Abstract
Marine-derived fungi continue to be a prolific source of secondary metabolites showing diverse bioactivities. Terpenoids from marine-derived fungi exhibit wide structural diversity including numerous compounds with pronounced biological activities. In this review, we survey the last five years’ reports on terpenoidal metabolites from marine-derived fungi with particular attention on those showing marked biological activities.
1. Introduction
Marine-derived fungi have proven to be a prolific source of secondary metabolites with interesting structural properties and biological activities [1,2,3,4]. In this review, we survey terpenes reported in literature from marine-derived fungi over the years from 2010 to 2014. The compounds covered include monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, and triterpenes in addition to prenylated polyketides known as “meroterpenes”.
2. Different Classes of Terpenoids
2.1. Monoterpenes (C10)
Monoterpenes have been rarely isolated from marine-derived fungi over the last decade. To the best of our knowledge, the only example reported till now dates back to 2006 and it was identified as a chlorinated monoterpene derivative, (1S,2S,3S,4R)-3-chloro-4-(2-hydroxypropan-2-yl)-1-methylcyclohexane-1,2-diol (1) (Figure 1). It was obtained from the extract of a fermentation broth of the mangrove-derived endophytic fungus Tryblidiopycnis sp. isolated from Kandelia woody tissue in Hong Kong [5].
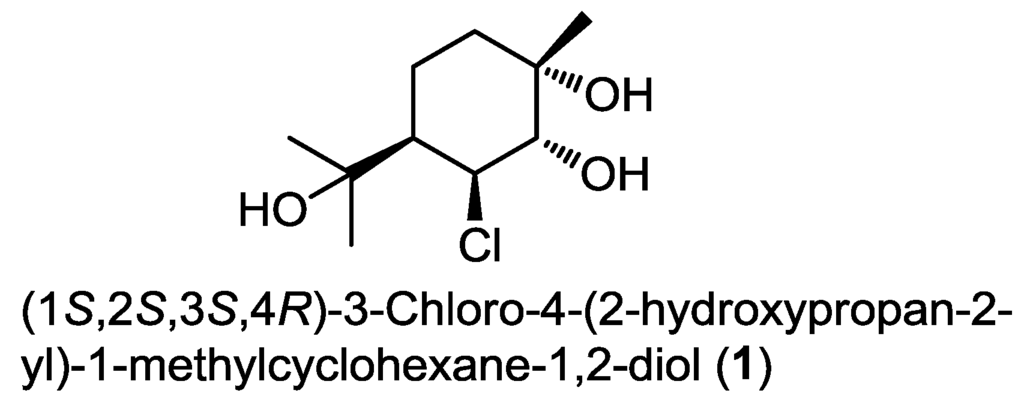
Figure 1.
The only monoterpene derivative isolated from marine fungi since 2006.
Figure 1.
The only monoterpene derivative isolated from marine fungi since 2006.
2.2. Sesquiterpenes (C15)
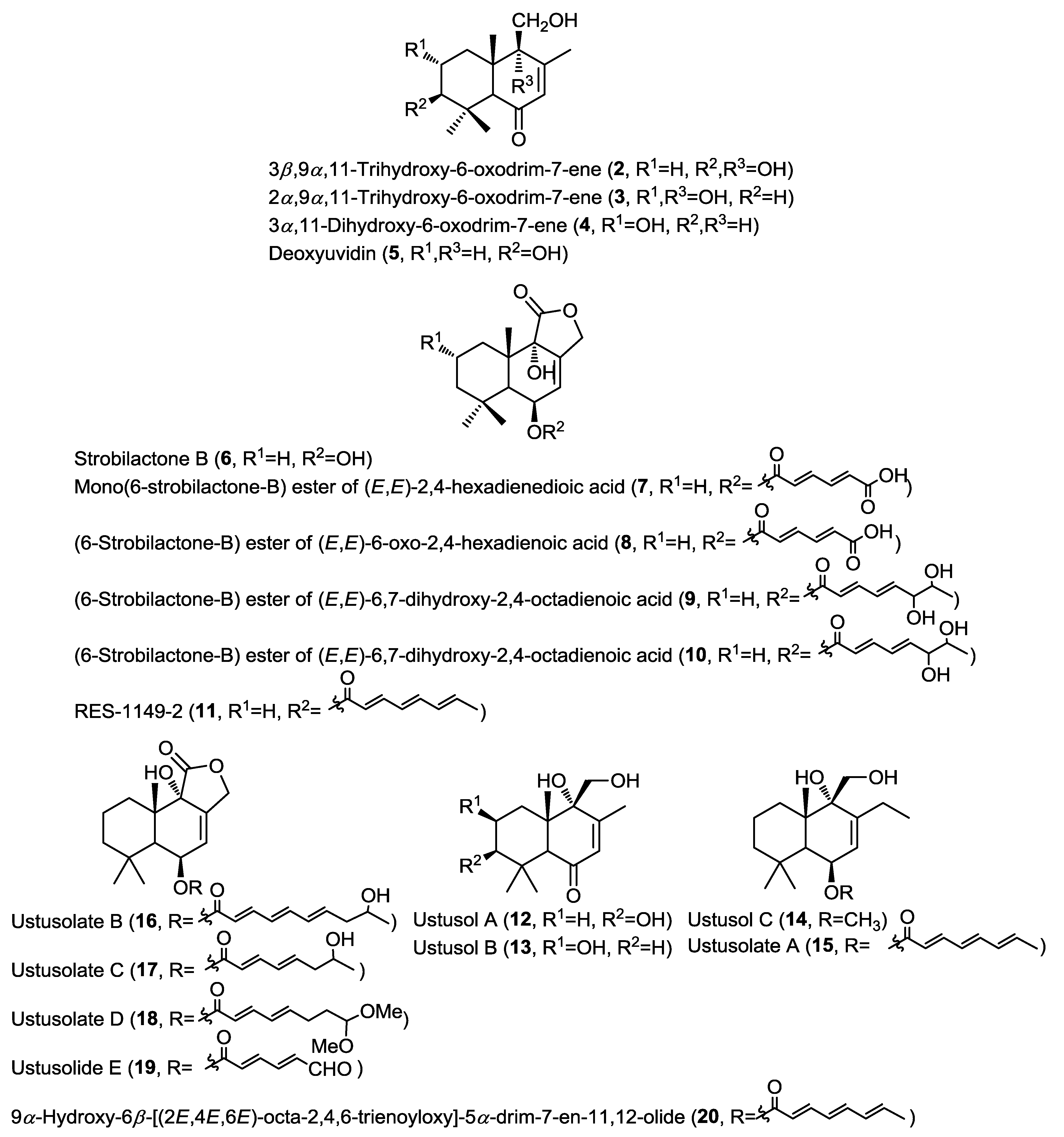
Purification of the cytotoxic ethyl acetate extract of the fermentation broth of the fungus Aspergillus ustus isolated from the marine sponge Suberites domuncula collected from the Adriatic Sea yielded new drimane-type sesquiterpenoids (2–4 and 7–10) together with the known related compounds deoxyuvidin B (5), strobilactone B (6) and RES-1149-2 (11) (Figure 2) [6]. Compounds 5 and 6 were previously reported from terrestrial fungi and this was the first report from a marine-derived fungus [7,8]. Compounds 2–5 were identified as hydroxylated derivatives of drim-7-ene-6-one, whereas compounds 7–11 were identified as esters of 6β,9α-dihydroxy-5α-drim-7-en-11,12-olide with polyunsaturated acid substituents at C-6. Cytotoxicity of compounds 2–10 has been evaluated against a panel of cancer cell lines, including mouse lymphoma (L5178Y), human cervical cancer (HeLa), and rat pheochromocytoma (PC12) cells. Results indicated that only compounds 7, 8 and 10 showed cytotoxicity with compound 10 being the most active (IC50 = 1.6, 15.8, and 19.3 µM, respectively) and it exhibited a higher selectivity toward mouse lymphoma (L5178Y) cells. This activity was strongly related to the esterification with polyunsaturated acids at C-6.
Simultaneous report on a different isolate of the same fungus A. ustus, isolated from the rhizospheric soil of the mangrove plant Bruguiera gymnorrhiza, revealed the isolation of the structurally closely related drimane sesquiterpenoids ustusols A–C (12–14) and the ester derivatives ustusolates A–E (15–19) together with the known compound 9α-hydroxy-6β-[(2E,4E,6E)-octa-2,4,6-trienoyloxy]-5α-drim-7-en-11,12-olide (20) (Figure 2) [9]. New compounds were evaluated for their cytotoxic activity against A549 and HL-60 cancer cell lines. Only ustusolates C (17) and E (19) showed cytotoxicity against A549 and HL-60 cell lines with IC50 values of 10.5 and 9.0 µM, respectively, whereas ustusolate A (14) showed weak growth inhibition against both A549 and HL-60 cell lines with IC50 values of 30.0 and 20.6 µM, respectively. These results may indicate that the length and substituents on the unsaturated side chain can influence the cytotoxicity of the ustusolates.
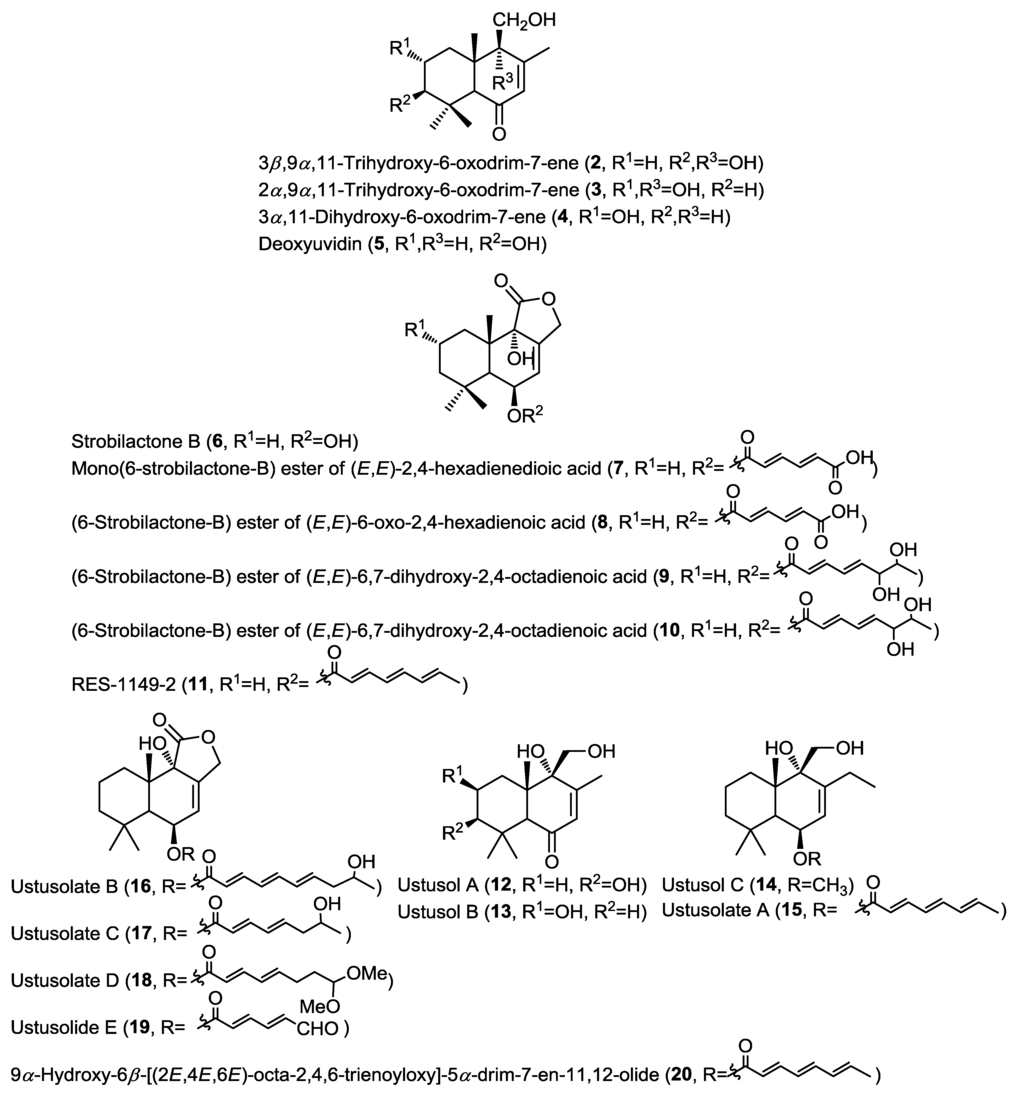
Figure 2.
Sesquiterpenes isolated from Aspergillus ustus and A. ustus.
Figure 2.
Sesquiterpenes isolated from Aspergillus ustus and A. ustus.
Four new phenolic bisabolane sesquiterpenoids (21–24) were reported from the endophytic marine fungus Penicillium expansum 091006 isolated from the roots of the mangrove plant Excoecaria agallocha (China) (Figure 3) [10]. Expansols A (21) and B (22) contain a diphenyl ether moiety related to diorcinol linked with the phenolic bisabolane sesquiterpene through a methylene bridge, whereas (S)-(+)-11-dehydrosydonic acid (23) and (7S,11S)-(+)-12-acetoxysydonic acid (24) are derivatives of sydonic acid differing from the latter compound by unsaturation and acetylation, respectively. Bisabolane sesquiterpenoids are uncommon fungal metabolites and only a limited number of reports so far indicated their occurrence in fungi [11,12]. Moreover, this was the first report of phenolic bisabolane coupled with diphenyl ethers. Compounds 21–24 were subjected to cytotoxicity studies against A549 and HL-60 cancer cell lines. Results revealed that only expansol A (21) exhibited moderate cytotoxicity against the HL-60 cell line with IC50 of 15.7 µM, whereas, expansol B (22) inhibited the proliferation of A549 and HL-60 cells with IC50 values of 1.9 and 5.4 µM, respectively. These results highlighted the importance of coupling diphenyl ethers with the phenolic bisabolane sesquiterpenoids for enhancing their cytotoxic activity.
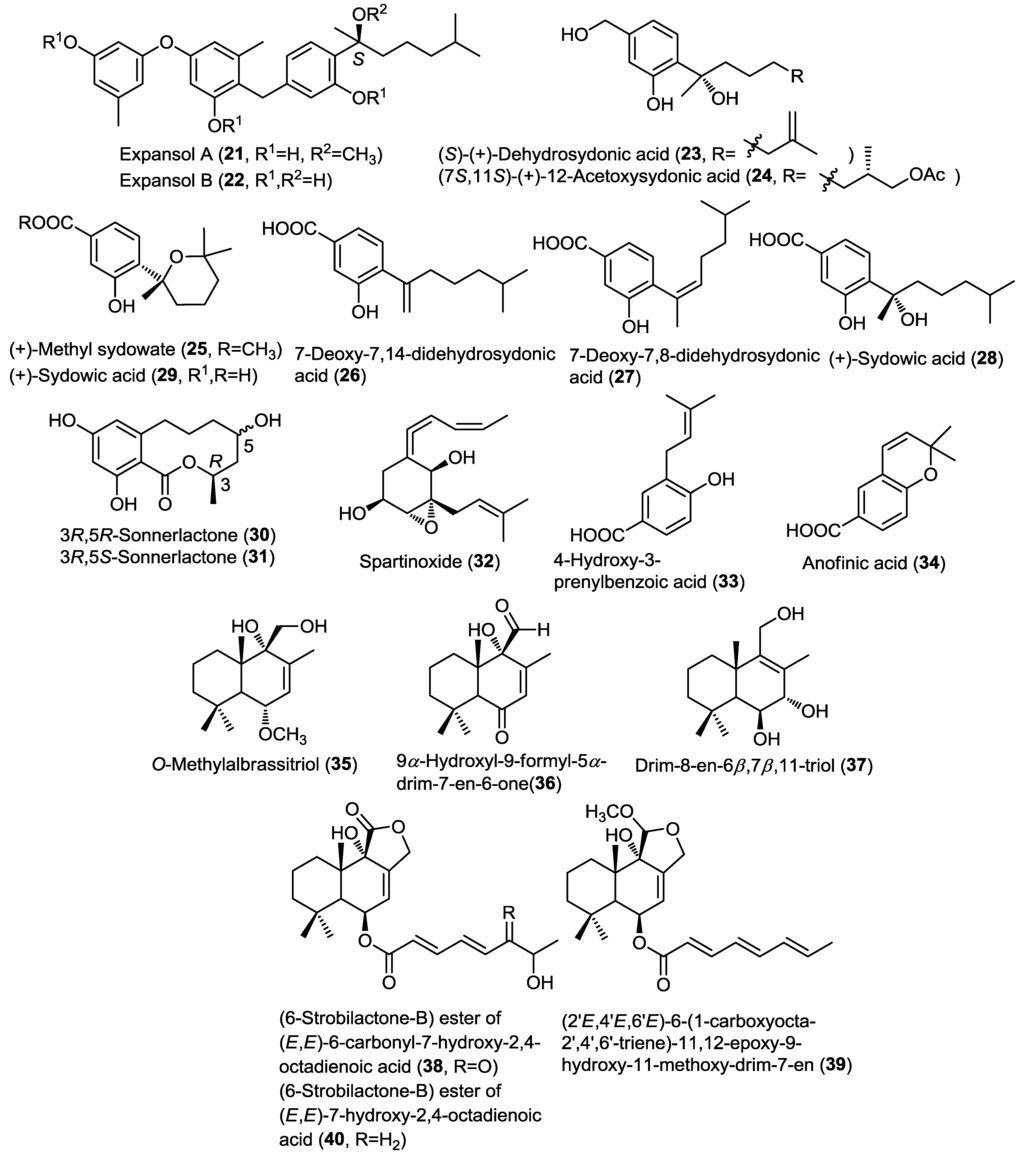
Figure 3.
Sesquiterpenes isolated from Penicillium expansum, Aspergillus sp., unidentified endophytic fungal strain Zh6-B1, Phaeosphaeria spartinae and Aspergillus ustus.
Figure 3.
Sesquiterpenes isolated from Penicillium expansum, Aspergillus sp., unidentified endophytic fungal strain Zh6-B1, Phaeosphaeria spartinae and Aspergillus ustus.
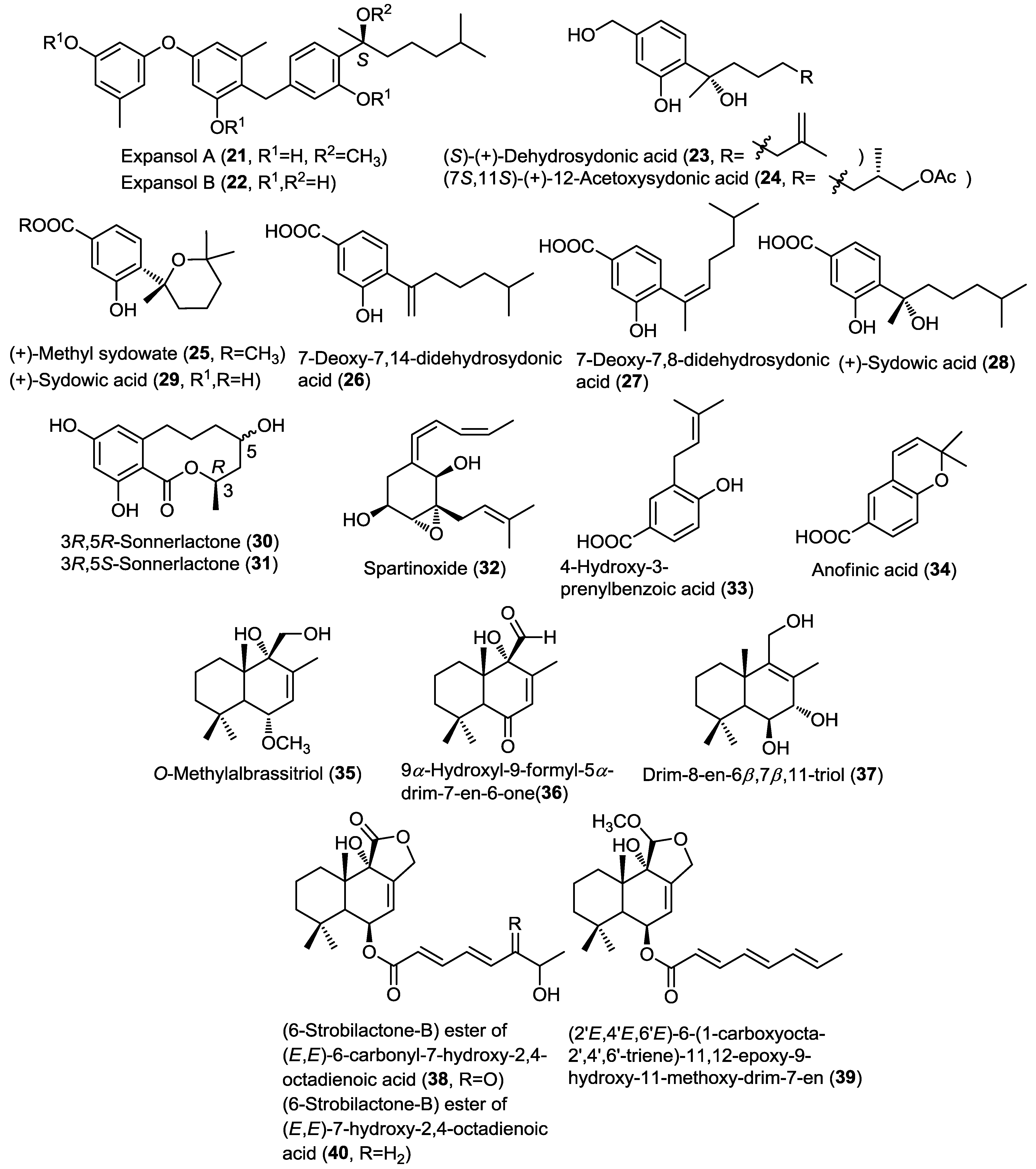
A simultaneous report indicated the isolation of three new structurally related bisabolane sesquiterpenoids (25–27) together with the known sesquiterpenes (+)-sydowic acid (28) and (+)-sydonic acid (29), from the fungus Aspergillus sp. cultured from the marine gorgonian Dichotella gemmacea (Figure 3) [13]. This was the first report of natural products from gorgonian-derived fungus. Methyl sydowate (25) is an ester of 28, and the authors implemented HPLC to ensure that 25 is a genuine natural product and not an artifact. Similarly, 7-deoxy-7,14-didehydrosydonic acid (26), and 7-deoxy-7,8-didehydrosydonic acid (27) were confirmed as genuine natural products by treating the crude extract with mild acidic conditions which failed to provoke the formation of 26 and 27. This is the first report for the isolation of (+)-sydowic acid and (+)-sydonic acid which were previously only known as (−) isomers. Antimicrobial evaluation of compounds 25, 28 and 29 showed weak activity against Staphylococcus aureus and no activity against methicillin resistant S. aureus [13].
Two new isomeric sesquiterpene lactones, 3R,5R-sonnerlactone (30) and 3R,5S-sonnerlactone (31), were isolated from an unidentified endophytic fungal strain Zh6-B1 cultured from the bark of the mangrove plant Sonneratia apetala (Figure 3) [14]. The two compounds possess identical 1D NMR and MS spectra and the differentiation was achieved using X-ray crystallography of 30 and through comparing their NOESY spectra. Cytotoxicity investigation of both compounds was conducted on multi-drug resistant human oral floor carcinoma cell lines KV/MDR and revealed weak cytotoxic activity for both compounds [14].
Spartinoxide (32), a new sesquiterpene, was isolated from the fungus Phaeosphaeria spartinae cultured from the marine alga Ceramium sp. collected in North Sea, Germany, together with the known compounds 4-hydroxy-3-prenyl-benzoic acid (33) and anofinic acid (34) (Figure 3) [15]. Spartinoxide (32) is an optical isomer of the known fungal metabolite A82775C, having identical 1D, 2D and MS spectra whereas it differs in the optical rotation. Compounds 32–34 were investigated for their enzymatic inhibitory activity against a panel of human enzymes including human leukocyte elastase (HLE), trypsin, acetylcholinesterase and cholesterolesterase. Compounds 32 and 33 showed potent inhibitory activity against Human leukocyte elastase (HLE), responsible for inflammatory conditions such as pulmonary emphysema and cystic fibrosis [15].
A further report on the fungus Aspergillus ustus cultured from the rhizospheric soil of the mangrove plant Acrostichum aureurm yielded five new drimane-type sesquiterpenoids (35–39), O-methylalbrassitriol (35), 9α-hydroxyl-9-formyl-5α-drim-7-en-6-one (36), drim-8-en-6β,7β,11-triol (37), (6-strobilactone-B) ester of (E,E)-6-carbonyl-7-hydroxy-2,4-octadienoic acid (38), 11-methoxy-drim-7-ene (39) together with the known compound (6-strobilactone-B) ester of (E,E)-7-hydroxy-2,4-octadienoic acid (40) (Figure 3) [16]. Compounds 35–40 were investigated for their cytotoxic activity against a panel of cancer cell lines, and only 38 showed cytotoxic activity against P388 cell line with IC50 value of 8.7 µM, whereas other compounds exhibited no activity [16]. The difference in the cytotoxic activity between 38 and 40 hinted to the possible role of the carbonyl group at C-6' in the activity of compound 38.
Three new azaphilone sesquiterpenoids, chermesinones A–C (41–43), were obtained from the endophytic fungus Penicillium chermesinum (ZH4-E2) isolated from the stems of the mangrove plant Kandelia candel (Figure 4) [17]. The absolute configuration of 41 was determined by X-ray crystallography. Compounds 41–43 were investigated for their inhibitory activity against α-glucosidase and acetylcholinesterase enzymes, and only chermesinone A (41) showed mild α-glucosidase inhibitory activity.
Four new norsesquiterpene peroxides, talaperoxides A–D (44–47), were isolated from the endophytic fungus Talaromyces flavus isolated from the leaves of the mangrove plant Sonneratia apetala, together with the known compound steperoxide B (merulin A) (48) (Figure 4) [18]. Talaperoxides A–D constitute two isomeric pairs. The absolute configuration of the new compounds 44 and 45 together with the known 48 was determined through single crystal X-ray diffraction analysis. Compounds 44–48 were investigated for cytotoxic activity against five human cancer cell lines, including HepG2 (liver), HeLa (cervix), PC-3 (prostate), MCF-7 and MDA-MB-435 (breast). Compounds 45 and 47 showed potent cytotoxic activity against the five cell lines being most active against PC-3 cell line with IC50 values 3.00 and 2.78 µM, respectively. This variation in activity is most properly due to the R configuration at C-7 compared to the S configuration for their congeners 44 and 46, and due to the presence of an acetyl or carbonyl groups at C-3 as compared to steperoxide B (merulin A) (48). Therefore, both the R configuration at C-7 and the presence of a carbonyl or acetyl groups at C-3 were assumed to be responsible for the potent cytotoxic activity.
Simultaneously, another research group isolated a group of structurally related sesquiterpene endoperoxides including merulin D (51) together with the known merulins B (49) and C (50), and steperoxide A (52) from the basidiomycete fungus XG8D obtained from the leaves of the mangrove plant Xylocarpus granatum (Figure 4) [19]. Merulin D (steperoxide B) (51) was found to be an epimer of 49 differentiated mainly through the 2D NOESY experiment. The antiangiogenic activity for compounds 49–52 was investigated revealing that only compounds 50 and 52 possess activity with compound 50 (IC50 = 2.5 µM) being ten times more potent than 52 (IC50 = 25 µM). These results provided evidence for the possible roles of the C4–C7 endoperoxide linkage as well as for the α,β-unsaturated ketone and hydroxymethyl functionalities. The antiangiogenic activity of compound 50 was further evaluated through a series of in vitro and in vivo experiments. Compound 50 was found to inhibit neovascularization through suppression of VEGF-induced endothelial cell proliferation and migration via the reduction in Erk1/2 phosphorylation.
Asperaculin A (53), a new sesquiterpenoid, was isolated from the sponge derived fungus Aspergillus aculeatus CRI323-04 obtained from the sponge Xestospongia testudinaria (Figure 4) [20]. Structurally, 53 was revealed to exhibit a new [5,5,5,6] fenestrane sesquiterpenoid ring system, which was given the trivial name aspergillane. The authors proposed a scheme for the biosynthesis of 53 through double bond migration of the known sesquiterpenoid silphinene. Asperaculin A (53) was assessed for its antiproliferative activity against HepG2, MOLT-3, A549, and HuCCA-1 cancer cell lines; however, it didn’t exhibit activity up to 50 µg/mL (180 µM) concentration [20].
Four drimane-type sesquiterpenoid lactones (54–57) were isolated from the fungus Aspergillus insuetus (OY-207) isolated from the Mediterranean sponge Psammocinia sp. (Figure 4) [21]. The isolated compounds were found to be derivatives of strobilactone A including the new compound, (E)-6-(4' hydroxy-2'-butenoyl)-strobilactone A (54), the known derivatives strobilactone A (56) and (E,E)-6-(6',7'-dihydroxy-2',4'-octadienoyl)-strobilactone A (57) together with 2α,9α,11-trihydroxy-6-oxodrim-7-ene (55) [21]. The cytotoxic and antifungal activities of compounds 54–57 were investigated and results showed that 54 and 57 exhibited weak cytotoxic activity against MOLT-4 cancer cell lines whereas 56 and 57 showed mild antifungal activity against the fungus Neurospora crassa.
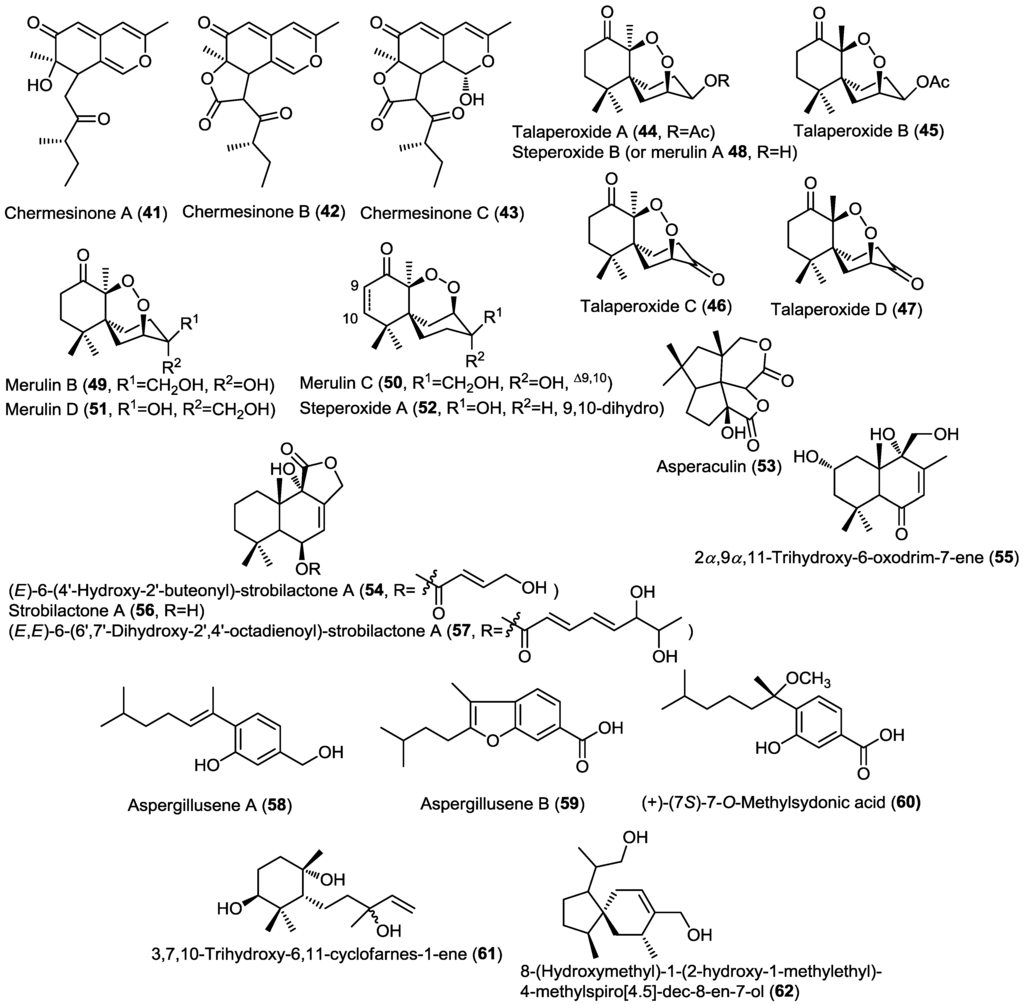
Figure 4.
Sesquiterpenes isolated from Penicillium chermesinum (ZH4-E2), Talaromyces flavus, basidiomycete fungus XG8D, Aspergillus aculeatus, Aspergillus insuetus (OY-207), Aspergillus sydowii PSU-F154, and Eutypella scoparia FS26.
Figure 4.
Sesquiterpenes isolated from Penicillium chermesinum (ZH4-E2), Talaromyces flavus, basidiomycete fungus XG8D, Aspergillus aculeatus, Aspergillus insuetus (OY-207), Aspergillus sydowii PSU-F154, and Eutypella scoparia FS26.
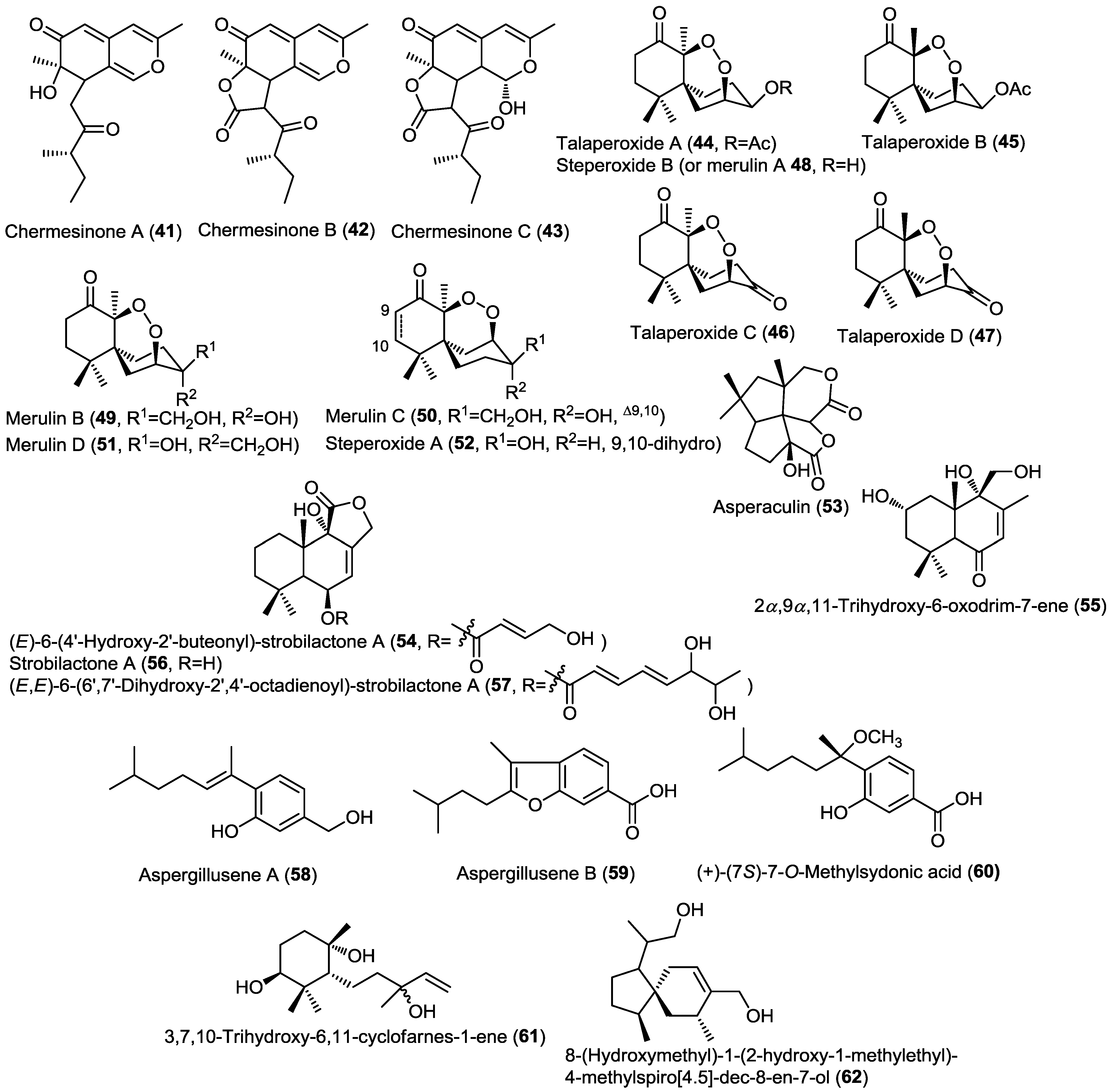
Three new bisabolane sesquiterpenoids, aspergillusene A,B and (+)-(7S)-7-O-methylsydonic acid (58–60) were isolated from the fungus Aspergillus sydowii PSU-F154 isolated from a sea fan, Annella sp. (Figure 4) [22]. The isolated compounds were found to be derivatives of the known (+)-sydonic acid (29). Compounds 58–60 were found inactive in the antioxidant DPPH assay.
A new monocyclic farnesyl sesquiterpene named 3,7,10-trihydroxy-6,11-cyclofarnes-1-ene (61) together with a new acorane sesquiterpene, 8-(hydroxymethyl)-1-(2-hydroxy-1-methylethyl)-4-methylspiro[4.5]-dec-8-en-7-ol (62), were isolated from the marine-derived fungus Eutypella scoparia FS26 isolated from a sediment sample from the South China Sea (Figure 4) [23]. Compounds 61 and 62 were investigated for cytotoxicity against a panel of cancer cell lines but they only showed a weak cytotoxic activity against MCF-7 cell line.
Three dimeric bisabolane sesquiterpenoids, disydonols A–C (63–65), together with the known precursor (+)-S-sydonol (66), were isolated from the marine-derived fungus Aspergillus sp., isolated from the sponge Xestospongia testudinaria (Figure 5) [24]. The absolute configuration of the new compounds at C-7 and C-7' was estimated tentatively by comparing their optical rotation to the known precursor S-sydonol. This was the second report for the isolation of dimeric bisabolane sesquiterpenes from nature. Disydonol C (65) revealed selective in vitro cytotoxic activity toward HepG-2 and Caski cancer cell lines with IC50 values of 6.0 and 21.0 µM, respectively. However, disydonol A (63) displayed moderate cytotoxicity toward these two cell lines with IC50 values of 19.1 and 25.5 µM, respectively, where disydonol B (64) was found to be relatively noncytotoxic at a concentration up to 200 µM. It was observed that disydonols A (63) and C (65), possessing the 7S and 7'S configurations, displayed more potent cytotoxicity toward the tumor cell lines than 64, with the 7S and 7'R configurations. These results indicated that the cytotoxic activity might be weakened due to mesomeric effect and thus, the activity of compounds is stereoselective.
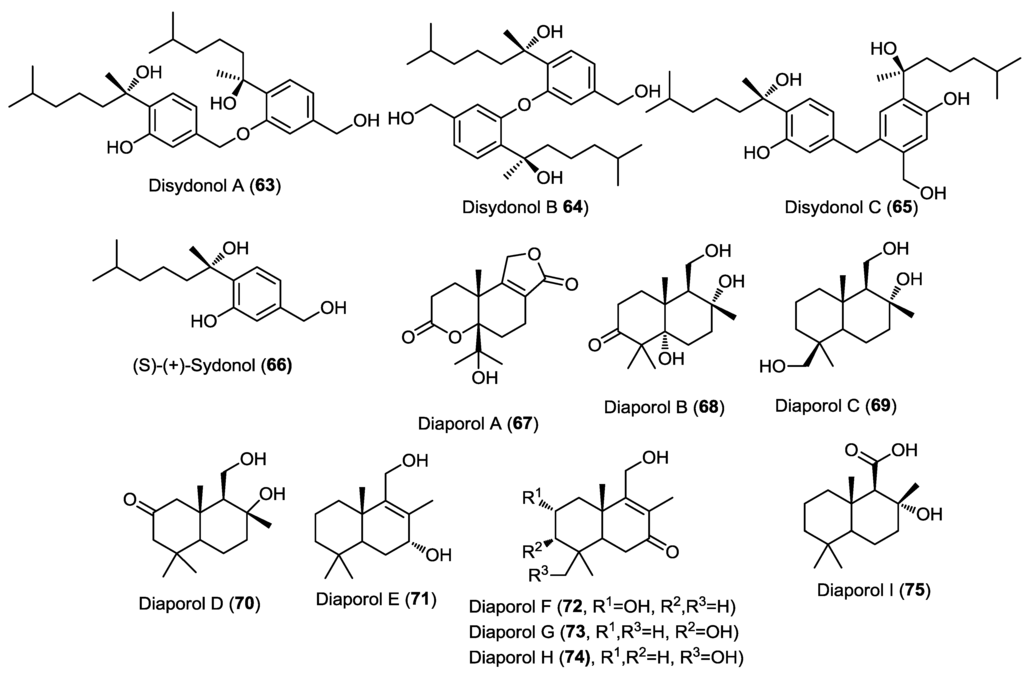
Figure 5.
Sesquiterpenes isolated from Aspergillus sp. and Diaporthe sp. IFB-3lp-10.
Figure 5.
Sesquiterpenes isolated from Aspergillus sp. and Diaporthe sp. IFB-3lp-10.
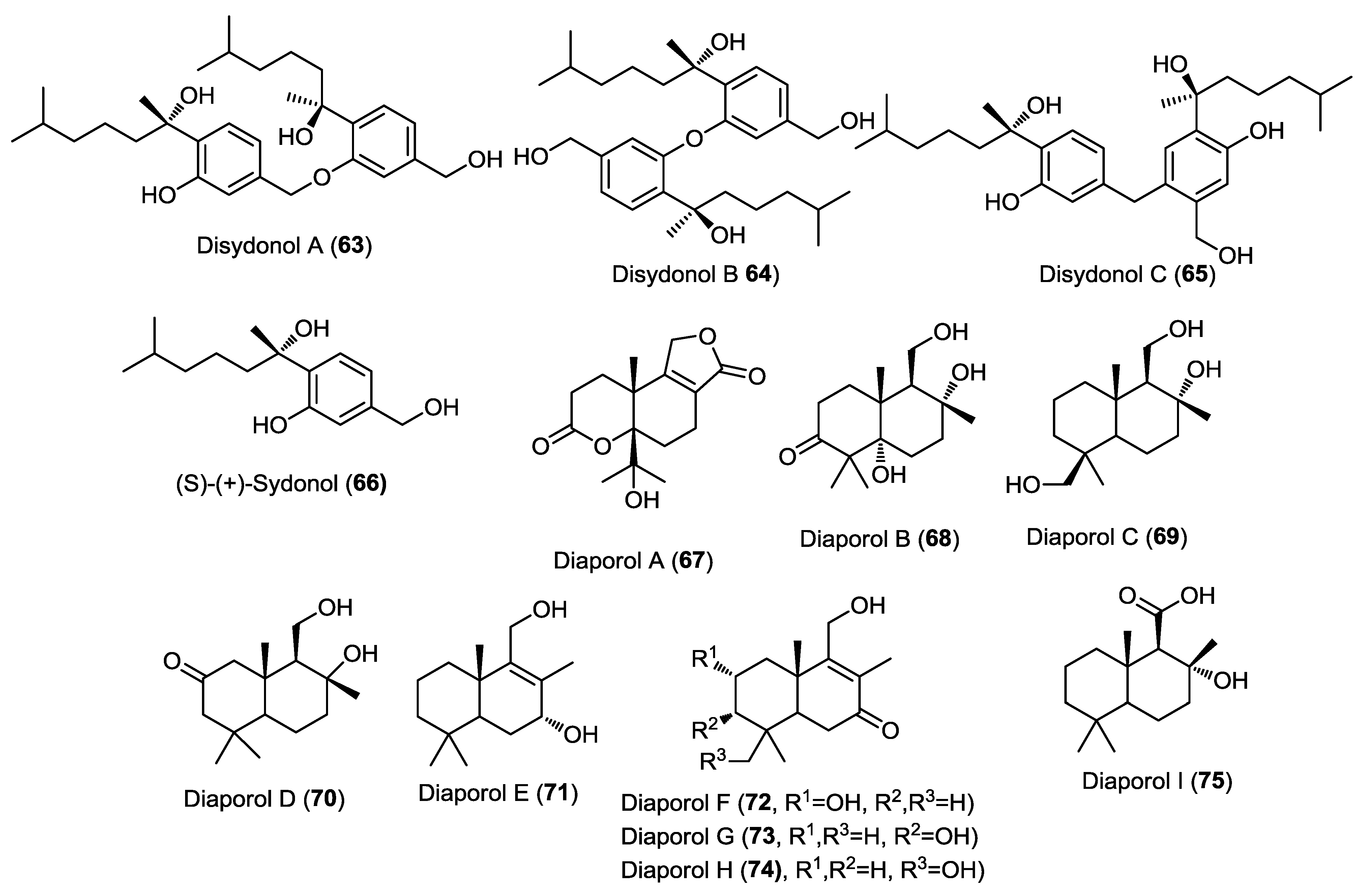
Nine new sesquiterpenoids, diaporols A–I (67–75), were isolated from the endophytic fungus Diaporthe sp. IFB-3lp-10 isolated from the leaves of the mangrove Rhizophora stylosa (Figure 5) [25]. The parent compound, diaporol A (67), constitutes a unique tricyclic lactone sesquiterpene while the other diaporols belong to the drimane type sesquiterpenoids. The absolute configuration of compounds 67–71 was determined using single crystal X-ray diffraction. All isolated compounds were subjected to a cytotoxicity assay against a panel of cancer cell lines but none of them showed remarkable activity.
A new drimane sesquiterpene lactone, 9α-hydroxy-5α-drim-7-ene-6-one-11,12-olide (76), was isolated from the endophytic fungus Aspergillus carneus KMM 4638, obtained from the marine brown alga Laminaria sachalinensis (Figure 6) [26]. Compound 76 is strongly related to the previously reported lactone derivatives such as strobilactone 56.
A new bicyclic sesquiterpene with unusual bicyclo[3.2.1]octane skeleton, (5E)-2-methyl-5-[(1'R*,5'R*)-2-methylidene-7-oxobicyclo[3.2.1]oct-6-ylidene]-4-oxopentanoic acid (77), was isolated from the sponge-associated fungus Emericellopsis minima obtained from the marine sponge Hyrtios erecta (Figure 6) [27]. Compound 77 showed neither cytotoxic nor antimicrobial activities.
Three new eremophilane-type sesquiterpenoids (78–80), together with the known congener 07H239-A (81), were isolated from the mangrove associated fungus Xylaria sp. BL321 (Figure 6) [28]. A cytotoxicity assay of compounds 78–80 revealed no activity against different cancer cell lines in contrast to the related 81 which was previously reported to possess cytotoxicity [29]. Compound 81 showed dose-dependent activation followed by gradual inhibition of α-glucosidase.
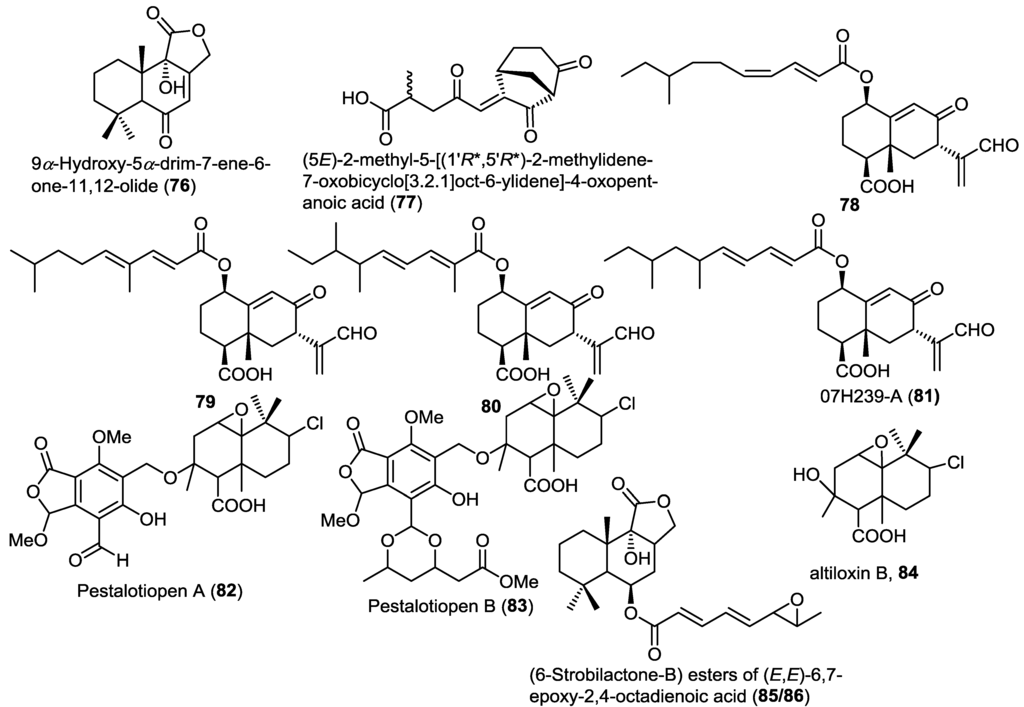
Figure 6.
Sesquiterpenes isolated from Aspergillus carneus KMM 4638, Emericellopsis minima, Xylaria sp. BL321, Pestalotiopsis sp. and Aspergillus ustus.
Figure 6.
Sesquiterpenes isolated from Aspergillus carneus KMM 4638, Emericellopsis minima, Xylaria sp. BL321, Pestalotiopsis sp. and Aspergillus ustus.
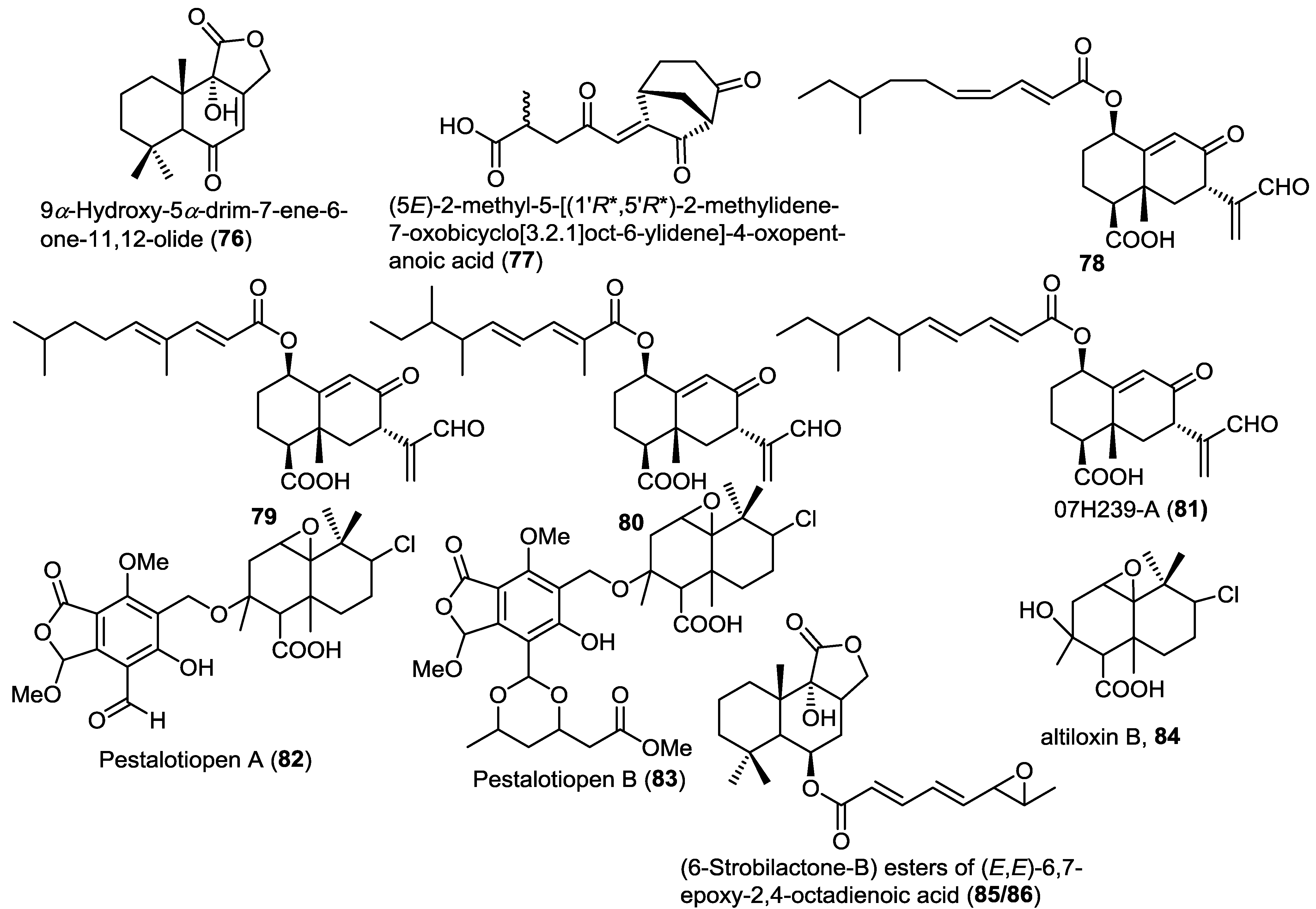
Two new hybrid drimane sesquiterpene-cyclopaldic acids, pestalotiopens A,B (82 and 83), were isolated from the mangrove derived fungus Pestalotiopsis sp. obtained from leaves of the Chinese mangrove Rhizophora mucronata (Figure 6) [30]. Pestalotiopens were identified as ethers of the known altiloxin B (84), which was also isolated in the same report with cyclopaldic acid. Compound 83 possesses an extra triketide moiety. The authors proposed a biogenetic pathway for the new compounds involving altiloxin B as a common precursor. The antibacterial activity of the new compounds was assessed. Pestalotiopen A (82) showed a moderate activity against Enterococcus faecalis.
Further investigation of the fungus Aspergillus ustus obtained from the marine alga Codium fragile resulted in the isolation of two new isomeric strobilactone B esters of (E,E)-6,7-epoxy-2,4-octadienoic acid (85 and 86) (Figure 6) [31]. The new compounds displayed potent lethal activity against brine shrimp.
Four new chlorinated eremophilane-type sesquiterpenoids were isolated from the fungus Penicillium sp. PR19N-1 obtained from an Antarctic deep sea marine sludge [32]. The new compounds were identified as 1-chloro-3β-acetoxy-7-hydroxytrinoreremophil-1,6,9-trien-8-one (87), 1-chloro-3β-hydroxy-7-epoxyeremophil-1,9-dien-8-one (88), 1α-chloro-2β-hydroxyeremophil-7(11),9-dien-8-one (89), and epoxy-tetrahydrofuran eremophilane (90). The authors proposed a biosynthetic pathway for compounds 87–90 involving biochemical modifications of the key intermediate 1α-hydroxy-7βH-eremophil-9,11-dien-8-one (93), that was subsequently reported by the same group in another study (Figure 7). Compounds 87–90 were evaluated for their cytotoxic activity against HL-60 and A549 cancer cells. Only 87 showed moderate cytotoxicity.
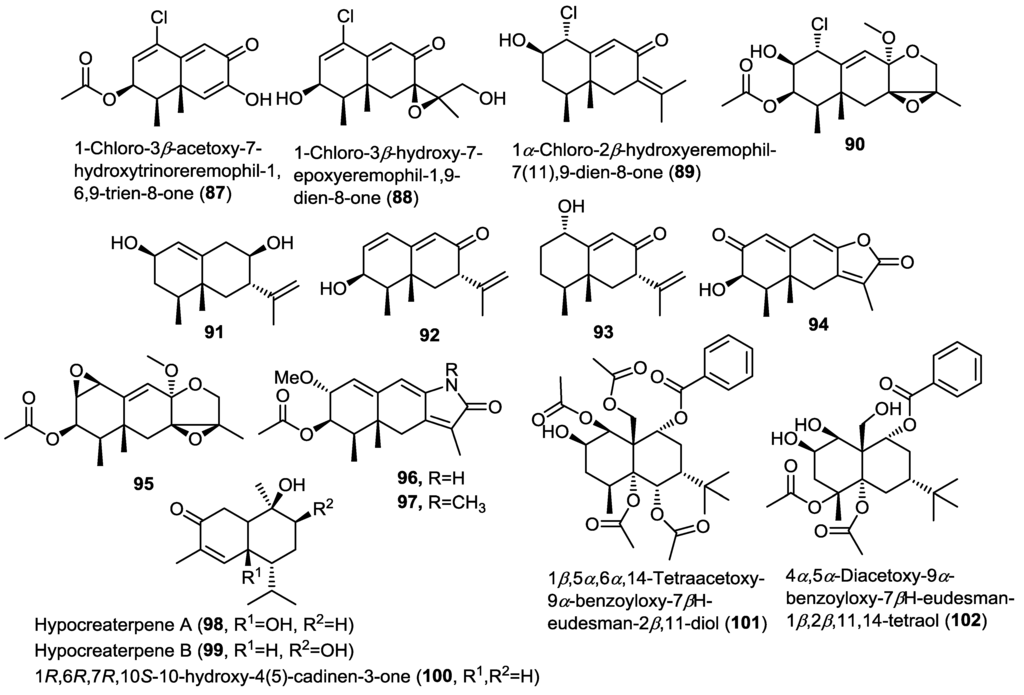
Figure 7.
Sesquiterpenes isolated from Penicillium sp. PR19N-1, Hypocreales sp. HLS-104 and Pestalotiopsis sp.
Figure 7.
Sesquiterpenes isolated from Penicillium sp. PR19N-1, Hypocreales sp. HLS-104 and Pestalotiopsis sp.
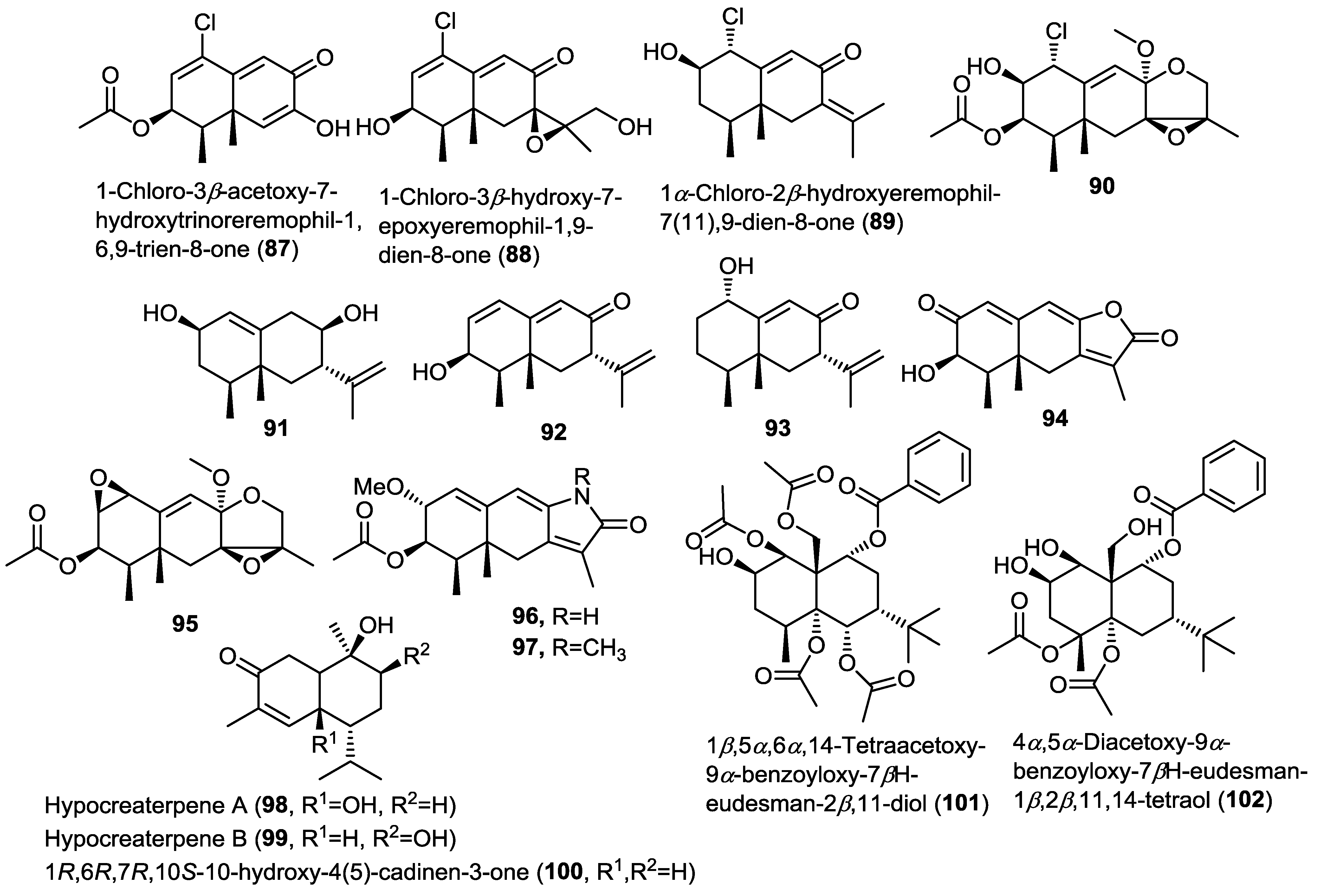
The same research group conducted further investigation on the same fungal material leading to the isolation of six additional new eremophilane sesquiterpenoids (91–96) (Figure 7) [33]. Compound 96 belongs to the rare eremophilane lactam-type that is strongly related to the semi-synthetic compound 97 [31]. New compounds were evaluated for their cytotoxic activity against HL-60 and A549 cell lines but only compounds 91 and 95 were found to possess moderate cytotoxicity.
Investigation of the fungus Hypocreales sp. HLS-104 isolated from the marine sponge Gelliodes carnosa led to the isolation of two new cadinane-type sesquiterpenoids hypocreaterpenes A and B (98 and 99) along with the known 1R,6R,7R,10S-10-hydroxy-4(5)-cadinen-3-one (100) (Figure 7) [34]. HPLC chromatograms of the extracts obtained from fungal cultures prepared with distilled water and with sea water were compared revealing that sea water based media were superior with regard to the induction of natural products accumulation. Compounds 98–100 were tested for their anti-inflammatory activity via estimating the inhibitory activity on nitric oxide production in RAW 264.7 cell assay. Only 100 showed moderate inhibition, highlighting the possible role of the 6- or 9-hydroxyl group in decreasing or inhibiting nitric oxide production.
A report on the endophytic fungus Pestalotiopsis sp. isolated from the marine alga Sargassum horneri showed the isolation of two new highly esterified sesquiterpenoids 1β,5α,6α,14-tetraacetoxy-9α-benzoyloxy-7βH-eudesman-2β,11-diol (101) and 4α,5α-diacetoxy-9α-benzoyloxy-7βH-eudesman-1β,2β,11,14-tetraol (102) (Figure 7) [35]. Compounds 101 and 102 were produced as a result of abiotic stress on the fungus in the culture media using CuCl2 as revealed by comparison of the TLC chromatograms of stressed and non-stressed fungal cultures. Tyrosinase inhibitory activity of both 101 and 102 was evaluated revealing their potent enzyme inhibitory activity with IC50 values of 14.8 and 22.3 µM, respectively. These results point to the possibility of their progress as future candidates for clinical trials in hyperpigmentation conditions.
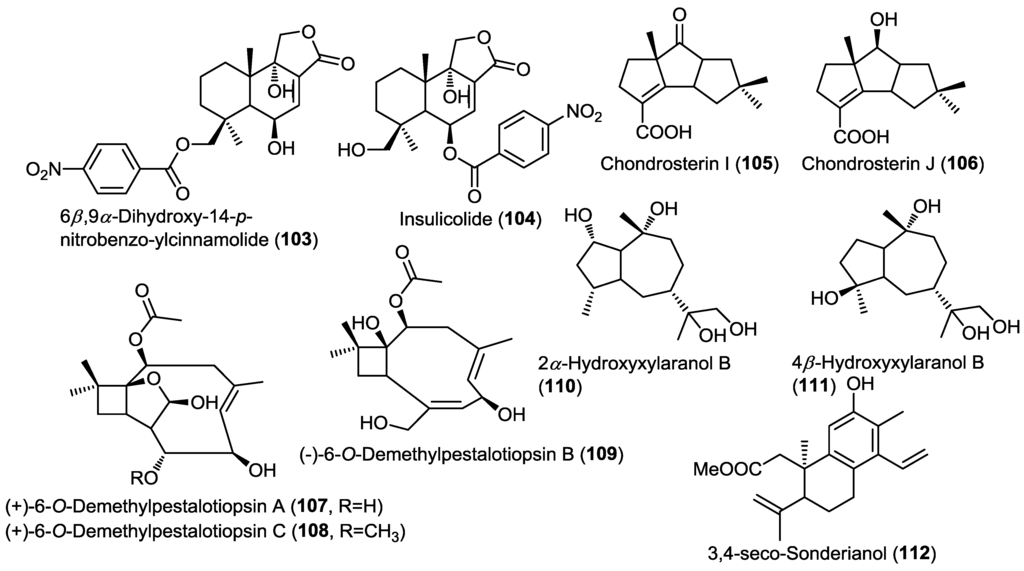
Figure 8.
Sesquiterpenes isolated from Aspergillus ochraceus Jcma1F17, Chondrostereum sp., Ascotricha sp. ZJ-M-5 and endophytic fungus J3.
Figure 8.
Sesquiterpenes isolated from Aspergillus ochraceus Jcma1F17, Chondrostereum sp., Ascotricha sp. ZJ-M-5 and endophytic fungus J3.
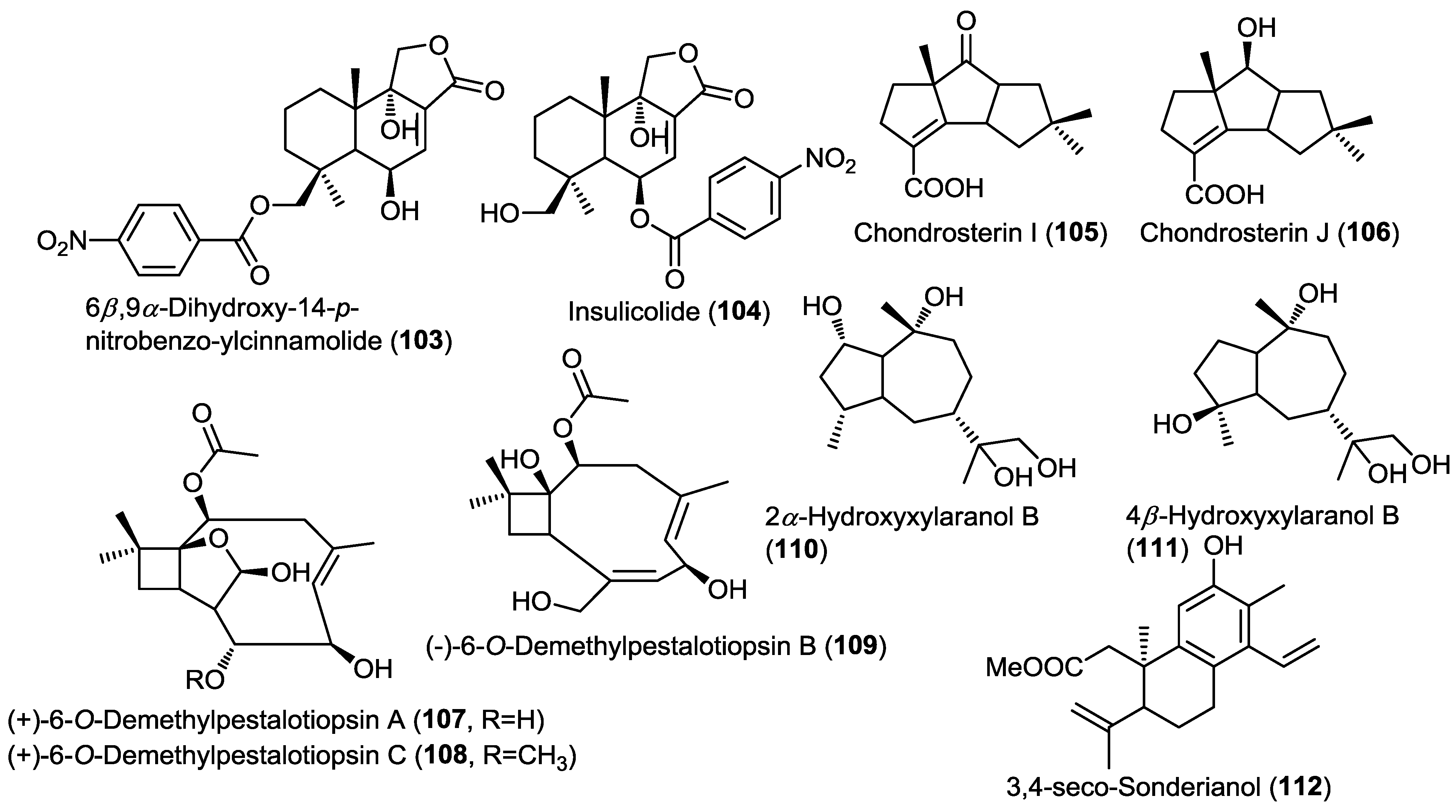
Investigation of the endophytic fungus Aspergillus ochraceus Jcma1F17 isolated from the marine alga Coelarthrum sp. led to the isolation of the new nitrobenzoyl sesquiterpene 6β,9α-dihydroxy-14-p-nitrobenzoylcinnamolide (103) and the known positional isomer insulicolide A (104) (Figure 8) [36]. Nitrobenzoyl sesquiterpenoids are very rare natural compounds and to the best of our knowledge they are restricted to Aspergillus sp. and considered as chemotaxonomic markers for few Aspergillus species.
Compounds 103 and 104 showed potent cytotoxic activity against a panel of 10 human cancer cell lines (H1975, U937, K562, BCG-823, Molt-4, MCF-7, A549, HeLa, HL60, and Huh-7), with IC50 values of 1.95 to 6.35 µM. In addition, 103 showed potent antiviral activity against influenza virus H3N2 and human enterovirus EV71 with IC50 values of 17.0 and 9.4 µM, respectively [36].
Two new unique hirsutane sesquiterpenoids were isolated from the fungus Chondrostereum sp. isolated from the soft coral Sarcophyton tortuosum (Figure 8) [37]. The new compounds were identified as chondrosterin I (105) and J (106) based on 1D, 2D NMR, MS and single crystal X-ray diffraction [37]. The authors proposed that changing the carbon source in the culture media from glucose to glycerol was the main reason for the dramatic changes in the hirsutane nucleus observed in the new compounds when compared to their previously reported congeners isolated from the same fungus, with a migration of C-2 methyl to C-6 and the introduction of carboxyl group at C-3. This experimental approach of changing the components of culture media in order to trigger the stimulation or inhibition of certain genes and subsequently changing the secondary metabolites profile is a well-known technique called One Strain Many Compounds Analysis (OSMAC). Compound 106 showed potent cytotoxic activity against human nasopharyngeal cancer cell lines CNE-1 and 2 with IC50 values of 1.32 and 0.56 µM, respectively; whereas 105 was found to be inactive. These results point out to the importance of the 7-OH group for the activity of the compound.
The OSMAC approach was also employed by another research group by changing the MgCl2 concentration in the culture media of the fungus Ascotricha sp. ZJ-M-5 that had been obtained from a mud sample collected on the coastline of Fenghua, China [38]. Modifications of the Czapek Dox broth culture media by adding different concentrations of MgCl2 or completely removing it resulted in the isolation of three new caryophyllene-type sesquiterpenes, (+)-6-O-demethylpestalotiopsin A and C together with (−)-6-O-demethylpestalotiopsin B (107–109) (Figure 8) [38]. Compounds 107 and 108 were produced in response to the absence of MgCl2 in the culture media whereas the addition of Mg2+ resulted in suppression of their production but stimulated the production of 109. Further increase of the Mg2+ concentration in the broth inhibited the production of compounds 107–109. Compounds 107 and 108 showed potent growth inhibitory activity against HL-60 and K562 with IC50 values ranging between 6.9 and 12.3 µM; while 109 showed no activity at all.
Two new sesquiterpenoids, 2α-hydroxyxylaranol B (110) and 4β-hydroxyxylaranol B (111), together with the known diterpene 3,4-seco-sonderianol (112) were isolated from the endophytic fungus J3 obtained from leaves of the mangrove Ceriops tagal (Figure 8) [39]. Evaluation of the cytotoxic activities of compounds 110–112 against K562, SGC-7901, and BEL-7402 cancer cell lines revealed that compound 112 exhibited moderate cytotoxic activity against all the three cell lines whereas the new compounds did not show any cytotoxic activity.
2.3. Diterpenes (C20)
Six new diterpenes were isolated from the marine-derived fungus Penicillium sp. strain F23-2 cultured from deep ocean sediment. The new diterpenes were found to belong to the very rare conidiogenone class and were identified as conidiogenone B–G (113–118) (Figure 9) [40]. Conidiogenone G (118) was postulated by the authors as a biosynthetic precursor for the new diterpene alkaloid meleagrin B (119) co-isolated in the same report. The proposed biosynthetic scheme comprises several steps with a Michael addition reaction as a key reaction. Compounds 113–119 were evaluated for their cytotoxic activity against HL-60, A549, BEL-7402 and MOLT-4 cancer cell lines. Conidiogenone C (114) showed potent cytotoxicity against HL-60 and BEL-7402 cells with IC50 values of 0.038 and 0.97 µM whereas meleagrin B (119) showed moderate cytotoxicity against all cell lines investigated.
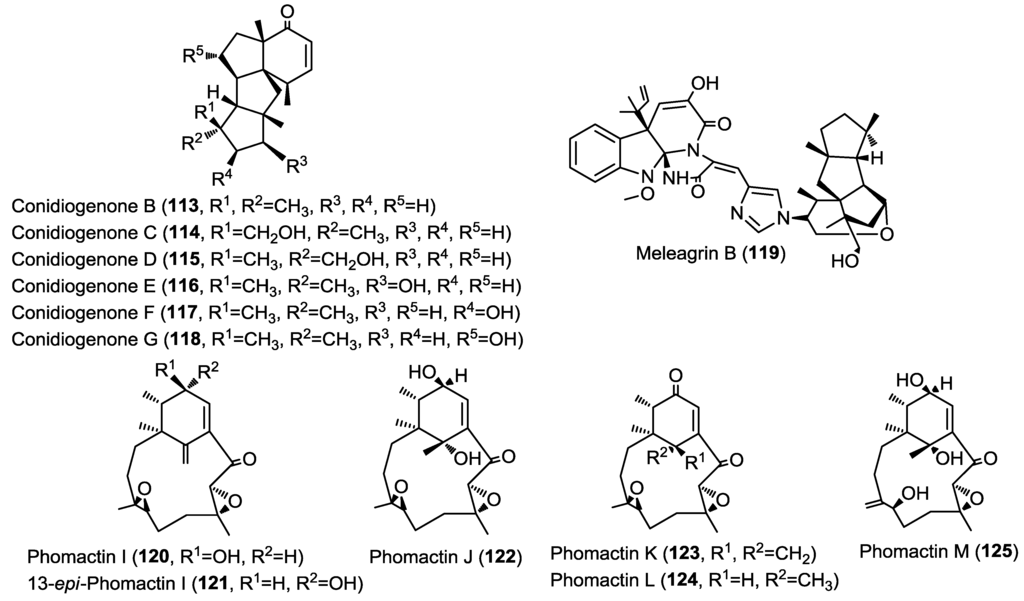
Figure 9.
Diterpenes isolated from Penicillium sp., unidentified fungal strain (MPUC 046) and Phoma sp.
Figure 9.
Diterpenes isolated from Penicillium sp., unidentified fungal strain (MPUC 046) and Phoma sp.
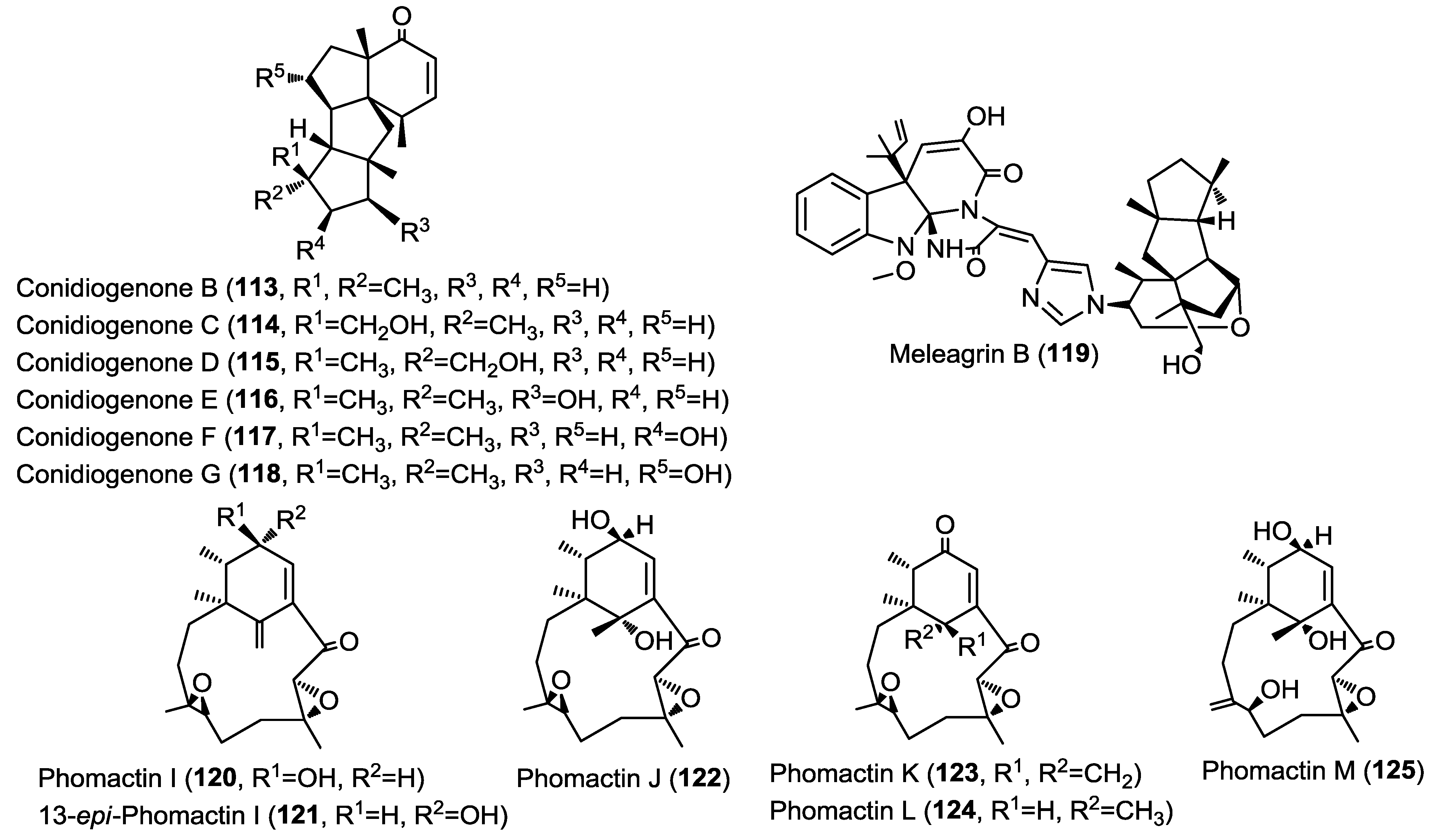
Three new macrocyclic epoxy-diterpenes were isolated from an unidentified fungal strain (MPUC 046) obtained from the marine brown alga Ishige okamurae [41]. The isolated compounds are similar to the known platelet activating factor (PAF) antagonists, phomactins and were identified as phomactin I, 13-epi-phomactin I and phomactin J (120–122) (Figure 9) [41]. The absolute configuration was assessed with the aid of the single crystal X-ray diffraction experiment. The same authors had previously reported the unique phomactin H from the same fungal material and it is worth noting that the earlier members of this series were isolated from a phylogenetically different fungal species, Phoma sp. [42].
Further investigation of the unidentified fungal strain (MPUC 046) obtained from the marine brown alga Ishige okamurae yielded three macrocyclic diterpenes, phomactins K–M (123–125) (Figure 9) [43]. Identification of the isolated compounds was achieved using single X-ray crystal diffraction analysis and by comparing the obtained spectra with the previously reported data of related congeners. It is worth noting that phomactins were found to possess a significant platelet activating factor inhibitory effect. Additionally, most of these compounds were inactive in several other biological assays suggesting their high selectivity towards PAF inhibitory effect and their potential as future candidates for clinical trials.
The same research group had reported the isolation of three new diterpenes myrocin D, libertellenone E and F (126–128), together with the known congeners myrocin A (129) and libertellenone C (130), from the fungus Arthrinium sacchari obtained from an unidentified sponge collected from the coast of Atami-shi (Figure 10) [44]. The absolute configuration of the new compounds was determined using single crystal X-ray diffraction analysis.
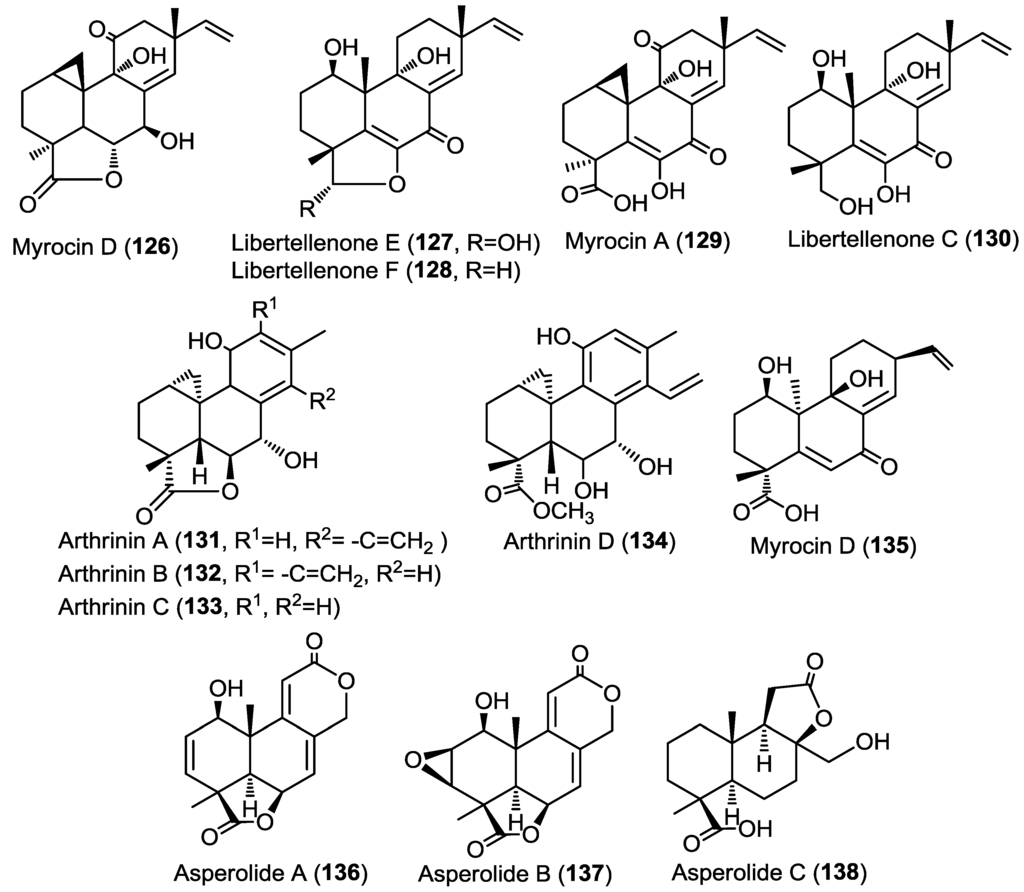
Figure 10.
Diterpenes isolated from Arthrinium sacchari, Libertella sp., Arthrinium sp. and Aspergillus wentii EN-48.
Figure 10.
Diterpenes isolated from Arthrinium sacchari, Libertella sp., Arthrinium sp. and Aspergillus wentii EN-48.
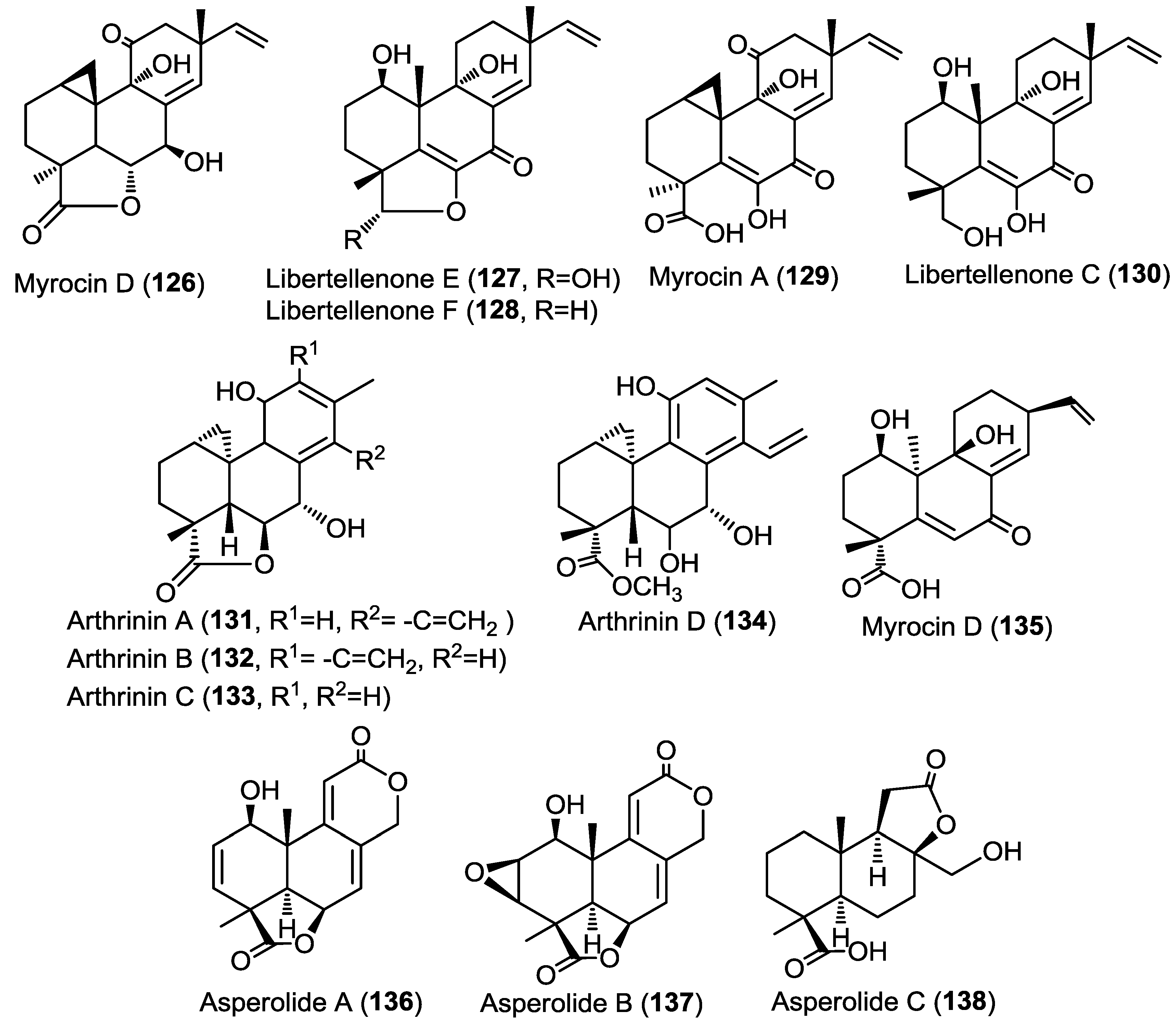
Earlier members of the libertellenone series, libertellenone A–D, were previously reported from the fungus Libertella sp. as a response to an induced stress caused by co-culturing the fungus with a marine α-proteobacterium (strain CNJ-328) [45]. Compounds 126–130 were evaluated for their antiangiogenic activity through measuring their ability to inhibit the proliferation of human umbilical vein endothelial cells (HUVECs) and human umbilical artery endothelial cells (HUAECs) (Figure 10). Only 129 and 130 showed inhibitory activity whereas the other compounds were inactive.
In a parallel study on the marine fungus Arthrinium sp. derived from the Mediterranean sponge Geodia cydonium, four novel diterpenoids, arthrinins A–D (131–134), were identified from its methanol extract. In addition, one new diterpenoid, myrocin D (135) along with five known compounds including myrocin A and two xanthone derivatives, norlichexanthone and anomalin A were purified from the same extract (Figure 10) [46]. The structures of arthrinins A–D (131–134) were recognized as being of hybrid origin and derived from cleistanthane and pimarane diterpenes. The absolute configuration of arthrinins A–D (131–134) was established by the modified Mosher’s method and by ROESY spectra. Antiproliferative activity of the isolated compounds was evaluated toward four different tumor cell lines, namely, L5178Y, K562, A2780, and A2780CisR cell lines. Results revealed that norlichexanthone and anomalin featured the strongest activities (IC50 values of 0.40–74.0 µM) [46]. These findings are in accordance with results from protein kinase activity assays that included aurora-B, PIM-1, and VEGF-R2 kinases which were inhibited by norlichexanthone and anomalin A with IC50 values between 0.3 and 11.7 µM [46]. Furthermore, in the in vitro angiogenesis assay against HUVECs sprouting induced by VEGF-A, myrocins D (135), A, and anomalin A inhibited endothelial cell sprouting with IC50 values of 2.6, 3.7, and 1.8 µM, respectively.
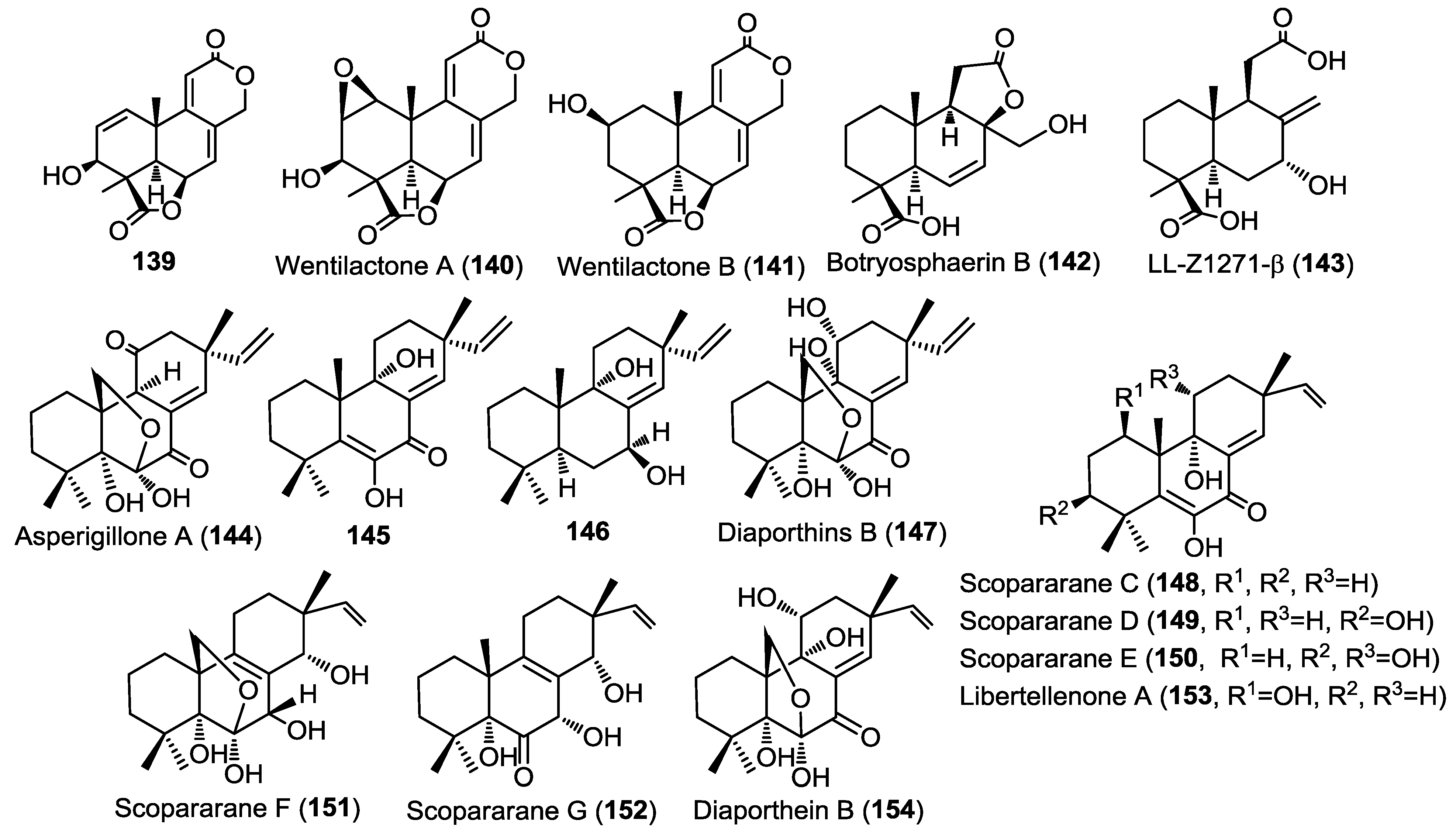
Three new tetranorlabdane diterpenoids, asperolides A–C (136–138) (Figure 10), together with five known derivatives, a tetranorditerpenoid derivative (139), wentilactones A (140) and B (141), botryosphaerin B (142) and LL-Z1271-β (143) (Figure 11) were isolated from the endophytic fungus Aspergillus wentii EN-48 cultured from the marine algae Sargassum sp. [47]. All isolated compounds were evaluated for their cytotoxic and antimicrobial activities against a panel of cancer cell lines and microbial strains, respectively. Compounds 136, 137, and 139–141 showed moderate cytotoxicity with wentilactone B (141) as the most potent among the tested compounds (IC50 = 17 µM). In the antimicrobial assay, compound 139 showed considerable antifungal activity against Candida albicans with an MIC value of 55.6 µM [47].
Three new pimarane diterpenoids (144–146) together with the known diaporthins B (147) were isolated from the fungal strain HS-1 cultured from the sea cucumber Apostichopus japonicus (Figure 11) [48]. Diaporthins B (147) was used as a reference in the determination of the absolute configuration of the new compounds via comparison of the circular dichroism (CD) spectra.
All isolated compounds were evaluated for their cytotoxic activities against KB and KBv200 cell lines. Compounds 144 and 147 showed potent activity against both cell lines with IC50 of 10.1, 6.8 µM and 10.6, 17.9 µM, respectively. Compound 145 was only weakly active (IC50 > 45 µM) whereas compound 146 showed no activity, pointing out to the possible role of carbonyl group at C-7 in the cytotoxic activity.
Five new pimarane diterpenoids, scopararanes C–G (148–152), together with six known pimarane diterpenes, were isolated from the marine-derived fungus Eutypella scoparia FS26 that had been obtained from sediment collected in the South China Sea (Figure 11) [49]. All isolated compounds were assessed for their antiproliferative activity using a cytotoxicity (MTT) assay against three different human cancer cell lines; including MCF-7 (breast), NCI-H460 (lung), and SF-268 (brain). Among the tested compounds, scopararane D (149) showed only mild antiproliferative activity with IC50 values between 25.6 and 46.0 µM, whereas, the known compounds, libertellenone A (153) and diaporthein B (154), revealed potent antiproliferative activities with IC50 values ranging from 4.4–20.0 µM, compared to cisplatin (IC50 = 1.5–9.2 µM) (Figure 11) [49].
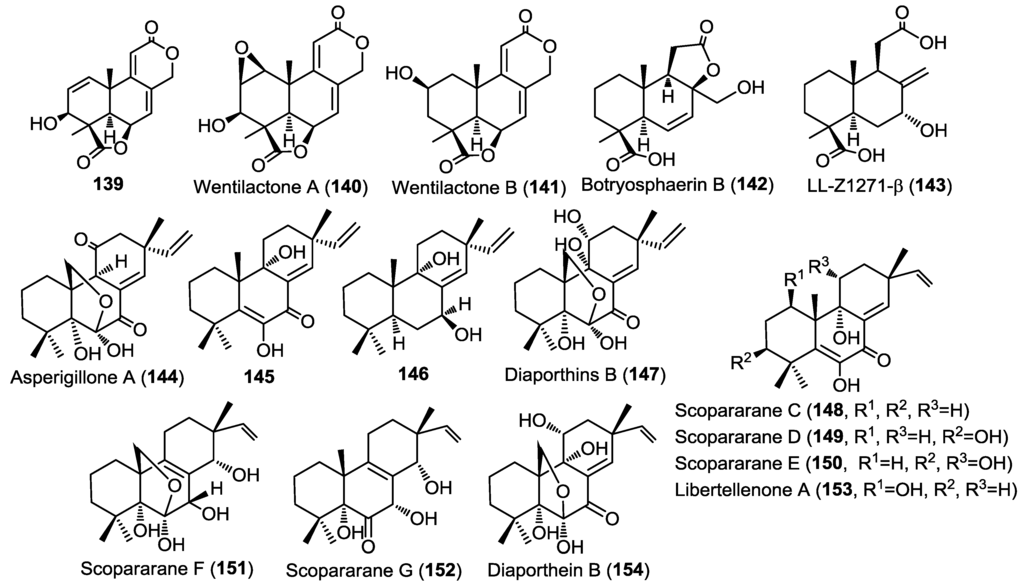
Figure 11.
Diterpenes isolated from Aspergillus wentii EN-48, fungal strain HS-1, Eutypella scoparia FS26.
Figure 11.
Diterpenes isolated from Aspergillus wentii EN-48, fungal strain HS-1, Eutypella scoparia FS26.
2.4. Sesterterpenes (C25)
Three new sesterterpenes belonging to the ophiobolin class were isolated from the marine derived fungus Emericella variecolor strain GF-10 obtained from sediment collected at 70 m depth from the Gokasyo Gulf, Japan [50]. The isolated compounds were identified as ophiobolin K, 6-epi-ophiobolin K and 6-epi-ophiobolin G (155–157) (Figure 12) [50] and were found to inhibit biofilm formation of Mycobacterium smegmatis with compound 155 being the most active (MIC = 4.1 µM). However, isolated compounds failed to show remarkable antimicrobial activity. 6-epi-Ophiobolin G (157) was further studied using M. bovis BCG and found to inhibit its biofilm formation at a MIC = 8.2 µM [50]. Moreover, the compound showed the ability to restore the antimycobacterial effect of isoniazid against biofilm forming M. smegmatis [50].
Five ophiobolin sesterterpenes were obtained from the fungus Aspergillus ustus isolated from the marine alga Codium fragile [31]. The compounds were identified as (6α)-21-deoxyophiobolin G, (6α)-16,17-dihydro-21-deoxyophiobolin G, and ophiobolins U–W (158–162) (Figure 12) [31]. All compounds were evaluated for their antibacterial and antifungal activity and for their lethal effect on brine shrimp eggs. Only compound 160 showed a remarkable antibacterial activity against Escherichia coli and Staphylococcus aureus as well as >75% lethality in the brine shrimp toxicity assay [31].
Investigation of the mangrove-derived fungus Aspergillus sp. 16-5C resulted in the isolation of a new pentacyclic sesterterpene, asperterpenoid A (163) (Figure 12) [51]. The absolute configuration of 163 was determined with the X-ray single crystal diffraction analysis. Compound 163 was found to possess an unprecedented pentacyclic nucleus; the authors had proposed a biosynthetic scheme involving an initial geranyl farnesyl pyrophosphate (GFPP) moiety undergoing a series of cyclization, group migration and oxidation reactions. Asperterpenoid A (163) showed potent inhibitory activity against Mycobacterium tuberculosis protein tyrosine phosphatase B (mPTPB) with IC50 = 2.2 µM [51].
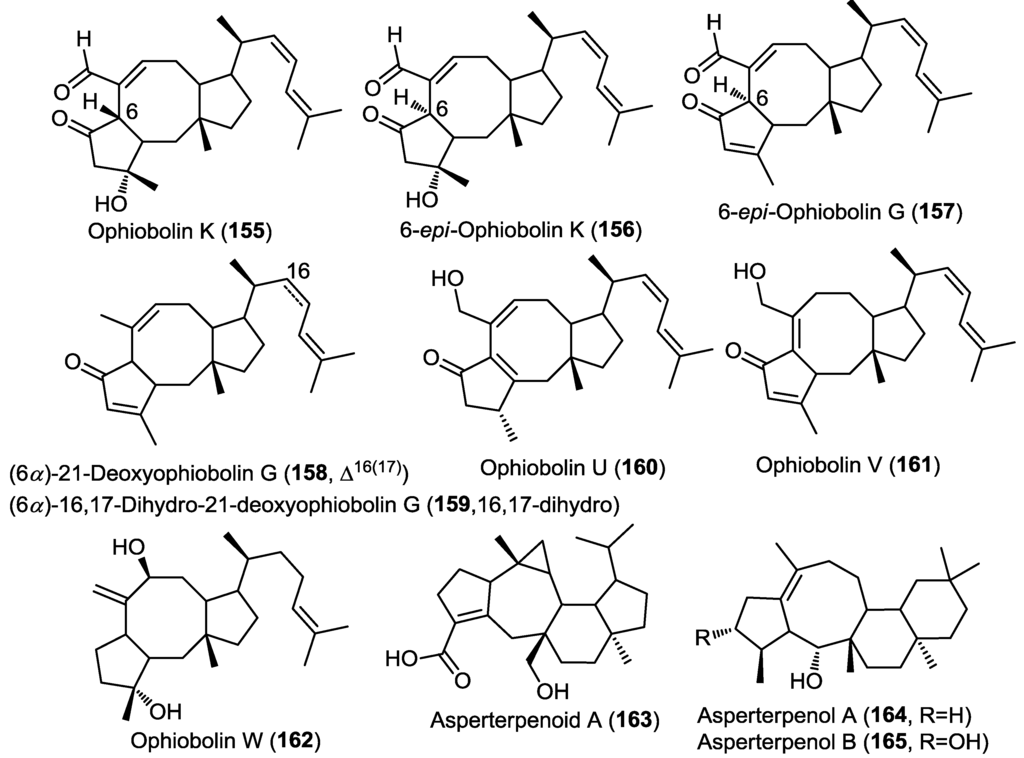
Figure 12.
Sesterterpenes isolated from Emericella variecolor strain GF-10, Aspergillus ustus, Aspergillus sp. 16-5C and Aspergillus sp. 085242.
Figure 12.
Sesterterpenes isolated from Emericella variecolor strain GF-10, Aspergillus ustus, Aspergillus sp. 16-5C and Aspergillus sp. 085242.
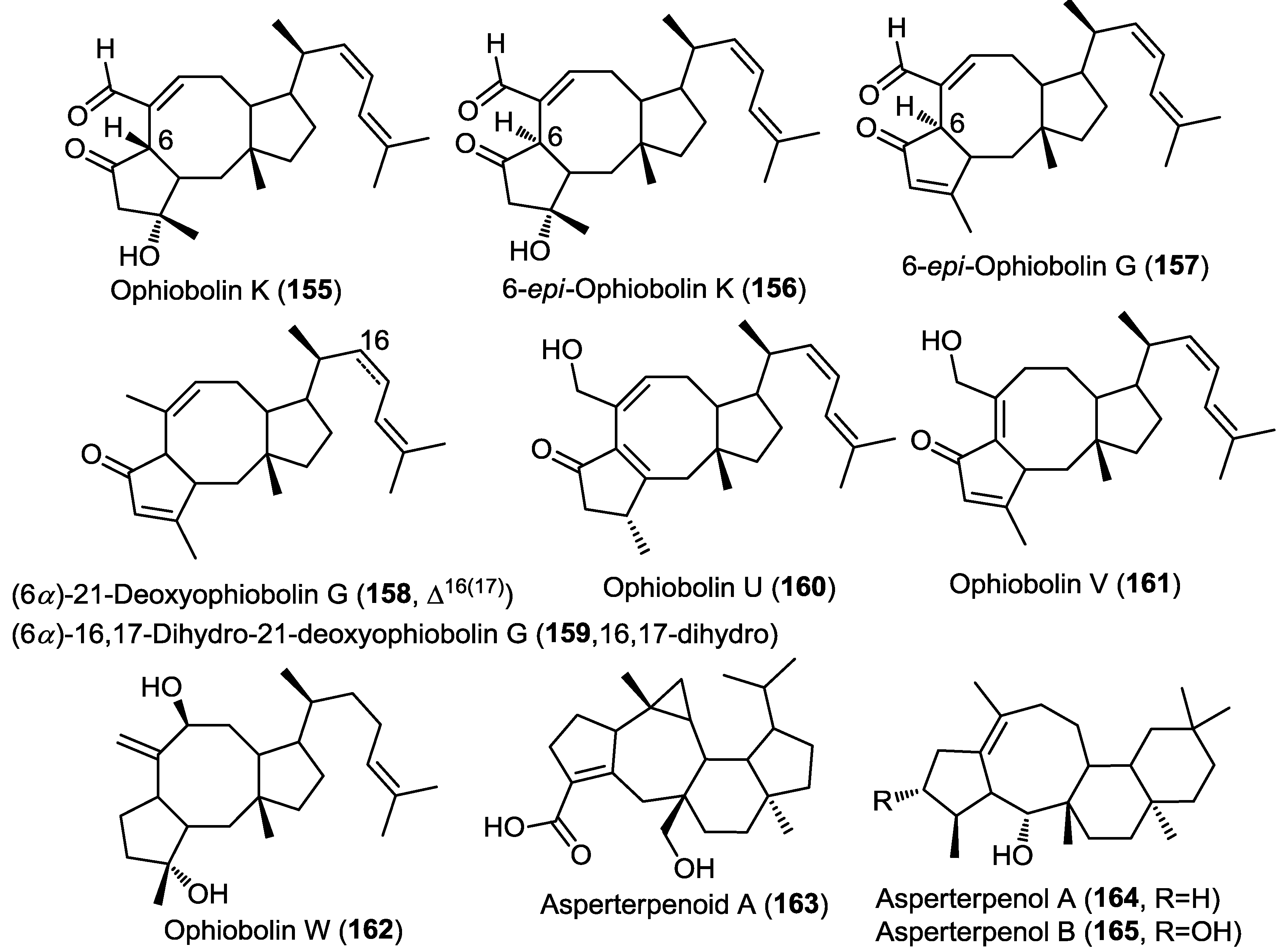
The same authors reported two additional new tetracyclic sesterterpenes, asperterpenol A (164) and B (165) from the mangrove-derived fungus Aspergillus sp. 085242 (Figure 12) [52]. The new compounds were found to possess an unprecedented 5/8/6/6 tetracarbocyclic nucleus; similarly the authors proposed a biosynthetic scheme for 164 and 165 involving a geranyl farnesyl pyrophosphate (GFPP) substrate undergoing a series of cyclization, group migration and oxidation reactions. Compounds 164 and 165 were evaluated for their anticholinesterase activity against acetylcholinesterase (AChE) and butyrylcholinesterase enzymes (BuChE). Both compounds showed potent inhibition of AChE at IC50 values of 2.3 and 3.0 µM, respectively. However, neither of them showed inhibition of BuChE, pointing to a selective activity.
2.5. Triterpenes (C30)
Two new oxidized ergosterols, 22E-7α-methoxy-5α,6α-epoxy-ergosta-8(14),22-dien-3β-ol (166) and 22E-3β-hydroxy-5α,6α,8α,14α-diepoxy-ergosta-22-en-7-one (167) were isolated from the fungus Aspergillus awamori obtained from soil surrounding the mangrove plant Acrostichum speciosum (Figure 13) [53]. Analysis of the ROESY spectrum of 166 showed that the endo-boat conformation of ring B is the most reasonable conformation instead of the half-chair conformation generally adopted for oxidized cyclohexene ring systems. It was suggested that this conformation was adopted due to the stabilization effect of C8/C14 double bond. Compounds 166 and 167 exhibited weak cytotoxic activity against A549 cancer cell lines.
Three ergosterol derivatives were isolated from the fungus Aspergillus ochraceus EN-31 obtained from the marine brown alga Sargassum kjellmanianum [54]. The isolated compounds were identified as 7-nor-ergosterolide (168), 3β,11α-dihydroxyergosta-8,24(28)-dien-7-one (169) and 3β-hydroxyergosta-8,24(28)-dien-7-one (170) (Figure 13) [54]. The absolute configuration of the isolated compounds was determined by the modified Mosher’s method. Compound 168 possesses an unprecedented pentalactone B-ring system. The presence of this unusual lactone moiety in ring B was suggested on the basis of a missing signal from the usual 28 signals of ergosterol as revealed by the 13C spectra and the structure was confirmed through HMBC correlations. The authors proposed a biosynthetic scheme for the unusual compound 168 involving the co-isolated 170 as a precursor through the ring-B opening, decarboxylation, oxidation and final intermolecular esterification. Compounds 168–170 were evaluated for their cytotoxicity as well as for their antibacterial and antifungal activities. Compound 168 showed selective cytotoxicity against NCI-H460, SMMC-7721, and SW1990 cell lines, whereas 169 showed activity against SMMC-7721 cell line. However, none of the tested compounds showed any antimicrobial activity.
Investigation of the endophytic fungus Penicillium chrysogenum QEN-24s, isolated from the marine red alga Laurenica sp., led to the isolation of two polyoxygenated new sterols, penicisteroid A (171) and B (172), together with the previously reported steroid, anicequol (173) (Figure 13) [55]. Compounds 171–173 shared the unique structural feature of having 11-OH and 16-acetoxy functionalities; to the best of our knowledge no other natural products possess this feature. The isolated compounds were subjected to cytotoxic and antifungal evaluation. Results disclosed that penicisteroid A (171) showed antifungal activity against both Aspergillus niger and Alternaria brassicae whereas anicequol (173) showed activity only against A. brassicae while penicisteroid B (172) did not exhibit activity against the two fungi. This may point to the role of the 6-OH function in the activity against A. niger and the role of OH-substitution in ring B for the activity against A. brassicae. Similarly, 171 exhibited cytotoxicity against a panel of cell lines HeLa, SW1990, and NCI-H460 (IC50 values of 29.6–79.0 µM) with no activity observed for the other compounds.
6β,16β-Diacetoxy-25-hydroxy-3,7-dioxo-29-nordammara-1,17(20)-dien-21,24-lactone (174), a new nor-dammarane triterpene, was isolated from the fungus Aspergillus fumigatus KMM4631 obtained from the soft coral Sinularia sp. (Figure 13) [56]. The isolated compound was evaluated for its antibacterial and antifungal activities but did not show any remarkable activity.
Spartopregnenolone (175), a new unique pregnane-type sterol, was isolated from the marine derived endophytic fungus Phaeosphaeria spartinae cultured from the marine red alga Ceramium sp. (Figure 13) [57]. Spartopregnenolone shares structural features common to lanosterol triterpenes, mainly the presence of a ∆8,9 double bond, whereas the other features are more common to pregnane-type sterols, mainly the presence of 17-acetyl side chain instead of the usual isoprene unit. The authors argued that 175 is an intermediate in the biosynthesis of sterols from lanosterol, the presence of the unusual 4-carboxylic group confirmed this assumption.
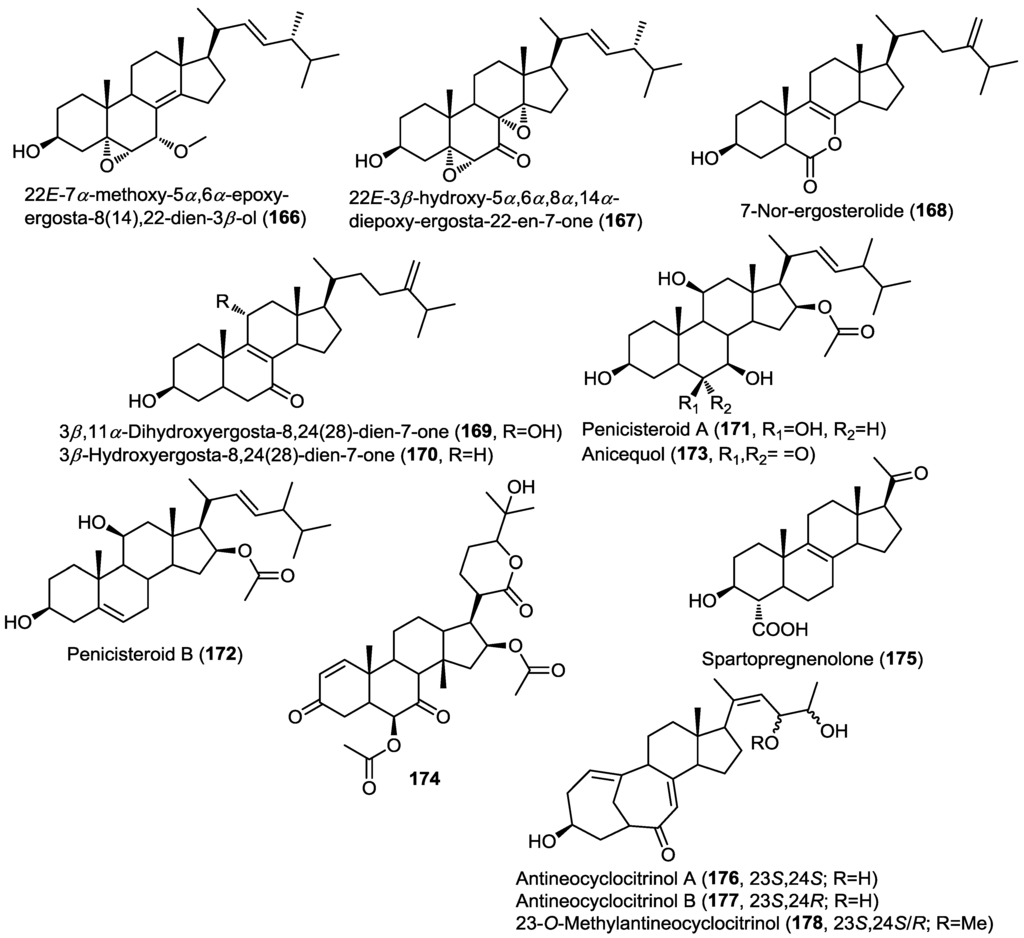
Figure 13.
Triterpenes isolated from Aspergillus awamori, Aspergillus ochraceus EN-31, Penicillium chrysogenum QEN-24s, Aspergillus fumigatus KMM4631, Phaeosphaeria spartinae and mutant AD-1-2 Penicillium purpurogenum G59.
Figure 13.
Triterpenes isolated from Aspergillus awamori, Aspergillus ochraceus EN-31, Penicillium chrysogenum QEN-24s, Aspergillus fumigatus KMM4631, Phaeosphaeria spartinae and mutant AD-1-2 Penicillium purpurogenum G59.
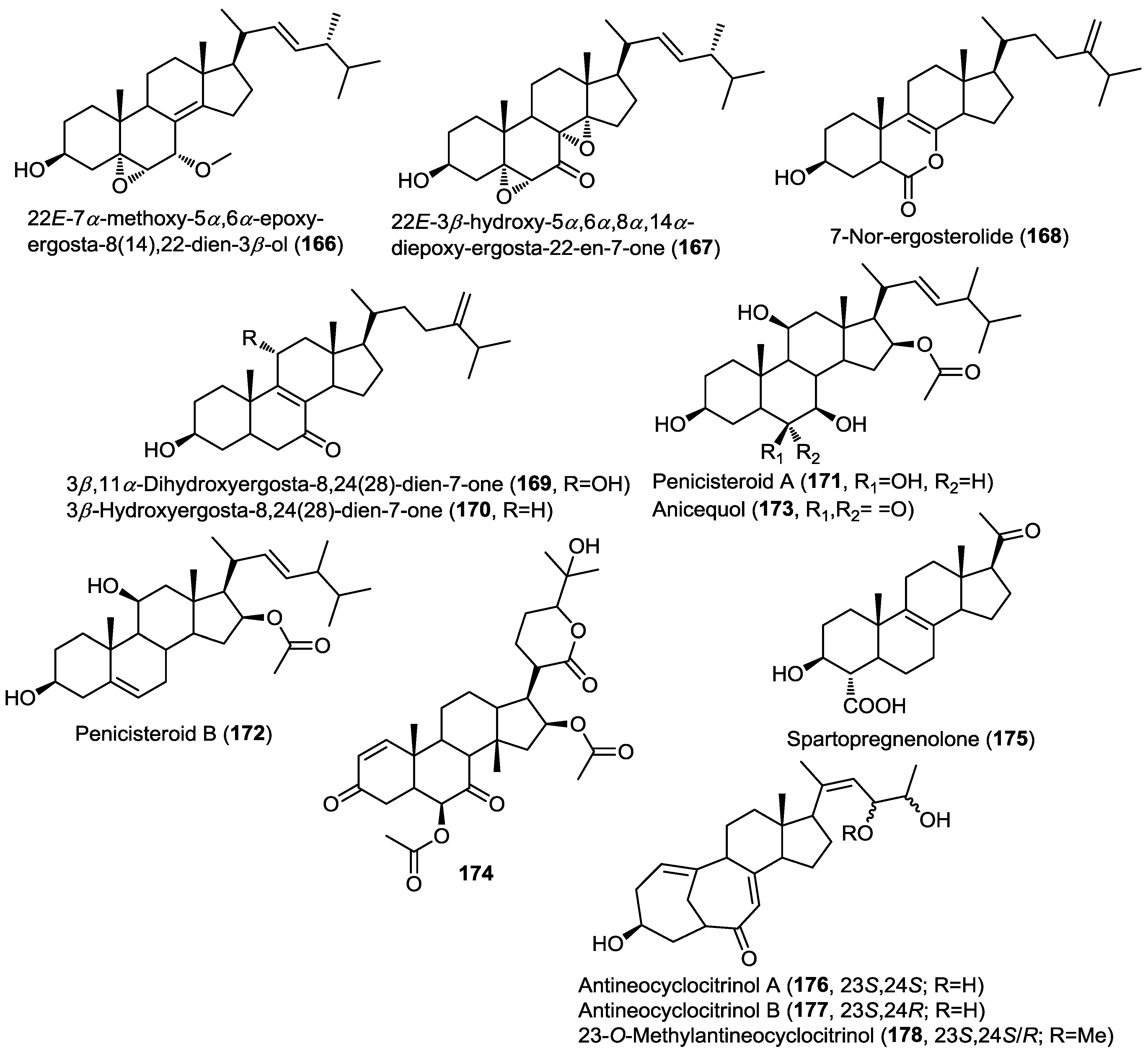
Three new steroids, antineocyclocitrinols A, B and 23-O-methylantineocyclocitrinol (176–178), were isolated from the mutant AD-1-2. This mutant was obtained by mutagenesis of the fungal strain Penicillium purpurogenum G59 collected from a soil sample at the tideland of Bohai Bay, China (Figure 13) [58]. LC/MS and HPLC-PDA chromatograms of the mutant strain revealed the presence of metabolites not detectable in the original extract of G59. Moreover, the ethyl acetate extract of AD-1-2 showed significant cytotoxicity whereas the wild type did not. Mutagenesis is a well-established technique for activation of silent genes and thus the production of unusual metabolites. In this report, the authors used the known diethyl sulfate (DES) as a mutagen. The isolated sterols shared the unusual bicyclo[4.4.1]A/B ring system and showed weak cytotoxicity against a panel of cancer cell lines, including K562, HL-60 and HeLa cells.
2.6. Meroterpenes
Three new meroterpenoids were obtained from the fungus Aspergillus insuetus (OY-207) isolated from the Mediterranean sponge Psammocinia sp. [21]. The isolated compounds were identified as insuetolides A–C (179–181) (Figure 14) [21]. Insuetolides constitute a new carbon skeleton in which a drimane sesquiterpene lactone is linked with tetraketide-3,5-dimethylorsellinic acid. The main difference between the insuetolides and their common congeners isolated from marine-derived fungi lies in the uncommon presence of perhydropyran ring C instead of the common cyclopentane ring. The authors pointed out to the possible additional oxidation step in the sesquiterpenoid prior to coupling of C-12 to the tetraketide moiety orsellinic acid. Compound 179 showed antifungal activity against the fungus Neurospora crassa, whereas compound 181 showed moderate cytotoxicity against MOLT-4 cancer cell lines.
Three new meroterpenoids were isolated from the fermentation broth of the fungus Penicillium sp. MA-37 obtained from sediment close to the mangrove plant Bruguiera gymnorrhiza [59]. The compounds were found to be derivatives of miniolutelide B and were identified as 4,25-dehydro-miniolutelide B (182), 4,25-dehydro-22-deoxy-miniolutelide B (183) and isominiolutelide A (184) (Figure 14) [59]. It is worth noting that the organism yielded a completely different class of compounds upon shaking of the cultures belonging to diphenyl ether derivatives including three new congeners, namely, ∆1',3'-1'-dehydropenicillide, 7-O-acetylsecopenicillide C, and hydroxytenellic acid B.
Investigation of the fungus A1 isolated from the mangrove plant Scyphiphora hydrophyllacea led to the isolation of six structurally related meroterpenoids sharing a guignardone nucleus. The isolated compounds comprised four new members, guignardone F–I (185–188) together with the previously reported congeners, guignardone A (189) and B (190) (Figure 14) [60]. The authors proposed a biosynthetic scheme involving compound 189 as a common precursor. All isolated compounds were evaluated for their antibacterial activity against MRSA and Staphylococcus aureus. Only compounds 188 and 189 showed activity.
The fungus Aspergillus ustus obtained from the marine alga Codium fragile yielded two related meroterpenes including the structurally revised terretonin F (191) and the new derivative 1,2-dihydroterretonin F (192) (Figure 14) [31]. Compound 191 was previously reported but in this study the authors revised its structure and confirmed the presence of a lactone moiety not included in the original structure. Compounds 191 and 192 showed toxicity to brine shrimp (>75% lethality at 49.6 and 138.6 µM, respectively).
Penicillipyrones A (193) and B (194) are two new meroterpenes isolated from the fungus Penicillium sp. F446 obtained from a 25 m deep marine sediment collected from Geomun-do island, Korea (Figure 14) [61]. The new compounds constituted an unprecedented sesquiterpenoid-γ-pyrone skeleton that differs from the previously reported prenylated γ-pyrones in both the linkage and orientation of the pyrone moiety relative to the sesquiterpenoid. In the isolated compounds, the sesquiterpene is connected to the pyrone through C-10 instead of the common C-12 connectivity. Compounds 193 and 194 did not show any remarkable cytotoxic or antibacterial activity, however, 194 showed dosage-dependent induction of quinone reductase in Hepa 1c1c7 cells which may play an important role as a chemopreventive agent.
Investigation of the sponge-derived fungus Aspergillus sp. OPMF00272, Japan, resulted in the isolation of an additional terretonin-type meroterpenes, terretonin G (195), together with the known terretonin (196) (Figure 14) [62]. Compound 195 showed potent antibacterial activity against gram-positive bacteria, however, it did not show activity against gram negative bacteria or fungi, whereas the parent compound, terretonin (196) did not show antibacterial or antifungal activity.
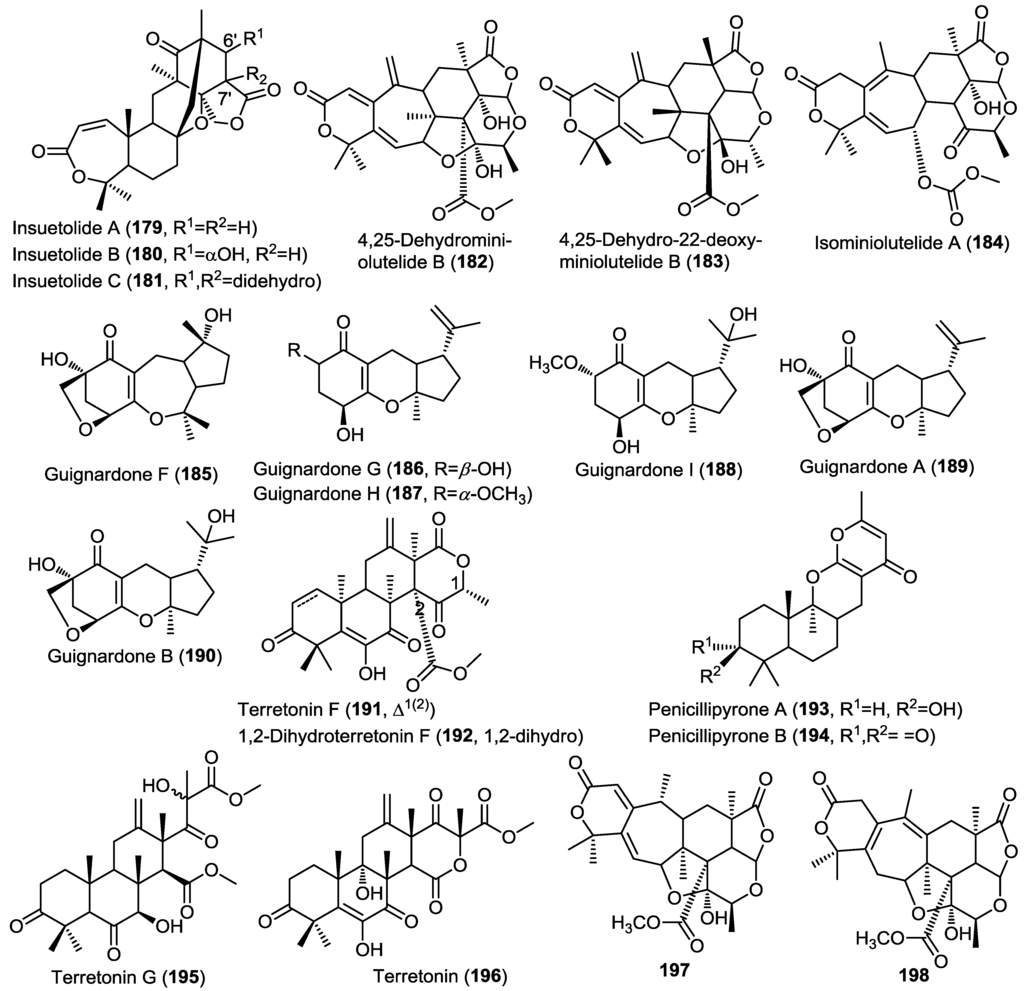
Figure 14.
Meroterpenes isolated from Aspergillus insuetus (OY-207), Penicillium sp. MA-37, fungus A1, Aspergillus ustus, Penicillium sp. F446, Aspergillus sp. OPMF00272 and Penicillium sp. 303.
Figure 14.
Meroterpenes isolated from Aspergillus insuetus (OY-207), Penicillium sp. MA-37, fungus A1, Aspergillus ustus, Penicillium sp. F446, Aspergillus sp. OPMF00272 and Penicillium sp. 303.
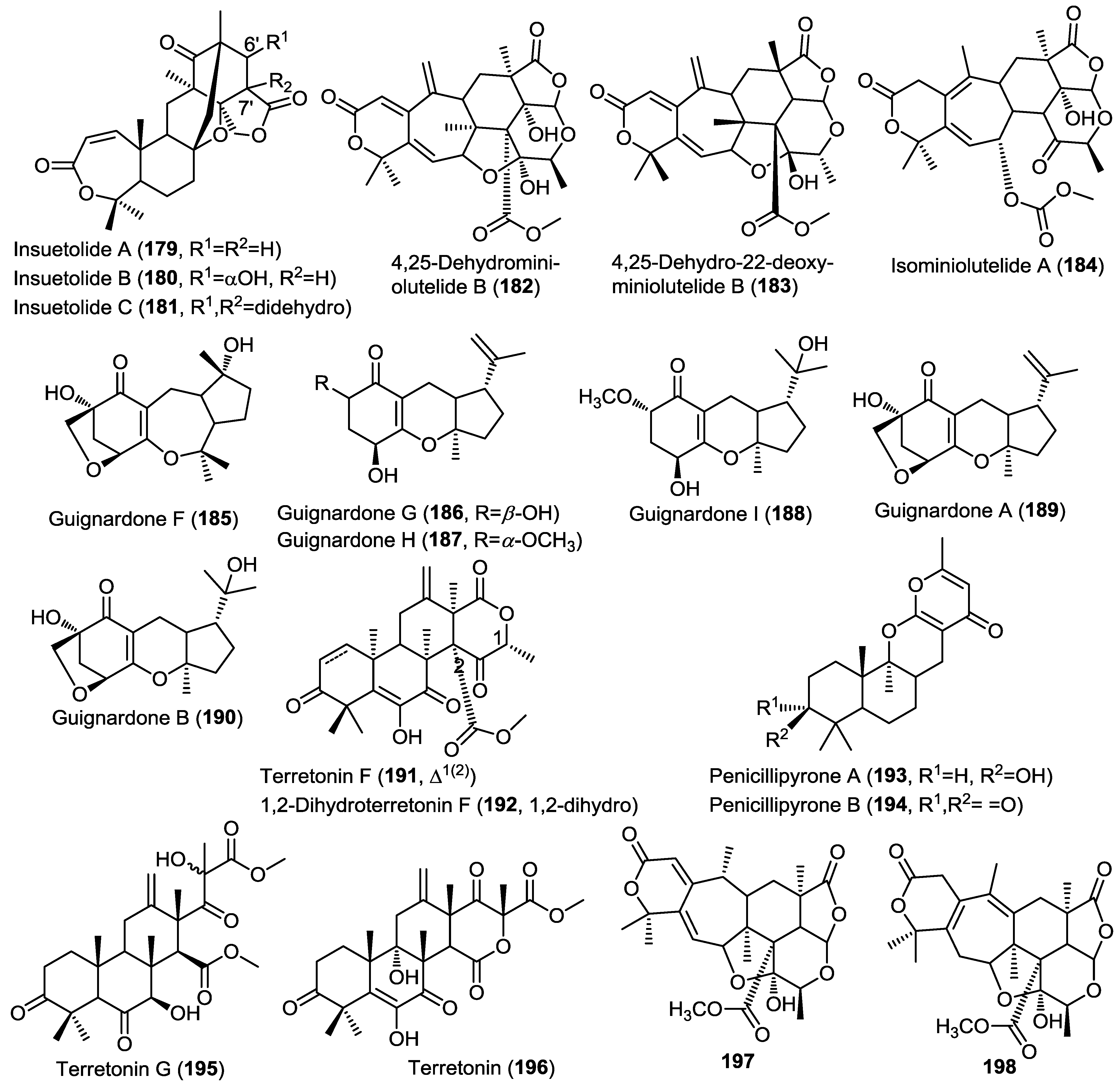
Two new meroterpenes (197 and 198) were isolated from the marine fungus Penicillium sp. 303 cultured from sea water sampled from Zhanjiang Mangrove National Nature Reserve in Guangdong Province, China (Figure 14) [63]. The isolated compounds are structurally related to the miniolutelide class of meroterpenoids and were identified as derivatives of miniolutelide B. Compounds 197 and 198 showed moderate cytotoxic activity against a panel of cancer cell lines including MDA-MB-435 (breast), HepG2 (liver), HCT-116 (colon) and A549 (lung) cells.
3. Conclusions
The isolation of terpenoids from marine-derived fungi with unique structures and interesting biological activity continued over the last five years with fascinating results. Different monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, triterpenes and meroterpenes were identified. Based on the summarized reports, a large library of sesquiterpenes was isolated possessing unique structures and potent biological activities including drimane, endoperoxides, nitrobenzoyl and bisabolane sesquiterpenes. Interestingly, changing the growth medium composition had a significant effect on the structures of the isolated sesquiterpenoids and the application of OSMAC strategy led to the isolation of novel compounds. Interesting diterpenes were also isolated such as the rare conidiogenone-type diterpenes and the potent inhibitor of platelet activating factor, phomactins. The conidiogenone-type exhibited the most potent cytotoxic activity against a large panel of cancer cell lines with conidiogenone C as the most potent congener showing IC50 values of 0.038 and 0.97 µM against HL-60 and BEL-7402 cells, respectively.
Unique triterpenes with polyoxygenated skeleton as well as 11-OH and 16-acetoxy functionalities were identified and found to possess potent antifungal activity. Meroterpenes with a unique sesquiterpenoid-γ-pyrone skeleton were purified and their chemopreventive activity represents an interesting topic for future research. The richness of marine-derived fungi with diverse terpenoids the feasibility of these organisms to be cultivated on different growth media offer a rare opportunity to expand the natural product chemical space with novel compounds possessing unprecedented chemical structures and biological activities.
Acknowledgments
Peter Proksch thanks German Ministry of Education and Research (BMBF) for its continued support of his research efforts. Ahmed M. Elissawy, Mohamed El-Shazly, Sherif S. Ebada, and AbdelNasser B. Singab acknowledge Egyptian Science and Technology Development Funds (STDF) for its financial support through grant No. 5251 entitled “Center for Drug Discovery and Development Research”.
Author Contributions
Ahmed M. Elissawy was responsible for writing the manuscript. Sherif S. Ebada collected literature data and drew the chemical structures in its final state. Mohamed El-Shazly was responsible for organizing the manuscript in accordance with the instructions for authors. AbdelNasser B. Singab and Peter Proksch performed the final editing of the manuscript before submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Proksch, P. Bioactive secondary metabolites from marine-derived fungi. In Marine Pharmacognosy: Trends and Applications, 1st ed.; Kim, S.-K., Ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2012; pp. 27–51. [Google Scholar]
- Ebel, R. Terpenes from marine-derived fungi. Mar. Drugs 2010, 8, 2340–2368. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.R.; Xia, X.K.; She, Z.G.; Lin, Y.C.; Vrijmoed, L.L.P.; Jones, E.B.G. A new chloro-monoterpene from the mangrove endophytic fungus Tryblidiopycnis sp. (4275). J. Asian Nat. Prod. Res. 2006, 8, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Edrada-Ebel, R.A.; Ebel, R.; Wang, Y.; Schulz, B.; Draeger, S.; Müller, W.E.G.; Wray, V.; Lin, W.H.; Proksch, P. Drimane sesquiterpenoids from the fungus Aspergillus ustus isolated from the marine sponge Suberites domuncula. J. Nat. Prod. 2009, 72, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Ayer, W.A.; Pena-Rodriguez, L.M. Metabolites produced by Alternaria brassicae, the black spot pathogen of Canola, Part 2, sesquiterpenoid metabolites. J. Nat. Prod. 1987, 50, 408–417. [Google Scholar] [CrossRef]
- Shiono, Y.; Hiramatsu, F.; Murayama, T.; Koseki, T.; Funakoshi, T.; Ueda, K.; Yasuda, H. Two drimane-type sesquiterepenes, strobilactones A and B, from the liquid culture of the edible mushroom Strobilurus ohshimae. Z. Naturforsch. B Chem. Sci. 2007, 62, 1585–1589. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Y.; Miao, C.; Liu, P.; Hong, K.; Zhu, W. Sesquiterpenoids and benzofuranoids from the marine-derived fungus Aspergillus ustus 094102. J. Nat. Prod. 2009, 72, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhu, H.; Fu, P.; Wang, Y.; Zhang, Z.; Lin, H.; Liu, P.; Zhuang, Y.; Hong, K.; Zhu, W. Cytotoxic polyphenols from the marine-derived fungus Penicillium expansum. J. Nat. Prod. 2010, 73, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Murakami, T.; Miyanishi, J.; Tanaka, K.; Takada, N.; Hashimoto, M. Isolation and the absolute stereochemistry of optically active sydonic acid from Glonium sp. (Hysteriales, Ascomycota). Biosci. Biotechnol. Biochem. 2009, 73, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Mülhaupt, T.; Kaspar, H.; Otto, S.; Reichert, M.; Bringmann, G.; Lindel, T. Isolation, structural elucidation, and synthesis of curcutetrol. Eur. J. Org. Chem. 2005, 2005, 334–341. [Google Scholar] [CrossRef]
- Wei, M.-Y.; Wang, C.-Y.; Liu, Q.-A.; Shao, C.-L.; She, Z.-G.; Lin, Y.-C. Five sesquiterenoids from a marine-derived fungus Aspergillus sp. isolated from a gogonian Dichotella gemmacea. Mar. Drugs 2010, 8, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-K.; Lu, X.-H.; Song, X.-H.; She, Z.-G.; Wu, X.-W.; An, L.-K.; Ye, C.-X.; Lin, Y.-C. The metabolites of mangrove endophytic fungus Zh6-B1 from the South China Sea. Bioorg. Med. Chem. Lett. 2010, 20, 3326–3328. [Google Scholar] [CrossRef] [PubMed]
- Elsebai, M.F.; Kehraus, S.; Gütschow, M.; König, G.M. Spartinoxide, a new enantiomer of A82775C with inhibitiory activity toward HLE from the marine-derived fungus Phaeosphaeria spatinae. Nat. Prod. Commun. 2010, 5, 1071–1076. [Google Scholar] [PubMed]
- Zhou, H.; Zhu, T.; Cai, S.; Gu, Q.; Li, D. Drimane sesquiterpenoids from the mangrove-derived fungus Aspergillus ustus. Chem. Pharm. Bull. 2011, 59, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Feng, X.; Xiao, Z.; Liu, L.; Li, H.; Ma, L.; Lu, Y.; Ju, J.; She, Z.; Lin, Y. Azaphilones and p-terphenyls from the mangrove endophytic fungus Penicillium chermesinum (Zh4-E2) isolated from the South China Sea. J. Nat. Prod. 2011, 74, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, H.; Shao, C.; Huang, H.; Jiang, J.; Zhu, X.; Liu, Y.; Liu, L.; Lu, Y.; Li, M.; et al. Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Chokpaiboon, S.; Sommit, D.; Bunyapaiboonsri, T.; Matsubara, K.; Pudhom, K. Antiangiogenic effect of chamigrane endoperoxides from a Thai mangrove-derived fungus. J. Nat. Prod. 2011, 74, 2290–2294. [Google Scholar] [CrossRef] [PubMed]
- Ingavat, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Asperaculin A, a sesquiterpenoid from a marine-derived fungus, Aspergillus aculeatus. J. Nat. Prod. 2011, 74, 1650–1652. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Koch, L.; Thu, K.M.; Rahamim; Aluma, Y.; Ilan, M.; Yarden, O.; Carmeli, S. Novel terpenoids of the fungus Aspergillus insuetus isolated from the Mediterranean sponge Psammaocinia sp. collected along the coast of Israel. Bioorg. Med. Chem. 2011, 19, 6587–6593. [Google Scholar] [CrossRef] [PubMed]
- Trisuwan, K.; Rukachaisirikul, V.; Kaewpet, M.; Phongpaichit, S.; Hutadilok-Towatana, N.; Preedanon, S.; Sakayaroj, J. Sesquiterpene and xanthone derivatives from the sea fan-derived fungus Aspergillus sydowii PSU-F154. J. Nat. Prod. 2011, 74, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, D.-L.; Tao, M.-H.; Dan, F.-J.; Zhang, W.-M. Two new sesquiterpenes from the marine fungus Eutypella scoparia FS26 from the South China Sea. Helv. Chim. Acta 2012, 95, 157–162. [Google Scholar] [CrossRef]
- Sun, L.-L.; Shao, C.-L.; Chen, J.-F.; Guo, Z.-Y.; Fu, X.-M.; Chen, M.; Chen, Y.-Y.; Li, R.; de Voogd, N.J.; She, Z.-G.; et al. New bisabolane sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from the sponge Xestospongia testudinaria. Bioorg. Med. Chem. Lett. 2012, 22, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.Y.; Wei, W.; Guo, Y.; Wang, T.; Jiao, R.H.; Ng, S.W.; Tan, R.X.; Ge, H.M. Sesquiterpenoids from the mangrove-derived endophytic fungus Diaporthe sp. J. Nat. Prod. 2012, 75, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Afiyatullov, S.S.; Denisenko, V.A.; Ermakova, S.P.; Slinkina, N.N.; Dmitrenok, P.S.; Kim, N.Y. Secondary metabolites from a marine-derived fungus Aspergillus carneus Blochwitz. Phytochemistry 2012, 80, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.; Dethoup, T.; Bessa, J.; Silva, A.M.S.; Kijjoa, A. A new bicyclic sesquiterpene from the marine sponge associated fungus Emericellopsis minima. Phytochem. Lett. 2012, 5, 68–70. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Huang, H.; Ma, L.; Wang, J.; Gu, Y.; Liu, L.; Lin, Y. Four eremophilane sesquiterpenes from the mangrove endophytic fungus Xylaria sp. BL321. Mar. Drugs 2012, 10, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.X.; Cheng, B.; Zhu, X.; Qiao, L.-T.; Wang, J.-J.; Gu, Y.-C.; Li, M.-F.; Liu, L.; Lin, Y.-C. Synthesis and cytotoxic evaluation of eremophilane sesquiterpene 07H239-A derivatives. Chem. Pharm. Bull. 2011, 59, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Hemberg, Y.; Xu, J.; Wray, V.; Proksch, P.; Wu, J.; Bringmann, G. Pestaliopens A and B: Stereochemically challenging flexible sesquiterpene-cyclopaldic acid hybrids from Pestalotiopsis sp. Chem. Eur. J. 2013, 19, 15556–15564. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Miao, F.-P.; Qiao, M.-F.; Cichewicz, R.H.; Ji, N.-Y. Terretonin, ophiobolin, and drimane terpenes with the absolute configurations from an algicolous Aspergillus ustus. RSC Adv. 2013, 3, 588–595. [Google Scholar] [CrossRef]
- Wu, G.; Lin, A.; Gu, Q.; Zhu, T.; Li, D. Four new chloro-eremophilane sesquiterpenes from an Antarctic deep-sea derived fungus, Penicillium sp. PR19N-1. Mar. Drugs 2013, 11, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Wu, G.; Gu, Q.; Zhu, T.; Li, D. New eremophilane-type sesquiterepenes from an Antarctic deep-sea derived fungus, Penicillium sp. PR19N-1. Arch. Pharm. Res. 2014, 37, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hua, X.-X.; Gong, T.; Pang, J.; Hou, Q.; Zhu, P. Hypocreaterpenes A and B, cadinane-type sesquiterpenes from a marine-derived fungus, Hypocreales sp. Phytochem. Lett. 2013, 6, 392–396. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Sun, M.; Li, M. Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233. Mar. Drugs 2013, 11, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Lin, X.; Zhou, X.; Wan, J.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.; Tu, Z.; et al. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus Aspergillus ochraceus Jcma1F17. Med. Chem. Commun. 2014, 5, 701–705. [Google Scholar] [CrossRef]
- Li, H.-J.; Jiang, W.-H.; Liang, W.-L.; Huang, J.-X.; Mo, Y.-F.; Ding, Y.-Q.; Lam, C.-K.; Qian, X.-J.; Zhu, X.-F.; Lan, W.-J. Induced marine fungus Chondrostereum sp. as a means of producing new sesquiterpenoids chondrosterins I and J by using glycerol as the carbon source. Mar. Drugs 2014, 12, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Li, D.-Y.; Li, Y.-C.; Hua, H.-M.; Ma, E.-L.; Li, Z.-L. Caryophyllene sesquiterpenes from the marine-derived fungus Ascotricha sp. ZJ-M-5 by the one strain-many compounds strategy. J. Nat. Prod. 2014, 77, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-B.; Gu, H.-G.; Zuo, W.-J.; Zhang, L.-L.; Bai, H.-J.; Guo, Z.-K.; Proksch, P.; Mei, W.-L.; Dai, H.-F. Two new sesquiterpenoids from endophytic fungus J3 isolated from mangrove plant Ceriops tagal. Arch. Pharm. Res. 2014. [Google Scholar] [CrossRef]
- Du, L.; Li, D.; Zhu, T.; Cai, S.; Wang, F.; Xiao, X.; Gu, Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Ishino, M.; Kiyomichi, N.; Takatori, K.; Sugita, T.; Shiro, M.; Kinoshita, K.; Takahashi, K.; Koyama, K. Phomactin I, 13-epi-phomactin I, and phomactin J, three novel diterpenes from a marine-derived fungus. Tetrahedron 2010, 66, 2594–2597. [Google Scholar] [CrossRef]
- Koyama, K.; Ishino, M.; Takatori, K.; Sugita, T.; Kinoshita, K.; Takahashi, K. Phomactin H, a novel diterpene from an unidentified marine-derived fungus. Tetrahedron Lett. 2004, 45, 6947–6948. [Google Scholar] [CrossRef]
- Ishino, M.; Kinoshita, K.; Takahashi, K.; Sugita, T.; Shiro, M.; Hasegawa, K.; Koyama, K. Phomactins K–M, three novel phomactin-type diterpenes from a marine-derived fungus. Tetrahedron 2012, 68, 8572–8576. [Google Scholar] [CrossRef]
- Tsukada, M.; Fukai, M.; Miki, K.; Shiraishi, T.; Suzuki, T.; Nishio, K.; Sugita, T.; Ishino, M.; Kinoshita, K.; Takahashi, K.; et al. Chemical constituents of a marine fungus Arthrinium sacchari. J. Nat. Prod. 2011, 74, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-C.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Libertellenones A–D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorg. Med. Chem. 2005, 13, 5267–5273. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Schulz, B.; Wray, V.; Totzke, F.; Kubbutat, M.H.G.; Müller, W.E.G.; Hamacher, A.; Kassack, M.U.; Lin, W.H.; Proksch, P. Arthrinins A–D: Novel diterpenoids and further constituents from the sponge derived fungus Arthrinium sp. Bioorg. Med. Chem. 2011, 19, 4644–4651. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-F.; Li, X.-M.; Meng, L.; Cui, C.-M.; Gao, S.-S.; Li, C.-S.; Huang, C.-G.; Wang, B.-G. Asperolides A–C, tetranorlabdene diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. J. Nat. Prod. 2012, 75, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, J.; Zhang, Y.; Wie, F.; Liu, X.; Jia, A.; Liu, C.; Li, W.; She, Z.; Lin, Y. Pimarane diterpenes from the fungus Epicoccum sp. HS-1 associated with Apostichopus japonicus. Bioorg. Med. Chem. Lett. 2012, 22, 3017–3019. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, D.; Tao, M.; Chem, Y.; Dan, F.; Zhang, W. Scopararanes C–G: New oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella scoparia FS26. Mar. Drugs 2012, 10, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Niikawa, H.; Kobayashi, M. Marine-derived fungal sesterterpenes, ophiobolins, inhibit biofilm formation of Mycobacterium species. J. Nat. Med. 2013, 67, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, H.; Li, H.; Sun, X.; Huang, H.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B from the culture of Aspergillus sp. 16-5c. Org. Lett. 2013, 15, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Huang, H.; Shao, C.; Xia, X.; Lin, M.; Huang, X.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenols A and B, new sesterterpenoids isolated from a mangrove endophytic fungus Aspergillus sp. 085242. Org. Lett. 2013, 15, 2522–2525. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Hong, K.; Chen, G.-D.; Wang, C.-X.; Tang, J.-S.; Yu, Y.; Jiang, M.-M.; Li, M.-M.; Wang, N.-L.; Yao, X.-S. New oxidized sterols from Aspergillus awamori and the endo-boat conformation adopted by the cyclohexene oxide system. Magn. Reson. Chem. 2010, 48, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.-M.; Li, X.-M.; Meng, L.; Li, C.-S.; Huang, C.-G.; Wang, B.-G. 7-Nor-ergosterolide, a pentalactone-containing norsteroid and related steroids from the marine-derived endophytic Aspegillus ochraceus EN-31. J. Nat. Prod. 2010, 73, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-S.; Li, X.-M.; Li, C.-S.; Proksch, P.; Wang, B.-G. Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum QEN-24S. Bioorg. Med. Chem. Lett. 2011, 21, 2894–2897. [Google Scholar] [CrossRef] [PubMed]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Kalinovsky, A.I.; Pivkin, M.V.; Menchinskaya, E.S.; Aminin, D.L. New metabolites from the marine-derived fungus Aspergillus fumigatus. Nat. Prod. Commun. 2012, 7, 497–500. [Google Scholar] [PubMed]
- Elsebai, M.F.; Kehraus, S.; König, G.M. Caught between triterpene- and steroid-metabolism: 4α-carboxylix pregnane-derivative from the marine alga-derived fungus Phaeosphaeria spartinae. Steroids 2013, 78, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.-W.; Cui, C.-B.; Li, C.-W.; Wu, C.-J. Three new and eleven known unusual C25 steroids: Activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulfate. Mar. Drugs 2014, 12, 1545–1568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.-M.; Shang, Z.; Li, C.-S.; Ji, N.-Y.; Wang, B.-G. Meroterpenoid and diphenyl ether derivatives from Penicillium sp. MA-37, a fungus isolated from marine mangrove rhizospheric soil. J. Nat. Prod. 2012, 75, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.-L.; Zheng, B.; Zhao, Y.-X.; Zhong, H.-M.; Chen, X.-L.W.; Zeng, Y.-B.; Dong, W.-H.; Huang, J.-L.; Proksch, P.; Dai, H.-F. Meroterpenes from endophytic fungus A1 of mangrove plant Scyphiphora hydrophyllacea. Mar. Drugs 2012, 10, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Lee, J.-H.; You, M.; Choi, T.-J.; Park, W.; Lee, S.K.; Oh, D.-C.; Oh, K.-B.; Shin, J. Penicillipyrones A and B, meroterpenoids from a marine-derived Penicillium sp. fungus. J. Nat. Prod. 2014, 77, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kurihara, Y.; Kanamoto, A.; Tomoda, H. Terretonin G, a new sesterterpenoid antibiotic from marine-derived Aspergillus sp. OPMF00272. J. Antibiot. 2014, 67, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Lin, Y.; Yuan, J.; Lu, Y.; Zhu, X.; Li, J.; Li, M.; Lin, Y.; He, J.; et al. Meroterpenes and azaphilones from marine mangrove endophytic fungus Penicillium 303#. Fitoterapia 2014, 97, 241–246. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
