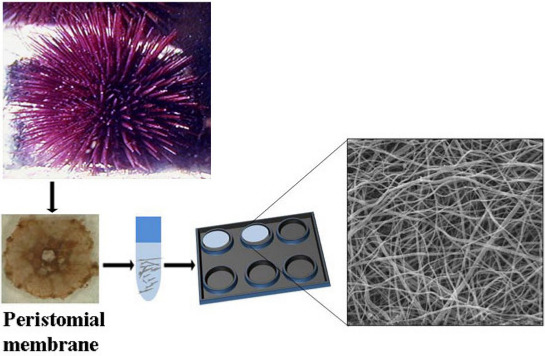
Production, Characterization and Biocompatibility of Marine Collagen Matrices from an Alternative and Sustainable Source: The Sea Urchin Paracentrotus lividus
Abstract
:1. Introduction

2. Results and Discussion
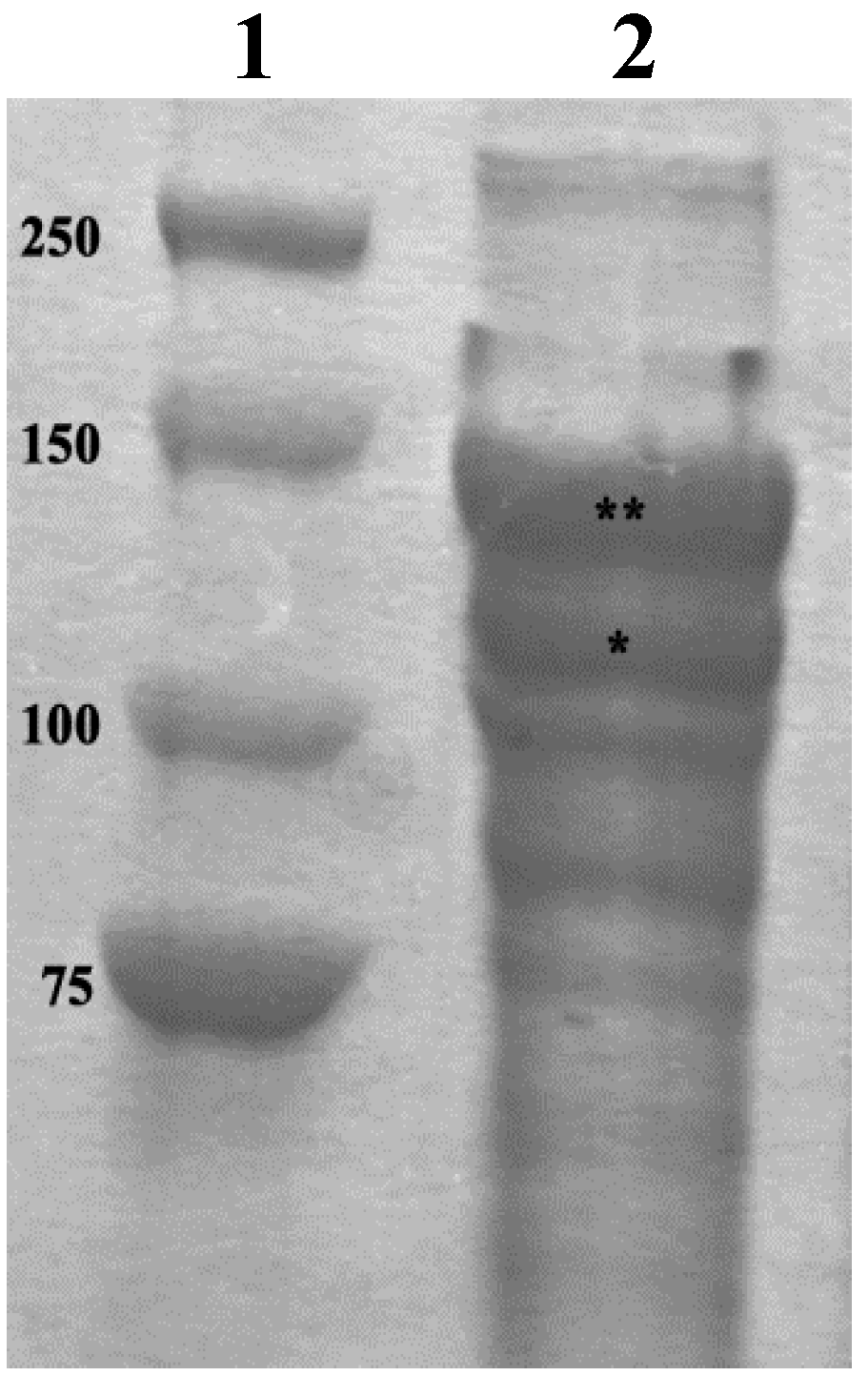
2.1. Collagen Extraction
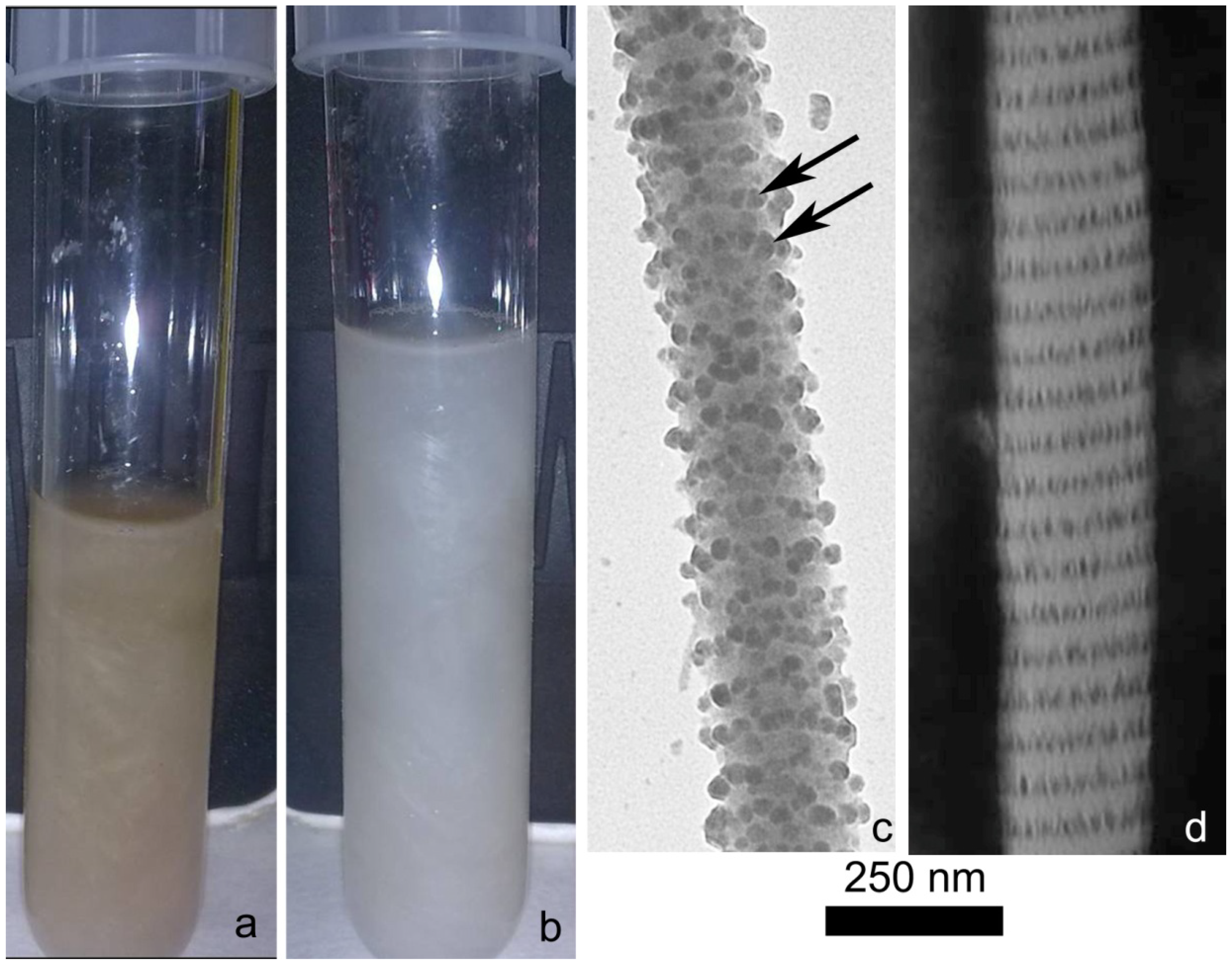
2.2. Collagen Matrix Production
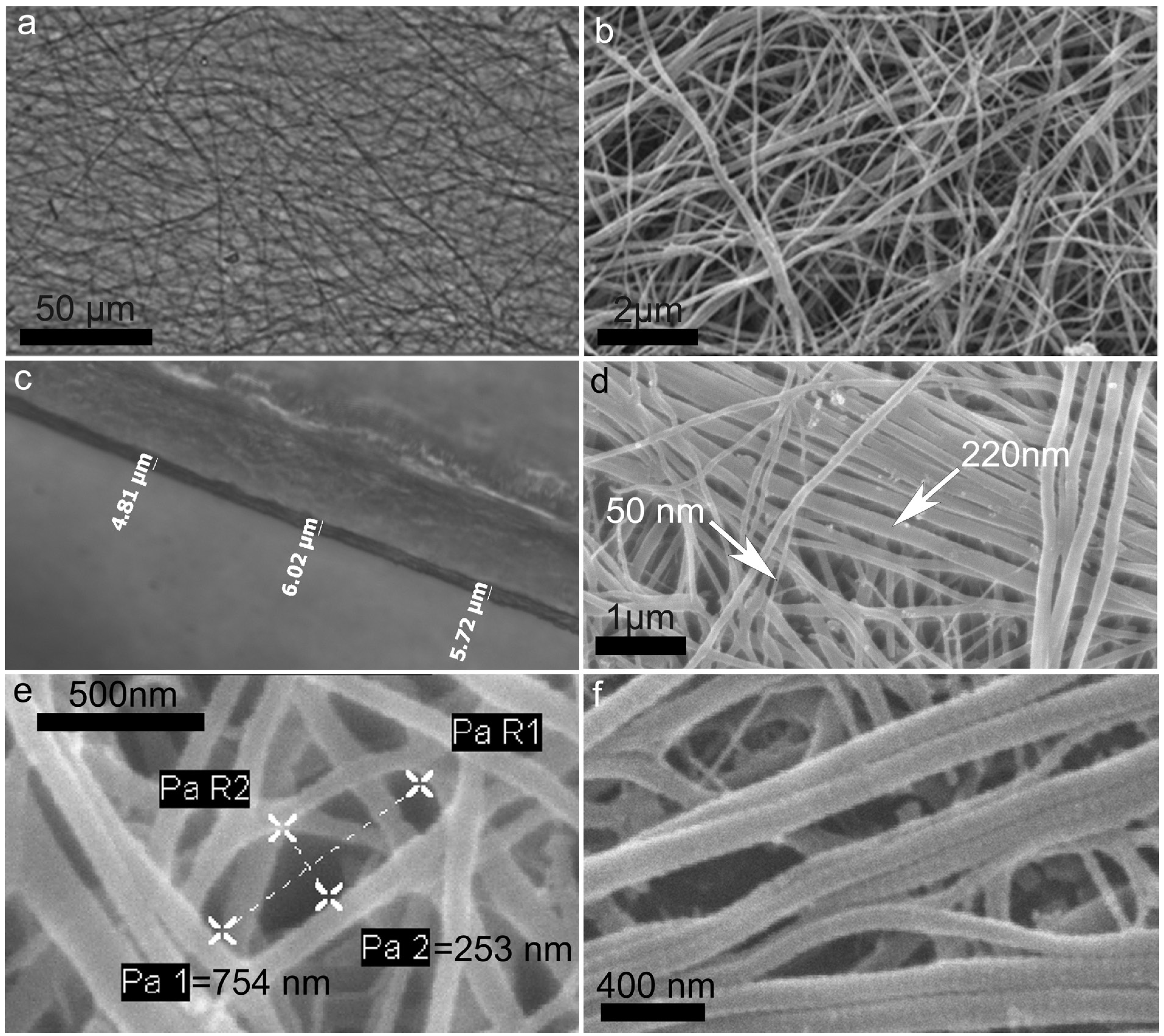
2.3. Characterization of Collagen Matrices
2.4. Biomechanics
2.4.1. Creep Tests
| Structure | Viscosity (MPa·s) | References |
|---|---|---|
| Sea urchin compass depressor ligament (P. lividus) | 560.6 ± 364.7 | [50] |
| Sea urchin spine joint ligament (Diadema setosum) | 20–5860 | [51] |
| Sea cucumber dermis (Stichopus japonicus) | 3.0 ± 5.6 | [52] |
| Sea cucumber dermis (Holothuria leucospita) | 11 | [52] |
| Sea cucumber dermis (Actinopyga echinites) | 100 | [52] |
| Sea cucumber dermis (Thyone inermis) | 5100 | [53] |
| Brittle star intervertebral ligament (Ophiocomina nigra) | 2260 ± 1940 | [54] |
| Feather star stalk (Cenocrinus asterius) | 16,700 | [55] |
| Human patellar tendon | 438.13 ± 232.2 | [56] |
2.4.2. Force-Extension Tests
| Structure | Stiffness (Elastic or Young’s Modulus) | References |
|---|---|---|
| Sea urchin compass depressor ligament (P. lividus) | 16.65 ± 8.93 (Mpa) | [50] |
| Sea urchin spine catch apparatus (Anthocidaris crassispina) | 90 ± 0.87 (Mpa) | [58] |
| Sea cucumber dermis (Actinopyga mauritiana) | 1 (Mpa) | [59] |
| Sea cucumber single native collagen fibril (C. frondosa) | 1–2 (Gpa) | [36] |
| Bovine single collagen fibril | 0.2–0.8 (Gpa) | [60] |
| Rat Achille’s tendon (Rattus norvegicus) | 310 (Mpa) | [61] |
| Pig liver | 6.9–34.7 (kPa) | [62] |
| Human skin | 98.97 ± 97 (Mpa) | [63] |
| Human cornea | 0.3–7 (Mpa) | [64] |
| Human articular cartilage (hip joints) | 1.816 ± 0.868 (Mpa) | [65] |
| Human cortical bone (femoral diaphysis) | 17.9 (Gpa) | [65] |
| Bovine Trabecular bone material | 0.76 ± 0.39 (Gpa) | [66] |
| EDC crosslinked bovine collagen | 31 ± 4.4 (Mpa) | [67] |
| Soluble rat tail collagen (1–3 mg/mL) | 1–28 (kPa) | [68] |
| PCL/collagen scaffold crosslinked with glutaraldheyde | 11 (Mpa) | [69] |
| Sea Urchin (P. lividus) Collagen Matrices (SCM) | ||
|---|---|---|
| Mechanical Properties | Mean ± SD | n |
| Viscosity | 60.98 ± 52.07 GPa·s | 36 |
| Breaking load | 17± 2.8 MPa | |
| Stiffness (Elastic or Young’s modulus) | 146 ± 48 MPa | 19 |
| Tensile strength | 44.58 ± 9.56 MPa | |
| Tensile strain | 32.3% ± 5.8% | |
2.5. In Vitro Biocompatibility
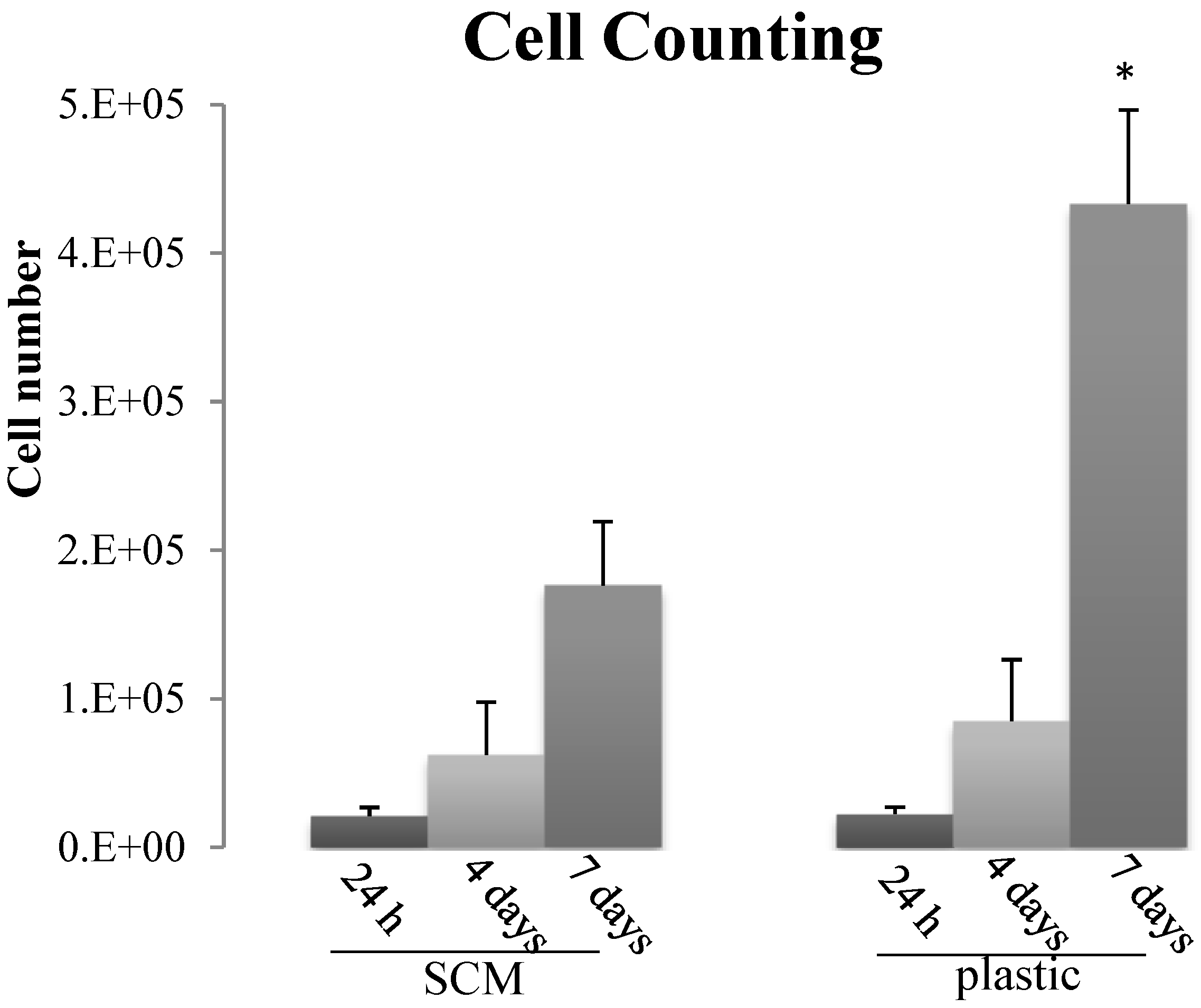

3. Experimental Section
3.1. Extraction of Collagen from the PM of P. lividus
3.2. Ultrastructural Analysis of Isolated Collagen Fibrils
3.3. Cuprolinic Blue Staining for GAG Visualization in Isolated Collagen Fibrils
3.4. SDS-PAGE Analyses of the Collagen Suspension
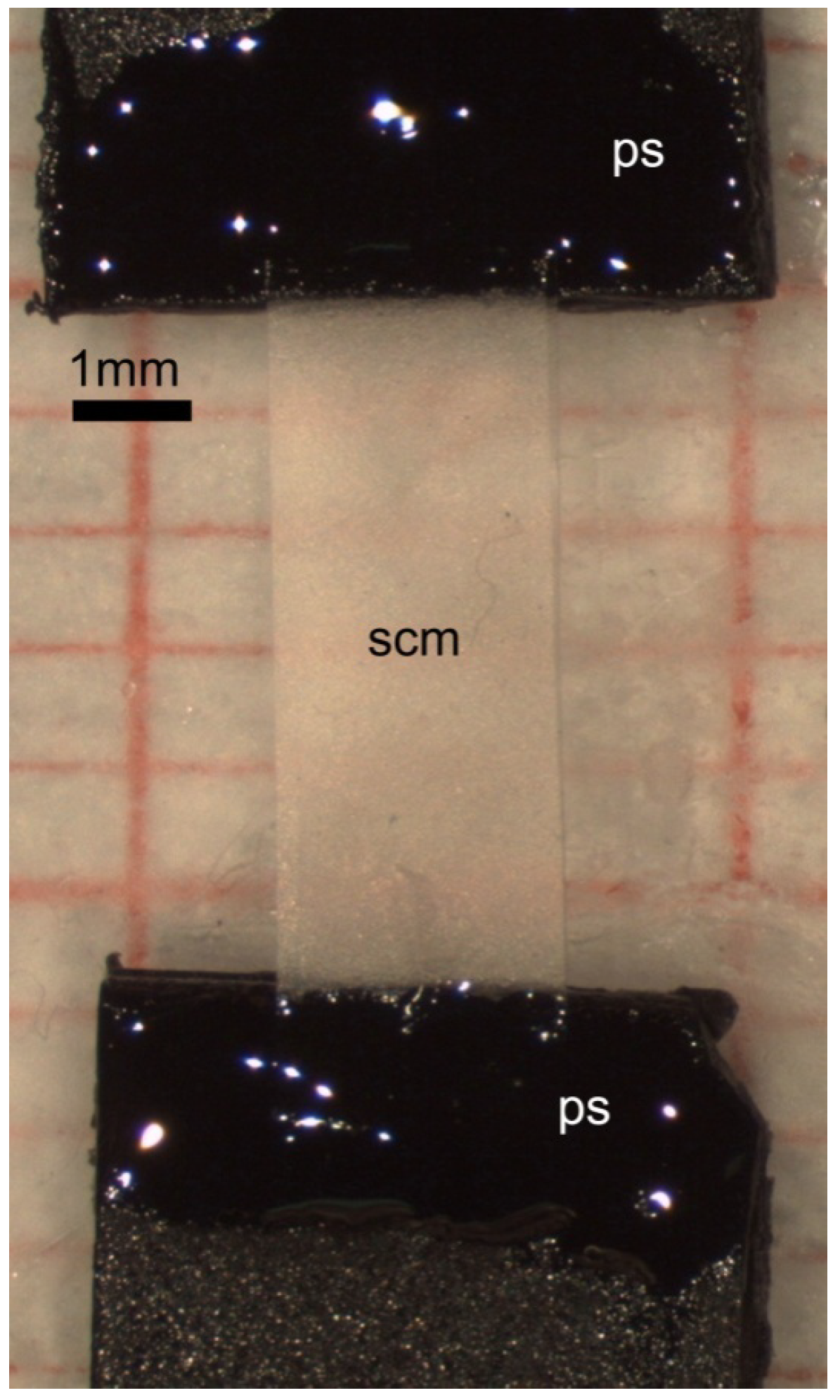
3.5. Production of SCM for Cell Cultures
3.6. Production of SCM for Mechanical Tests
3.7. Scanning Electron Microscopy (SEM)
3.8. Mechanical Tests
3.8.1. Creep Tests
3.8.2. Force-Extension Test
3.9. Biocompatibility
3.9.1. Mesenchymal Stromal Cell Cultures
3.9.2. Cell Counting
3.9.3. Proliferation Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barnes, C.P.; Pemble, C.W.; Brand, D.D.; Simpson, D.G.; Bowlin, G.L. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007, 13, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Stamov, D.R.; Pompe, T. Structure and function of ecm-inspired composite collagen type I scaffolds. Soft Matter 2012, 8, 10200–10212. [Google Scholar] [CrossRef]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Arias-Moscoso, J.L.; Soto-Valdez, H.; Plascencia-Jatomea, M.; Vidal-Quintanar, R.L.; Rouzaud-Sández, O.; Ezquerra-Brauer, J.M. Composites of chitosan with acid-soluble collagen from jumbo squid (Dosidicus gigas) by-products. Polym. Int. 2011, 60, 924–931. [Google Scholar] [CrossRef]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O. Natural marine sponge fiber skeleton: A biomimetic scaffold for human osteoprogenitor cell attachment, growth, and differentiation. Tissue Eng. 2003, 9, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Chitin and Collagen as Universal and Alternative Templates in Biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Ehrlich, H.; Deutzmann, R.; Capellini, E.; Koon, H.; Solazzo, C.; Yang, Y.; Ashford, D.; Thomas-Oates, J.; Lubeck, M.; Baessmann, C.; et al. Mineralization of the Meter-long Biosilica Structures of Glass Sponges is template on Hydroxylated Collagen. Nat. Chem. 2010, 2, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Guille, M.M.; Besseau, L.; Chopin, C.; Durand, P.; Herbage, D. Structural aspects of fish skin collagen which forms ordered arrays via liquid crystalline states. Biomaterials 2000, 21, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yamashita, E.; Taniguchi, K.; Kanamori, N.; Suzuki, N. Isolation and characterisation of collagen from the outer skin waste material of cuttlefish (Sepia lycidas). Food Chem. 2001, 72, 425–429. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Carnevali, M.D.C.; Andrietti, F. Microarchitecture and mechanics of the sea-urchin peristomial membrane. Ital. J. Zool. 1994, 61, 39–51. [Google Scholar]
- Bonasoro, F.; Carnevali, M.D.C.; Wilkie, I.C. The peristomial membrane of regular sea-urchins—Functional-morphology of the epidermis and coelomic lining in Paracentrotus lividus (Lamarck). Ital. J. Zool. 1995, 62, 121–135. [Google Scholar]
- Wilkie, I.C. Mutable collagenous tissue: overview and biotechnological perspective. Prog. Mol. Subcell. Biol. 2005, 39, 221–250. [Google Scholar] [PubMed]
- Barbaglio, A.; Department of Biosciences, University of Milan, Italy. The PM collagen is similar to the mammalian type-I, 2014. personal communication.
- Ribeiro, A.R.; Barbaglio, A.; Benedetto, C.D.; Ribeiro, C.C.; Wilkie, I.C.; Carnevali, M.D.C.; Barbosa, M.A. New insights into mutable collagenous tissue: Correlations between the microstructure and mechanical state of a sea-urchin ligament. PLos One 2011, 6, e24822. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, C.F.; Verlaque, M. Paracentrotus lividus. Dev. Aquac. Fish Sci. 2013, 38, 297–327. [Google Scholar]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bonnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Matsiko, A.; Haugh, M.G.; Gleeson, J.P.; O’Brien, F.J. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen-glycosaminoglycan scaffolds. J. Mech. Behav. Biomed. 2012, 11, 53–62. [Google Scholar] [CrossRef]
- Matsumura, T. Collagen fibrils of the sea cucumber, Stichopus japonicus: Purification and morphological study. Connect. Tissue Res. 1974, 2, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Trotter, J.A.; Koob, T.J. Collagen and proteoglycan in a sea urchin ligament with mutable mechanical properties. Cell Tissue Res. 1989, 258, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Barbaglio, A.; Tricarico, S.; Ribeiro, A.; Ribeiro, C.; Sugni, M.; Di Benedetto, C.; Wilkie, I.; Barbosa, M.; Bonasoro, F.; Candia Carnevali, M.D. The mechanically adaptive connective tissue of echinoderms: Its potential for bio-innovation in applied technology and ecology. Mar. Environ. Res. 2012, 76, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.T.; Tona, A.; Woodward, J.T.; Jones, P.L.; Plant, A.L. Thin films of collagen affect smooth muscle cell morphology. Langmuir 2003, 19, 1506–1514. [Google Scholar] [CrossRef]
- Chung, K.H.; Bhadriraju, K.; Spurlin, T.A.; Cook, R.F.; Plant, A.L. Nanomechanical properties of thin films of type I collagen fibrils. Langmuir 2010, 26, 3629–3636. [Google Scholar] [CrossRef] [PubMed]
- Steven, F.S.; Jackson, D.S.; Schofield, J.D.; Bard, J.B. Polymeric collagen isolated from the human intestinal submucosa. Gut 1969, 10, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Tajima, S.; Nagai, Y. Isolation and partial characterization of collagen fibrils, fibers and fiber-bundles from insoluble calf dermis. Connect. Tissue Res. 1980, 7, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.S.; Birtles, M.J.; Conway, J.F.; Parry, D.A.D. An estimate of the mean length of collagen fibrils in rat tail-tendon as a function of age. Connect. Tissue Res. 1989, 19, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Birk, D.E.; Hahn, R.A.; Linsenmayer, C.Y.; Zycband, E.I. Characterization of collagen fibril segments from chicken embryo cornea, dermis and tendon. Matrix Biol. 1996, 15, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Friess, W.; Lee, G. Basic thermoanalytical studies of insoluble collagen matrices. Biomaterials 1996, 17, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- De Vente, J.E.; Lester, G.E.; Trotter, J.A.; Dahners, L.E. Isolation of intact collagen fibrils from healing ligament. J. Electron Microscopy 1997, 46, 353–356. [Google Scholar]
- White, J.F.; Werkmeister, J.A.; Darby, I.A.; Bisucci, T.; Birk, D.E.; Ramshaw, J.A.M. Collagen fibril formation in a wound healing model. J. Struct. Biol. 2002, 137, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Crabb, R.A.B.; Chau, E.P.; Evans, M.C.; Barocas, V.H.; Hubel, A. Biomechanical and microstructural characteristics of a collagen film-based corneal stroma equivalent. Tissue Eng. 2006, 12, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Bozec, L.; van der Heijden, G.; Horton, M. Collagen fibrils: Nanoscale ropes. Biophys. J. 2007, 92, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Amyot, F.; Small, A.; Boukari, H.; Sackett, D.; Elliott, J.; McDaniel, D.; Plant, A.; Gandjbakhche, A. Thin films of oriented collagen fibrils for cell motility studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, F.A.; Trotter, J.A. Morphology and biomechanics of the microfibrillar network of sea cucumber dermis. J. Exp. Biol. 1996, 199, 1817–1828. [Google Scholar] [PubMed]
- Omura, Y.; Urano, N.; Kimura, S. Occurrence of fibrillar collagen with structure of (α1)2α2 in the test of sea urchin Asthenosoma ijimai. Comp. Biochem. Phys. B 1996, 115, 63–68. [Google Scholar] [CrossRef]
- Cluzel, C.; Lethias, C.; Garrone, R.; Exposito, J.Y. Distinct maturations of n-propeptide domains in fibrillar procollagen molecules involved in the formation of heterotypic fibrils in adult sea urchin collagenous tissues. J. Biol. Chem. 2004, 279, 9811–9817. [Google Scholar] [CrossRef] [PubMed]
- Heim, A.J.; Matthews, W.G.; Koob, T.J. Determination of the elastic modulus of native collagen fibrils via radial indentation. Appl. Phys. Lett. 2006, 89, 181902. [Google Scholar] [CrossRef]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef] [PubMed]
- Damink, L.H.H.O.; Dijkstra, P.J.; vanLuyn, M.J.A.; vanWachem, P.B.; Nieuwenhuis, P.; Feijen, J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials 1996, 17, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Scotchford, C.A.; Cascone, M.G.; Downes, S.; Giusti, P. Osteoblast responses to collagen-pva bioartificial polymers in vitro: The effects of cross-linking method and collagen content. Biomaterials 1998, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.R. Enhanced physicochemical properties of collagen by using EDC/NHS-crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamaoka, H.; Nishizawa, S.; Nagata, S.; Ogasawara, T.; Asawa, Y.; Fujihara, Y.; Takato, T.; Hoshi, K. The optimization of porous polymeric scaffolds for chondrocyte/atelocollagen based tissue-engineered cartilage. Biomaterials 2010, 31, 4506–4516. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, G.; Selvakumar, R.; Sastry, T.P.; Mandal, A.B.; Doble, M. Effect of d-amino acids on collagen fibrillar assembly and stability: Experimental and modelling studies. Biochem. Eng. J. 2013, 75, 92–100. [Google Scholar] [CrossRef]
- Di Benedetto, C. Progenitor Cells and Regenerative Potential in Echinoderms: An in Vivo and in Vitro Approach; Lambert Academic Publishing: Saarbrucken, Germany, 2011. [Google Scholar]
- Tricarico, S.; Barbaglio, A.; Burlini, N.; Del Giacco, L.; Ghilardi, A.; Sugni, M.; di Benedetto, C.; Bonasoro, F.; Wilkie, I.; Candia Carnevali, M.D. New insights into the mutable collagenous tissue of Paracentrotus lividus: Preliminary results. Zoosymposia 2012, 7, 7. [Google Scholar]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Kinoshita, T.; Izumi, S.; Tomino, S.; Yoshizato, K. ; Characterizations of sea urchin fibrillar collagen and its cDNA clone. Biochim. Biophys. Acta 1994, 1217, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Mayne, J.; Robinson, J.J. Comparative analysis of the structure and thermal stability of sea urchin peristome and rat tail tendon collagen. J. Cell. Biochem. 2002, 84, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, I.C.; School of Biological and Biomedical Sciences, Glasgow Caledonian University, Glasgow, Scotland. The viscosity of the sea urchin compass depressor ligament (P. Lividus) is 560.6 ± 364.7 (MPa•s). The stiffness of the sea urchin compass depressor ligament (P. Lividus) is 16.65 ± 8.93 (Mpa). personal communication, 2014. [Google Scholar]
- Motokawa, T. Mechanical properties and structure of the spine-joint central ligament of the sea urchin, Diadema setosum (echinodermata, echinoidea). J. Zool. 1983, 201, 223–235. [Google Scholar] [CrossRef]
- Motokawa, T. Connective tissue catch in echinoderms. Biol. Rev. 1984, 59, 255–270. [Google Scholar] [CrossRef]
- Eylers, J.P. Ion-dependent viscosity of holothurian body wall and its implications for the functional morphology of echinoderms. J. Exp. Biol. 1982, 99, 1–8. [Google Scholar]
- Wilkie, I. Design for disaster: The ophiuroid intervertebral ligament as a typical mutable collagenous structure. In Echinoderm Biology; Burke, R.D., Mladenov, P.V., Lambert, P., Parsley, R.L., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1988; pp. 25–38. [Google Scholar]
- Baumiller, T.K.; Labarbera, M. Mechanical properties of the stalk and cirri of the sea lily Cenocrinus asterius. Comparative Biochem. Physiol. Part A Physiol. 1993, 106, 91–95. [Google Scholar] [CrossRef]
- Pioletti, D.P.; Rakotomanana, L.R.; Benvenuti, J.F.; Leyvraz, P.F. Viscoelastic constitutive law in large deformations: Application to human knee ligaments and tendons. J. Biomech. 1998, 31, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.O.; Lin, C.W.; Sheu, M.T. Diffusion characteristics of collagen film. J. Control. Release Off. J. Control. Release Soc. 2001, 77, 97–105. [Google Scholar] [CrossRef]
- Hidaka, M. Effects of certain physico-chemical agents on the mechanical properties of the catch apparatus of the sea-urchin spine. J. Exp. Biol. 1983, 103, 15. [Google Scholar]
- Motokawa, T.; Tsuchi, A. Dynamic mechanical properties of body-wall dermis in various mechanical states and their implications for the behavior of sea cucumbers. Biol. Bull. 2003, 205, 261–275. [Google Scholar] [CrossRef] [PubMed]
- van der Rijt, J.A.; van der Werf, K.O.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Micromechanical testing of individual collagen fibrils. Macromol. Biosci. 2006, 6, 697–702. [Google Scholar]
- Levental, I.; Georges, P.C.; Janmey, P.A. Soft biological materials and their impact on cell function. Soft Matter 2007, 3, 299–306. [Google Scholar] [CrossRef]
- Kerdok, A.E.; Ottensmeyer, M.P.; Howe, R.D. Effects of perfusion on the viscoelastic characteristics of liver. J. Biomech. 2006, 39, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.J.; Ní Annaidh, A.; Bruyere, K.; Otténio, M.; Xie, H.; Gilchrist, M.D. Dynamic tensile properties of human skin. In Proceedings of Ircobi Conference, Dublin, Ireland, 12–14 September 2012; International Research Council on the Biomechanics of Injury: Dublin, Ireland, 2012; pp. 494–502. [Google Scholar]
- Duan, X.; Sheardown, H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: Mechanical properties and corneal epithelial cell interactions. Biomaterials 2006, 27, 4608–4617. [Google Scholar] [CrossRef] [PubMed]
- Brandl, F.; Sommer, F.; Goepferich, A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials 2007, 28, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.D.; Williams, J.L. Tensile testing of rodlike trabeculae excised from bovine femoral bone. J. Biomech. 1989, 22, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and composition influence the surface properties, mechanical stiffness and cell reactivity of collagen-based films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, M.D.; Beebe, D.J.; Crone, W.C. Mechanical interactions of mouse mammary gland cells with collagen in a three-dimensional construct. Ann. Biomed. Eng. 2010, 38, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Criswell, T.; Sapoznik, E.; Lee, S.; Soker, S. The influence of cross-linking methods on the mechanical and biocompatible properties of vascular scaffold. J. Sci. Appl. Biomed. 2013, 1, 1–7. [Google Scholar]
- Martinello, T.; Bronzini, I.; Maccatrozzo, L.; Iacopetti, I.; Sampaolesi, M.; Mascarello, F.; Patruno, M. Cryopreservation does not affect the stem characteristics of multipotent cells isolated from equine peripheral blood. Tissue Eng. Part C Methods 2010, 16, 771–781. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Benedetto, C.D.; Barbaglio, A.; Martinello, T.; Alongi, V.; Fassini, D.; Cullorà, E.; Patruno, M.; Bonasoro, F.; Barbosa, M.A.; Carnevali, M.D.C.; et al. Production, Characterization and Biocompatibility of Marine Collagen Matrices from an Alternative and Sustainable Source: The Sea Urchin Paracentrotus lividus. Mar. Drugs 2014, 12, 4912-4933. https://doi.org/10.3390/md12094912
Benedetto CD, Barbaglio A, Martinello T, Alongi V, Fassini D, Cullorà E, Patruno M, Bonasoro F, Barbosa MA, Carnevali MDC, et al. Production, Characterization and Biocompatibility of Marine Collagen Matrices from an Alternative and Sustainable Source: The Sea Urchin Paracentrotus lividus. Marine Drugs. 2014; 12(9):4912-4933. https://doi.org/10.3390/md12094912
Chicago/Turabian StyleBenedetto, Cristiano Di, Alice Barbaglio, Tiziana Martinello, Valentina Alongi, Dario Fassini, Emanuele Cullorà, Marco Patruno, Francesco Bonasoro, Mario Adolfo Barbosa, Maria Daniela Candia Carnevali, and et al. 2014. "Production, Characterization and Biocompatibility of Marine Collagen Matrices from an Alternative and Sustainable Source: The Sea Urchin Paracentrotus lividus" Marine Drugs 12, no. 9: 4912-4933. https://doi.org/10.3390/md12094912
APA StyleBenedetto, C. D., Barbaglio, A., Martinello, T., Alongi, V., Fassini, D., Cullorà, E., Patruno, M., Bonasoro, F., Barbosa, M. A., Carnevali, M. D. C., & Sugni, M. (2014). Production, Characterization and Biocompatibility of Marine Collagen Matrices from an Alternative and Sustainable Source: The Sea Urchin Paracentrotus lividus. Marine Drugs, 12(9), 4912-4933. https://doi.org/10.3390/md12094912