The Effect of Dissolved Polyunsaturated Aldehydes on Microzooplankton Growth Rates in the Chesapeake Bay and Atlantic Coastal Waters
Abstract
:1. Introduction
2. Results
2.1. Water Column Conditions and Ambient PUA Concentrations
| Exp. | Date | Location | Temp. °C | Salinity | Heptadienal | Octadienal | |||
|---|---|---|---|---|---|---|---|---|---|
| Dissolved nM | Particulate nM | Dissolved nM | Particulate nM | ||||||
| 1 | Apr-13 | Chesapeake Bay | Choptank River, HPL | 14.0 | 10.0 | nd | nd | nd | nd |
| 2 | May-14 | Choptank River, HPL | 16.7 | 9.4 | 0.021 | 0.002 | 0.011 | 0.000 | |
| 3 | May-14 | Ragged Point | 16.5 | 10.0 | 0.004 | 0.002 | 0.003 | 0.000 | |
| 4 | Sep-14 | Atlantic waters | Wachapreague, ESL | 23.2 | 29.8 | 0.014 | 0.055 | 0.004 | 0.004 |
| 5 | Sep-14 | Wachapreague, Inlet | 24.0 | 30.0 | 0.060 | 0.017 | 0.001 | 0.002 | |
2.2. Microplankton Composition and Biomass
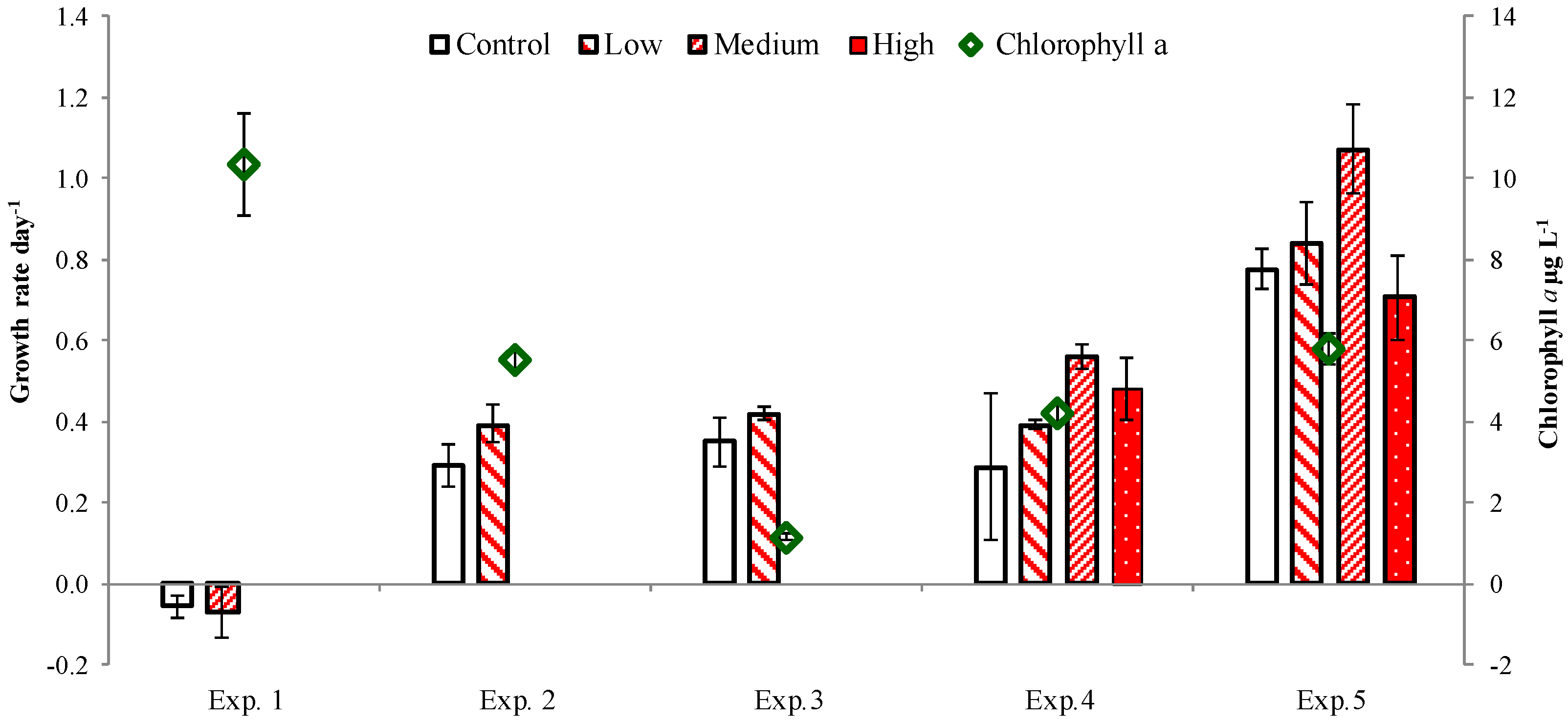
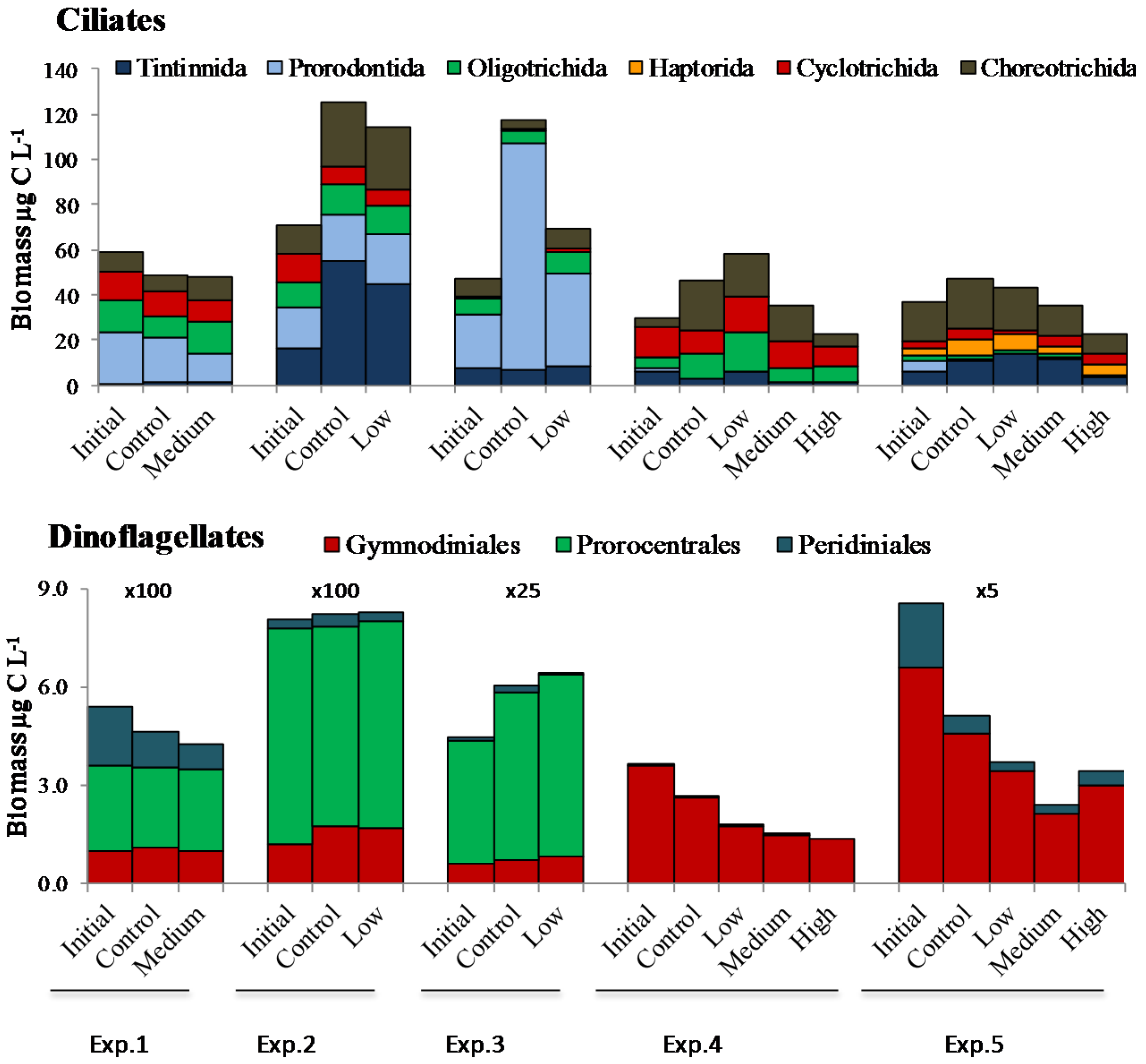
2.3. PUA and Community Growth Rates
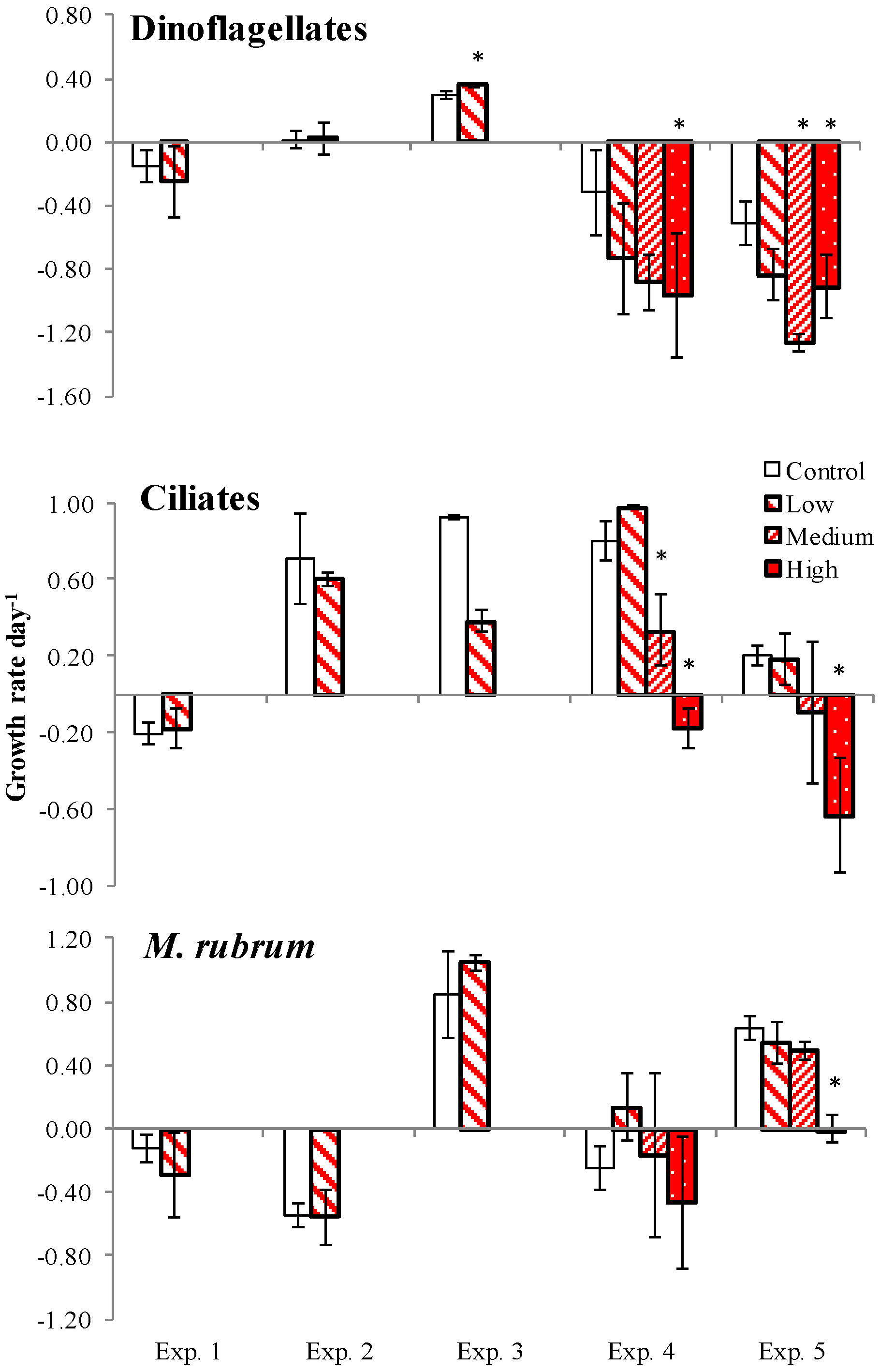
2.4. PUA and Species-Specific Growth Rates
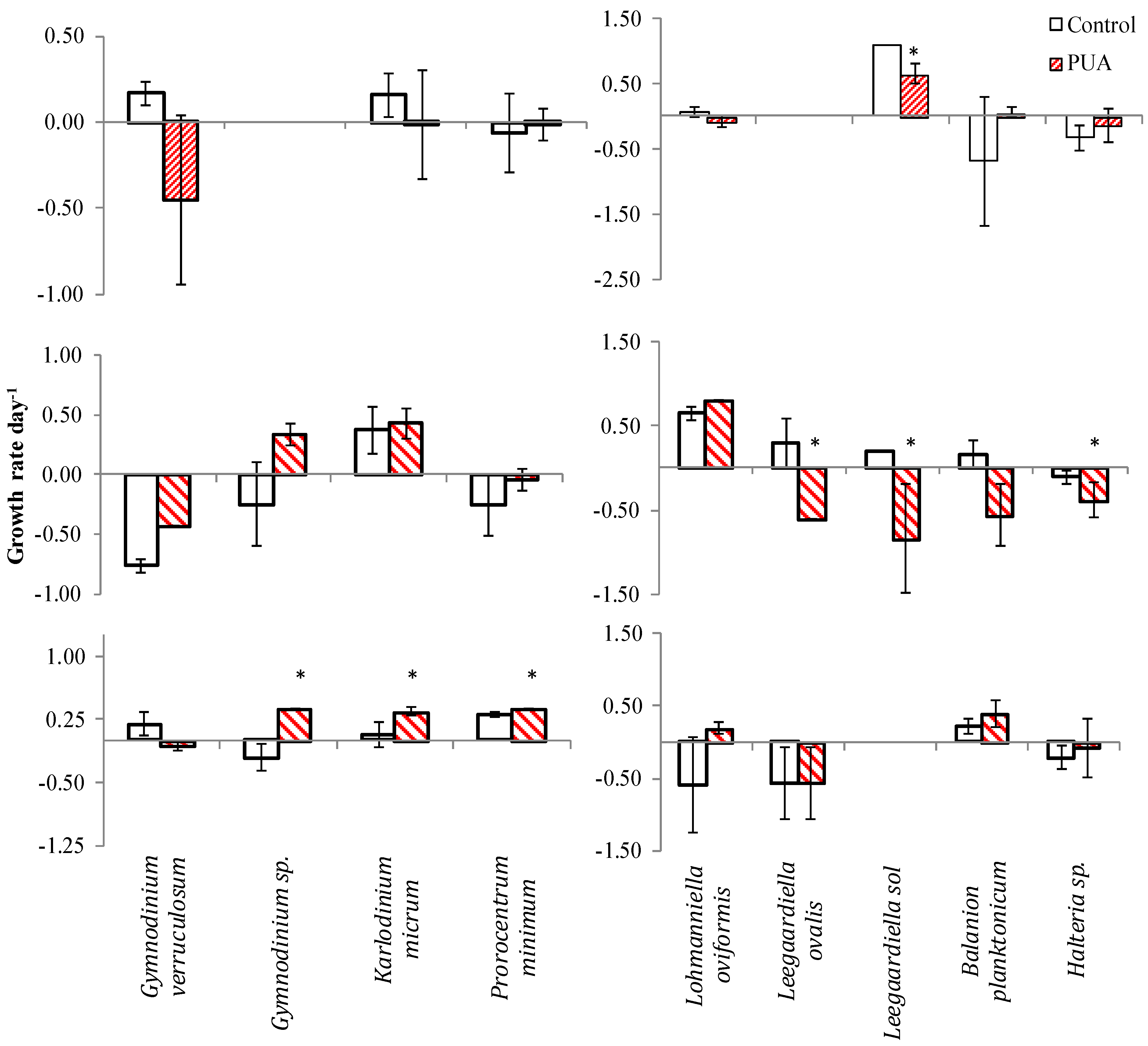
| Species | Control | Low | Medium | High | |||||
|---|---|---|---|---|---|---|---|---|---|
| Exp. 4 | Balanion planktonicum | −1.06 ± 0.23 | ab | −0.41 ± 0.15 | a | −1.61 ± 0.10 | b | −1.54 ± 0.15 | b |
| Codonella sp. | −1.33 ± 0.10 | a | −2.82 ± 0.12 | b | −2.83 ± 0.17 | b | none | - | |
| Cyrtostrombidium sp. | 1.43 ± 0.05 | a | 1.01 ± 0.07 | a | −0.56 ± 0.52 | b | −0.37 ± 0.12 | b | |
| Favella panamensis | −0.75 ± 0.08 | b | 0.23 ± 0.11 | a | −1.73 ± 0.13 | c | −1.50 ± 0.16 | c | |
| Gymnodinium verruculosum | −0.12 ± 0.48 | a | −0.85 ± 0.47 | b | −1.05 ± 0.42 | b | −1.21 ± 0.47 | b | |
| Gymnodinium sp. | −0.29 ± 0.05 | a | −0.35 ± 0.05 | a | −0.43 ± 0.20 | a | −0.35 ± 0.22 | a | |
| Gyrodinium spirale | 0.65 ± 0.05 | a | −0.33 ± 0.14 | b | −1.35 ± 0.10 | c | −1.62 ± 0.23 | c | |
| Lohmanniella oviformis | 0.62 ± 0.26 | a | 0.80 ± 0.23 | a | 0.44 ± 0.16 | a | 0.02 ± 0.11 | a | |
| Strobilidium neptuni | 2.50 ± 0.07 | a | 2.44 ± 0.17 | a | 2.08 ± 0.19 | a | 0.84 ± 0.25 | b | |
| Strombidium conicum | 0.83 ± 0.15 | b | 1.76 ± 0.17 | a | 0.90 ± 0.18 | b | 1.03 ± 0.06 | b | |
| Strombidium lynni | 1.62 ± 0.18 | ab | 2.14 ± 0.32 | a | 0.09 ± 0.14 | bc | −0.43 ± 0.82 | c | |
| Strombidium sp. | 0.96 ± 0.05 | a | 1.11 ± 0.31 | a | 0.18 ± 0.20 | a | 0.69 ± 0.57 | a | |
| Strombidium acutum | 0.01 ± 0.15 | ab | 0.74 ± 0.18 | a | −0.80 ± 0.45 | bc | −1.35 ± 0.00 | c | |
| Exp. 5 | Cyclotrichium gigas | 0.46 ± 0.23 | a | 0.46 ± 0.46 | a | 0.35 ± 0.28 | a | 0.55 ± 0.45 | a |
| Cyrtostrombidium sp. | −0.47 ± 0.00 | a | 0.77 ± 0.12 | a | −0.47 ± 0.00 | a | −0.12 ± 0.28 | a | |
| Favella panamensis | 1.10 ± 0.00 | a | 1.10 ± 0.40 | a | 0.64 ± 0.23 | a | none | - | |
| Gymnodinium verruculosum | −0.57 ± 0.07 | a | −0.77 ± 0.09 | ab | −1.19 ± 0.07 | ab | −0.99 ± 0.16 | b | |
| Gyrodinium spirale | −0.39 ± 0.09 | a | −0.40 ± 0.08 | a | −1.72 ± 0.07 | c | −1.19 ± 0.10 | b | |
| Leegaardiella sol | 1.41 ± 0.04 | a | 0.85 ± 0.16 | ab | 0.84 ± 0.00 | ab | 0.59 ± 0.22 | b | |
| Lohmanniella oviformis | 0.19 ± 0.05 | a | 0.33 ± 0.12 | a | −0.47 ± 0.05 | b | −1.72 ± 0.21 | c | |
| Strobilidium neptuni | −0.61 ± 0.12 | a | 0.08 ± 0.08 | a | −0.82 ± 0.35 | ab | −1.28 ± 0.17 | b |

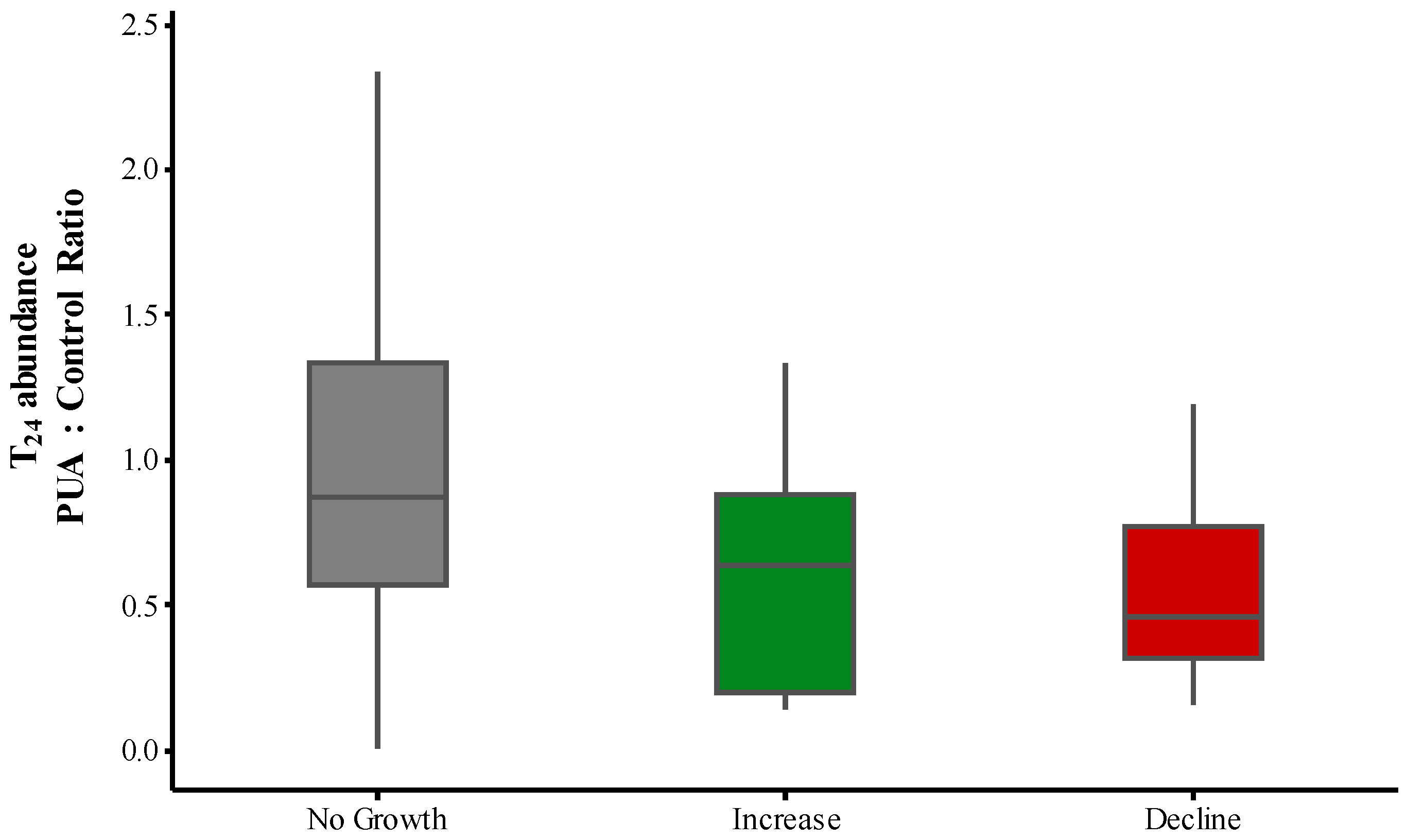
2.5. Methanol Addition Effect
| Species | Control | Methanol | p-Value |
|---|---|---|---|
| Akashiwo sanguinea | −0.11 ± 0.15 | −0.15 ± 0.11 | ns |
| Balanion comatum | −0.19 ± 0.13 | 0.18 ±0.06 | 0.06 |
| Balanion planktonicum | 0.17 ± 0.23 | 0.56 ± 0.08 | ns |
| Cyclotrichium gigas | 0.22 ± 0.11 | −0.38 ± 0.28 | ns |
| Eutintinnus pectinis | −0.48 ± 0.37 | 0.70 ± 0.25 | 0.05 |
| Gymnodinium verruculosum | −0.62 ± 0.04 | −0.45 ± 0.07 | ns |
| Gymnodinium sp. | −0.93 ± 0.17 | 0.10 ± 0.06 | 0.00 |
| Gyrodinium dominans | −1.15 ± 0.23 | −0.65 ± 0.14 | ns |
| Gyrodinium uncatenatum | −0.84 ± 0.41 | 0.10 ± 0.04 | ns |
| Leegaardiella sol | 0.60 ± 0.24 | 1.12 ± 0.07 | ns |
| Lohmanniella oviformis | 0.72 ± 0.22 | 1.29 ± 0.12 | ns |
| Mesodinium acarus | −0.50 ± 0.14 | −0.41 ± 0.20 | ns |
| Polykrikos schwartzii | 0.16 ± 0.09 | −0.21 ± 0.32 | ns |
| Strombidium acutum | 0.43 ± 0.12 | 0.69 ± 0.07 | ns |
| Strombidium conicum | −0.09 ± 0.16 | −0.91 ±0.42 | ns |
| Strombidium sp. | −0.74 ± 0.45 | −0.80 ± 0.50 | ns |
| Tintinnopsis beroidea | −0.14 ± 0.07 | 0.81 ± 0.00 | <0.01 |
| Tintinnopsis campanula | −0.79 ± 0.32 | −0.12 ± 0.17 | ns |
| Tintinnopsis coronata | 1.23 ± 0.42 | 1.83 ± 0.37 | ns |
| Tintinnopsis denticulata | −1.90 ± 0.00 | −0.97 ± 0.46 | ns |
| Tintinnopsis minuta | −0.54 ± 0.33 | 0.08 ± 0.07 | ns |
| Tontonia gracillima | −0.16 ± 0.09 | 0.31 ± 0.12 | 0.03 |
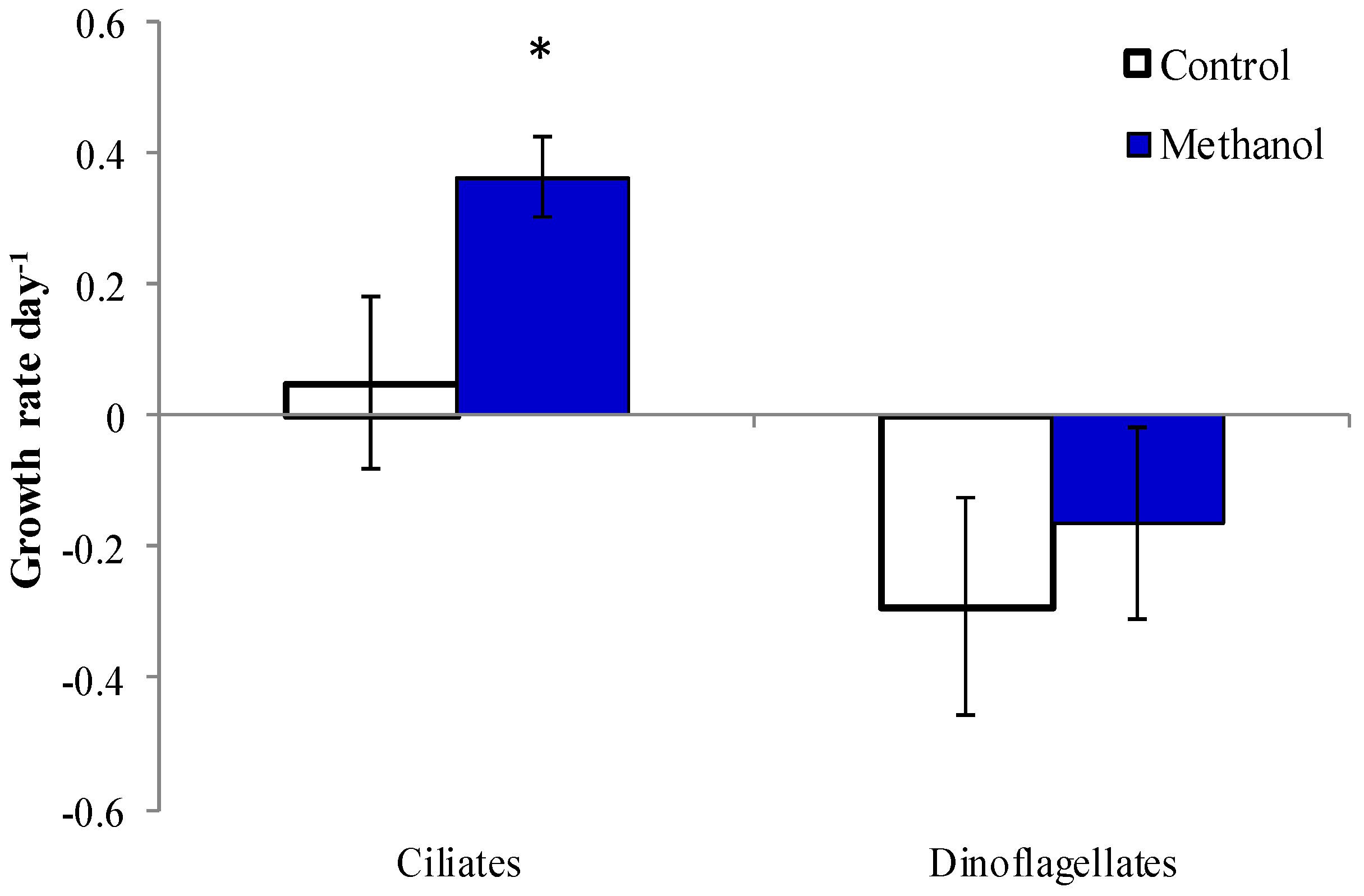
3. Discussion
4. Experimental Section
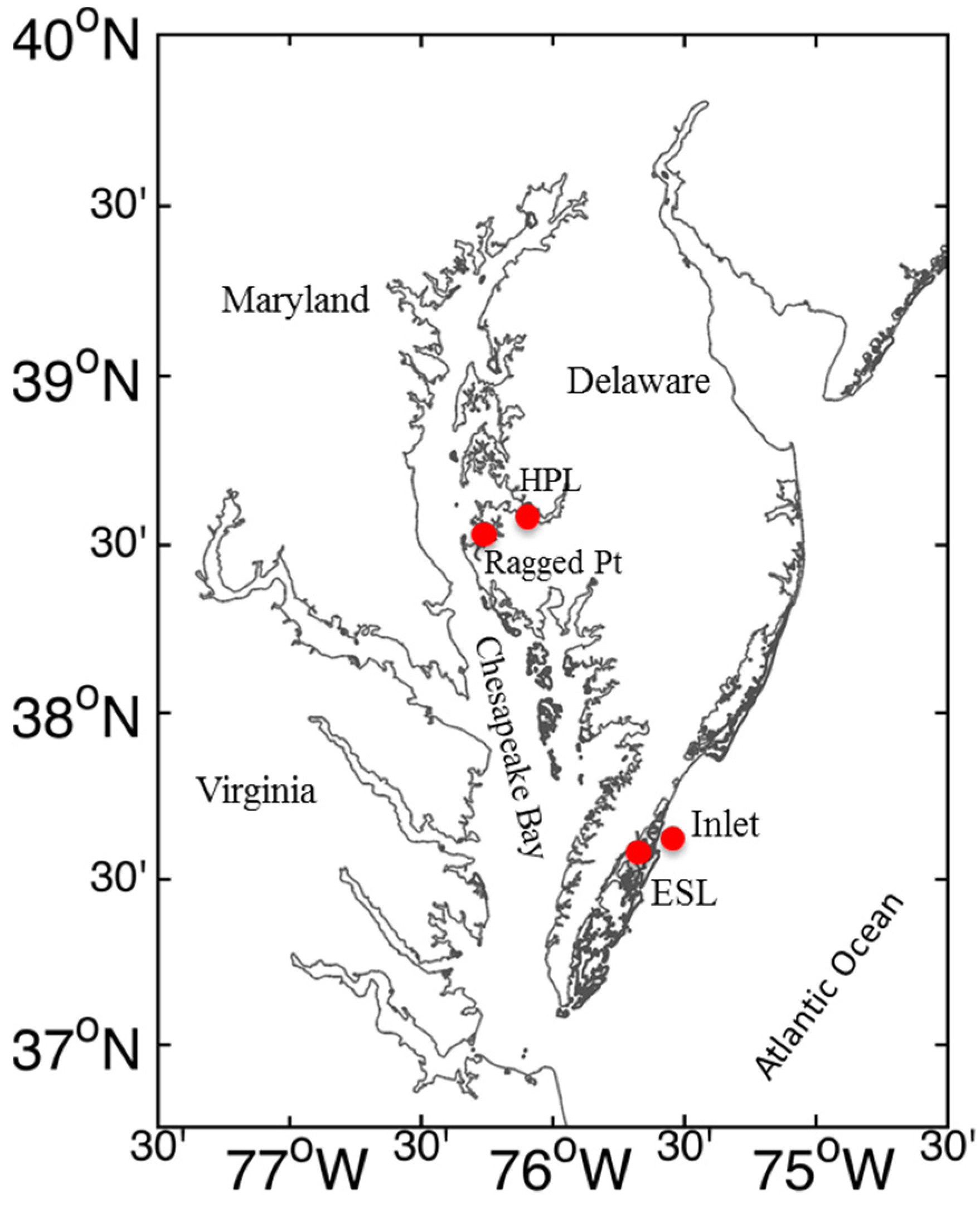
4.1. Field Sampling
4.2. Experimental Setup
4.3. Chlorophyll A Analysis
4.4. Microzooplankton Analysis
4.5. Rate Calculations
4.6. Ambient PUA Analyses
4.7. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mann, D.G. The species concept in diatoms. Phycologia 1999, 38, 437–495. [Google Scholar] [CrossRef]
- Nelson, D.M.; Treguer, P.; Brzezinski, M.A.; Leynaert, A.; Queguiner, B. Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob. Biogeochem. Cycles 1995, 9, 359–372. [Google Scholar] [CrossRef]
- Smetacek, V. Diatoms and the ocean carbon cycle. Protist 1999, 150, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sarthou, G.; Timmermans, K.R.; Blain, S.; Tréguer, P. Growth physiology and fate of diatoms in the ocean: A review. J. Sea Res. 2005, 53, 25–42. [Google Scholar] [CrossRef]
- Wassmann, P.; Slagstad, D.; Riser, C.W.; Reigstad, M. Modelling the ecosystem dynamics of the Barents Sea including the marginal ice zone. J. Mar. Syst. 2006, 59, 1–24. [Google Scholar] [CrossRef]
- Buesseler, K.O. The decoupling of production and particulate export in the surface ocean. Glob. Biogeochem. Cycles 1998, 12, 297–310. [Google Scholar] [CrossRef]
- Ianora, A.; Bentley, M.G.; Caldwell, G.S.; Casotti, R.; Cembella, A.D.; Engstrom-Ost, J.; Halsband, C.; Sonnenschein, E.; Legrand, C.; Llewellyn, C.A.; et al. The relevance of marine chemical ecology to plankton and ecosystem function: An emerging field. Mar. Drugs 2011, 9, 1625–1648. [Google Scholar] [CrossRef] [PubMed]
- Strom, S.L. Microbial ecology of ocean biogeochemistry: A community perspective. Science 2008, 320, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Miralto, A.; Barone, G.; Romano, G.; Poulet, S.A.; Ianora, A.; Russo, G.L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; et al. The insidious effect of diatoms on copepod reproduction. Nature 1999, 402, 173–176. [Google Scholar] [CrossRef]
- Wichard, T.; Poulet, S.A.; Halsband-Lenk, C.; Albaina, A.; Harris, R.; Liu, D.Y.; Pohnert, G. Survey of the chemical defence potential of diatoms: Screening of fifty one species for α,β,γ,δ-unsaturated aldehydes. J. Chem. Ecol. 2005, 31, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Ianora, A.; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: A review. Ecotoxicology 2010, 19, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Ernstsen, A.; Eilertsen, H.C. Isolation and characterisation of a cytotoxic polyunsaturated aldehyde from the marine phytoplankter Phaeocystis pouchetii (Hariot) Lagerheim. Toxicology 2004, 199, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Sarno, D.; Kooistra, W.H.C.F.; Medlin, L.K.; Percopo, I.; Zingone, A. Diversity in the genus Skeletonema (Bacillariophyceae). II. An assessment of the taxonomy of S. costatum-like species with the description of four new species. J. Phycol. 2005, 41, 151–176. [Google Scholar] [CrossRef]
- Ribalet, F.; Berges, J.A.; Ianora, A.; Casotti, R. Growth inhibition of cultured marine phytoplankton by toxic algal-derived polyunsaturated aldehydes. Aquat. Toxicol. 2007, 85, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Pohnert, G. Wound-activated chemical defense in unicellular planktonic algae. Angew. Chem. Int. Ed. 2000, 39, 4352–4354. [Google Scholar] [CrossRef]
- Ribalet, F.; Wichard, T.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms. Phytochemistry 2007, 68, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Wichard, T.; Poulet, S.A.; Pohnert, G. Determination and quantification of α,β,γ,δ-unsaturated aldehydes as pentafluorobenzyl-oxime derivates in diatom cultures and natural phytoplankton populations: Application in marine field studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 814, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ribalet, F.; Bastianini, M.; Vidoudez, C.; Acri, F.; Berges, J. Phytoplankton cell lysis associated with polyunsaturated aldehyde release in the Northern Adriatic Sea. PLoS ONE 2014, 9, e98727. [Google Scholar]
- Fontana, A.; d’Ippolito, G.; Cutignano, A.; Miralto, A.; Ianora, A.; Romano, G.; Cimino, G. Chemistry of oxylipin pathways in marine diatoms. Pure Appl. Chem. 2007, 79, 481–490. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A.; Poulet, S.A.; Carotenuto, Y.; Buttino, I.; Romano, G.; Casotti, R.; Pohnert, G.; Wichard, T.; Colucci-D’Amato, L.; et al. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature 2004, 429, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Carotenuto, Y.; Miralto, A.; Procaccini, G.; Ianora, A. Copepod population-specific response to a toxic diatom diet. PLoS ONE 2012, 7, e47262. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Miralto, A.; Ianora, A. Teratogenic effects of diatom metabolites on sea urchin Paracentrotus lividus embryos. Mar. Drugs 2010, 8, 950–967. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, G.S. The influence of bioactive oxylipins from marine diatoms on invertebrate reproduction and development. Mar. Drugs 2009, 7, 367–400. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.T.; Ianora, A.; Miralto, A.; Laabir, M.; Esposito, F. Decoupling of copepod grazing rates, fecundity and egg-hatching success on mixed and alternating diatom and dinoflagellate diets. Mar. Ecol. Progr. Ser. 2001, 220, 187–199. [Google Scholar] [CrossRef]
- Calbet, A.; Landry, M.R. Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol. Oceanogr. 2004, 49, 51–57. [Google Scholar] [CrossRef]
- Buitenhuis, E.T.; Rivkin, R.B.; Sailley, S.; Le Quere, C. Biogeochemical fluxes through microzooplankton. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Jacobson, D.M.; Anderson, D.M. Thecate heterotrophic dinoflagellates—Feeding-behavior and mechanisms. J. Phycol. 1986, 22, 249–258. [Google Scholar] [CrossRef]
- Jeong, H.J.; Yoo, Y.D.; Kim, J.S.; Seong, K.A.; Kang, N.S.; Kim, T.H. Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean. Sci. J. 2010, 45, 65–91. [Google Scholar] [CrossRef]
- Nejstgaard, J.C.; Gismervik, I.; Solberg, P.T. Feeding and reproduction by Calanus finmarchicus, and microzooplankton grazing during mesocosm blooms of diatoms and the coccolithophore Emiliania huxleyi. Mar. Ecol. Progr. Ser. 1997, 147, 197–217. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F. Capacity of herbivorous protists to control initiation and development of mass phytoplankton blooms. Aquat. Microb. Ecol. 2009, 57, 253–262. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F.; Ross, C. Microzooplankton grazing impact in the Bering Sea during spring sea ice conditions. Deep Sea Res. Part. II 2013, 94, 57–67. [Google Scholar] [CrossRef]
- Strom, S.L.; Fredrickson, K.A. Intense stratification leads to phytoplankton nutrient limitation and reduced microzooplankton grazing in the southeastern Bering Sea. Deep Sea Res. Part. II 2008, 55, 1761–1774. [Google Scholar] [CrossRef]
- Flynn, K.J.; Irigoien, X. Aldehyde-induced insidious effects cannot be considered as a diatom defence mechanism against copepods. Mar. Ecol. Progr. Ser. 2009, 377, 79–89. [Google Scholar] [CrossRef]
- Smith, K.L.; Kaufmann, R.S. Long-term discrepancy between food supply and demand in the deep eastern North Pacific. Science 1999, 284, 1174–1177. [Google Scholar] [CrossRef] [PubMed]
- Bergholtz, T.; Daugbjerg, N.; Moestrup, O.; Fernandez-Tejedor, M. On the identity of Karlodinium veneficum and description of Karlodinium armiger sp. nov. (Dinophyceae), based on light and electron microscopy, nuclear-encoded LSU rDNA, and pigment composition. J. Phycol. 2006, 42, 170–193. [Google Scholar] [CrossRef]
- Franzè, G.; Lavrentyev, P.J. Microzooplankton growth rates examined across a temperature gradient in the Barents Sea. PLoS ONE 2014, 9, e86429. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.A.; Ricke, S.C. Effect of ethanol and methanol on growth of ruminal bacteria Selenomonas ruminantium and Butyrivibrio fibrisolvens. J. Environ. Sci. Health B 2015, 50, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.L.; Sargeant, S.; Nightingale, P.D.; Murrell, J.C. Gradients in microbial methanol uptake: productive coastal upwelling waters to oligotrophic gyres in the Atlantic Ocean. ISME J. 2013, 7, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Mincer, T.; Woods Hole Oceanographic Institution, Woods Hole, MA, USA. Personal Communication, 2014.
- Dittami, S.M.; Wichard, T.; Malzahn, A.M.; Pohnert, G.; Boersma, M.; Wiltshire, K.H. Culture conditions affect fatty acid content along with wound-activated production of polyunsaturated aldehydes in Thalassiosira rotula (Coscinodiscophyceae). Nova Hedwig. 2010, 231–248. [Google Scholar]
- Strom, S.; Western Washington University, Washington, WA, USA. Personal Communication, 2013.
- Lavrentyev, P.J.; Franzè, G.; University of Akron, Akron, OH, USA. 2013; Unpublished work.
- Hansen, E.; Eilertsen, H.C. Do the polyunsaturated aldehydes produced by Phaeocystis pouchetii (Hariot) Lagerheim influence diatom growth during the spring bloom in Northern Norway? J. Plankton Res. 2006, 29, 87–96. [Google Scholar] [CrossRef]
- Vidoudez, C.; Casotti, R.; Bastianini, M.; Pohnert, G. Quantification of dissolved and particulate polyunsaturated aldehydes in the Adriatic sea. Mar. Drugs 2011, 9, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Morillo-Garcia, S.; Valcarcel-Perez, N.; Cozar, A.; Ortega, M.J.; Macias, D.; Ramirez-Romero, E.; Garcia, C.M.; Echevarria, F.; Bartual, A. Potential Polyunsaturated Aldehydes in the Strait of Gibraltar under Two Tidal Regimes. Mar. Drugs 2014, 12, 1438–1459. [Google Scholar] [CrossRef] [PubMed]
- Bartual, A.; Arandia-Gorostidi, N.; Cozar, A.; Morillo-Garcia, S.; Jesus Ortega, M.; Vidal, M.; Maria Cabello, A.; Ignacio Gonzalez-Gordillo, J.; Echevarria, F. Polyunsaturated aldehydes from large phytoplankton of the Atlantic Ocean surface (42° N to 33° S). Mar. Drugs 2014, 12, 682–699. [Google Scholar] [CrossRef] [PubMed]
- Balestra, C.; Alonso-Saez, L.; Gasol, J.M.; Casotti, R. Group-specific effects on coastal bacterioplankton of polyunsaturated aldehydes produced by diatoms. Aquat. Microb. Ecol. 2011, 63, 123–131. [Google Scholar] [CrossRef]
- Casotti, R.; Mazza, S.; Brunet, C.; Vantrepotte, V.; Ianora, A.; Miralto, A. Growth inhibition and toxicity of the diatom aldehyde 2-trans,4-trans-decadienal on Thalassiosira weissflogii (Bacillariophyceae). J. Phycol. 2005, 41, 7–20. [Google Scholar] [CrossRef]
- Ka, S.; Carotenuto, Y.; Romano, G.; Hwang, J.S.; Buttino, I.; Ianora, A. Impact of the diatom-derived polyunsaturated aldehyde 2-trans,4-trans decadienal on the feeding, survivorship and reproductive success of the calanoid copepod Temora stylifera. Mar. Environ. Res. 2014, 93, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ribalet, F.; Intertaglia, L.; Lebaron, P.; Casotti, R. Differential effect of three polyunsaturated aldehydes on marine bacterial isolates. Aquat. Toxicol. 2008, 86, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Costantini, M.; Buttino, I.; Ianora, A.; Palumbo, A. Nitric oxide mediates the stress response induced by diatom aldehydes in the sea urchin Paracentrotus lividus. PLoS ONE 2011, 6, e25980. [Google Scholar] [CrossRef] [PubMed]
- Vidoudez, C.; Nejstgaard, J.C.; Jakobsen, H.H.; Pohnert, G. Dynamics of dissolved and particulate polyunsaturated aldehydes in mesocosms inoculated with different densities of the diatom Skeletonema marinoi. Mar. Drugs 2011, 9, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Ribalet, F.; Vidoudez, C.; Cassin, D.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. High plasticity in the production of diatom-derived polyunsaturated aldehydes under nutrient limitation: Physiological and ecological implications. Protist 2009, 160, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, G.S.; Bentley, M.G.; Olive, P.J. First evidence of sperm motility inhibition by the diatom aldehyde 2E,4E-decadienal. Mar. Ecol. Progr. Ser. 2004, 273, 97–108. [Google Scholar] [CrossRef]
- First, M.R.; Miller, H.L.; Lavrentyev, P.J.; Pinckney, J.L.; Burd, A.B. Effects of microzooplankton growth and trophic interactions on herbivory in coastal and offshore environments. Aquat. Microb. Ecol. 2009, 54, 255–267. [Google Scholar] [CrossRef]
- Franzè, G.; Modigh, M. Experimental evidence for internal predation in microzooplankton communities. Mar. Biol. 2013, 160, 3103–3112. [Google Scholar]
- Modigh, M.; Franzè, G. Changes in phytoplankton and microzooplankton populations during grazing experiments at a Mediterranean coastal site. J. Plankton Res. 2009, 31, 853–864. [Google Scholar] [CrossRef]
- Johnson, M.D.; Rome, M.; Stoecker, D.K. Microzooplankton grazing on Prorocentrum minimum and Karlodinium micrum in Chesapeake Bay. Limnol. Oceanogr. 2003, 48, 238–248. [Google Scholar] [CrossRef]
- Rychert, K. A modified dilution method reveals higher protozoan growth rates than the size fractionation method. Eur. J. Protistol. 2013, 49, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.B.; Lessard, E.J. The influence of the Pseudo-nitzschia toxin, domoic acid, on microzooplankton grazing and growth: A field and laboratory assessment. Harmful Algae 2010, 9, 540–547. [Google Scholar] [CrossRef]
- Prince, E.K.; Irmer, F.; Pohnert, G. Domoic Acid improves the competitive ability of Pseudo-nitzschia delicatissima against the diatom Skeletonema marinoi. Mar. Drugs 2013, 11, 2398–2412. [Google Scholar] [CrossRef] [PubMed]
- Herfort, L.; Peterson, T.D.; Campbell, V.; Futrell, S.; Zuber, P. Myrionecta rubra (Mesodinium rubrum) bloom initiation in the Columbia River estuary. Estuar. Coast. Shelf Sci. 2011, 95, 440–446. [Google Scholar] [CrossRef]
- Yih, W.; Kim, H.S.; Jeong, H.A.; Myung, G.; Kim, Y.G. Ingestion of cryptophyte cells by the marine photosynthetic ciliate Mesodinium rubrum. Aquat. Microb. Ecol. 2004, 36, 165–170. [Google Scholar] [CrossRef]
- Chen, B.; Landry, M.R.; Huang, B.; Liu, H. Does warming enhance the effect of microzooplankton grazing on marine phytoplankton in the ocean? Limnol. Oceanogr. 2012, 57, 519–526. [Google Scholar] [CrossRef]
- Gifford, D.J. Laboratory culture of marine planktonic Oligotrichs (Ciliophora, Oligotrichida). Mar. Ecol. Progr. Ser. 1985, 23, 257–267. [Google Scholar] [CrossRef]
- Arar, E.J.; Collins, G.B. Method 445.0. In Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence; National Exposure Research Laboratory: Cincinnati, OH, USA, 1997. [Google Scholar]
- Welschmeyer, N.A. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 1994, 39, 1985–1992. [Google Scholar] [CrossRef]
- Bérard-Therriault, L.; Poulin, M.; Bossé, L. Guide D’identification du Phytoplancton Marin de L’estuaire et du Golfe du Saint-Laurent Incluant Également Certains Protozoaires; NRC Research Press: Ottawa, Canada, 1999. [Google Scholar]
- Kofoid, C.A.; Campbell, A.S. A Conspectus of the Marine and Freshwater Ciliata Belonging to the Suborder Tintinnoinea, with Description of New Species Principally from the Agassiz Expedition to the Eastern Tropical Pacific 1904–1905; University of California Press: Berkeley, CA, USA, 1929; Volume 34, pp. 1–264. [Google Scholar]
- Kofoid, C.A.; Swezy, O. The Free-Living Unarmored Dinoflagellata; University of California Press: Berkeley, CA, USA, 1921. [Google Scholar]
- Steidinger, K.; Tangen, K. Dinoflagellates. In Identifying Marine Phytoplankton; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Strüder-Kypke, M.; Kypke, E.R.; Agatha, S.; Warwick, J.; Montagnes, D.J.S. Guide to UK Coastal Planktonic Ciliates. Available online: http://www.liv.ac.uk/ciliate/site/index.htm (accessed on 6 April 2015).
- Møller, E.F.; Nielsen, T.G.; Richardson, K. The zooplankton community in the Greenland Sea: Composition and role in carbon turnover. Deep Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 76–93. [Google Scholar] [CrossRef]
- Myung, G.; Yih, W.; Kim, H.S.; Park, J.S.; Cho, B.C. Ingestion of bacterial cells by the marine photosynthetic ciliate Myrionecta rubra. Aquat. Microb. Ecol. 2006, 44, 175–180. [Google Scholar] [CrossRef]
- Stoecker, D. K.; Johnson, M.D.; de Vargas, C.; Not, F. Acquired phototrophy in aquatic protists. Aquat. Microb. Ecol. 2009, 57, 279–310. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D.Y. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrentyev, P.J.; Franzè, G.; Pierson, J.J.; Stoecker, D.K. The Effect of Dissolved Polyunsaturated Aldehydes on Microzooplankton Growth Rates in the Chesapeake Bay and Atlantic Coastal Waters. Mar. Drugs 2015, 13, 2834-2856. https://doi.org/10.3390/md13052834
Lavrentyev PJ, Franzè G, Pierson JJ, Stoecker DK. The Effect of Dissolved Polyunsaturated Aldehydes on Microzooplankton Growth Rates in the Chesapeake Bay and Atlantic Coastal Waters. Marine Drugs. 2015; 13(5):2834-2856. https://doi.org/10.3390/md13052834
Chicago/Turabian StyleLavrentyev, Peter J., Gayantonia Franzè, James J. Pierson, and Diane K. Stoecker. 2015. "The Effect of Dissolved Polyunsaturated Aldehydes on Microzooplankton Growth Rates in the Chesapeake Bay and Atlantic Coastal Waters" Marine Drugs 13, no. 5: 2834-2856. https://doi.org/10.3390/md13052834
APA StyleLavrentyev, P. J., Franzè, G., Pierson, J. J., & Stoecker, D. K. (2015). The Effect of Dissolved Polyunsaturated Aldehydes on Microzooplankton Growth Rates in the Chesapeake Bay and Atlantic Coastal Waters. Marine Drugs, 13(5), 2834-2856. https://doi.org/10.3390/md13052834





