The Cytoprotective Effect of Petalonia binghamiae Methanol Extract against Oxidative Stress in C2C12 Myoblasts: Mediation by Upregulation of Heme Oxygenase-1 and Nuclear Factor-Erythroid 2 Related Factor 2
Abstract
:1. Introduction
2. Results and Discussion
2.1. PBME Reduces H2O2-Induced C2C12 Cytotoxicity
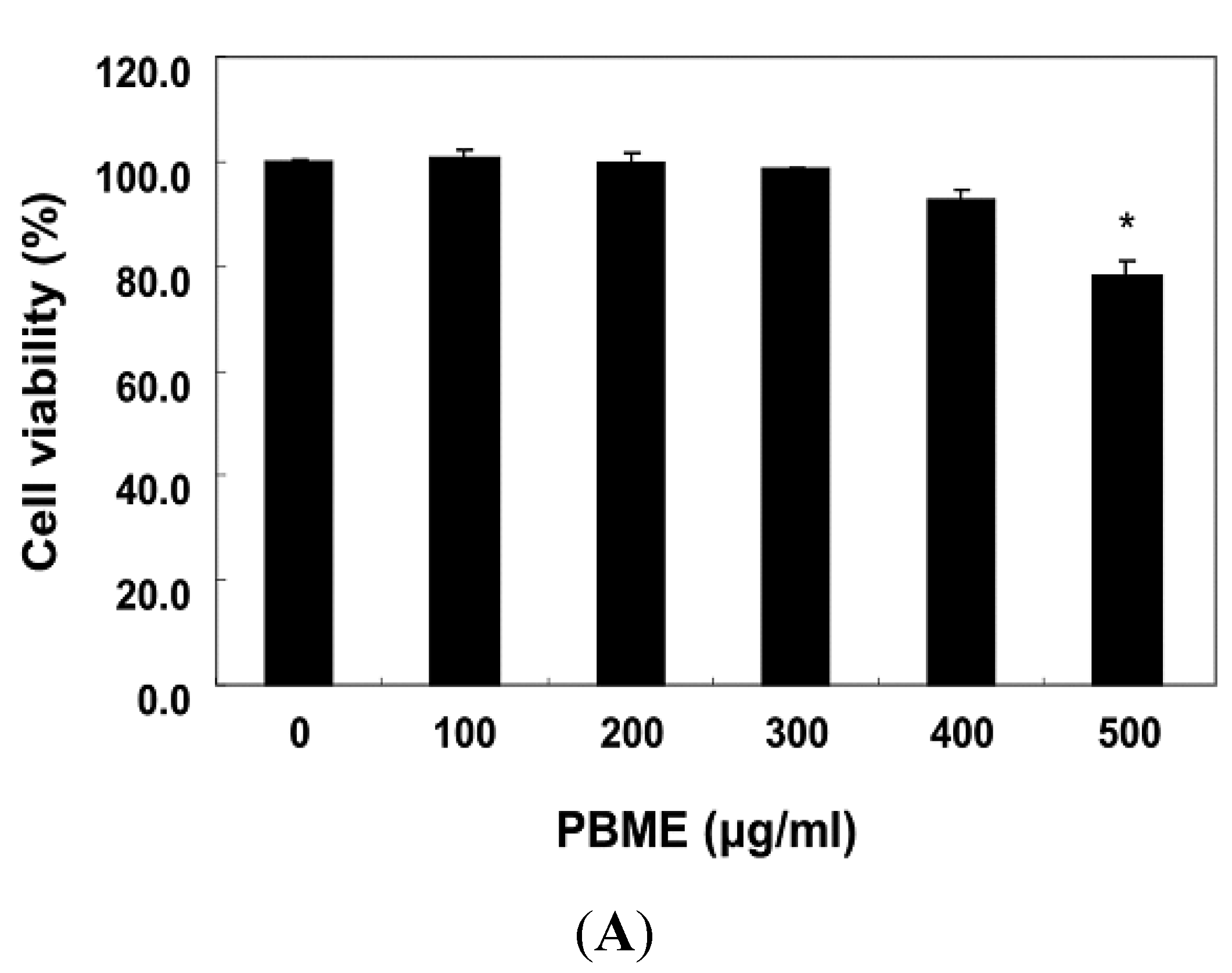
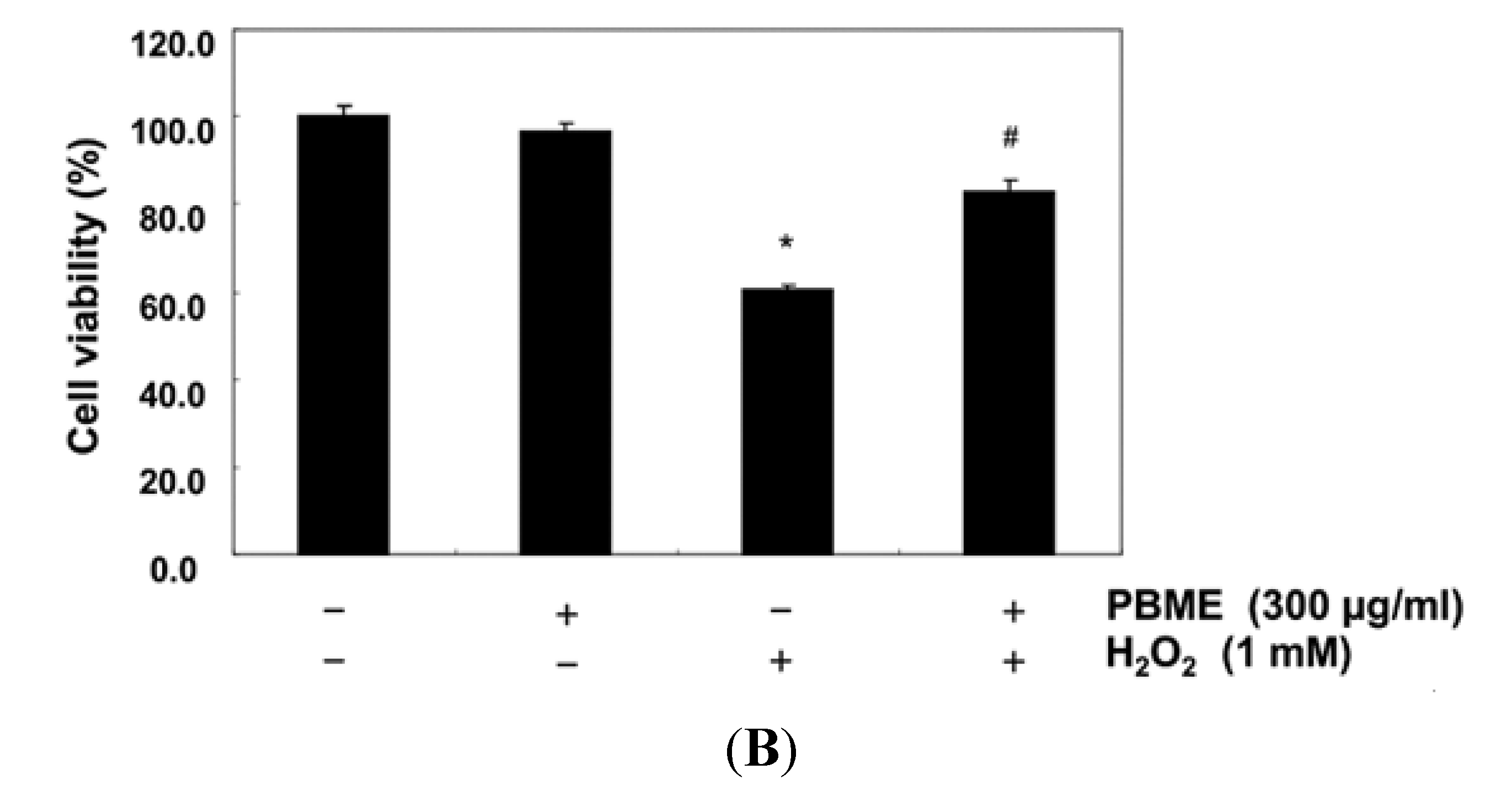
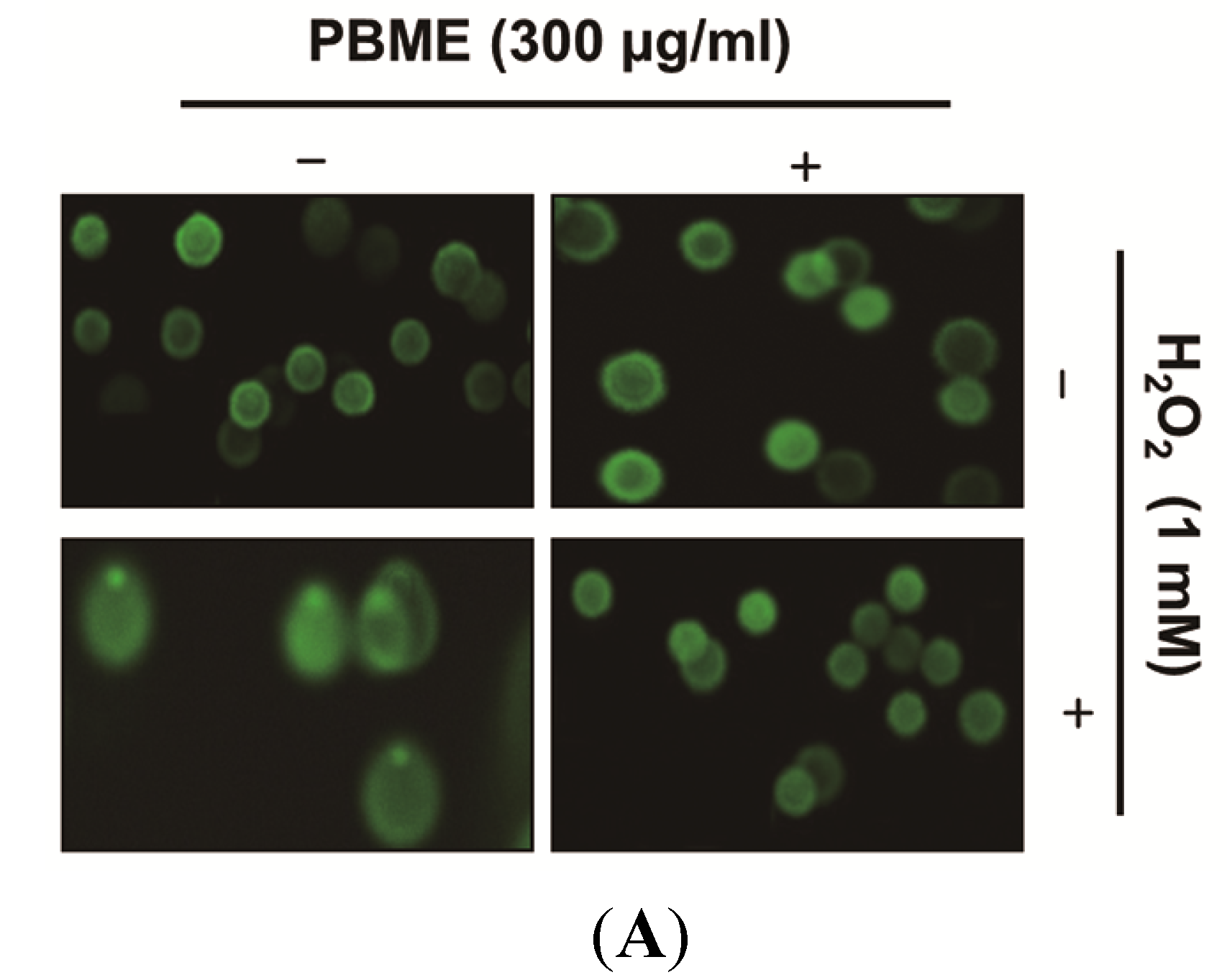
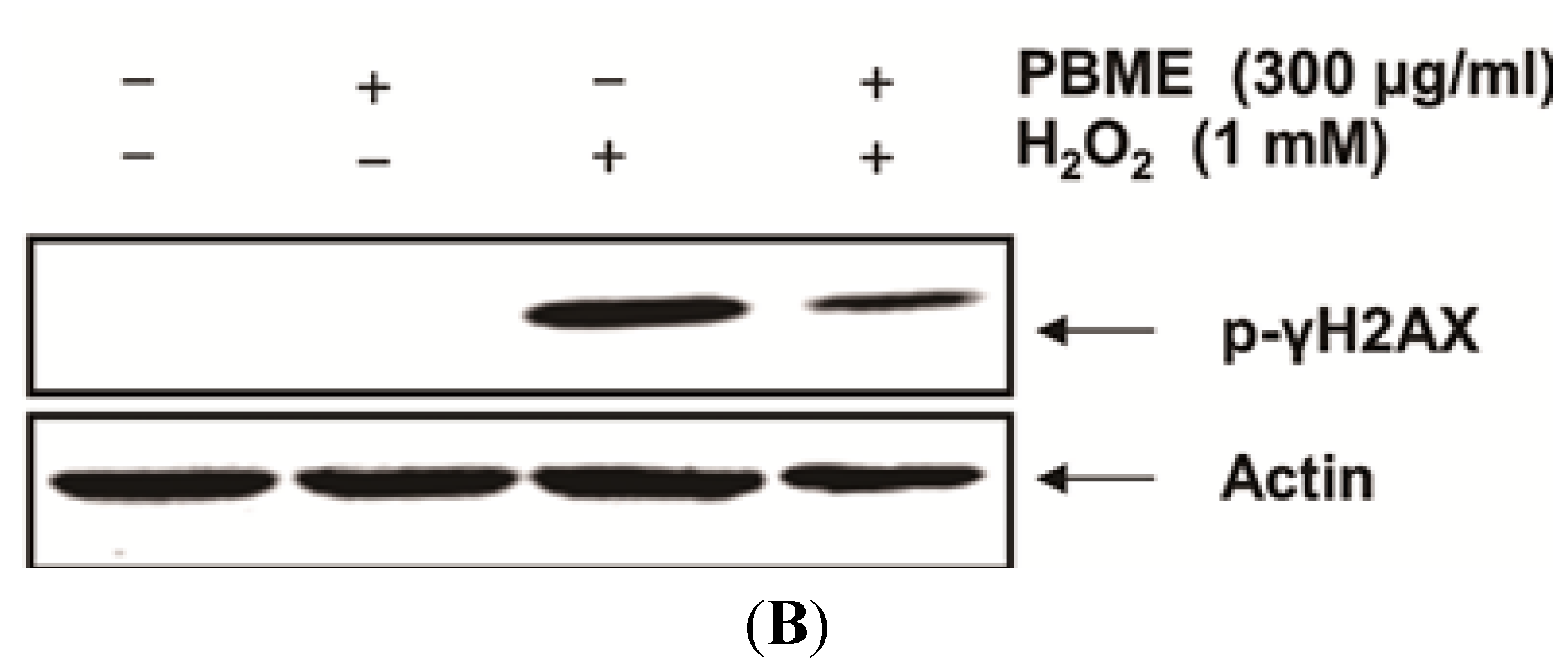
2.2. PBME Inhibits H2O2-Induced DNA Damage
2.3. PBME Attenuates H2O2-Induced ROS Accumulation and Apoptosis
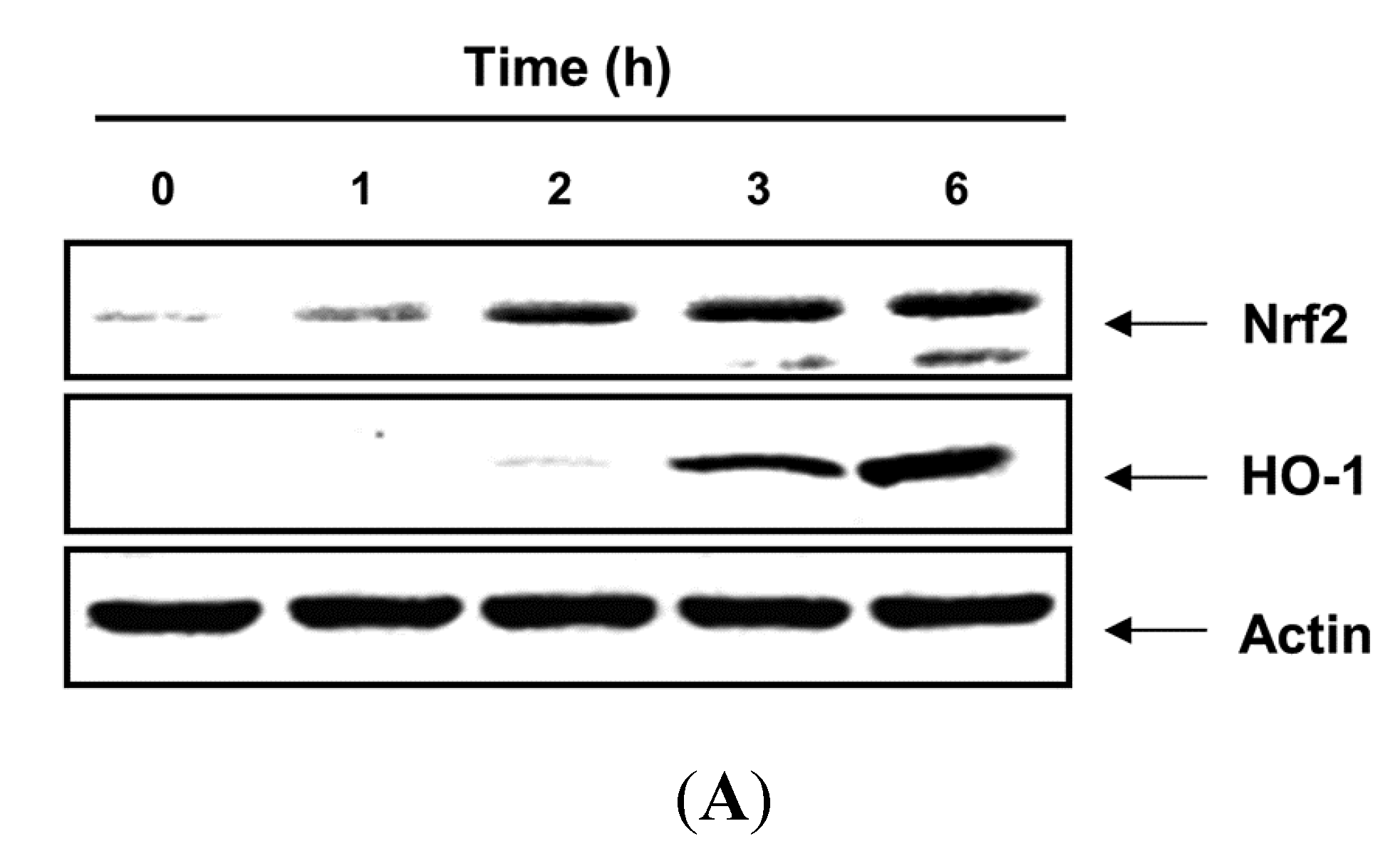
2.4. PBME Upregulates the Expression of HO-1 and Nrf2 Proteins
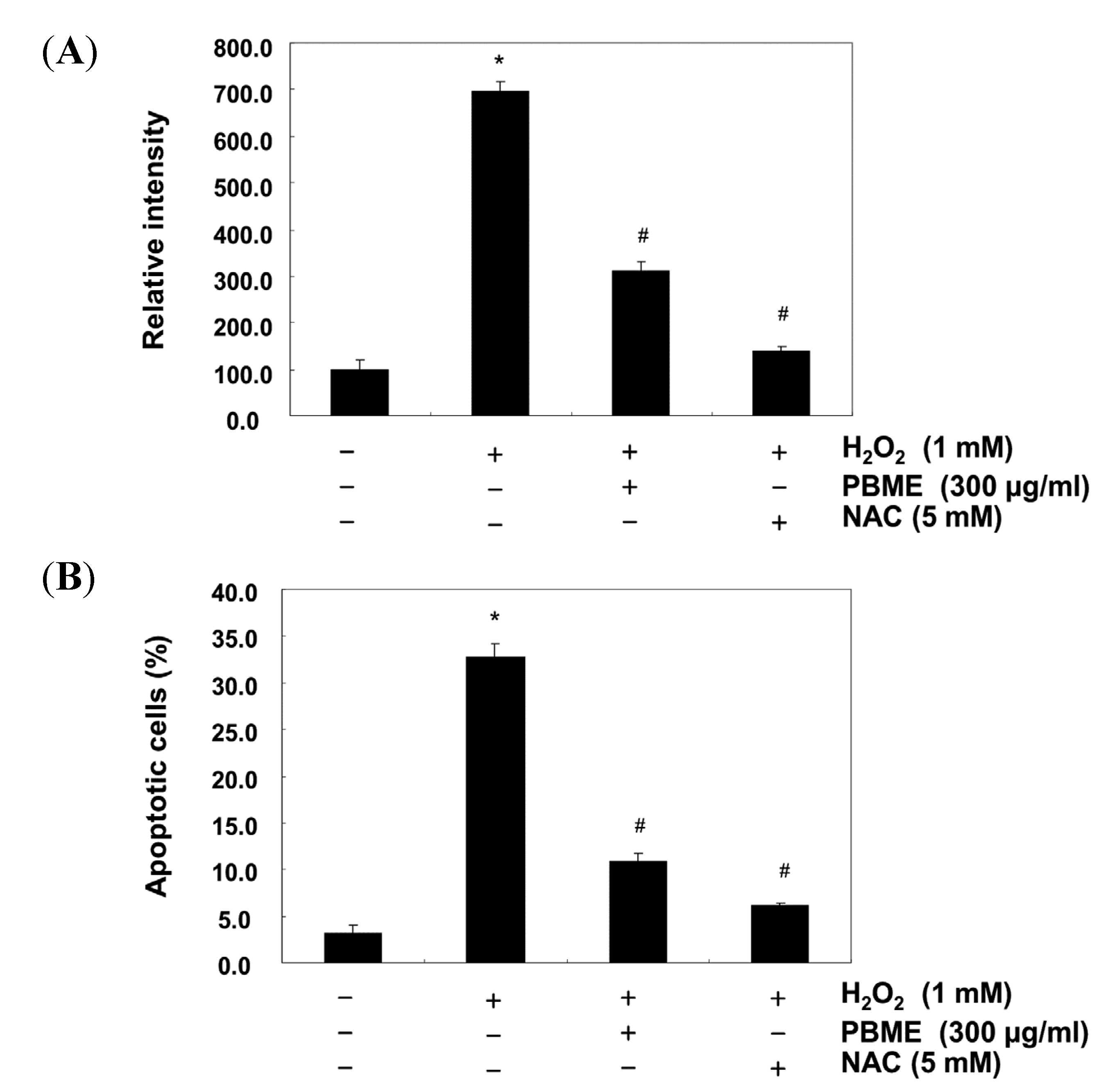

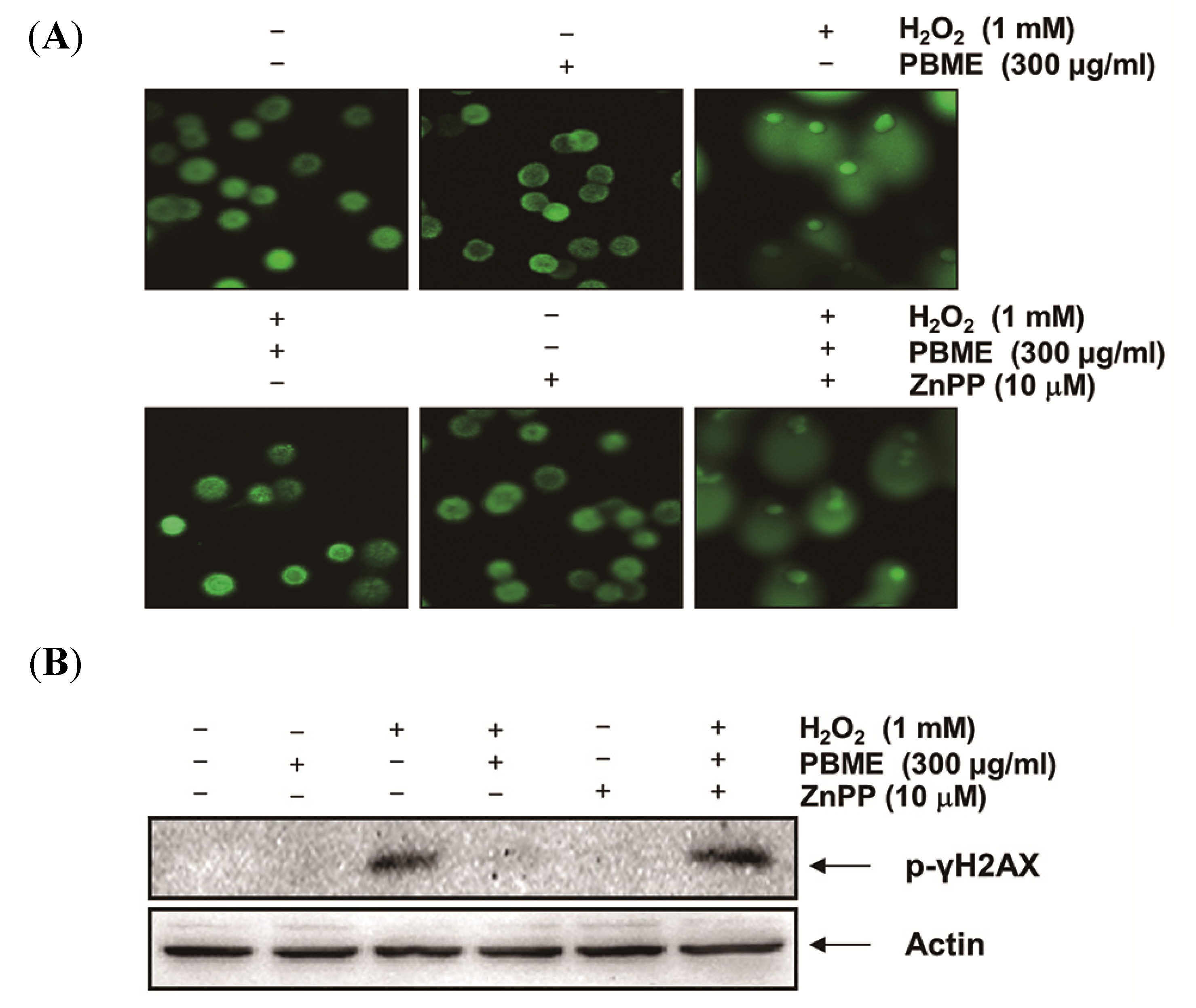
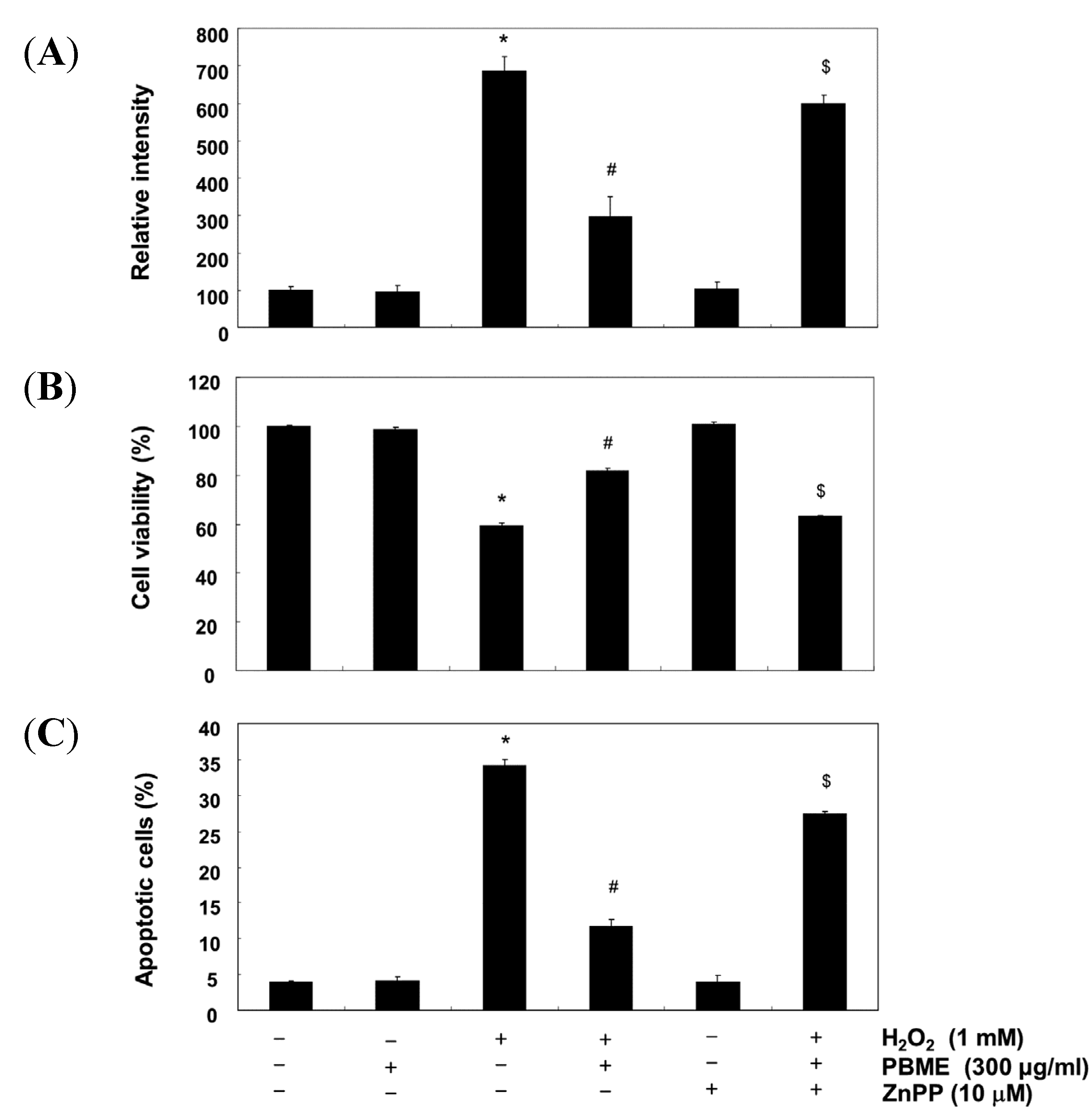
2.5. The Nrf2/HO-1 Pathway Is Involved in the Protection of PBME against H2O2 Treatment
2.6. Global Discussion
3. Experimental Section
3.1. Preparation of PBME
3.2. Cell Culture and PBME Treatment
3.3. Cell Viability Assay
3.4. Comet Assay (Single-Cell Gel Electrophoresis)
3.5. Western Blot Analysis
3.6. Measurement of ROS
3.7. Assessment of Apoptosis by Flow Cytometry
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Kregel, K.C.; Zhang, H.J. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R18–R36. [Google Scholar] [CrossRef] [PubMed]
- Vesely, M.J.; Exon, D.J.; Clark, J.E.; Foresti, R.; Green, C.J.; Motterlini, R. Heme oxygenase-1 induction in skeletal muscle cells: Hemin and sodium nitroprusside are regulators in vitro. Am. J. Physiol. 1998, 275, C1087–C1094. [Google Scholar]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Cook, J.L. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr. Pharm Des. 2003, 9, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Bai, L.; Zhu, L.; Yang, L.; Zhang, X. Marine algae-derived bioactive peptides for human nutrition and health. J. Agric. Food Chem. 2014, 62, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Kim, M.H.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Park, J.G.; Ko, H.C.; Lee, N.H.; Chung, W.S.; Kim, S.J. A water-soluble extract of Petalonia binghamiae inhibits the expression of adipogenic regulators in 3T3-L1 preadipocytes and reduces adiposity and weight gain in rats fed a high-fat diet. J. Nutr. Biochem. 2010, 21, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. Petalonia binghamiae extract and its constituent fucoxanthin ameliorate high-fat diet-induced obesity by activating AMP-activated protein kinase. J. Agric. Food Chem. 2012, 60, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Jin, Y.J.; Ko, H.C.; Choi, S.Y.; Hwang, J.H.; Whang, I.; Kim, M.H.; Shin, H.S.; Jeong, H.B.; Kim, S.J. Petalonia improves glucose homeostasis in streptozotocin-induced diabetic mice. Biochem. Biophys. Res. Commun. 2008, 373, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Kuda, T.; Hishi, T.; Maekawa, S. Antioxidant properties of dried product of “haba-nori”, an edible brown alga, Petalonia binghamiae (J. Agaradh) Vinogradova. Food Chem. 2006, 98, 545–550. [Google Scholar] [CrossRef]
- Orrenius, S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab. Rev. 2007, 39, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Chao, T.C.; Ros, A. Insulator-based dielectrophoretic single particle and single cancer cell trapping. Electrophoresis 2011, 32, 2550–2558. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2—an update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Abuarqoub, H.; Foresti, R.; Green, C.J.; Motterlini, R. Heme oxygenase-1 mediates the anti-inflammatory actions of 2′-hydroxychalcone in RAW 264.7 murine macrophages. Am. J. Physiol. Cell Physiol. 2006, 290, C1092–C1099. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Goatly, H.; Green, C.J.; Motterlini, R. Role of heme oxygenase-1 in hypoxia-reoxygenation: Requirement of substrate heme to promote cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2 2001, 281, H1976–H1984. [Google Scholar]
- Kang, J.S.; Han, M.H.; Kim, G.Y.; Kim, C.M.; Kim, B.W.; Hwang, H.J.; Hyun, Y. Nrf2-mediated HO-1 induction contributes to antioxidant capacity of a Schisandrae Fructus ethanol extract in C2C12 myoblasts. Nutrients 2014, 6, 5667–5678. [Google Scholar] [CrossRef] [PubMed]
- Abuarqoub, H.; Green, C.J.; Foresti, R.; Motterlini, R. Curcumin reduces cold storage-induced damage in human cardiac myoblasts. Exp. Mol. Med. 2007, 39, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Teng, L.; Vu, D.; He, J.Q.; Guo, Y.; Li, Q.; Tang, X.L.; Rokosh, G.; Bhatnagar, A.; Bolli, R. The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. J. Biol. Chem. 2012, 287, 33720–33732. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.; Foresti, R.; Sarathchandra, P.; Kaur, H.; Green, C.J.; Motterlini, R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H643–H651. [Google Scholar] [PubMed]
- Clark, J.E.; Foresti, R.; Green, C.J.; Motterlini, R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem. J. 2000, 348, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, C.A.; Carraway, M.S.; Babiker, A.; Suliman, H.B. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ. Res. 2008, 103, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Oh, I.; Kim, J.H.; Jeon, J.E.; Jeon, B.; Shin, J.; Kim, T.Y. Anti-inflammatory activity of compounds isolated from Astragalus sinicus L. in cytokine-induced keratinocytes and skin. Exp. Mol. Med. 2014, 46, e87. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Han, J.S. Padina arborescens extract protects high glucose-induced apoptosis in pancreatic β cells by reducing oxidative stress. Nutr. Res. Pract. 2014, 8, 494–500. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.S.; Choi, I.-W.; Han, M.H.; Lee, D.-S.; Kim, G.-Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Yoo, Y.H.; Choi, Y.H. The Cytoprotective Effect of Petalonia binghamiae Methanol Extract against Oxidative Stress in C2C12 Myoblasts: Mediation by Upregulation of Heme Oxygenase-1 and Nuclear Factor-Erythroid 2 Related Factor 2. Mar. Drugs 2015, 13, 2666-2679. https://doi.org/10.3390/md13052666
Kang JS, Choi I-W, Han MH, Lee D-S, Kim G-Y, Hwang HJ, Kim BW, Kim CM, Yoo YH, Choi YH. The Cytoprotective Effect of Petalonia binghamiae Methanol Extract against Oxidative Stress in C2C12 Myoblasts: Mediation by Upregulation of Heme Oxygenase-1 and Nuclear Factor-Erythroid 2 Related Factor 2. Marine Drugs. 2015; 13(5):2666-2679. https://doi.org/10.3390/md13052666
Chicago/Turabian StyleKang, Ji Sook, Il-Whan Choi, Min Ho Han, Dae-Sung Lee, Gi-Young Kim, Hye Jin Hwang, Byung Woo Kim, Cheol Min Kim, Young Hyun Yoo, and Yung Hyun Choi. 2015. "The Cytoprotective Effect of Petalonia binghamiae Methanol Extract against Oxidative Stress in C2C12 Myoblasts: Mediation by Upregulation of Heme Oxygenase-1 and Nuclear Factor-Erythroid 2 Related Factor 2" Marine Drugs 13, no. 5: 2666-2679. https://doi.org/10.3390/md13052666
APA StyleKang, J. S., Choi, I.-W., Han, M. H., Lee, D.-S., Kim, G.-Y., Hwang, H. J., Kim, B. W., Kim, C. M., Yoo, Y. H., & Choi, Y. H. (2015). The Cytoprotective Effect of Petalonia binghamiae Methanol Extract against Oxidative Stress in C2C12 Myoblasts: Mediation by Upregulation of Heme Oxygenase-1 and Nuclear Factor-Erythroid 2 Related Factor 2. Marine Drugs, 13(5), 2666-2679. https://doi.org/10.3390/md13052666






