID-MS2 Analysis of Oligosaccharides from ZCMP-E
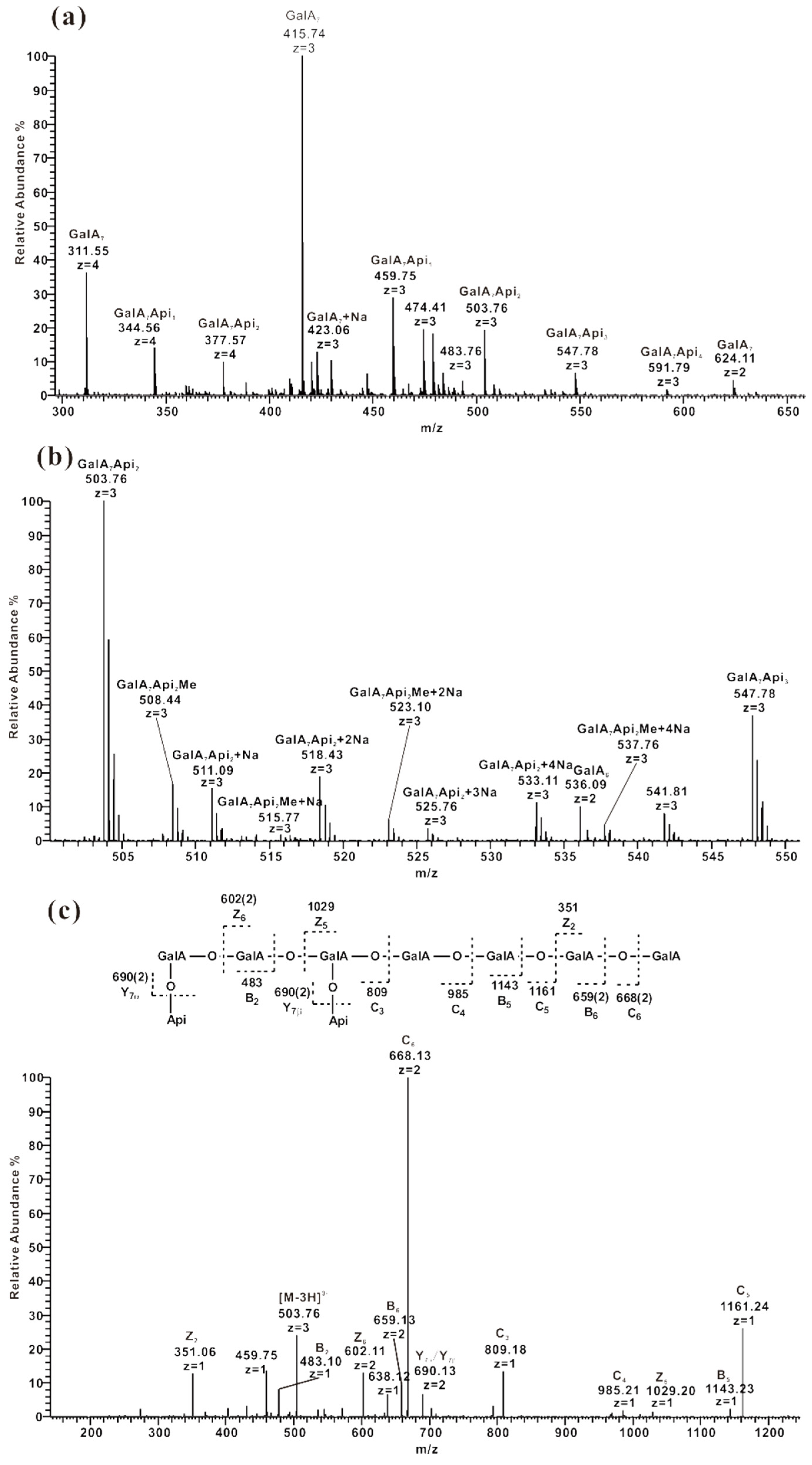
2.4. Methylation Analysis of ZCMP
| Permethylated Alditol Acetate | Primary Mass Fragments (m/z) | Linkages | Molar Ratio |
|---|---|---|---|
| 2,3,3′-Me3-Api f | 118, 132, 161 | Api f-(1→ | 12.81 |
| 2,3-Me2-Ara f | 118, 129, 189 | →5)-Ara f-(1→ | 14.26 |
| 2,3-Me2-Xyl | →4)-Xyl-(1→ | ||
| 3,4-Me2-Rha | 131, 190, 234, 304 | →2)-Rha-(1→ | 17.75 |
| 2,3,6-Me3-Gal | 118, 131, 173, 233 | →4)-Gal-(1→ | 6.55 |
| 2,3,6-Me3-Galred | 118, 174, 234 | →4)-GalA-(1→ | 32.40 |
| 2,6-Me2-Galred | 118, 130, 186, 306 | →3,4)-GalA-(1→ | 8.35 |
| 2-Me-Ara f | 118, 159, 201, 261 | →3,5)-Ara f-(1→ | 3.07 |
| 2,3-Me2-Api f | 118, 129, 189, 234 | →3′)-Api f-(1→ | 4.82 |
2.5. NMR Analysis of ZCMP-SS
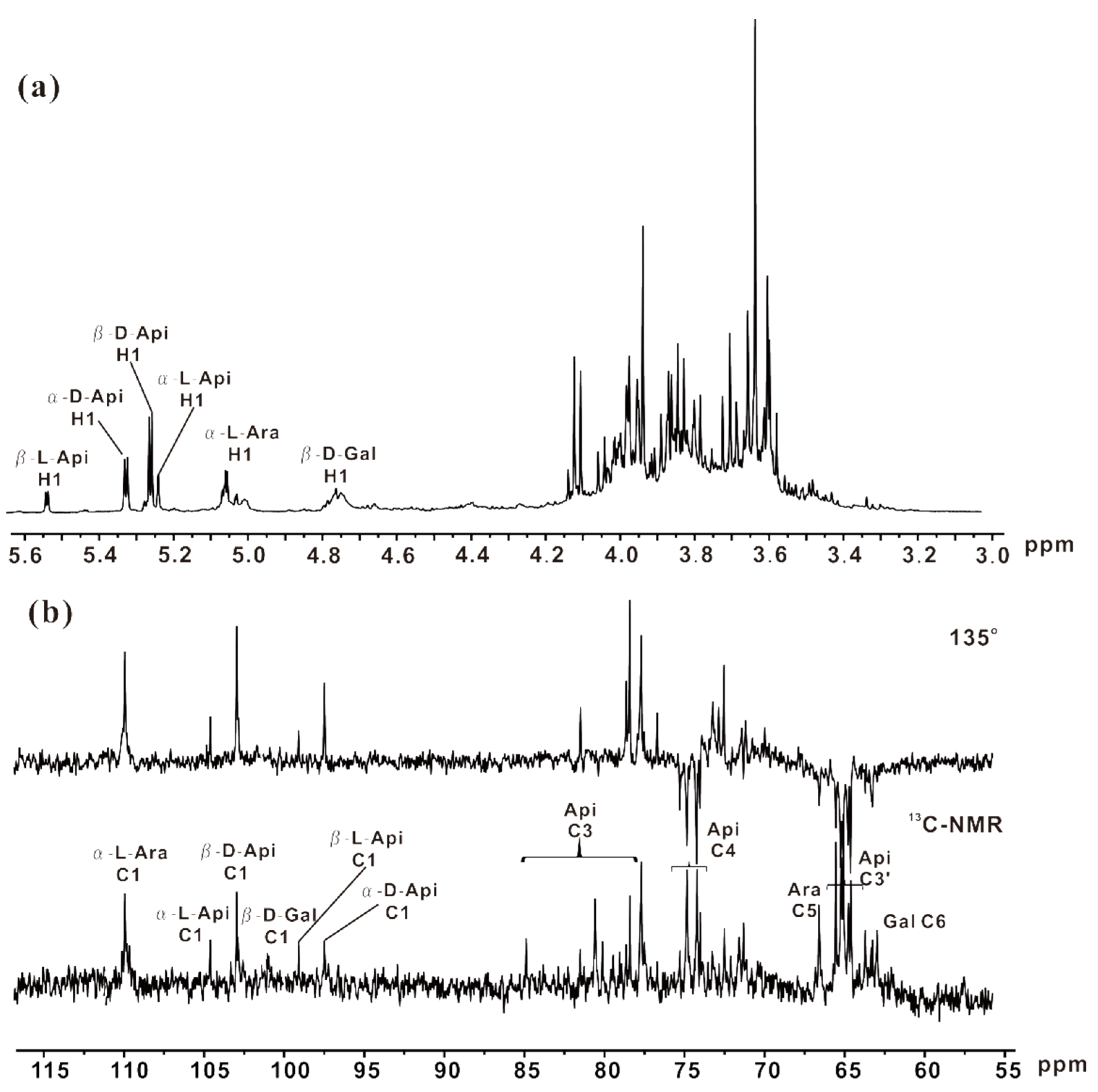
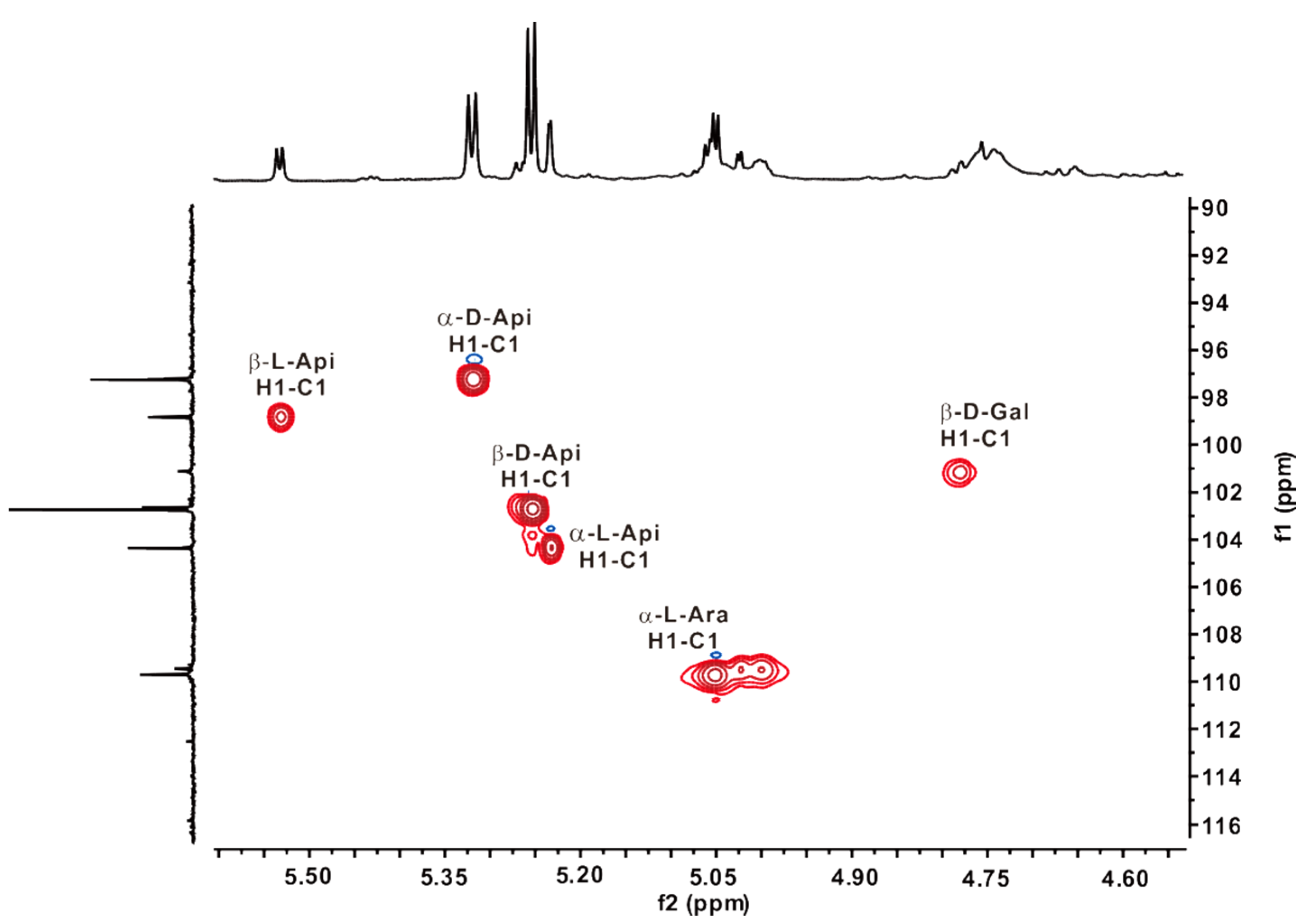
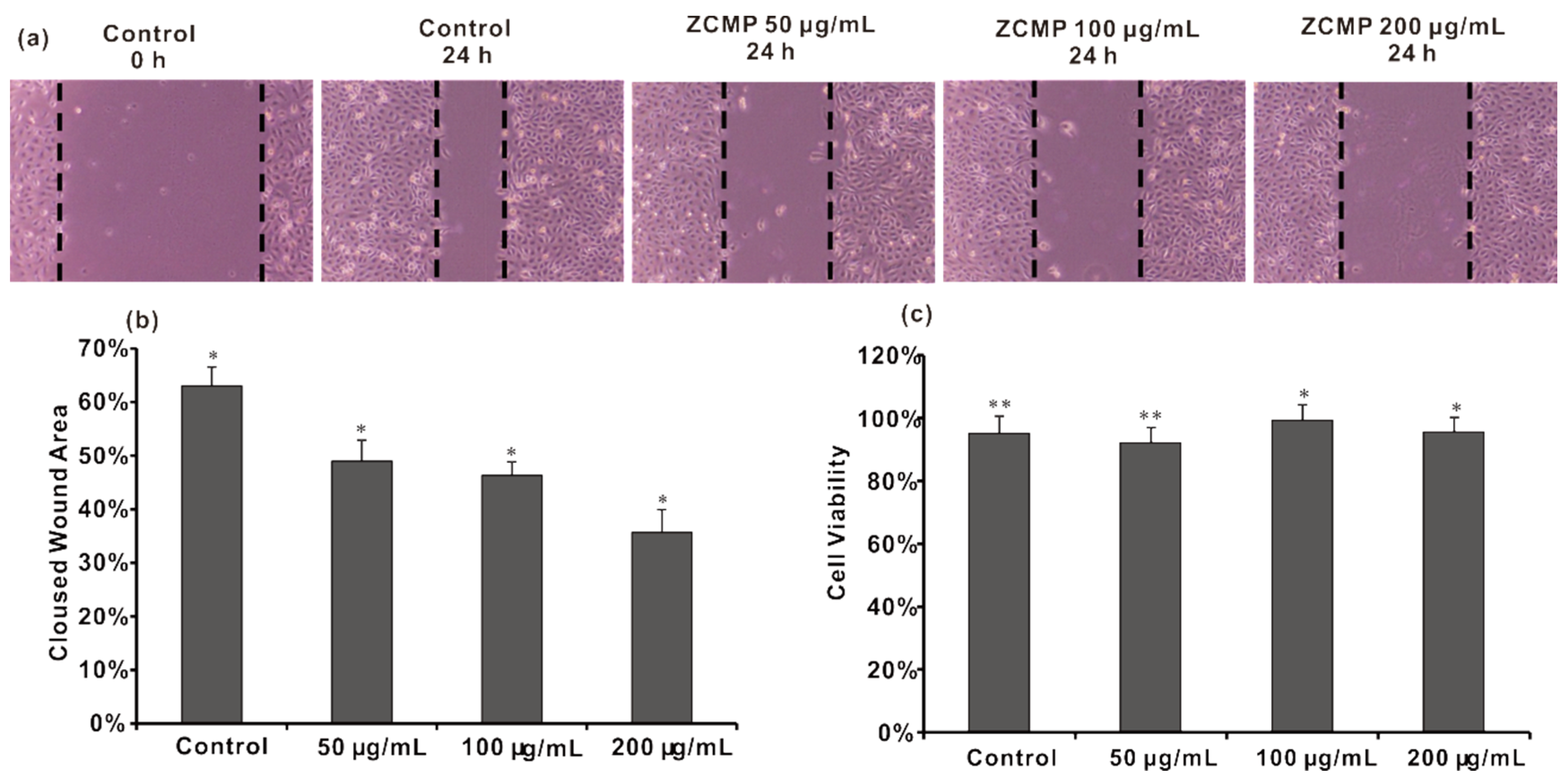
2.6. ZCMP Inhibited the Migration of HUVECs
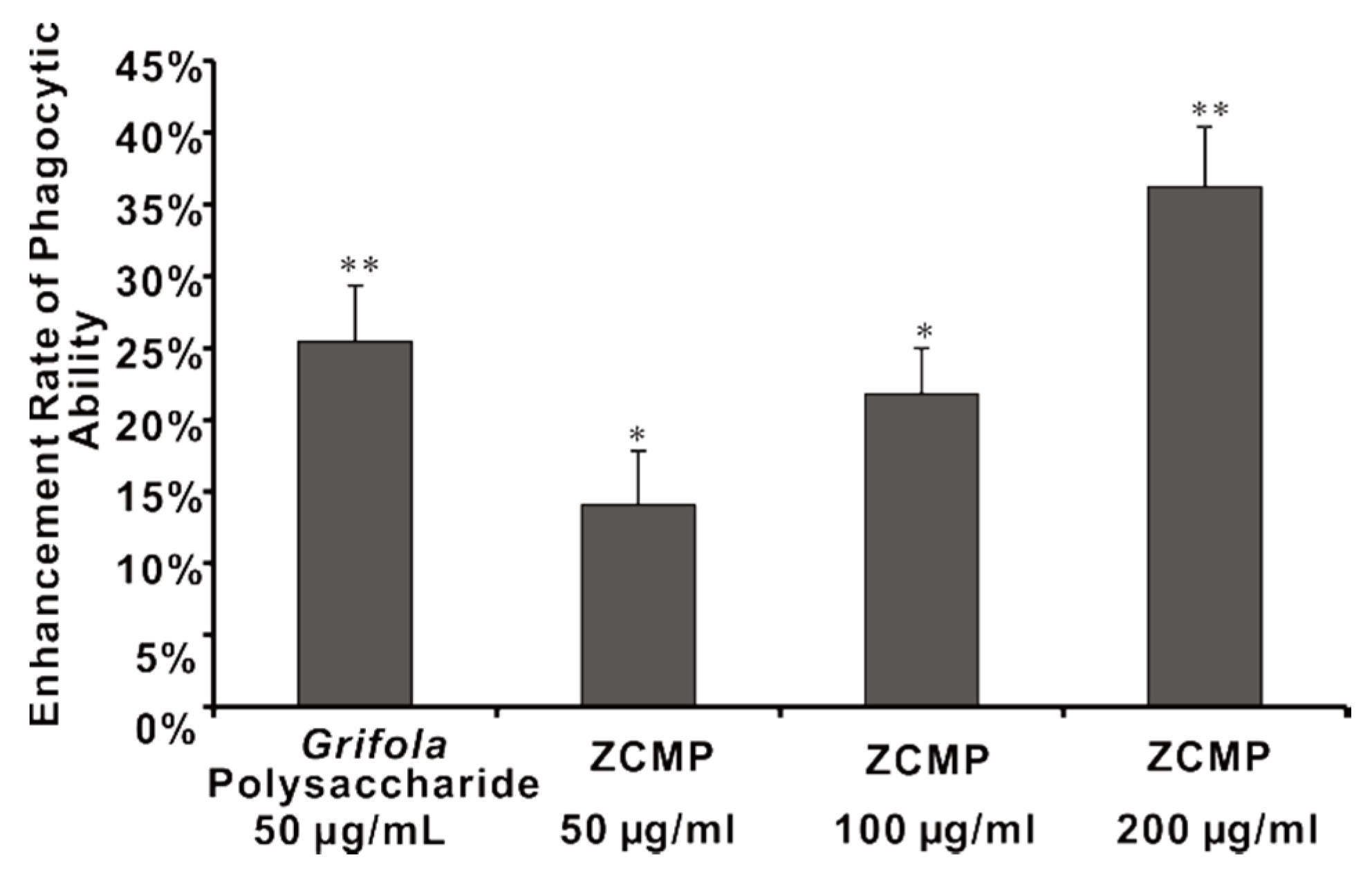
2.7. ZCMP Enhanced Macrophage Phagocytosis
3. Experimental Section
3.1. Samples and Materials
3.2. Extraction, Isolation, and Purification of ZCMP
3.3. General Analysis of ZCMP
3.4. Carboxyl-Group Reductions and Methylation Analysis of ZCMP
3.5. Preparation and Purification of Oligosaccharides of ZCMP
3.6. Oligosaccharide Reduction
3.7. MS Analysis of Oligosaccharides Derived from ZCMP
3.8. NMR Analysis of ZCMP-SS
3.9. Cell Lines and Culture Conditions
3.10. HUVEC Proliferation (MTT) Assays
3.11. HUVEC Migration Assays
3.12. Macrophage Phagocytosis Assays
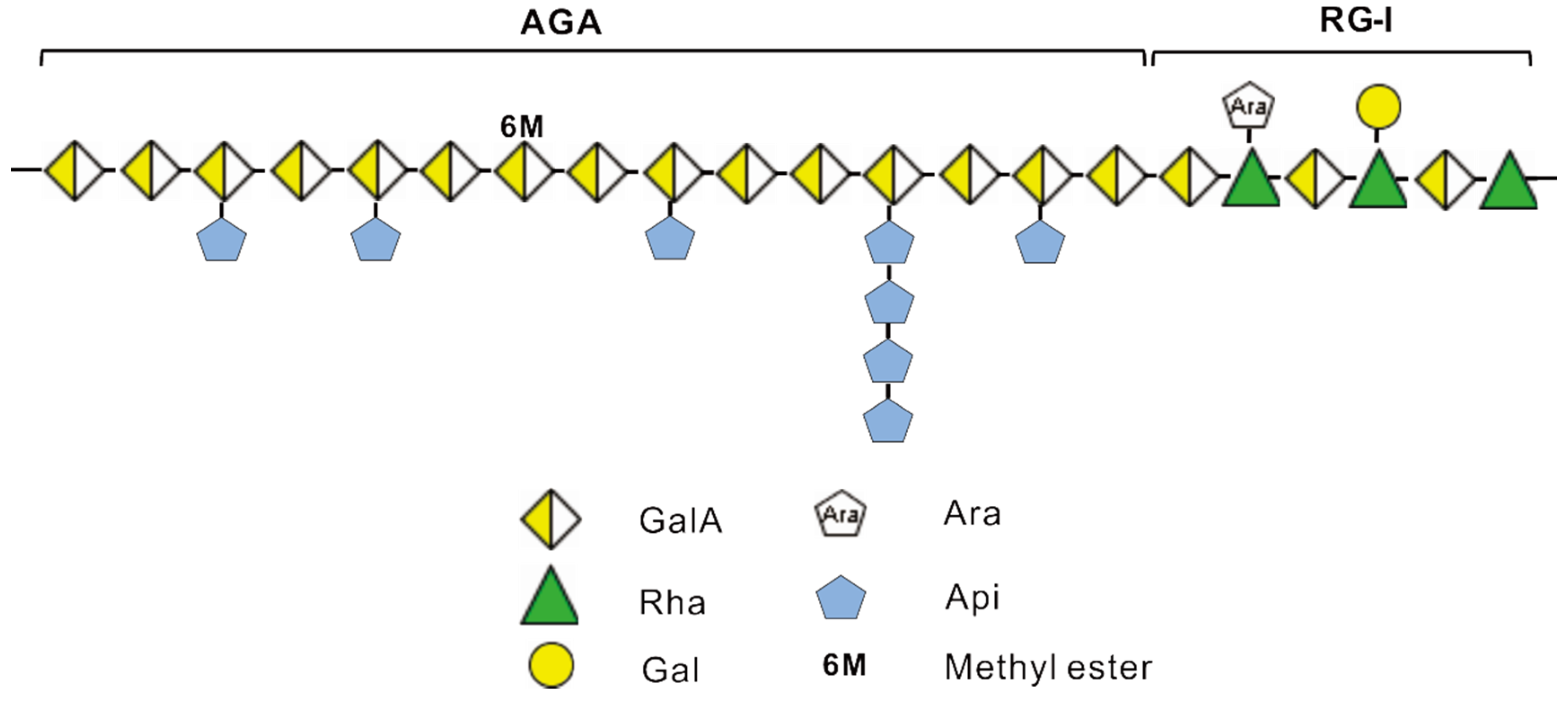
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ichihara, E.; Kiura, K.; Tanimoto, M. Targeting angiogenesis in cancer therapy. Acta Med. Okayama 2011, 65, 353–362. [Google Scholar] [PubMed]
- Huang, X.; Zhang, Q.; Jiang, Q.; Kang, X.; Zhao, L. Polysaccharides derived from Lycium barbarum suppress IGF-1-induced angiogenesis via PI3K/HIF-1α/VEGF signalling pathways in MCF-7 cells. Food Chem. 2012, 131, 1479–1484. [Google Scholar] [CrossRef]
- Zong, A.; Zhao, T.; Zhang, Y.; Song, X.; Shi, Y.; Cao, H.; Liu, C.; Cheng, Y.; Qu, X.; Cao, J.; et al. Anti-metastatic and anti-angiogenic activities of sulfated polysaccharide of Sepiella maindroni ink. Carbohydr. Polym. 2013, 91, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Rujitanaroj, P.; Aid-Launais, R.; Chew, S.Y.; le Visage, C. Polysaccharide electrospun fibers with sulfated poly (fucose) promote endothelial cell migration and VEGF-mediated angiogenesis. Biomater. Sci. 2014, 2, 843–852. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Yao, J.; Shi, Y.; Li, P.; Ding, K. An arabinogalactan from flowers of Panax notoginseng inhibits angiogenesis by BMP2/Smad/Id1 signaling. Carbohydr. Polym. 2015, 121, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Golovchenko, V.V.; Ovodova, R.G.; Shashkov, A.S.; Ovodov, Y.S. Structural studies of the pectic polysaccharide from duckweed Lemna minor L. Phytochemistry 2002, 60, 89–97. [Google Scholar] [CrossRef]
- Ovodova, R.G.; Golovchenko, V.V.; Shashkov, A.S.; Popov, S.V.; Ovodov, Y.S. Structural studies and physiological activity of lemnan, a pectin from Lemna minor L. Russ. J. Bioorganic Chem. 2000, 26, 743–751. [Google Scholar] [CrossRef]
- Hart, D.A.; Kindel, P.K. Isolation and partial characterization of apiogalacturonans from the cell wall of Lemna minor. Biochem. J. 1970, 116, 569–579. [Google Scholar] [PubMed]
- Gloaguen, V.; Brudieux, V.; Closs, B.; Barbat, A.; Krausz, P.; Sainte-Catherine, O.; Kraemer, M.; Maes, E.; Guerardel, Y. Structural Characterization and Cytotoxic Properties of an Apiose-Rich Pectic Polysaccharide Obtained from the Cell Wall of the Marine Phanerogam Zostera marina. J. Nat. Prod. 2010, 73, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Ovodov, Y.S.; Ovodova, R.G.; Bondarenko, O.D.; Krasikova, I.N. The pectic substances of zosteraceae: Part IV. Pectinase digestion of zosterine. Carbohydr. Res. 1971, 18, 311–318. [Google Scholar] [CrossRef]
- Ovodov, Y.S.; Mikheyskaya, L.V.; Ovodova, R.G.; Krasikova, I.N. The pectic substances of Zosteraceae: Part V. Smith degradation of zosterine. Carbohydr. Res 1971, 18, 319–322. [Google Scholar] [CrossRef]
- Ovodova, R.G.; Vaskovsky, V.E.; Ovodov, Y.S. The pectic substances of Zosferaceae. Carbohydr. Res. 1968, 6, 328–332. [Google Scholar] [CrossRef]
- Popov, S.V.; Ovodova, R.G.; Ovodov, Y.S. Effect of lemnan, pectin from Lemna minor L., and its fragments on inflammatory reaction. Phytother. Res. 2006, 20, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Sergushchenko, I.S.; Kovalev, V.V.; Bednyak, V.E.; Khotimchenko, Y.S. A comparative evaluation of the metal-binding activity of low-esterified pectin from the seagrass Zostera marine and other sorbents. Russ. J. Mar. Biol. 2004, 30, 70–72. [Google Scholar] [CrossRef]
- Sgrebneva, M.N.; Tsygankov, V.I.; Anisimov, A.P.; Khasina, E.I. Effect of zosterin on protein-synthesizing activity of hepatocytes. Bull. Exp. Biol. Med. 2005, 140, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Piskarev, V.; Lawson, A.M. Negative-ion electrospray mass spectrometry of neutral underivatized oligosaccharides. Anal. Chem. 2001, 73, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Zaia, J. Mass spectrum of oligosaccharides. Mass Spectrom. Rev. 2004, 23, 161–227. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Zhao, X.; Liu, L.; Yu, G. Applications of mass spectrometry to structural analysis of marine oligosaccharides. Mar. Drugs 2014, 12, 4005–4030. [Google Scholar] [CrossRef] [PubMed]
- Saad, O.M.; Leary, J.A. Delineating mechanisms of dissociation for isomeric heparin disaccharides using isotope labeling and ion trap tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, X.; Lv, Y.; Liu, Y.; Lang, Y.; Wu, J.; Liu, X.; Li, M.; Yu, G. Analysis of structural heterogeneity of fucoidan from Hizikia fusiforme by ES-CID-MS/MS. Carbohydr. Polym. 2012, 90, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Quemener, B.; Vigouroux, J.; Rathahao, E.; Tabet, J.C.; Dimitrijevic, A.; Lahaye, M. Negative electrospray ionization mass spectrometry: A method for sequencing and determining linkage position in oligosaccharides from branched hemicelluloses. J. Mass Spectrom. 2015, 50, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Everest-Dass, A.V.; Abrahams, J.L.; Kolarich, D.; Packer, N.H.; Campbell, M.P. Structural feature ions for distinguishing N- and O-linked glycan isomers by LC-ESI-IT MS/MS. J. Am. Soc. Mass Spectrom. 2013, 24, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhao, X.; Yang, B.; Ren, S.; Guan, H.; Zhang, Y.; Lawson, A.M.; Chai, W. Sequence determination of sulfated carrageenan-derived oligosaccharides by high-sensitivity negative-ion electrospray tandem mass spectrometry. Anal. Chem. 2006, 78, 8499–8505. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Mao, W.; Yan, M.; Zhao, C.; Li, N.; Shan, J.; Lin, C.; Liu, X.; Guo, T.; Guo, T.; et al. Galactomannan with novel structure produced by the coral endophytic fungus Aspergillus ochraceus. Carbohydr. Polym. 2014, 105, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Peau, M.C.; Thibault, J. Structure of the repeating units in the rhamnogalacturonic backbone of apple, beet and citrus pectin. Carbohydr. Res. 1995, 275, 155–165. [Google Scholar] [CrossRef]
- Coenen, G.J.; Bakx, E.J.; Verhoef, R.P.; Schols, H.A.; Voragen, A.G.J. Identification of the connecting linkage between hono- or xylogalacturonan and rhamnogalacturonan type Ι. Carbohydr. Polym. 2007, 70, 224–235. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, G.; Zhao, X.; Liu, H.; Guan, H.; Lawson, A.M.; Chai, W. Sequence analysis of alginate-derived oligosaccharides by negative-ion electrospray tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2006, 17, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Quéméner, B.; Cabrera Pino, J.C.; Ralet, M.C.; Bonnin, E.; Thibault, J.F. Assignment of acetyl groups to O-2 and/or O-3 of pectic oligogalacturonides using negative electrospray ionization ion trap mass spectrometry. J. Mass Spectrom. 2003, 38, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S. Mass spectrometry for characterizing plant cell wall polysaccharides. Front. Plant Sci. 2012, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.R.; Serianni, A.S. dl-apiose substituted with stable isotopes: Synthesis, NMR-spectral analysis, and furanose anomerization. Carbohydr. Res. 1987, 166, 85–99. [Google Scholar] [CrossRef]
- Yapo, B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Huang, Z.; Lin, H.; Wang, Y.; Cao, Z.; Lin, W.; Chen, Q. Studies on the anti-angiogenic effect of Marsdenia tenacissima extract in vitro and in vivo. Oncol. Lett. 2013, 5, 917–922. [Google Scholar] [PubMed]
- Masuda, Y.; Inoue, H.; Ohta, H.; Miyake, A.; Konishi, M.; Nanba, H. Oral administration of soluble beta-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int. J. Cancer 2013, 133, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, M.; Lamy, F. Determination of proteins and sulfobetaine with the folin-phenol reagent. Anal. Biochem. 1986, 157, 28–31. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106. [Google Scholar] [PubMed]
- Chen, S.; Xu, J.; Xue, C.; Dong, P.; Sheng, W.; Yu, G.; Chai, W. Sequence determination of a non-sulfated glycosaminoglycan-like polysaccharide from melanin-free ink of the squid Ommastrephes bartrami by negative-ion electrospray tandem mass spectrometry and NMR spectroscopy. Glycoconj. J. 2008, 25, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.L.; Conrad, H.E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl group. Biochemistry 1972, 11, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J. Biochem. 1964, 55, 205–208. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Shan, X.; Zhao, X.; Cai, C.; Zhao, X.; Lang, Y.; Zhu, H.; Yu, G. Extraction, Isolation, Structural Characterization and Anti-Tumor Properties of an Apigalacturonan-Rich Polysaccharide from the Sea Grass Zostera caespitosa Miki. Mar. Drugs 2015, 13, 3710-3731. https://doi.org/10.3390/md13063710
Lv Y, Shan X, Zhao X, Cai C, Zhao X, Lang Y, Zhu H, Yu G. Extraction, Isolation, Structural Characterization and Anti-Tumor Properties of an Apigalacturonan-Rich Polysaccharide from the Sea Grass Zostera caespitosa Miki. Marine Drugs. 2015; 13(6):3710-3731. https://doi.org/10.3390/md13063710
Chicago/Turabian StyleLv, Youjing, Xindi Shan, Xia Zhao, Chao Cai, Xiaoliang Zhao, Yinzhi Lang, He Zhu, and Guangli Yu. 2015. "Extraction, Isolation, Structural Characterization and Anti-Tumor Properties of an Apigalacturonan-Rich Polysaccharide from the Sea Grass Zostera caespitosa Miki" Marine Drugs 13, no. 6: 3710-3731. https://doi.org/10.3390/md13063710
APA StyleLv, Y., Shan, X., Zhao, X., Cai, C., Zhao, X., Lang, Y., Zhu, H., & Yu, G. (2015). Extraction, Isolation, Structural Characterization and Anti-Tumor Properties of an Apigalacturonan-Rich Polysaccharide from the Sea Grass Zostera caespitosa Miki. Marine Drugs, 13(6), 3710-3731. https://doi.org/10.3390/md13063710