Identification of Marine Neuroactive Molecules in Behaviour-Based Screens in the Larval Zebrafish
Abstract
:1. Introduction
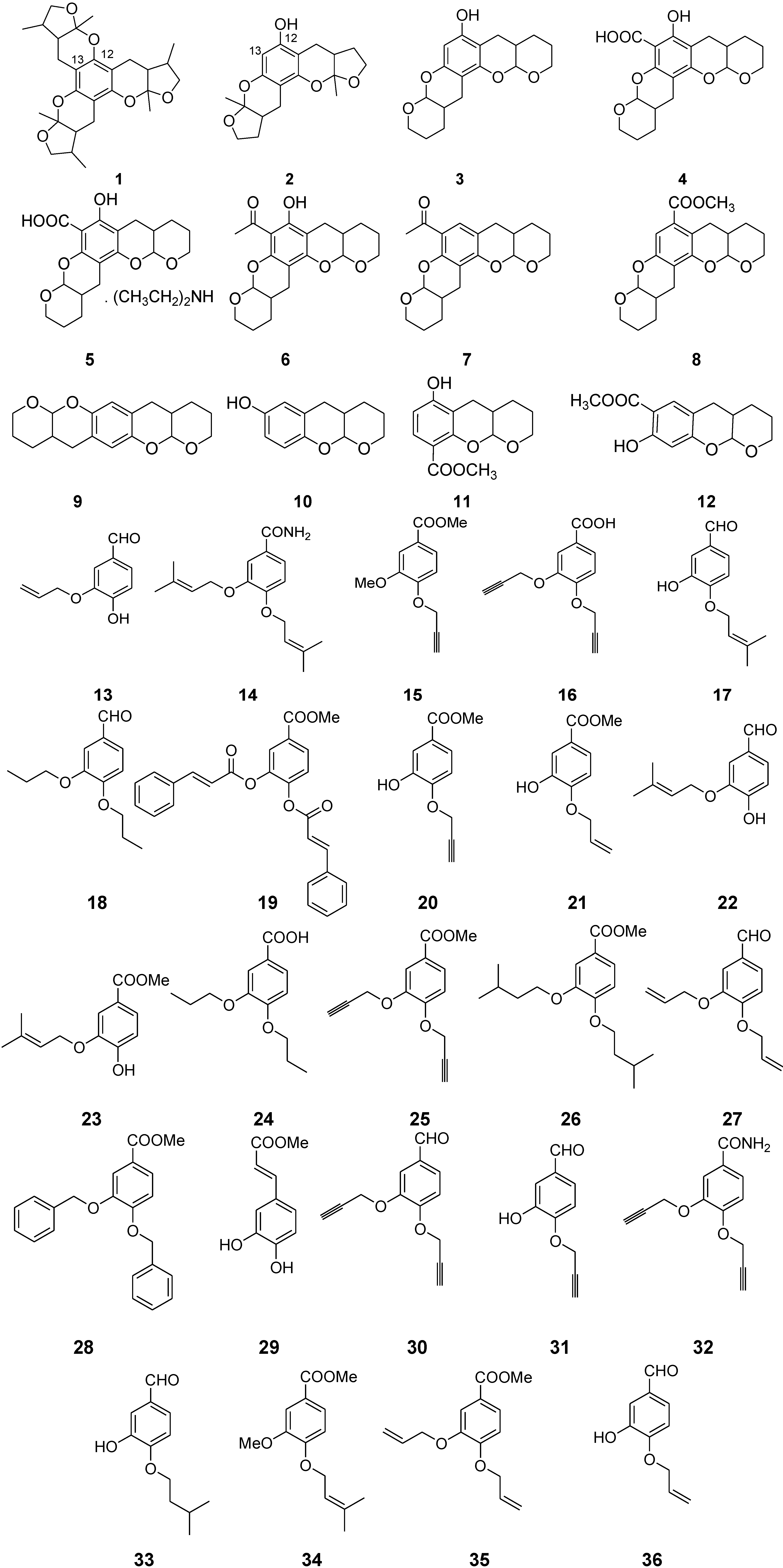
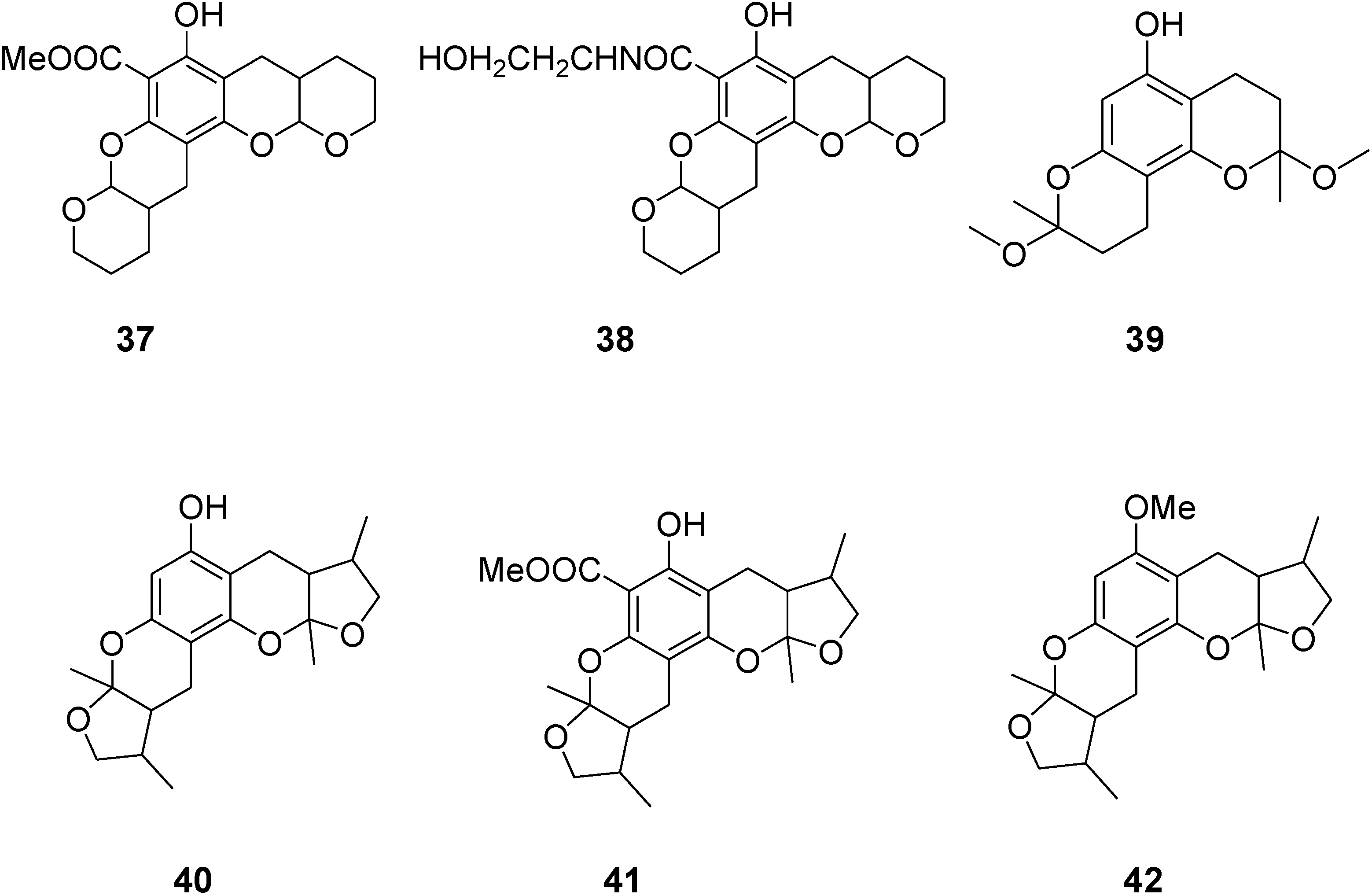
2. Results and Discussion
2.1. Chemistry
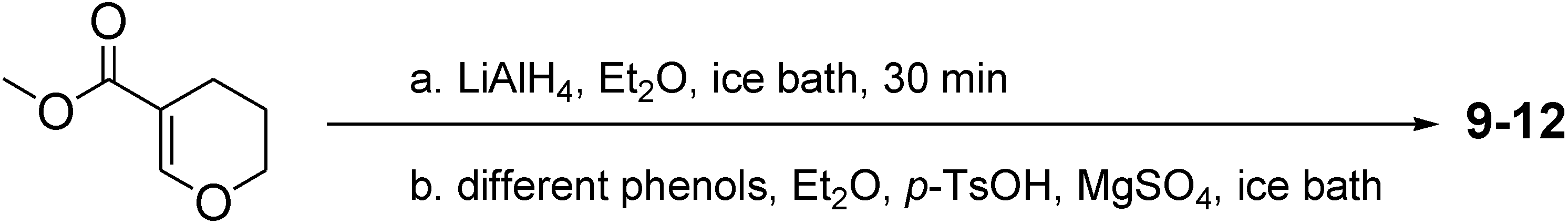
2.2. Neuroactive Activity of 36 Compounds were Evaluated in Zebrafish Behavioral Assay
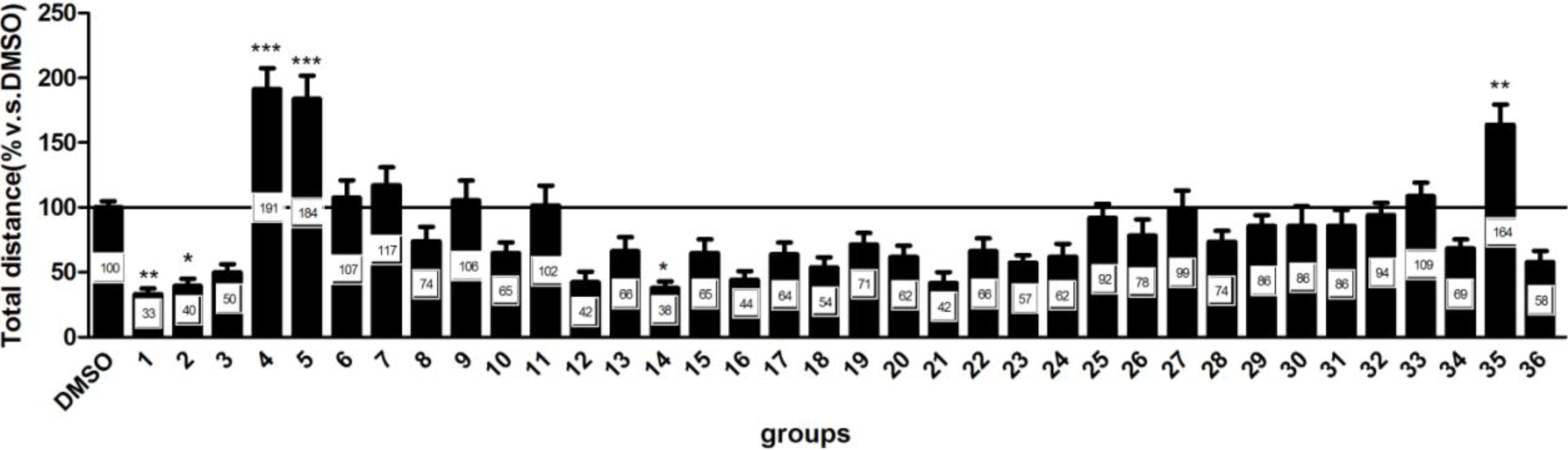
2.3. Neuroactive Compounds Exhibited Different Behavioral Patterns
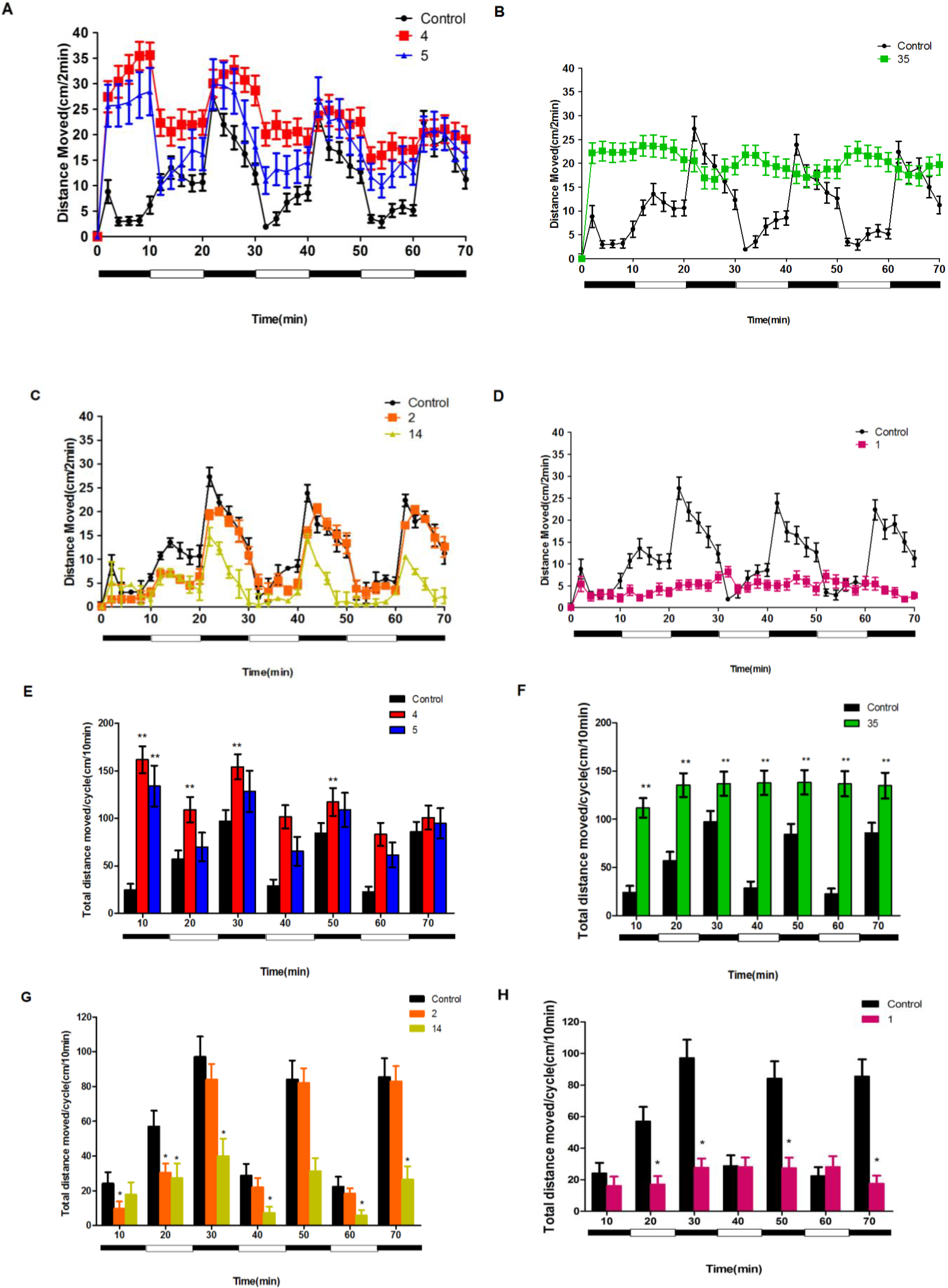
2.4. Compounds 1 and 37–41 Suppressed Locomotor Activity in Larval Zebrafish
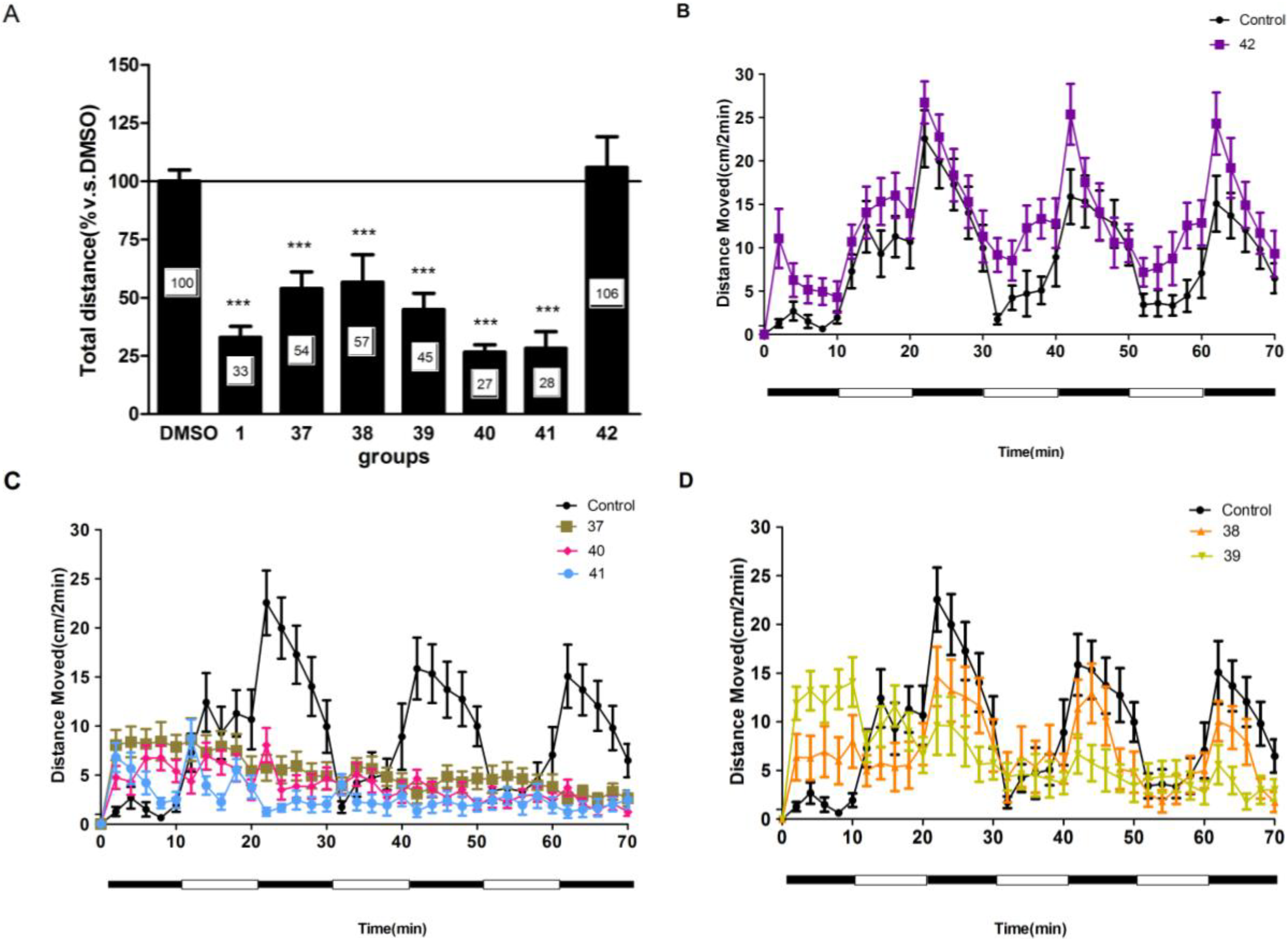
2.5. The Potential of Selected Compounds for New Antiepileptic Drug Development in PTZ-Induced Seizure Model
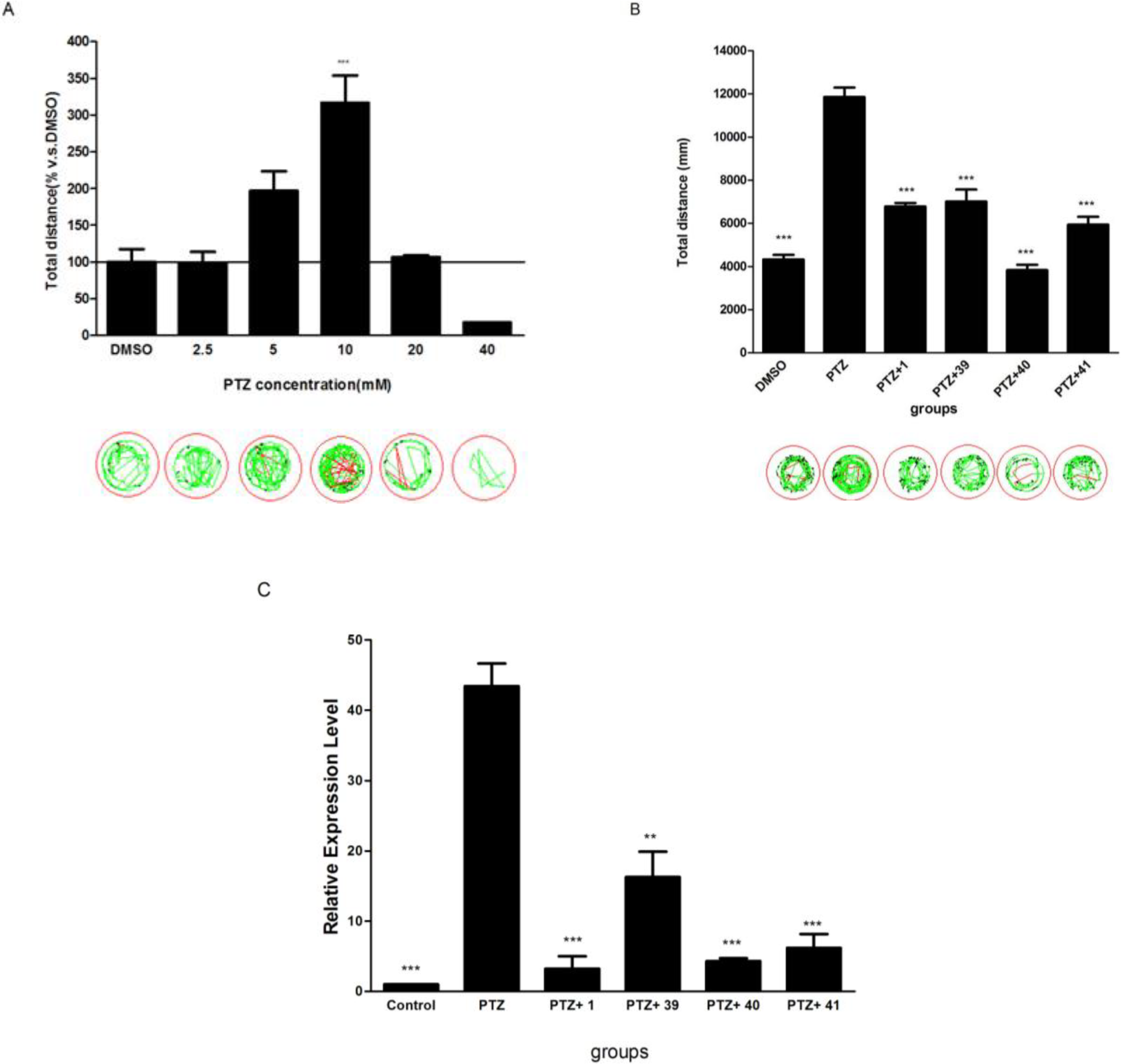
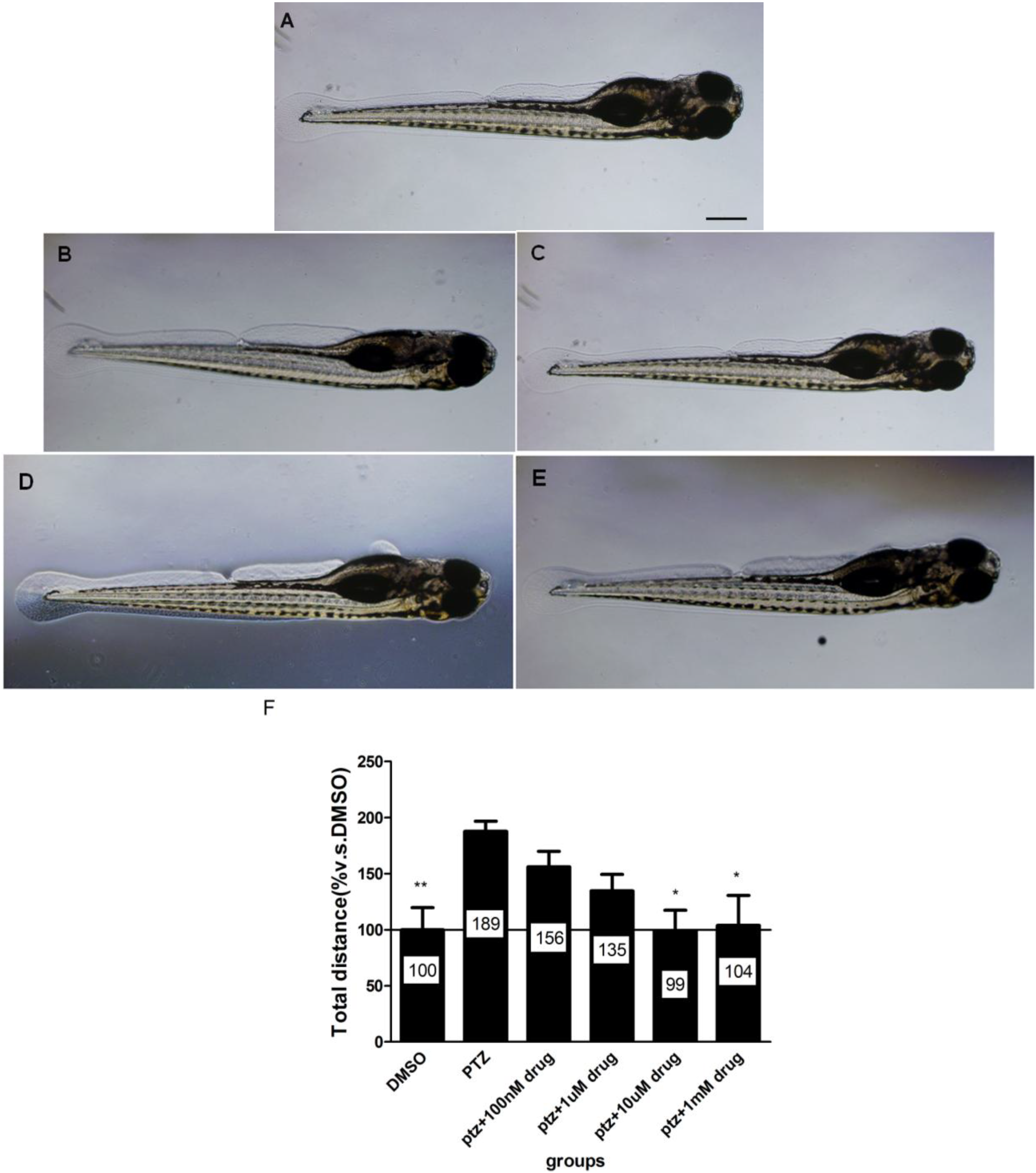
2.6. The Dose-Response Study of Compound 40 in PTZ Model
3. Experimental Section
3.1. Chemistry
3.2. General Procedure of Synthesizing Compounds 9–12; Take 9 for Example
3.2.1. Compound 9
3.2.2. Compound 10
3.2.3. Compound 11
3.2.4. Compound 12
3.3. Zebrafish Behavior-Based Activity Screen
3.3.1. Zebrafish Maintenance
3.3.2. Zebrafish Behavioral Assay
3.3.3. Morphology Assay
3.3.4. Quantitative RT Real Time PCR
- c-fos Forward primer: 5′-GCTCCATCTCAGTCCCAGAG-3′
- c-fos Reverse primer: 5′-AGAGTGGGCTCCAGATCAGA-3′
- β-actin Forward primer: 5′-CCGTTGCCCCGAGGCTCTCT-3′
- β-actin Reverse primer: 5′-CGCATCCTGAGTCAATGCGCCA-3′
3.3.5. Data Analysis
4. Conclusions
Abbreviations
| HT | high-throughput |
| PTZ | pentylenetetrazol |
| GABA | γ-Aminobutyric acid |
| 5-HT | 5-hydroxy-tryptamine |
| NMDA | N-Methyl-d-aspartic acid |
| CBZ | carbamazepine |
| DMSO | dimethyl sulfoxide |
| dpf | days post-fertilization |
| hpf | hours post fertilization |
| CNS | central nervous system |
| IEGs | immediate early genes |
| EEG | electroencephalography |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pangalos, M.N.; Schechter, L.E.; Hurko, O. Drug development for CNS disorders: Strategies for balancing risk and reducing attrition. Nat. Rev. Drug Discov. 2007, 6, 521–532. [Google Scholar] [CrossRef]
- Kokel, D.; Peterson, R.T. Chemobehavioural phenomics and behaviour-based psychiatric drug discovery in the zebrafish. Brief. Funct. Genomics Proteomics 2008, 7, 483–490. [Google Scholar] [CrossRef]
- Brockerhoff, S.E.; Hurley, J.B.; Janssen-Bienhold, U.; Neuhauss, S.C.; Driever, W.; Dowling, J.E. A behavioral screen for isolating zebrafish mutants with visual system defects. Proc. Natl. Acad. Sci. USA 1995, 92, 10545–10549. [Google Scholar] [CrossRef]
- Neuhauss, S.C.; Biehlmaier, O.; Seeliger, M.W.; Das, T.; Kohler, K.; Harris, W.A.; Baier, H. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J. Neurosci. 1999, 19, 8603–8615. [Google Scholar]
- Burgess, H.A.; Granato, M. Sensorimotor gating in larval zebrafish. J. Neurosci. 2007, 27, 4984–4894. [Google Scholar] [CrossRef]
- Prober, D.A.; Rihel, J.; Onah, A.A.; Sung, R.J.; Schier, A.F. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J. Neurosci. 2006, 26, 13400–13410. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Wang, S.Y.; Leclair, O.U.; Danilova, N.P. Melatonin promotes sleep-like state in zebrafish. Brain Res. 2001, 903, 263–268. [Google Scholar] [CrossRef]
- Kokel, D.; Bryan, J.; Laggner, C.; White, R.; Cheung, C.Y.; Mateus, R.; Healey, D.; Kim, S.; Werdich, A.A.; Haggarty, S.J.; et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 2010, 6, 231–237. [Google Scholar] [CrossRef]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 2010, 327, 348–351. [Google Scholar] [CrossRef]
- Li, S.; Shen, C.; Guo, W.; Zhang, X.; Liu, S.; Liang, F.; Xu, Z.; Pei, Z.; Song, H.; Qiu, L.; Lin, Y.; Pang, J. Synthesis and neuroprotective action of xyloketal derivatives in Parkinson’s disease models. Mar. Drugs 2013, 11, 5159–5189. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, Z.Y.; Jiang, M.Y.; Wang, F.; Liu, J.K. A new isoprenyl phenyl ether riboside from the culture of basidiomycete Laccaria amethystea. J. Asian Nat. Prod. Res. 2010, 12, 723–726. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Zhu, X.; He, Z.; Liu, S.; Li, M.; Pang, J.; Lin, Y. Studies on synthesis and structure-activity relationship (SAR) of derivatives of a new natural product from marine fungi as inhibitors of influenza virus neuraminidase. Mar. Drugs 2011, 9, 1887–1901. [Google Scholar] [CrossRef]
- Pettigrew, J.D.; Wilson, P.D. Synthesis of xyloketal A, B, C, D, and G analogues. J. Organ. Chem. 2006, 71, 1620–1625. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Xiang, Q.; Pei, Z.; Liu, X.; Lu, B.; Chen, L.; Wang, G.; Pang, J.; Lin, Y. Design and synthesis of novel xyloketal derivatives and their vasorelaxing activities in rat thoracic aorta and angiogenic activities in zebrafish angiogenesis screen. J. Med. Chem. 2010, 53, 4642–4653. [Google Scholar] [CrossRef]
- Guo, S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes Brain Behav. 2004, 3, 63–74. [Google Scholar] [CrossRef]
- Epilepsy Fact Sheet, World Health Organization. October 2012. Available online: http://www.who.int/mediacentre/factsheets/fs999/en/ (accessed on 10 May 2014).
- Mussulini, B.H.; Leite, C.E.; Zenki, K.C.; Moro, L.; Baggio, S.; Rico, E.P.; Rosemberg, D.B.; Dias, R.D.; Souza, T.M.; Calcagnotto, M.E.; et al. Seizures induced by pentylenetetrazole in the adult zebrafish: a detailed behavioral characterization. PLoS One 2013, 8, e54515. [Google Scholar] [CrossRef]
- Scallet, A.C.; Kowalke, P.K.; Rountree, R.L.; Thorn, B.T.; Binienda, Z.K. Electroencephalographic, behavioral, and c-fos responses to acute domoic acidexposure. Neurotoxicol. Teratol. 2004, 26, 331–342. [Google Scholar] [CrossRef]
- Fleming, A.; Diekmann, H.; Goldsmith, P. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS One 2013, 8, e77548. [Google Scholar] [CrossRef]
- Ghasemi, M.; Shafaroodi, H.; Nazarbeiki, S.; Meskar, H.; Heydarpour, P.; Ghasemi, A.; Talab, S.S.; Ziai, P.; Bahremand, A.; Dehpour, A.R. Voltage-dependent calcium channel and NMDA receptor antagonists augment anticonvulsant effects of lithium chloride on pentylenetetrazole-induced clonic seizures in mice. Epilepsy Behav. 2010, 18, 171–178. [Google Scholar] [CrossRef]
- Meyer, F.B.; Anderson, R.E.; Sundt, T.M., Jr.; Yaksh, T.L.; Sharbrough, F.W. Suppression of pentylenetetrazole seizures by oral administration of a dihydropyridine Ca2+ antagonist. Epilepsia 1987, 28, 409–414. [Google Scholar] [CrossRef]
- Moron, M.A.; Stevens, C.W.; Yaksh, T.L. The antiseizure activity of dihydropyridine calcium channel antagonists in the conscious rat. J. Pharmacol. Exp. Ther. 1990, 252, 1150–1155. [Google Scholar]
- Wu, X.Y.; Liu, X.H.; Lin, Y.C.; Luo, J.H.; She, Z.G.; Jin, L.H.; Chan, W.L.; Antus, S.; Kurtan, T.; Elsässer, B.; et al. Xyloketal F: A strong l-calcium channel blocker from the mangrove fungus xylaria sp. (#2508) from the South China Sea Coast. Eur. J. Org. Chem. 2005, 2005, 4061–4064. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Long, S.-M.; Liang, F.-Y.; Wu, Q.; Lu, X.-L.; Yao, X.-L.; Li, S.-C.; Li, J.; Su, H.; Pang, J.-Y.; Pei, Z. Identification of Marine Neuroactive Molecules in Behaviour-Based Screens in the Larval Zebrafish. Mar. Drugs 2014, 12, 3307-3322. https://doi.org/10.3390/md12063307
Long S-M, Liang F-Y, Wu Q, Lu X-L, Yao X-L, Li S-C, Li J, Su H, Pang J-Y, Pei Z. Identification of Marine Neuroactive Molecules in Behaviour-Based Screens in the Larval Zebrafish. Marine Drugs. 2014; 12(6):3307-3322. https://doi.org/10.3390/md12063307
Chicago/Turabian StyleLong, Si-Mei, Feng-Yin Liang, Qi Wu, Xi-Lin Lu, Xiao-Li Yao, Shi-Chang Li, Jing Li, Huanxing Su, Ji-Yan Pang, and Zhong Pei. 2014. "Identification of Marine Neuroactive Molecules in Behaviour-Based Screens in the Larval Zebrafish" Marine Drugs 12, no. 6: 3307-3322. https://doi.org/10.3390/md12063307
APA StyleLong, S.-M., Liang, F.-Y., Wu, Q., Lu, X.-L., Yao, X.-L., Li, S.-C., Li, J., Su, H., Pang, J.-Y., & Pei, Z. (2014). Identification of Marine Neuroactive Molecules in Behaviour-Based Screens in the Larval Zebrafish. Marine Drugs, 12(6), 3307-3322. https://doi.org/10.3390/md12063307



