Carotenoids of Sea Angels Clione limacina and Paedoclione doliiformis from the Perspective of the Food Chain
Abstract
:1. Introduction
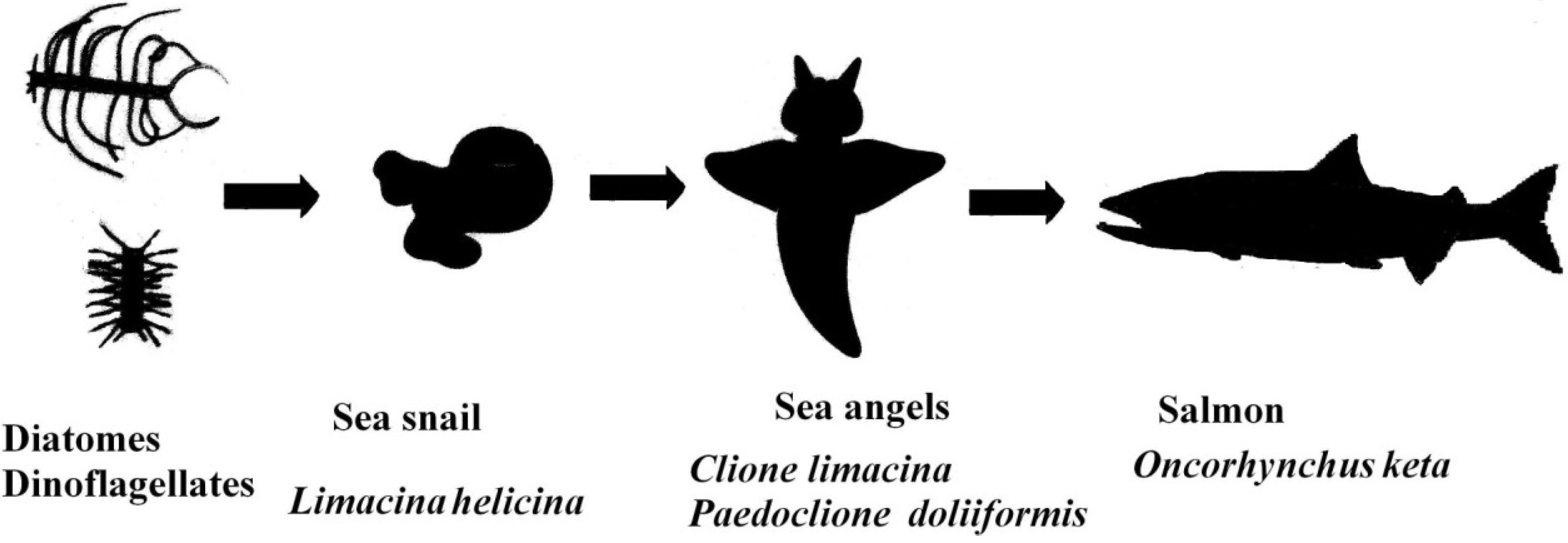
2. Results
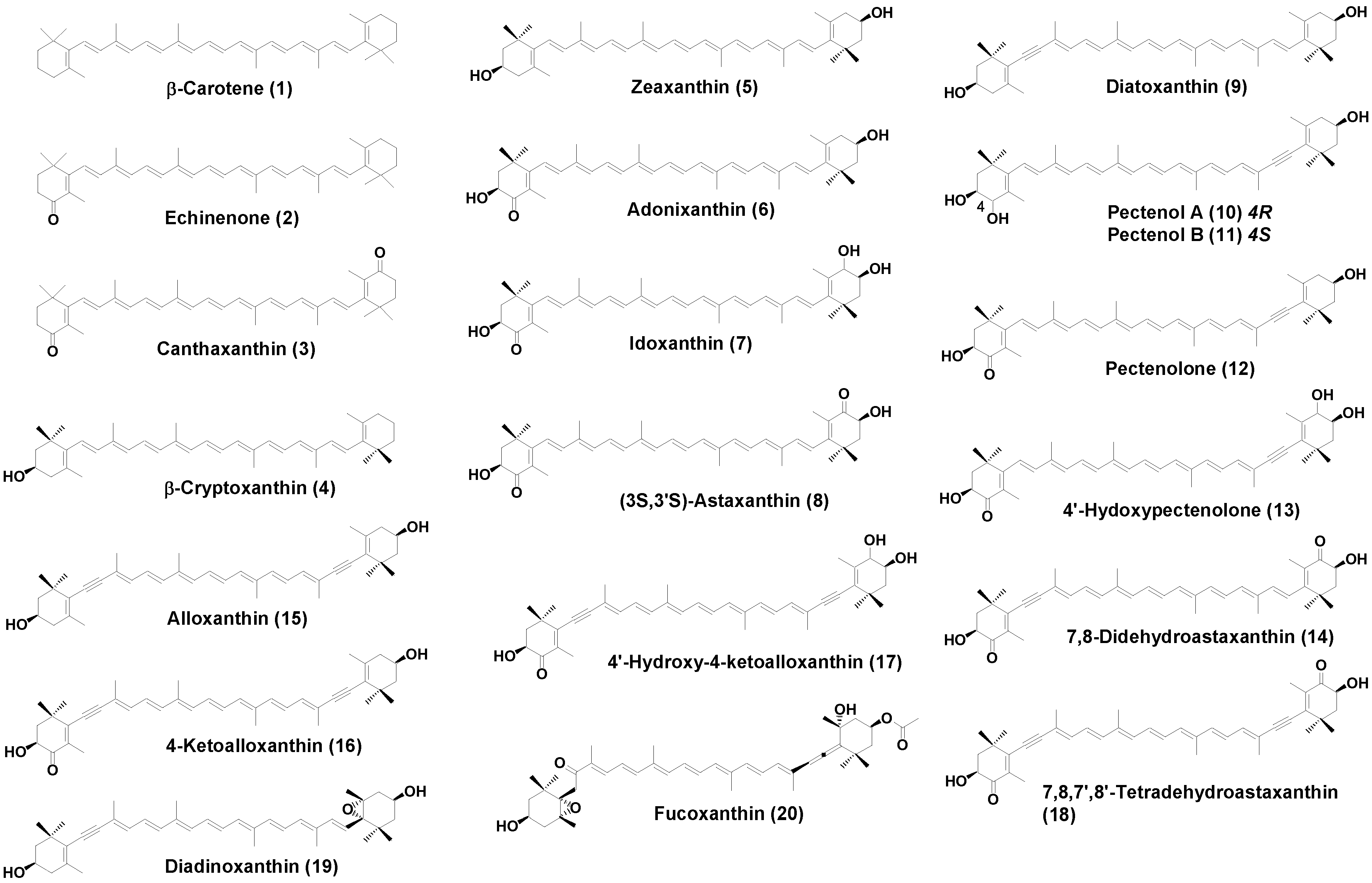
2.1. Carotenoids of L. helicina
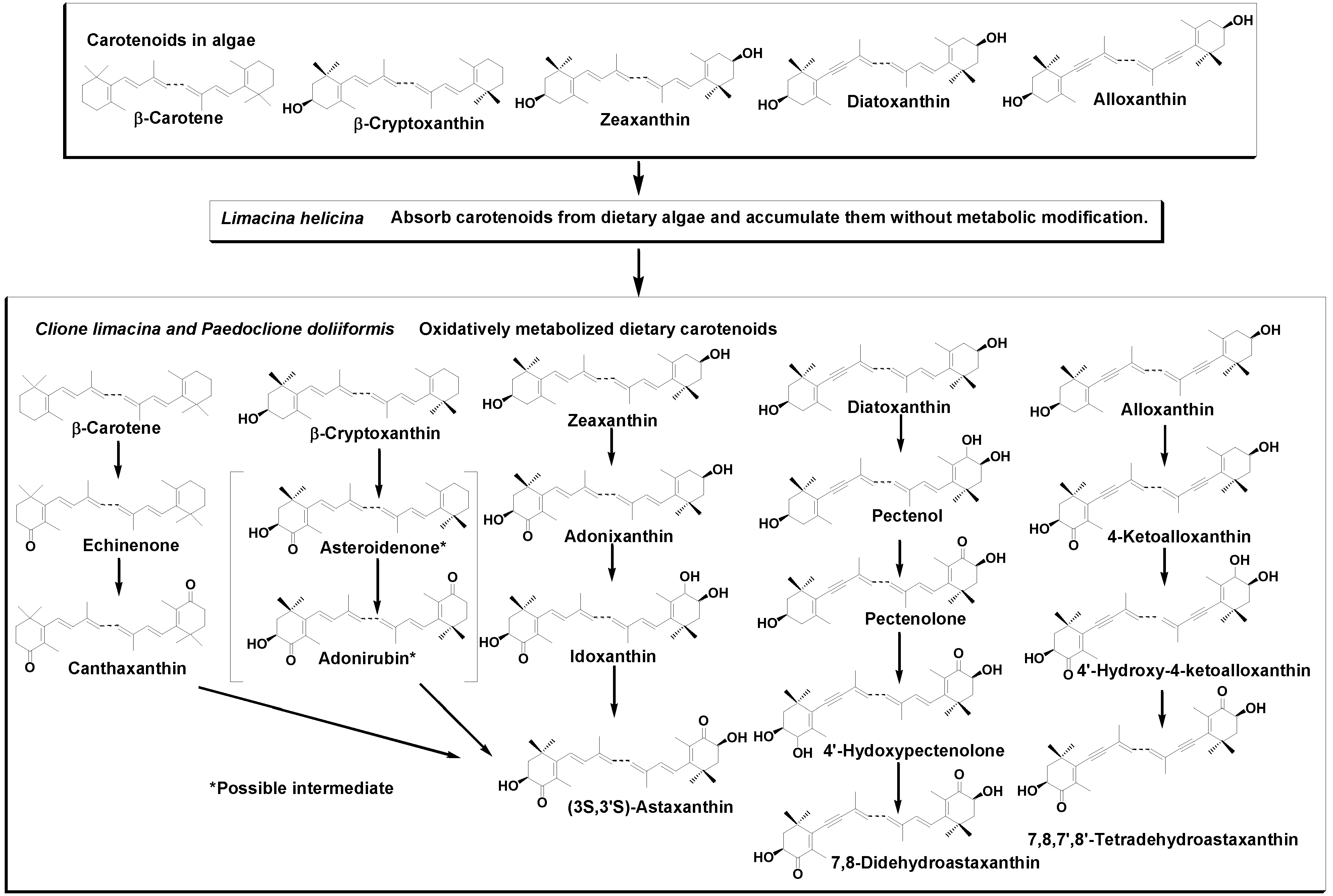
2.2. Carotenoids of C. limacinea
2.3. Carotenoids of P. doliiformis
| L. helicina | C. limacina | P. doliiformis | |
|---|---|---|---|
| Carotenoid content (μg/g wet weight) | 21.0 | 47.0 | 159.8 |
| (μg/specimen) | 0.70 | 0.75 | 2.68 |
| Composition | % | % | % |
| β-Carotene (1) | 32.2 | 27.1 | 10.2 |
| Echinenone (2) | 9.2 | 6.4 | |
| Canthaxanthin (3) | 3.3 | ||
| β-Cryptoxanthin (4) | 10.4 | 13.1 | 12.8 |
| Zeaxanthin (5) | 24.2 | 1.1 | 1.2 |
| Adonixanthin (6) | 9.1 | 1.4 | |
| Idoxanthin (7) | 2.5 | ||
| (3S,3′S)-Astaxanthin (8) | 1.1 | 5.5 | |
| Diatoxanthin (9) | 11.1 | 3.5 | 3.6 |
| Pectenol A (10) | 1.2 | 2.2 | |
| Pectenol B (11) | 1.2 | 2.2 | |
| Pectenolone (12) | 9.2 | 30.5 | |
| 4′-Hydroxypectenolone (13) | 4.2 | 2.5 | |
| 7,8-Didehydroastaxanthin (14) | 4.5 | 6.4 | |
| Alloxanthin (15) | 6.4 | 2.1 | 1.1 |
| 4-Ketoalloxanthin (16) | 3.5 | 2.3 | |
| 4′-Hydroxy-4-ketoalloxanthin (17) | 3.2 | 2.5 | |
| 7,8,7′,8′-Tetradehydroastaxanthin (18) | 4.5 | 1.2 | |
| Diadinoxanthin (19) | 2.4 | ||
| Fucoxanthin (20) | 5.2 | ||
| Others | 8.1 | 2.2 | 2.2 |
2.4. Carotenoids of the Chum Salmon O. keta
| Carotenoids Content and Composition of Flesh of the Chum Salmon O. keta | |
|---|---|
| Carotenoid content (μg/g wet weight) | 0.89 |
| Composition | % |
| Astaxanthin * | 83.5 |
| 9-cis-Astaxanthin * | 5.1 |
| 13-cis-Astaxanthin * | 2.5 |
| 7,8-Didehydroastaxanthin | 0.5 |
| Adonixanthin | 1.1 |
| Pectenolone | 2.5 |
| Others | 4.8 |
3. Discussion
4. Experimental Section
4.1. General
4.2. Animal Specimens
4.3. Analysis of Carotenoids
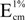 = 2100 [25] at λ max. The ether-hexane solution was evaporated. The residue was subjected to HPLC on silica gel. Carotenoid compositions were estimated by the peak area of the HPLC on silica gel with acetone–hexane (2:8)–(4:6) monitored at 450 nm.
= 2100 [25] at λ max. The ether-hexane solution was evaporated. The residue was subjected to HPLC on silica gel. Carotenoid compositions were estimated by the peak area of the HPLC on silica gel with acetone–hexane (2:8)–(4:6) monitored at 450 nm.4.4. Identification of Carotenoids
4.5. 1O2 Quenching Activity of Carotenoids
5. Conclusions
Acknowledgements
Authors Contributions
Conflicts of Interest
References
- Carol, M.; Ronald, L.; Gilmer, W. Pelagic Snails; Stanford University Press: Stanford, CA, USA, 1989. [Google Scholar]
- Gilmer, R.W.; Harbison, G.R. Diet of Limacina helicina (Gastropoda: Thecosomata) in arctic waters in midsummer. Mar. Ecol. Prog. Ser. 1991, 77, 125–134. [Google Scholar] [CrossRef]
- Azuma, T. Biological mechanisms enabling sympatry between salmoids with special reference to sockeye and chum salmon in oceanic water. Fish. Res. 1995, 24, 291–300. [Google Scholar] [CrossRef]
- Davis, N.D.; Volkov, A.V.; Efimkin, A.Y.; Kuznetsova, N.A.; Armstrong, J.L.; Sakai, O. Review of basis salmon food habits studies. N. Pac. Anadr. Fish Comm. Bull. 2009, 5, 197–208. [Google Scholar]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Hand Book; Birkhäuser: Basel, Switzerland, 2004. [Google Scholar]
- Liaaen-Jensen, S. Carotenoids in Food Chain. In Carotenoids Volume 3: Biosynthesis and Metabolism; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1998; pp. 359–371. [Google Scholar]
- Matsuno, T. Aquatic animal carotenoids. Fish. Sci. 2001, 67, 771–789. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Maoka, T. Recent progress in structural studies of carotenoids in animals and plants. Arch. Biochem. Biophys. 2009, 483, 191–195. [Google Scholar] [CrossRef]
- Maoka, T.; Akimoto, N.; Tsushima, M.; Komemushi, S.; Mezaki, T.; Iwase, F.; Takahashi, Y.; Sameshima, N.; Mori, M.; Sakagami, Y. Carotenoids in marine invertebrates living along the Kuroshio current coast. Mar. Drugs 2011, 9, 1419–1427. [Google Scholar] [CrossRef]
- Matsuno, T.; Katagiri, K.; Maoka, T.; Komori, T. Novel reductive metabolic pathways of 4-oxo-β-end group in carotenoids of the spindle shell Fushinus perplexus. Comp. Biochem. Physiol. 1985, 81B, 905–908. [Google Scholar]
- Katagiri, K.; Maoka, T.; Matsuno, T. Carotenoids of shellfishes VIII. Comparative biochemical studies of carotenoids in three species of spindle shell, Fushinus perplexus, F. perplexus ferrugineus and F. forceps. Comp. Biochem. Physiol. 1986, 84B, 473–476. [Google Scholar]
- Maoka, T.; Ochi, J.; Mori, M.; Sakagami, Y. Identification of Carotenoids in the Freshwater Shellfish Unio douglasiae nipponensis, Anodonta lauta, Cipangopaludina chinensis laeta, and Semisulcospira libertia. J. Oleo Sci. 2012, 61, 69–74. [Google Scholar] [CrossRef]
- Tsushima, M.; Katsuyama, M.; Matsuno, T. Metabolism of carotenoids in the apple snail Pomacea canaliculata. Comp. Biochem. Physiol. 1997, 118B, 431–436. [Google Scholar] [CrossRef]
- Maoka, T.; Katsuyama, M.; Kaneko, N.; Matsuno, T. Stereochemical investigation of carotenoids in the antarctic krill Euphausia superba. Bull. Jpn. Soc. Sci. Fish 1985, 51, 1671–1673. [Google Scholar] [CrossRef]
- Torrissen, O.J.; Christiansen, R. Requirements for carotenoids in fish diets. J. Appl. Ichthyol. 1995, 11, 225–230. [Google Scholar] [CrossRef]
- Tsushima, M.; Kawakami, T.; Mine, M.; Matsuno, T. The role of carotenoids in the development of the sea urchin Pseudocentrotus depressus. Invertebr. Reprod. Dev. 1997, 32, 149–153. [Google Scholar] [CrossRef]
- Terao, J. Antioxidative activity of beta-carotene-related carotenoids in solution. Lipids 1989, 24, 659–661. [Google Scholar] [CrossRef]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as singlet oxygen quenchers in marine organism. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Maoka, T.; Goto, Y.; Isobe, K.; Fujiwara, Y.; Hashimoto, K.; Mochida, K. Antioxidative activity of capsorubin and related compounds from paprika (Capsicum annuum). J. Oleo Sci. 2001, 50, 663–665. [Google Scholar] [CrossRef]
- Narita, M.; Maoka, T.; Ebitani, K.; Nishino, H. Characteristics of chemical constituents and red pigment of orange adductor muscle of scallop Mizuhopecten yessoensis in the Okhotsk Sea and anti-oxidative activity of the pigment. Nippon Suisan Gakkaishi 2013, 79, 48–54. [Google Scholar] [CrossRef]
- Maoka, T.; Komori, T.; Matsuno, T. Direct resolution of diastereomeric carotenoid-I. 3-oxo-β-end group. J. Chromatogr. 1985, 318, 122–124. [Google Scholar] [CrossRef]
- Maoka, T.; Akimoto, N. Natural product chemistry in carotenoid, some experimental techniques for structural elucidation and analysis of natural carotenoids. Carotenoid Sci. 2008, 13, 10–17. [Google Scholar]
- Britton, G. UV/Visible Spectroscopy. In Carotenoids; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1995; pp. 13–62. [Google Scholar]
- Maoka, T.; Yasui, H.; Ohmori, A.; Tokuda, H.; Suzuki, N.; Osawa, A.; Shindo, K.; Ishibashi, T. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J. Oleo Sci. 2013, 62, 181–186. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maoka, T.; Kuwahara, T.; Narita, M. Carotenoids of Sea Angels Clione limacina and Paedoclione doliiformis from the Perspective of the Food Chain. Mar. Drugs 2014, 12, 1460-1470. https://doi.org/10.3390/md12031460
Maoka T, Kuwahara T, Narita M. Carotenoids of Sea Angels Clione limacina and Paedoclione doliiformis from the Perspective of the Food Chain. Marine Drugs. 2014; 12(3):1460-1470. https://doi.org/10.3390/md12031460
Chicago/Turabian StyleMaoka, Takashi, Takashi Kuwahara, and Masanao Narita. 2014. "Carotenoids of Sea Angels Clione limacina and Paedoclione doliiformis from the Perspective of the Food Chain" Marine Drugs 12, no. 3: 1460-1470. https://doi.org/10.3390/md12031460
APA StyleMaoka, T., Kuwahara, T., & Narita, M. (2014). Carotenoids of Sea Angels Clione limacina and Paedoclione doliiformis from the Perspective of the Food Chain. Marine Drugs, 12(3), 1460-1470. https://doi.org/10.3390/md12031460




