Spongionella Secondary Metabolites Protect Mitochondrial Function in Cortical Neurons against Oxidative Stress
Abstract
:1. Introduction
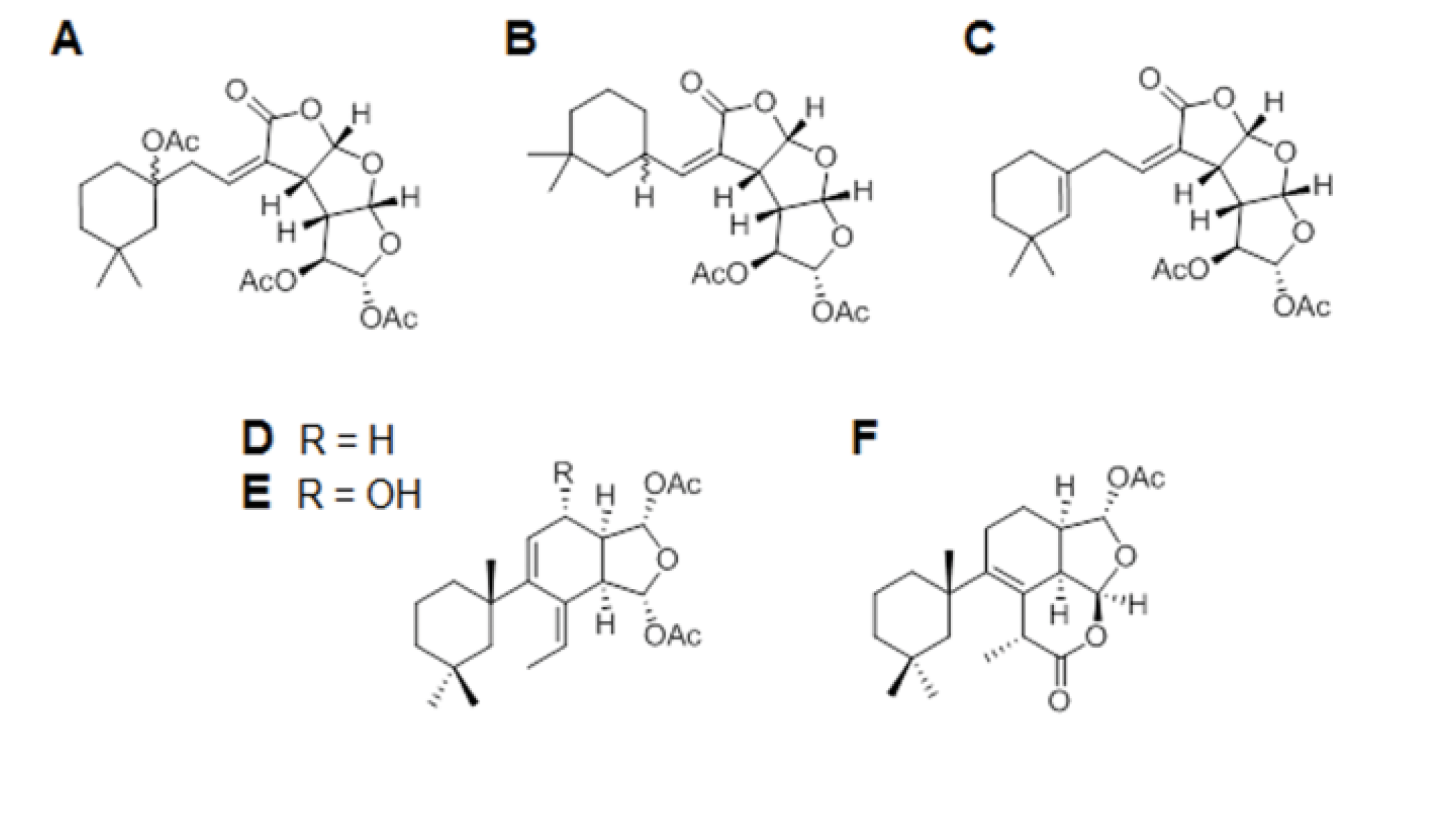
2. Results and Discussion
2.1. Spongionella Compound Effects on Cortical Neurons Viability
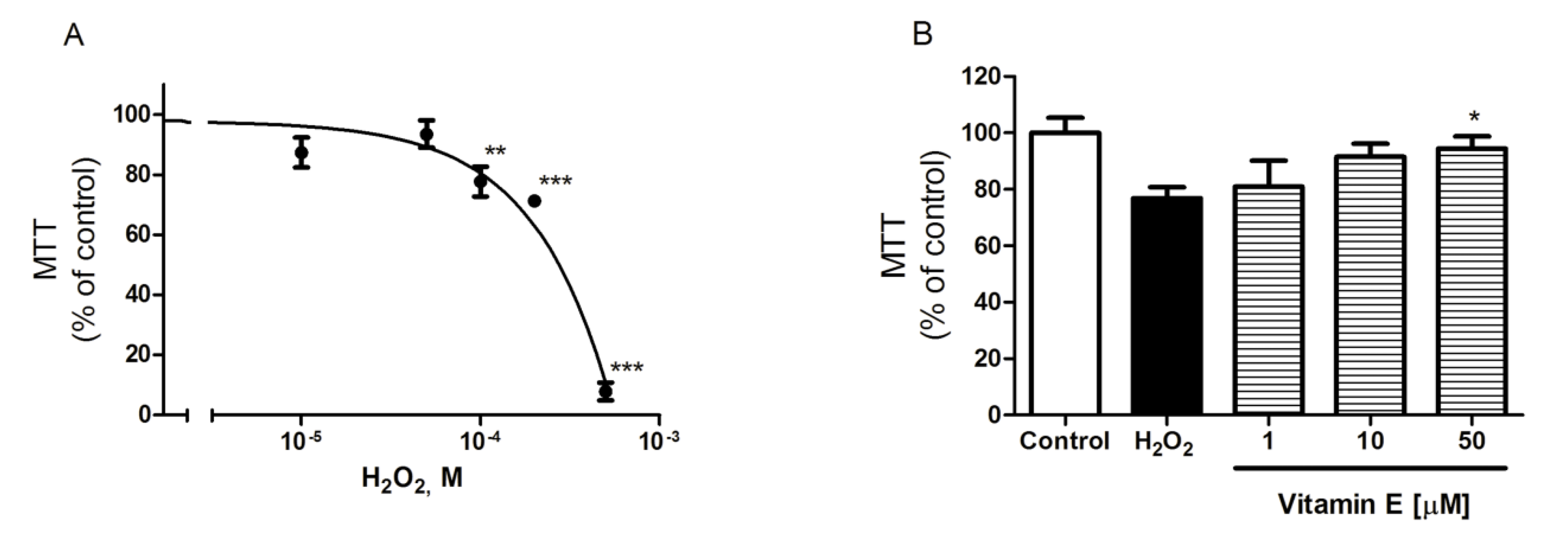
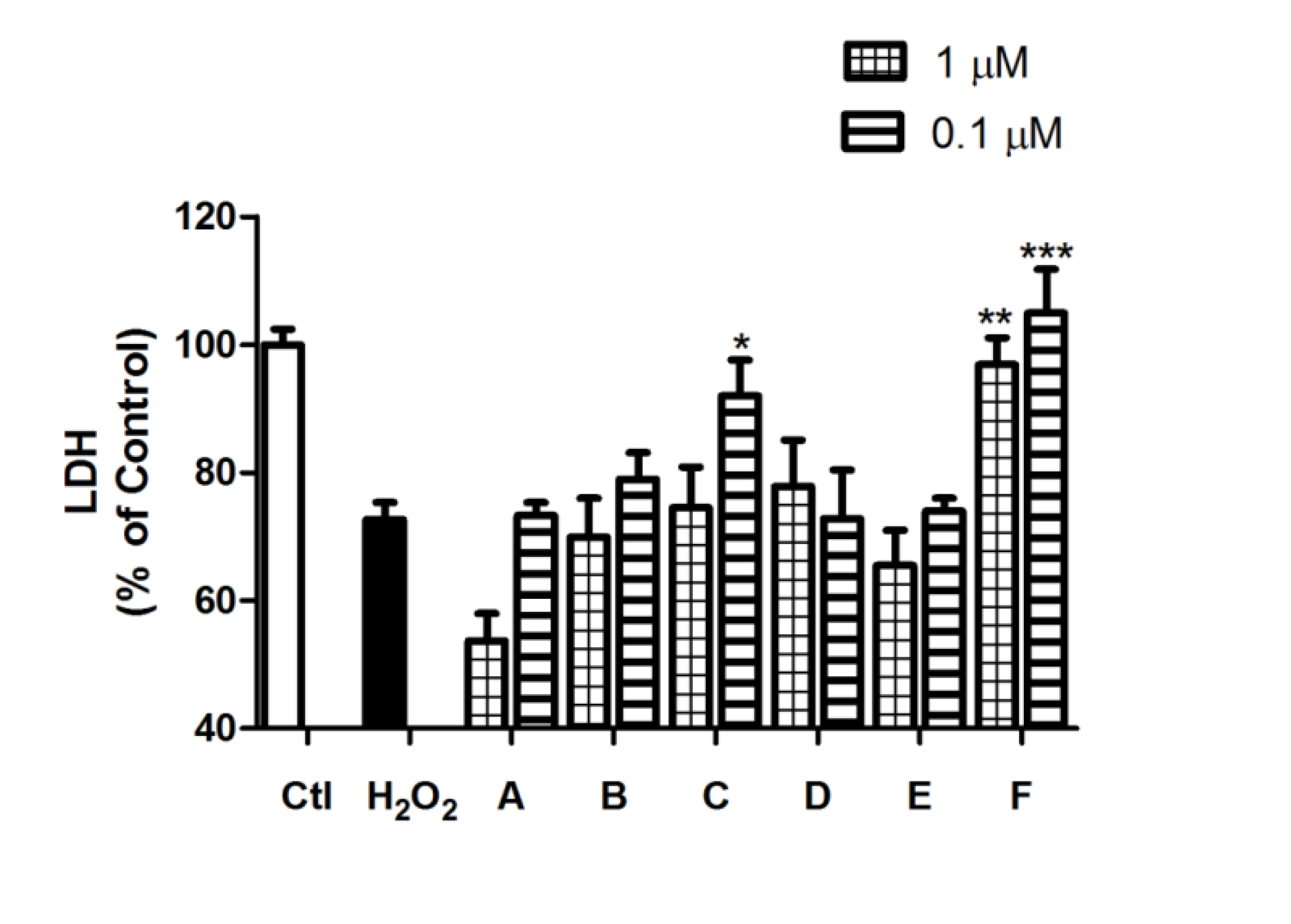
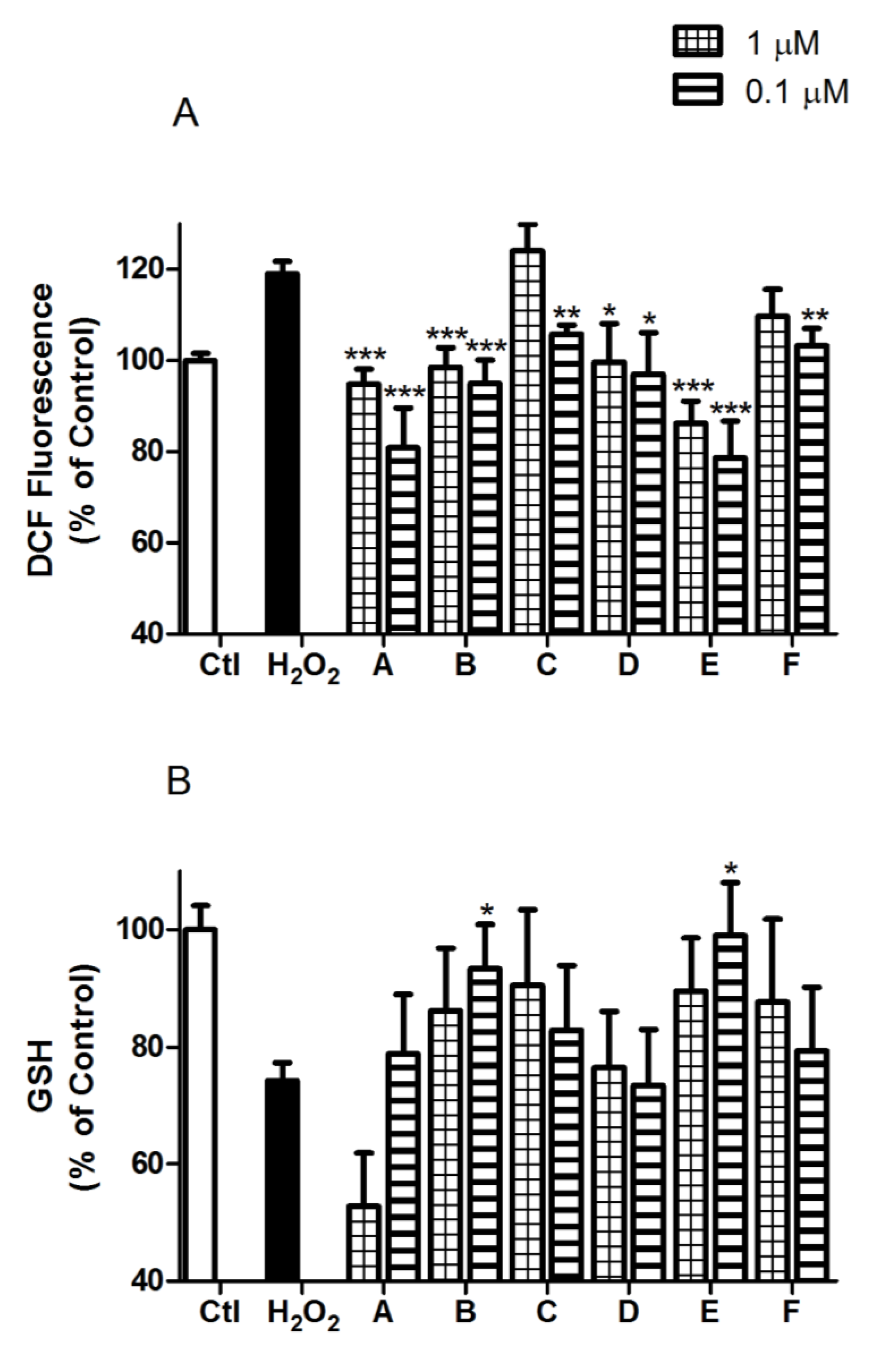
2.2. Spongionella Compounds Show Neuroprotection Activity against H2O2 Insults

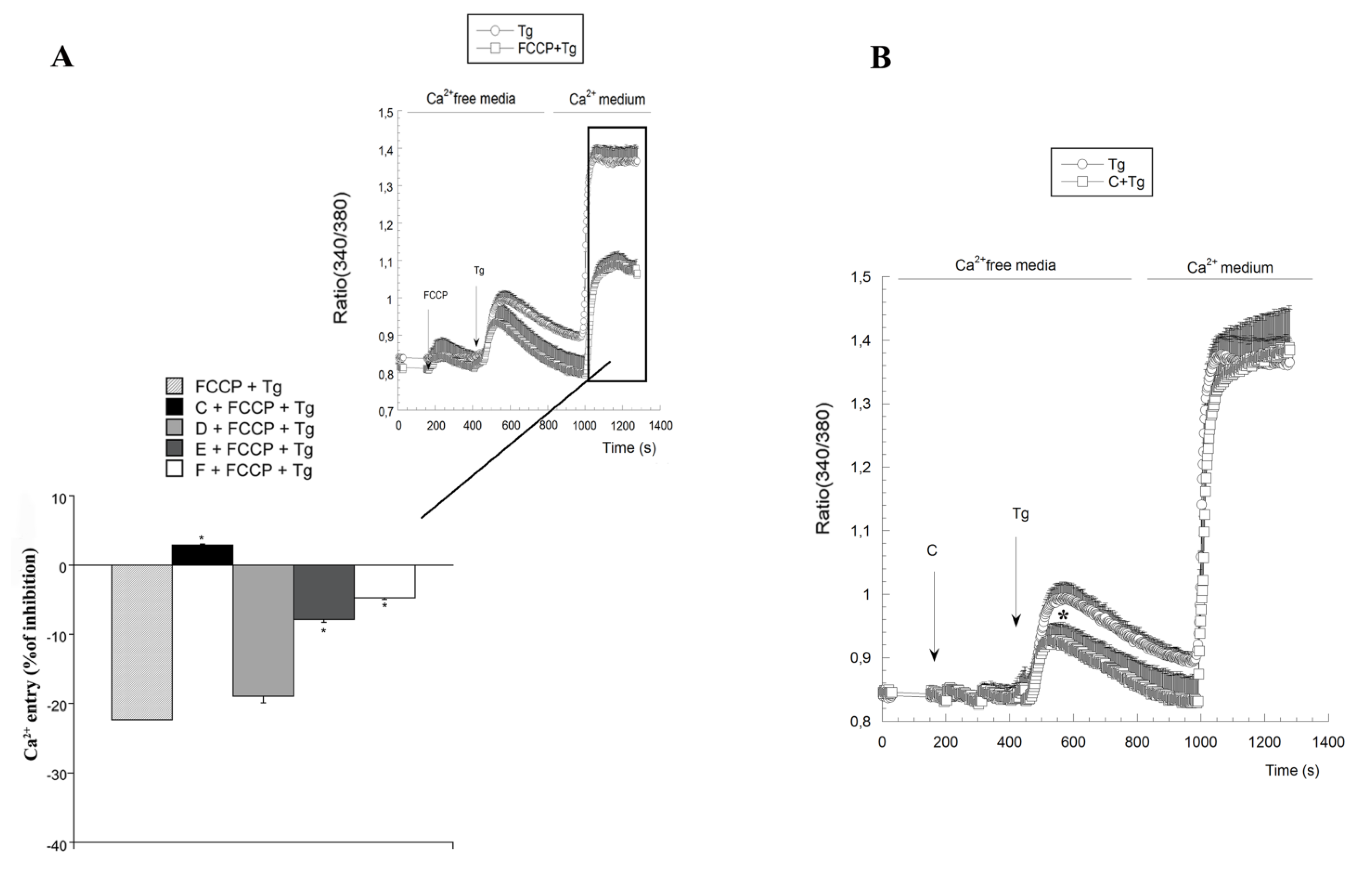
2.3. Variations on [Ca2+]c Induced by Spongionella Compounds
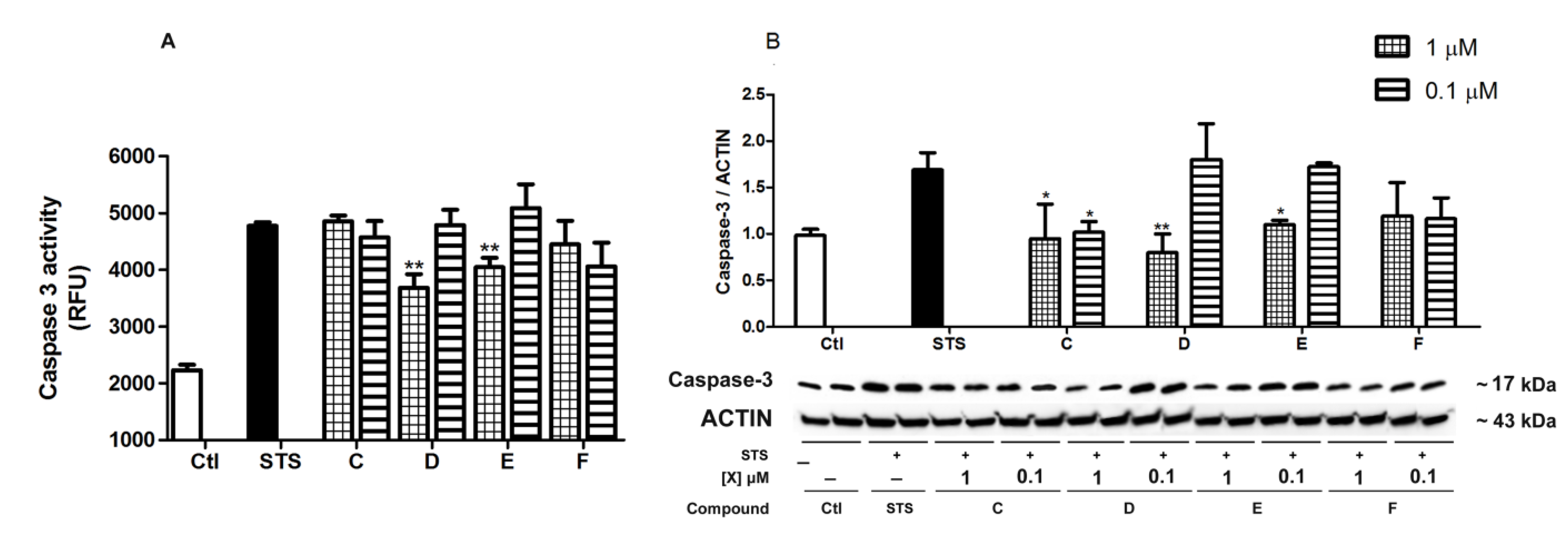
2.4. Spongionella Compounds Inhibit Caspase-3
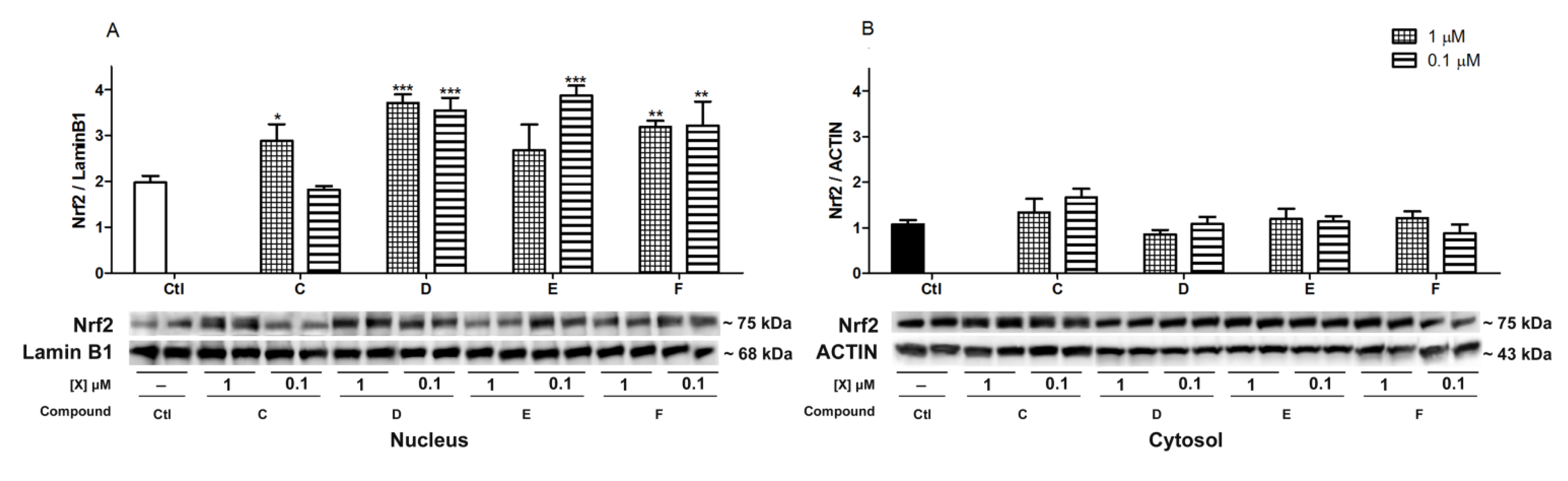
2.5. Nrf2 Translocation Induced by Spongionella Compounds
3. Experimental Section
3.1. Porifera Compounds Information
| Code | Chemical Name | Formula | MW | Source Organism |
|---|---|---|---|---|
| A | Gracilin J | C24H32O10 | 480.19 | Spongionella sp. |
| B | Gracilin K | C21H28O8 | 408.17 | Spongionella sp. |
| C | Gracilin H | C22H28O8 | 420.17 | Spongionella sp. |
| D | Gracilin A | C23H34O5 | 390.24 | Spongionella sp. |
| E | Gracilin L | C23H34O6 | 406.23 | Spongionella sp. |
| F | Tetrahydroaplysulphurin-1 | C22H32O5 | 376.22 | Spongionella sp. |
3.2. Primary Cortical Neurons
3.3. Neuroblastoma Cell Line
3.4. Chemicals and Solutions
3.5. Cytotoxicity Assay
3.6. Neuroprotection Assays
3.6.1. Mitochondrial Function and ∆Ψm (Membrane Potential Variation) Assays
3.6.2. Cell Survival Measurement
3.7. Evaluation of ROS Generation
3.8. Estimation of Glutathione Levels
3.9. Determination of the Cytosolic Calcium Concentration [Ca2+]c
3.10. Measurement of Caspase-3 Activity and Expression
3.11. Nrf2 Analysis by Western Blotting
3.12. Statistical Analysis
4. Conclusions
Abbreviations
| ROS | reactive oxygen species |
| GSH | glutathione |
Acknowledgments
Conflicts of Interest
References
- Thomas, T.R.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef]
- Van Soest, R.W.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; de Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N. Global diversity of sponges (Porifera). PLoS One 2012, 7, e35105. [Google Scholar] [CrossRef]
- Abbas, S.; Kelly, M.; Bowling, J.; Sims, J.; Waters, A.; Hamann, M. Advancement into the Arctic region for bioactive sponge secondary metabolites. Mar. Drugs 2011, 9, 2423–2437. [Google Scholar] [CrossRef]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef]
- Mollica, A.; Locatelli, M.; Stefanucci, A.; Pinnen, F. Synthesis and bioactivity of secondary metabolites from marine sponges containing dibrominated indolic systems. Molecules 2012, 17, 6083–6099. [Google Scholar] [CrossRef]
- Hong, S.; Kim, S.H.; Rhee, M.H.; Kim, A.R.; Jung, J.H.; Chun, T.; Yoo, E.S.; Cho, J.Y. In vitro anti-inflammatory and pro-aggregative effects of a lipid compound, petrocortyne A, from marine sponge. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 368, 448–456. [Google Scholar] [CrossRef]
- Lysek, N.; Kinscherf, R.; Claus, R.; Lindel, T. l-5-Hydroxytryptophan: Antioxidant and anti-apoptotic principle of the intertidal sponge Hymeniacidon heliophila. Z. Naturforsch. C 2003, 58, 568–572. [Google Scholar]
- Petri, S.; Korner, S.; Kiaei, M. Nrf2/ARE Signaling pathway: Key mediator in oxidative stress and potential therapeutic target in ALS. Neurol. Res. Int. 2012, 2012, 878030. [Google Scholar]
- Sasaki, S.; Tozawa, T.; van Wagoner, R.M.; Ireland, C.M.; Harper, M.K.; Satoh, T. Strongylophorine-8, a pro-electrophilic compound from the marine sponge Petrosia (Strongylophora) corticata, provides neuroprotection through Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2011, 415, 6–10. [Google Scholar] [CrossRef]
- Tahtouh, T.; Elkins, J.M.; Filippakopoulos, P.; Soundararajan, M.; Burgy, G.; Durieu, E.; Cochet, C.; Schmid, R.S.; Lo, D.C.; Delhommel, F.; et al. Selectivity, cocrystal structures, and neuroprotective properties of leucettines, a family of protein kinase inhibitors derived from the marine sponge alkaloid leucettamine B. J. Med. Chem. 2012, 55, 9312–9330. [Google Scholar] [CrossRef]
- Rateb, M.E.; Houssen, W.E.; Schumacher, M.; Harrison, W.T.; Diederich, M.; Ebel, R.; Jaspars, M. Bioactive diterpene derivatives from the marine sponge Spongionella sp. J. Nat. Prod. 2009, 72, 1471–1476. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar]
- Melo, A.; Monteiro, L.; Lima, R.M.; Oliveira, D.M.; Cerqueira, M.D.; El-Bacha, R.S. Oxidative stress in neurodegenerative diseases: Mechanisms and therapeutic perspectives. Oxid. Med. Cell. Longev. 2011, 2011, 467180. [Google Scholar]
- Zampagni, M.; Wright, D.; Cascella, R.; D’Adamio, G.; Casamenti, F.; Evangelisti, E.; Cardona, F.; Goti, A.; Nacmias, B.; Sorbi, S.; et al. Novel S-acyl glutathione derivatives prevent amyloid oxidative stress and cholinergic dysfunction in Alzheimer disease models. Free Radic. Biol. Med. 2012, 52, 1362–1371. [Google Scholar] [CrossRef]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; St Clair, D.; Batinic-Haberle, I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta 2012, 1822, 794–814. [Google Scholar] [CrossRef]
- Paupe, V.; Dassa, E.P.; Goncalves, S.; Auchere, F.; Lonn, M.; Holmgren, A.; Rustin, P. Impaired nuclear Nrf2 translocation undermines the oxidative stress response in Friedreich ataxia. PLoS One 2009, 4, e4253. [Google Scholar] [CrossRef]
- Jones, Q.R.; Warford, J.; Rupasinghe, H.P.; Robertson, G.S. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol. Sci. 2012, 33, 602–610. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Varming, T.; Drejer, J.; Frandsen, A.; Schousboe, A. Characterization of a chemical anoxia model in cerebellar granule neurons using sodium azide: Protection by nifedipine and MK-801. J. Neurosci. Res. 1996, 44, 40–46. [Google Scholar] [CrossRef]
- Triana-Vidal, L.E.; Carvajal-Varona, S.M. Protective effect of galantamine against oxidative damage using human lymphocytes: A novel in vitro model. Arch. Med. Res. 2013, 44, 85–92. [Google Scholar] [CrossRef]
- Facecchia, K.; Fochesato, L.A.; Ray, S.D.; Stohs, S.J.; Pandey, S. Oxidative toxicity in neurodegenerative diseases: Role of mitochondrial dysfunction and therapeutic strategies. J. Toxicol. 2011, 2011, 683728. [Google Scholar]
- Crouzin, N.; Ferreira, M.C.; Cohen-Solal, C.; Barbanel, G.; Guiramand, J.; Vignes, M. Neuroprotection induced by vitamin E against oxidative stress in hippocampal neurons: Involvement of TRPV1 channels. Mol. Nutr. Food Res. 2010, 54, 496–505. [Google Scholar] [CrossRef]
- Giordano, G.; Hong, S.; Faustman, E.; Costa, L. Measurements of Cell Death in Neuronal and Glial Cells. In In Vitro Neurotoxicology. Methods and Protocols, Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 758, pp. 171–178. [Google Scholar]
- Parekh, A.B.; Penner, R. Store depletion and calcium influx. Physiol. Rev. 1997, 77, 901–930. [Google Scholar]
- Putney, J.W., Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Dispersyn, G.; Nuydens, R.; Connors, R.; Borgers, M.; Geerts, H. Bcl-2 protects against FCCP-induced apoptosis and mitochondrial membrane potential depolarization in PC12 cells. Biochim. Biophys. Acta 1999, 1428, 357–371. [Google Scholar]
- Karami-Mohajeri, S.; Abdollahi, M. Mitochondrial dysfunction and organophosphorus compounds. Toxicol. Appl. Pharmacol. 2013, 270, 39–44. [Google Scholar] [CrossRef]
- Kajta, M.; Trotter, A.; Lason, W.; Beyer, C. Impact of 17beta-estradiol on cytokine-mediated apoptotic effects in primary hippocampal and neocortical cell cultures. Brain Res. 2006, 1116, 64–74. [Google Scholar]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.M.; Li, J.; Johnson, J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef]
- Joshi, G.; Johnson, J.A. The Nrf2-ARE pathway: A valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat. CNS Drug Discov. 2012, 7, 218–229. [Google Scholar] [CrossRef]
- Cagle, P.T.; Allen, T.C. Lung cancer genotype-based therapy and predictive biomarkers: Present and future. Arch. Pathol. Lab. Med. 2012, 136, 1482–1491. [Google Scholar] [CrossRef]
- Nirmal, N.; Praba, G.O.; Velmurugan, D. Modeling studies on phospholipase A2-inhibitor complexes. Indian J. Biochem. Biophys. 2008, 45, 256–262. [Google Scholar]
- Sun, G.Y.; Xu, J.; Jensen, M.D.; Simonyi, A. Phospholipase A2 in the central nervous system: Implications for neurodegenerative diseases. J. Lipid Res. 2004, 45, 205–213. [Google Scholar]
- Rueda, A.; Losada, A.; Fernandez, R.; Cabanas, C.; Garcia-Fernandez, L.F.; Reyes, F.; Cuevas, C. Gracilins G–I, Cytotoxic Bisnorditerpenes from Spongionella pulchella, and the Anti-Adhesive Properties of Gracilin B. Lett. Drug Des. Discov. 2006, 3, 753–760. [Google Scholar] [CrossRef]
- Vale, C.; Alonso, E.; Rubiolo, J.A.; Vieytes, M.R.; LaFerla, F.M.; Gimenez-Llort, L.; Botana, L.M. Profile for amyloid-beta and tau expression in primary cortical cultures from 3xTg-AD mice. Cell. Mol. Neurobiol. 2010, 30, 577–590. [Google Scholar] [CrossRef]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Gimenez-Llort, L.; Botana, L.M. 13-Desmethyl spirolide-C is neuroprotective and reduces intracellular Abeta and hyperphosphorylated tau in vitro. Neurochem. Int. 2011, 59, 1056–1065. [Google Scholar] [CrossRef]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Gimenez-Llort, L.; Botana, L.M. The cholinergic antagonist gymnodimine improves Abeta and tau neuropathology in an in vitro model of Alzheimer disease. Cell. Physiol. Biochem. 2011, 27, 783–794. [Google Scholar] [CrossRef]
- White, M.G.; Wang, Y.; Akay, C.; Lindl, K.A.; Kolson, D.L.; Jordan-Sciutto, K.L. Parallel high throughput neuronal toxicity assays demonstrate uncoupling between loss of mitochondrial membrane potential and neuronal damage in a model of HIV-induced neurodegeneration. Neurosci. Res. 2011, 70, 220–229. [Google Scholar] [CrossRef]
- Kim, E.J.; Kwon, K.J.; Park, J.Y.; Lee, S.H.; Moon, C.H.; Baik, E.J. Neuroprotective effects of prostaglandin E2 or cAMP against microglial and neuronal free radical mediated toxicity associated with inflammation. J. Neurosci. Res. 2002, 70, 97–107. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leirós, M.; Sánchez, J.A.; Alonso, E.; Rateb, M.E.; Houssen, W.E.; Ebel, R.; Jaspars, M.; Alfonso, A.; Botana, L.M. Spongionella Secondary Metabolites Protect Mitochondrial Function in Cortical Neurons against Oxidative Stress. Mar. Drugs 2014, 12, 700-718. https://doi.org/10.3390/md12020700
Leirós M, Sánchez JA, Alonso E, Rateb ME, Houssen WE, Ebel R, Jaspars M, Alfonso A, Botana LM. Spongionella Secondary Metabolites Protect Mitochondrial Function in Cortical Neurons against Oxidative Stress. Marine Drugs. 2014; 12(2):700-718. https://doi.org/10.3390/md12020700
Chicago/Turabian StyleLeirós, Marta, Jon A. Sánchez, Eva Alonso, Mostafa E. Rateb, Wael E. Houssen, Rainer Ebel, Marcel Jaspars, Amparo Alfonso, and Luis M. Botana. 2014. "Spongionella Secondary Metabolites Protect Mitochondrial Function in Cortical Neurons against Oxidative Stress" Marine Drugs 12, no. 2: 700-718. https://doi.org/10.3390/md12020700
APA StyleLeirós, M., Sánchez, J. A., Alonso, E., Rateb, M. E., Houssen, W. E., Ebel, R., Jaspars, M., Alfonso, A., & Botana, L. M. (2014). Spongionella Secondary Metabolites Protect Mitochondrial Function in Cortical Neurons against Oxidative Stress. Marine Drugs, 12(2), 700-718. https://doi.org/10.3390/md12020700






