Marine Structure Derived Calcium Phosphate–Polymer Biocomposites for Local Antibiotic Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Susceptibility Testing and Drug Encapsulation Efficiency (DEE)
2.1.2. Drug Loading to HAp

2.1.3. Tensile Strength and Elongation at Break of Composites

2.1.4. In-Vitro Gentamicin Release
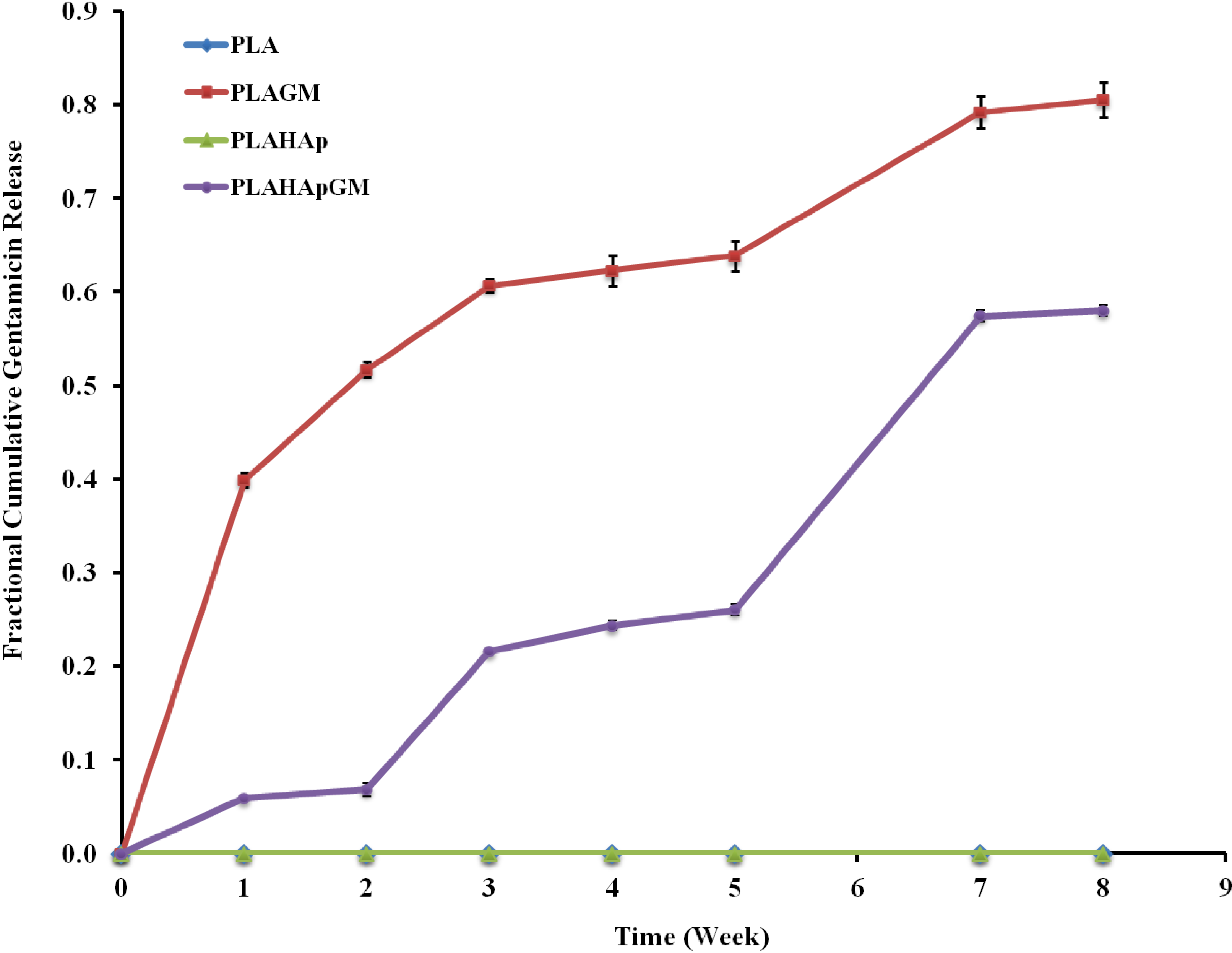
2.1.4.1. Release Kinetics—Model Dependent Method
| Model | Mathematical Expression | PLAGM | PLAHApGM | |
|---|---|---|---|---|
| Zero order | 0.440 | 0.937 | ||
| k0 | 0.125 | 0.070 | ||
| First order | 0.866 | 0.874 | ||
| k | 0.226 | 0.098 | ||
| Higuchi | 0.959 | 0.803 | ||
| a | 0.273 | 0.214 | ||
| Hixson- Crowell | 0.758 | 0.898 | ||
| k | 0.060 | 0.029 | ||
| Korsmeyer-Peppas | 0.992 | 0.962 | ||
| n | 0.282 | 1.315 | ||
| Baker Lonsdale | 0.945 | 0.727 | ||
| k | 0.107 | 0.038 | ||
| Reciprocal powered time | 0.971 | 0.835 | ||
| b | 0.744 | 1.037 | ||
| m | 1.524 | 17.190 |
2.1.4.2. Release Kinetics—Comparison of Drug Release Profiles
| PLAGM | PLAHApGM | |
|---|---|---|
| t50% (weeks) | 1.76 | 15.53 |
| f1 difference factor | 54.4 | |
| f2 similarity factor | 24.2 | |
2.1.5. Antibacterial Efficacy Test
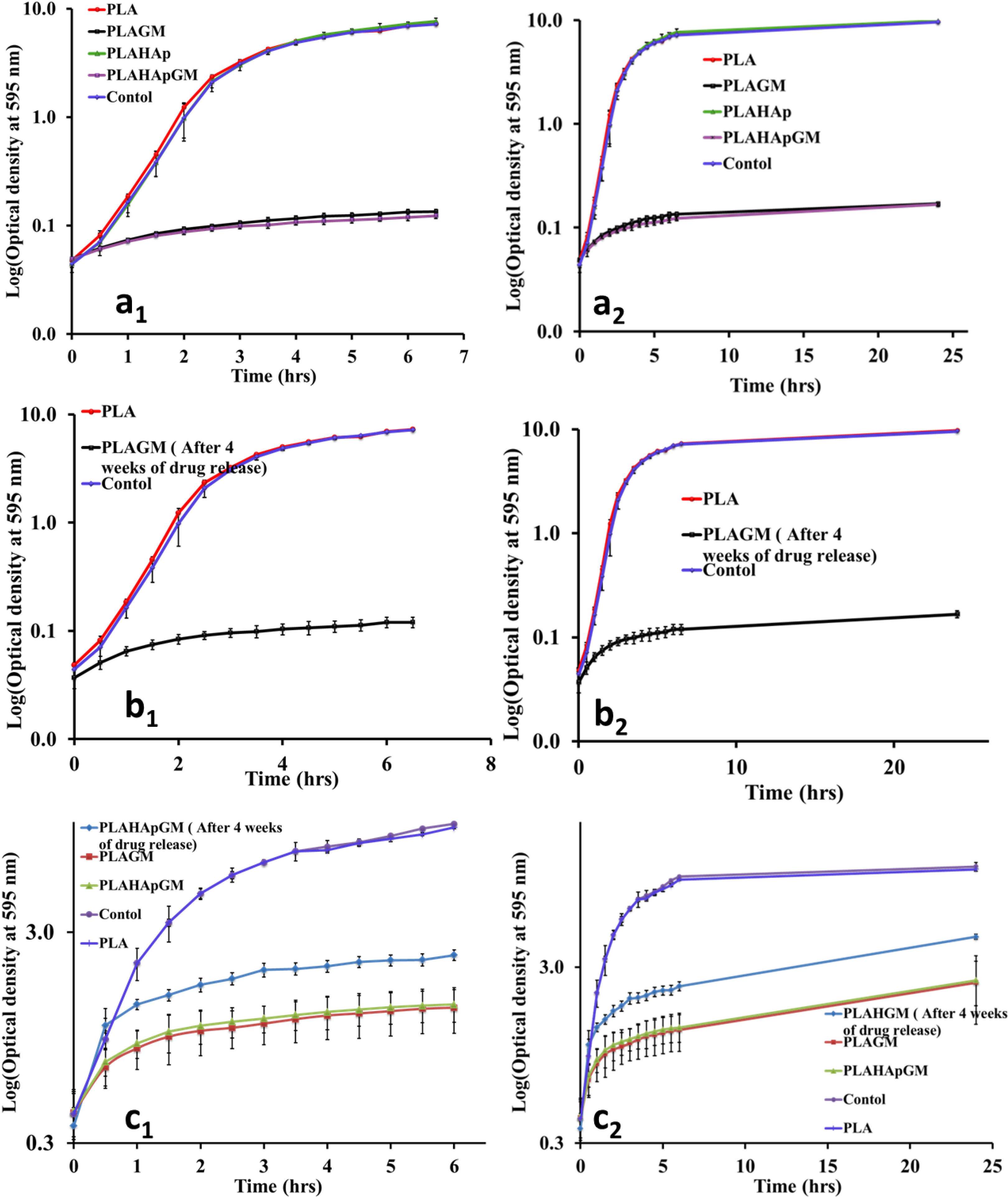
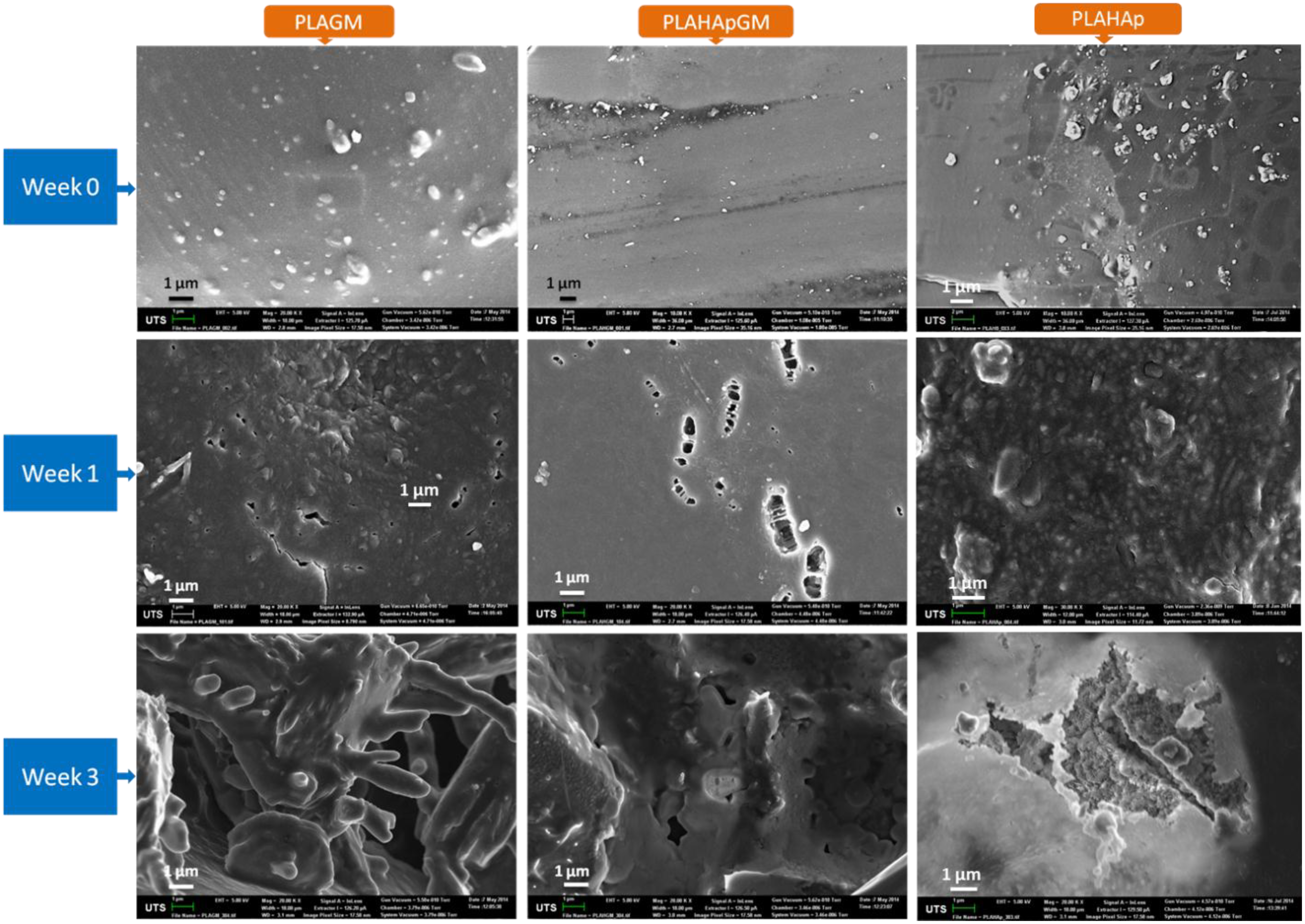
2.1.6. Morphology of Film Composites in Release Study
2.1.7. Statistical Analysis-Multivariate Approach (MANOVA)
2.2. Discussion
- Release of the drug contained on the exposed composite surface.
- Diffusion of the drug through the polymeric network and slow release of some part of drug contained into the surfaces of the HAp particles which is less accessible. The polymer plays a role of barrier that allows a slower release.
- Degradation/erosion and/or fragmentation of the polymeric composite which promotes accessibility to the “HAp-particle” porosity and facilitate release of gentamicin located inside porous network.
- Degradation of the HAp particles, which facilitates dissolution of Ca2+ and PO43− ions and exposure of further GM and dissolution by diffusion to the environment.
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Hydrothermal Conversion of Coral
3.2.2. Preparation of Film Composites
3.2.3. Minimal Inhibitory Concentration and Antimicrobial Efficacy
3.2.4. Drug Release and Release Kinetics
3.2.5. Morphology and Mechanical Properties
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control Release 2006, 113, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Habibe, A.F.; Maeda, L.D.; Souza, R.C.; Barboza, M.J.R.; Daguano, J.K.M.F.; Rogero, S.O.; Santos, C. Effect of bioglass additions on the sintering of Y-TZP bioceramics. Mater. Sci. Eng. C 2009, 29, 1959–1964. [Google Scholar] [CrossRef]
- Sivakumar, M.; Rao, K.P. Preparation, characterization and in vitro release of gentamicin from coralline hydroxyapatite-gelatin composite microspheres. Biomaterials 2002, 23, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, B. Natural bioceramics: From coral to bone and beyond. Curr. Opin. Solid State Mater. Sci. 2003, 7, 283–288. [Google Scholar] [CrossRef]
- Baro, M.; Sánchez, E.; Delgado, A.; Perera, A.; Evora, C. In vitro- in vivo characterization of gentamicin bone implants. J. Control Release 2002, 83, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Ben-Nissan, B.; Green, D.W.; Valenzuela, S.M.; Kohan, L. Targeting and dissolution characteristics of bone forming and antibacterial drugs by harnessing the structure of microspherical shells from coral beach sand. Adv. Eng. Mater. 2011, 13, 93–99. [Google Scholar] [CrossRef]
- Aviv, M.; Berdicevsky, I.; Zilberman, M. Gentamicin-loaded bioresorbable films for prevention of bacterial infections associated with orthopedic implants. J. Biomed. Mater. Res. Part A 2007, 83, 10–19. [Google Scholar] [CrossRef]
- Sampath, S.S.; Garvin, K.; Robinson, D.H. Preparation and characterization of biodegradable poly(l-lactic acid) gentamicin delivery systems. Int. J. Pharm. 1992, 78, 165–174. [Google Scholar] [CrossRef]
- Nelson, C.L.; Hickmon, S.G.; Skinner, R.A. Treatment of experimental osteomyelitis by surgical debridement and the implantation of bioerodable, polyanhydride-gentamicin beads. J. Orthop. Res. 1997, 15, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Macha, I.J.; Ben-Nissan, B.; Milthorpe, B. Improvement of elongation in nanosurface modified bioglass/pla thin film composites. Curr. Nanosci. 2014, 10, 200–204. [Google Scholar] [CrossRef]
- Mohammadi, G.; Barzegar-Jalali, M.; Valizadeh, H.; Nazemiyeh, H.; Barzegar-Jalali, A.; Siahi Shadbad, M.R.; Adibkia, K.; Zare, M. Reciprocal powered time model for release kinetic analysis of ibuprofen solid dispersions in oleaster powder, microcrystalline cellulose and crospovidone. J. Pharm. Pharm. Sci. 2010, 13, 152–161. [Google Scholar] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ford Versypt, A.N.; Pack, D.W.; Braatz, R.D. Mathematical modeling of drug delivery from autocatalytically degradable plga microspheres—A review. J. Controll. Release: Off. J. Controll. Release Soc. 2013, 165, 29–37. [Google Scholar]
- Buzarovska, A.; Grozdanov, A. Biodegradable poly(l-lactic acid)/TiO2 nanocomposites: Thermal properties and degradation. J. Appl. Polym. Sci. 2012, 123, 2187–2193. [Google Scholar] [CrossRef]
- Nelson, C.L.; McLaren, S.G.; Skinner, R.A.; Smeltzer, M.S.; Thomas, J.R.; Olsen, K.M. The treatment of experimental osteomyelitis by surgical debridement and the implantation of calcium sulfate tobramycin pellets. J. Orthop. Res. 2002, 20, 643–647. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; pp. 16–29. [Google Scholar]
- Chou, J.; Valenzuela, S.; Green, D.W.; Kohan, L.; Milthorpe, B.; Otsuka, M.; Ben-Nissan, B. Antibiotic delivery potential of nano- and micro-porous marine structure-derived beta-tricalcium phosphate spheres for medical applications. Nanomedicine (Lond.) 2014, 9, 1131–1139. [Google Scholar] [CrossRef]
- Hogan, J.; Shue, P.I.; Podczeck, F.; Newton, J.M. Investigations into the relationship between drug properties, filling, and the release of drugs from hard gelatin capsules using multivariate statistical analysis. Pharm. Res. 1996, 13, 944–949. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macha, I.J.; Cazalbou, S.; Ben-Nissan, B.; Harvey, K.L.; Milthorpe, B. Marine Structure Derived Calcium Phosphate–Polymer Biocomposites for Local Antibiotic Delivery. Mar. Drugs 2015, 13, 666-680. https://doi.org/10.3390/md13010666
Macha IJ, Cazalbou S, Ben-Nissan B, Harvey KL, Milthorpe B. Marine Structure Derived Calcium Phosphate–Polymer Biocomposites for Local Antibiotic Delivery. Marine Drugs. 2015; 13(1):666-680. https://doi.org/10.3390/md13010666
Chicago/Turabian StyleMacha, Innocent J., Sophie Cazalbou, Besim Ben-Nissan, Kate L. Harvey, and Bruce Milthorpe. 2015. "Marine Structure Derived Calcium Phosphate–Polymer Biocomposites for Local Antibiotic Delivery" Marine Drugs 13, no. 1: 666-680. https://doi.org/10.3390/md13010666
APA StyleMacha, I. J., Cazalbou, S., Ben-Nissan, B., Harvey, K. L., & Milthorpe, B. (2015). Marine Structure Derived Calcium Phosphate–Polymer Biocomposites for Local Antibiotic Delivery. Marine Drugs, 13(1), 666-680. https://doi.org/10.3390/md13010666








