Abstract
Six new tetraprenylated alkaloids, designated as malonganenones L–Q (1–6), were isolated from the gorgonian Echinogorgia pseudossapo, collected in Daya Bay of Guangdong Province, China. The structures of 1–6 featuring a methyl group at N-3 and a tetraprenyl chain at N-7 in the hypoxanthine core were established by extensive spectroscopic analyses. Compounds 1–6 were tested for their inhibitory activity against the phosphodiesterases (PDEs)-4D, 5A, and 9A, and compounds 1 and 6 exhibited moderate inhibitory activity against PDE4D with IC50 values of 8.5 and 20.3 µM, respectively.
1. Introduction
Tetraprenylated purine alkaloids and their derivatives are relatively uncommon in nature [1,2]. They are structurally characterized by a methyl group at N-3 and a tetraprenyl chain at N-7 in the hypoxanthine core. Malonganenone A [3], the first typical representative of this series, was isolated from the gorgonian Leptogorgia gilchristi, collected in Ponto Malongane, Mozambique in 2006. Until now, only 14 analogues have been reported from marine organisms [3,4,5], some of which exhibited antitumor activity [4,5]. Although the genus Echinogorgia is highly prolific in the South China Sea, only a few species of Echinogorgia have been chemically investigated, which led to the isolation of a series of metabolites including sterols [6,7,8,9,10,11,12], alkaloids [11,12,13,14], sesquiterpenes [14,15,16], ceramides [17], and coumarins [18]. In our screening program aimed at discovering new biologically active natural products from marine organisms of the South China Sea [19], a fraction of the CH2Cl2/MeOH extract of E. pseudosassapo showed inhibitory activity towards phosphodiesterases (PDEs)-4D, 5A, and 9A. Subsequent chemical investigation resulted in the purification of six new tetraprenylated alkaloids, malonganenones L–Q (1–6, Figure 1, Supplementary Figures S1–S31). The resulting inhibitory activity screening against PDE4D, PDE5A, and PDE9A showed that compounds 1 and 6 exhibited moderate activities against PDE4D with IC50 values of 8.5 and 20.3 µM, respectively. The present report describes the isolation, structure elucidation, and PDEs inhibitory activities of these tetraprenylated alkaloids.
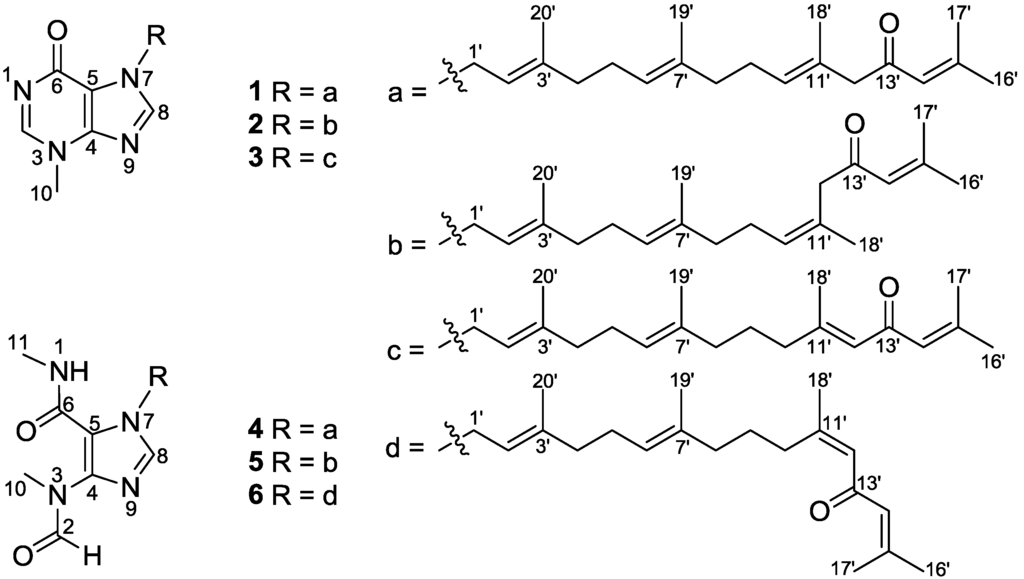
Figure 1.
Structures of malonganenones L–Q (1–6).
2. Results and Discussion
2.1. Structural Elucidation of New Compounds
The CH2Cl2/MeOH (v/v, 1:1) extract of the gorgonian was subjected to chromatography using Sephadex LH-20 followed by silica gel and HPLC separations to yield compounds 1–6.
Compound 1, a colorless oil, exhibited a molecular formula of C26H36N4O2 as determined by HRESIMS ([M + Na]+, 459.2721, calcd. 459.2736), implying 11 double bond equivalents (DBE). The IR absorption bands at 1709 and 1610 cm−1 indicated the presence of two carbonyls. The 1H NMR spectrum of 1 (Table 1) showed signals for two aromatic singlets [δH 8.26 (H-2) and 7.69 (H-8)], four olefinic protons [δH 6.07 (H-14′), 5.45 (H-2′), 5.19 (H-10′), and 5.05 (H-6′)], five vinylic methyls [δH 2.10 (H-17′), 1.84 (H-16′), 1.77 (H-20′), 1.58 (H-18′), and 1.56 (H-19′)], one heteroatom-functionalized methyl [δH 3.86 (H-10)], and a series of aliphatic methylene multiplets. The 13C NMR spectrum of 1 (Table 2) resolved 26 resonances attributable to five double bonds (δC 155.5 C, 122.9 CH; 147.3 C, 115.0 C; 143.4 C, 117.6 CH; 135.5 C, 123.5 CH; and 129.6 C, 129.0 CH), two carbonyls (δC 199.3 and 162.0), two imines (δC 147.7 and 140.3), five vinylic methyls (δC 27.7, 20.6, 16.5, 16.4, and 16.0), a N-methyl (δC 35.0), and six sp3 methylenes (δC 55.3, 44.5, 39.4, 39.3, 26.7, and 26.1). As nine of the eleven DBE were accounted for by abovementioned unsaturated functional groups, the remaining two DBE required that 1 was bicyclic. The collective spectroscopic information pointed clearly to a fused diterpene-N-methylhypoxanthine structure, which bore a high similarity to that of malonganenone D [4]. The N-methylhypoxanthine moiety of 1 was readily identified by comparison of its NMR data with that of malonganenone D, which gave almost identical 13C NMR data regarding to this portion. The tetraprenyl side-chain of 1 was deduced from detailed analysis of COSY and HMBC data (Figure 2). 1H–1H COSY correlations revealed four spin systems: (a) H-1′/H-2′/H-3′/H3-20′; (b) H-4′/H-5′/H-6′/H-7′/H3-19′; (c) H-8′/H-9′/H-10′/H-11′/H3-18′; and (d) H-14′/H-15′/H-16′ or H-17′. The connections from a to c were achieved by HMBC correlations of H-20′/C-4′ and H-19′/C-8′. The fragments c and d were linked via a methylene and a ketone (C-13) by HMBC correlations of H-18′/C-12′, H-12′/C-13′, and H-14′/C-13′. The geometries of the three olefins in the tetraprenyl side-chain were established from analysis of both 13C chemical shift and NOE data. The 13C chemical shifts of the vinylic methyls of C-18′, C-19′, and C-20′ (δC 16.4, 16.0, and 16.5, respectively) suggested the E geometries for ∆10′, ∆6′, and ∆2′ as the vinyl methyl corresponding to Z geometry are known to resonate at around 25 ppm [4]. This was further supported by NOE correlations (Figure 2) of H-9′/H-18′ and H-10′/H-12′, H-5′/H-19′ and H-6′/H-8′, and H-1′/H-20′ and H-2′/H-4′, respectively. Finally, the tetraprenyl side-chain was attached to N-7 by HMBC correlations from H-1′ to C-5 and C-8. Thus, compound 1 was determined as depicted and given the trivial name malonganenone L.

Table 1.
1H NMR spectroscopic data for malonganenones L–Q (1–6) (δ in ppm, J in Hz).
| No. | 1 a | 2 a | 3 a | 4 b | 5 b | 6 b |
|---|---|---|---|---|---|---|
| 1 | 7.23, brs | 7.22, brs | ||||
| 2 | 8.26, br s | 8.57, br s | 8.62, br s | 8.23, s | 8.23, s | 8.23, s |
| 8 | 7.69, s | 7.73, s | 7.73, s | 7.59, s | 7.59, s | 7.57, s |
| 10 | 3.86, s | 3.94, s | 3.95, s | 3.13, s | 3.13, s | 3.13, s |
| 11 | 2.84, s | 2.84, s | 2.84, d (4.7) | |||
| 1′ | 5.07, d (7.3) | 5.09, d (7.2) | 5.08, d (7.4) | 4.90, d (7.0) | 4.90, d (7.0) | 4.89, d (7.1) |
| 2′ | 5.45, t (7.0) | 5.47, t (6.9) | 5.47, t (6.9) | 5.40, t (7.1) | 5.41, t (7.0) | 5.39, t (7.0) |
| 4′ | 2.08, m | 2.11, m | 2.11, m | 2.07, m | 2.08, m | 2.09, m |
| 5′ | 2.09, m | 2.11, m | 2.12, m | 2.12, m | 2.13, m | 2.13, m |
| 6′ | 5.05, m | 5.08, m | 5.07, m | 5.14, t (6.2) | 5.14, t (6.1) | 5.14, t (6.3) |
| 8′ | 1.98, m | 1.99, m | 1.97, m | 2.01, m | 2.01, m | 2.02, m |
| 9′ | 2.04, m | 2.07, m | 1.55, m | 2.12, m | 2.12, m | 1.56, m |
| 10′ | 5.19, t (6.4) | 5.32, t (6.6) | 2.06, m | 5.26, t (6.8) | 5.30, t (6.3) | 2.55, br t, (7.9) |
| 12′ | 2.99, s | 3.09, s | 6.00, br s | 3.01, s | 3.12, s | 6.08, br s |
| 14′ | 6.07, br s | 6.06, br s | 6.04, br s | 6.17, br s | 6.15, br s | 6.10, br s |
| 16′ | 1.84, s | 1.87, s | 1.87, d (0.9) | 1.86, d (1.1) | 1.87, d (1.1) | 1.86, d (1.0) |
| 17′ | 2.10, s | 2.13, s | 2.13, d (1.0) | 2.09, d (1.1) | 2.09, d (1.0) | 2.12, d (1.0) |
| 18′ | 1.58, s | 1.69, s | 2.15, d (1.2) | 1.61, s | 1.66, d (1.2) | 1.87, d (1.3) |
| 19′ | 1.56, s | 1.58, s | 1.58, s | 1.59, s | 1.60, s | 1.62, s |
| 20′ | 1.77, s | 1.79, s | 1.80, s | 1.79, s | 1.79, s | 1.79, s |
a Measured at 400 MHz in CDCl3; b Measured at 400 MHz in Acetone-d6.

Table 2.
13C NMR spectroscopic data for malonganenones L–Q (1–6) (δ in ppm).
| No. | 1 a | 2 a | 3 a | 4 b | 5 b | 6 b |
|---|---|---|---|---|---|---|
| 2 | 147.7 | 148.1 | 148.1 | 163.3 | 163.3 | 163.3 |
| 4 | 147.3 | 147.2 | 147.2 | 141.8 | 141.8 | 141.8 |
| 5 | 115.0 | 115.2 | 115.2 | 118.2 | 118.2 | 118.2 |
| 6 | 162.0 | 160.4 | 160.3 | 160.9 | 160.9 | 161.0 |
| 8 | 140.3 | 140.7 | 140.8 | 137.6 | 137.6 | 137.6 |
| 10 | 35.0 | 35.5 | 35.5 | 31.9 | 31.9 | 31.9 |
| 11 | 26.1 | 26.1 | 26.3 | |||
| 1′ | 44.5 | 44.6 | 44.7 | 45.2 | 45.2 | 45.2 |
| 2′ | 117.6 | 117.3 | 117.4 | 120.5 | 120.5 | 120.4 |
| 3′ | 143.4 | 143.7 | 143.7 | 142.0 | 142.0 | 142.0 |
| 4′ | 39.4 | 39.5 | 39.4 | 40.1 | 40.2 | 40.2 |
| 5′ | 26.1 | 26.1 | 26.1 | 27.0 | 26.9 | 26.9 |
| 6′ | 123.5 | 123.6 | 123.9 | 124.8 | 124.8 | 124.8 |
| 7′ | 135.5 | 135.5 | 135.4 | 135.8 | 135.8 | 136.0 |
| 8′ | 39.3 | 39.5 | 39.1 | 40.1 | 40.3 | 40.6 |
| 9′ | 26.7 | 26.9 | 25.8 | 27.4 | 27.7 | 27.2 |
| 10′ | 129.0 | 128.2 | 40.8 | 129.4 | 128.7 | 33.8 |
| 11′ | 129.6 | 129.2 | 157.8 | 130.7 | 130.2 | 158.6 |
| 12′ | 55.3 | 47.9 | 125.7 | 55.8 | 48.3 | 126.9 |
| 13′ | 199.3 | 198.6 | 191.7 | 198.8 | 198.1 | 191.0 |
| 14′ | 122.9 | 123.0 | 126.3 | 123.7 | 124.0 | 127.0 |
| 15′ | 155.5 | 155.8 | 154.2 | 155.0 | 155.3 | 154.2 |
| 16′ | 27.7 | 27.7 | 27.7 | 27.5 | 27.5 | 27.6 |
| 17′ | 20.6 | 20.7 | 20.5 | 20.5 | 20.5 | 20.4 |
| 18′ | 16.4 | 24.1 | 19.1 | 16.5 | 24.4 | 25.5 |
| 19′ | 16.0 | 16.0 | 15.9 | 16.1 | 16.1 | 16.0 |
| 20′ | 16.5 | 16.6 | 16.6 | 16.5 | 16.5 | 16.5 |
a Measured at 100 MHz in CDCl3; b Measured at 100 MHz in Acetone-d6.
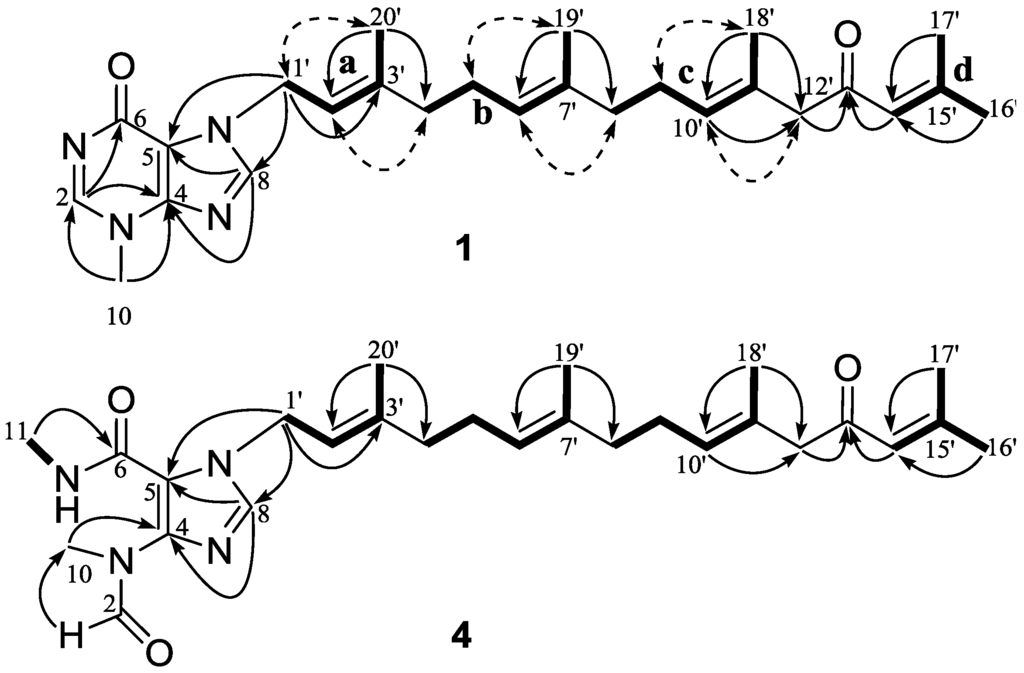
Figure 2.
Key 1H–1H COSY (–), HMBC (→) and NOESY (dashed arrows) correlations for 1 and 4.
Compound 2 exhibited the same molecular formula of C26H36N4O2 as 1 on the basis of the HRESIMS data ([M + Na]+, 459.2725, calcd. 459.2736). The NMR spectroscopic data of 2 (Table 1 and Table 2) was very similar to that of 1. In comparison with 1, the 13C NMR spectroscopic data for 2 differed significantly about ∆10′ moiety, with the upfield-shifted carbon at C-12′ and the downfield-shifted vinyl methyl at C-18′ (δC 55.3 and 16.3 in 1; δC 47.9 and 24.1 in 2, respectively). This indicated that ∆10′ in 2 adopts a Z configuration. Similar 13C NMR changes were also reported in malonganenone I [5], which possessed the same Z configuration of ∆10′ as 2. Thus, compound 2 was determined as depicted and named malonganenone M.
Compound 3 had a molecular formula of C26H36N4O2 as established by HRESIMS data. The 1H and 13C NMR data of 3 (Table 1 and Table 2) showed high similarity to those of 1 except that the ∆10′ double bond in 1 was migrated to ∆11′, forming a conjugated system with the C-13′ carbonyl. This was suggested by the significant downfield-shifted carbon at C-11′ and the upfield-shifted carbon at C-13′ as compared with those of 1 (δC 129.6 and 199.3 in 1; δC 157.8 and 191.7 in 3, respectively), and by the presence of a singlet olefinic signal (δH 6.00, H-12′) in the 1H NMR spectra of 3 instead of a triplet olefinic signal (δH 5.19, t, J = 6.4 Hz, H-10′) in 1. The configuration of ∆11′ in 3 was established to be E by the characteristic chemical shift of the vinylic methyl at C-18′ (δC 19.1) and by comparison of its NMR data with those of reported. Therefore, the structure of compound 3 was determined as depicted and given the trivial name malonganenone N.
Compound 4 exhibited an [M − H]− ion at m/z 467.3021 (calcd. for C27H39N4O3, 467.3022), suggesting the molecular formula C27H40N4O3 (ten DBE). The 1H and 13C NMR spectra of 4 (Table 1 and Table 2) bore a resemblance to those of 1, with the notable differences occurring in the hypoxanthine core. The NMR spectra of 4 showed the presence of an N-methylamide (δH 2.84, H-11; δC 26.1, C-11) and an N-methylformamide (δH 8.23, H-2 and 3.13, H-10; δC 163.3, C-2 and 31.9, C-10) groups, which were identical to those previously reported in malonganenones B, F, and G, indicating that 4 possessed the same trisubstituted imidazole ring. This was further supported by HMBC correlations (Figure 2) of H3-11/C-6, H3-10/C-4, and H-2/C-10. Thus, the structure of compound 4 was determined as depicted and given the trivial name malonganenone O.
Compound 5 had a molecular formula C27H40N4O3 by analysis of the HRESIMS data. Comparing the NMR data (Table 1 and Table 2) of 5 and 4, it appeared that the former had a Z configuration of ∆10′ instead of an E configuration of ∆10′ in 4. This was suggested by the upfield-shifted carbon at C-12′ and the downfield-shifted vinyl methyl at C-18′ (δC 48.3 and 24.4 in 5; δC 55.8 and 16.5 in 4, respectively). Thus, the structure of compound 5 was determined as depicted and given the trivial name malonganenone P.
The molecular formula of compound 6 was established as C27H40N4O3 by HRESIMS. The NMR data of 6 (Table 1 and Table 2) showed high similarity to that of 4 except that the ∆10′ double bond in 6 was migrated to ∆11′, forming a conjugated system with the C-13′ carbonyl. This was further suggested by the significant downfield-shifted carbon at C-11′ and the upfield-shifted carbon at C-13′ as compared with those of 4 (δC 130.7 and 198.8 in 4; δC 158.6 and 191.0 in 6, respectively), and by the presence of a singlet olefinic signal (δH 6.08, H-12′) in the 1H NMR spectra of 6 instead of a triplet olefinic signal (δH 5.26, t, J = 6.8 Hz, H-10′) in 4. The characteristic chemical shift of the vinylic methyl at C-18′ (δC 25.5) indicated the Z configuration of ∆11′ in 6. Thus, the structure of 6 was determined as depicted and given the trivial name malonganenone Q.
2.2. In Vitro Inhibitory Activity Screening against PDEs
Compounds 1–6 were screened for their inhibitory activities against PDE4D, PDE5A, and PDE9A by using our previously reported methods [20,21,22,23]. As shown in Table 3, all compounds exhibited inhibition at 50 µM against PDE4D with degree of inhibition from 72% to 85%, while displaying weaker activities against PDE5A and PDE9A. The two most active compounds, 1 and 6, were selected to test for the half maximal inhibitory concentration (IC50), which gave IC50 values of 8.5 and 20.3 µM, respectively (Figure 3).

Table 3.
Inhibitory activities of compounds 1–6 at 5 µM and 50 µM towards PDE4D, PDE5A and PDE9A.
| Compound | Inhibition (%) of Compounds at 50 µM | Inhibition (%) of Compounds at 5 µM | |||||
|---|---|---|---|---|---|---|---|
| PDE4D | PDE5A | PDE9A | PDE4D | PDE5A | PDE9A | ||
| 1 | 85 | 53 | 18 | 17 | 11 | <10 | |
| 2 | 72 | 32 | 15 | <10 | <10 | <10 | |
| 3 | 81 | 35 | 27 | 17 | <10 | 10 | |
| 4 | 79 | 38 | <10 | 18 | <10 | <10 | |
| 5 | 75 | 36 | 11 | 14 | <10 | <10 | |
| 6 | 85 | 38 | 15 | 18 | <10 | <10 | |
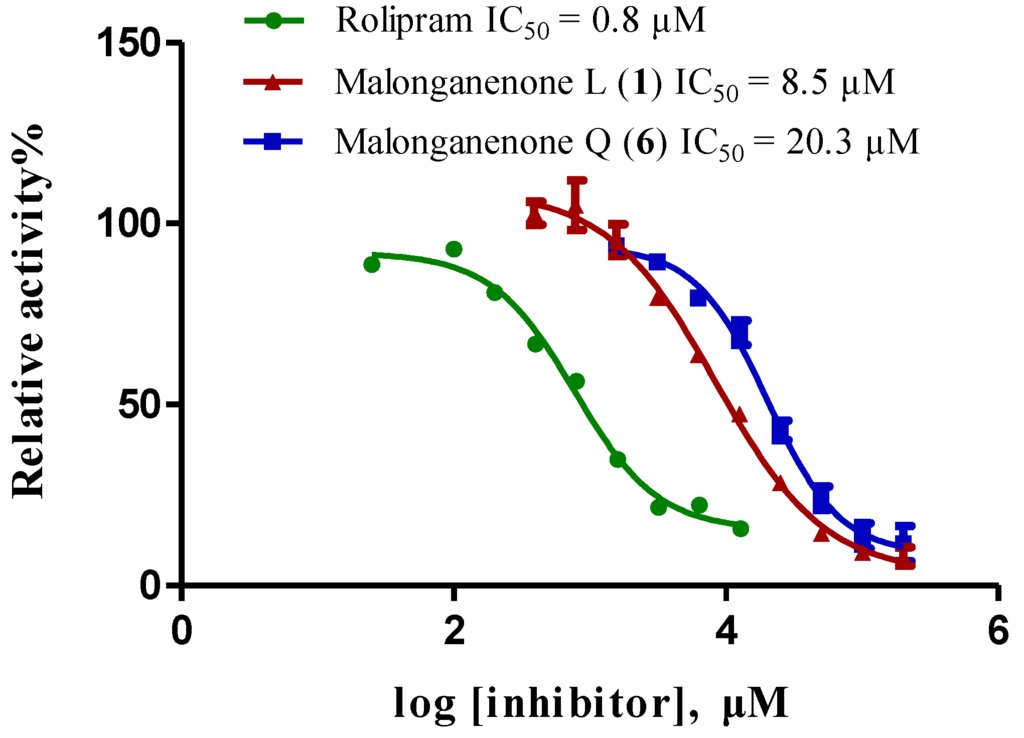
Figure 3.
Inhibition of phosphodiesterase-4D by compounds 1 and 6 (rolipram as positive control).
3. Experimental Section
3.1. General Experimental Procedures
UV spectra were recorded on a Shimadzu UV-2450 spectrophotometer. IR spectra were determined on a Bruker Tensor 37 infrared spectrophotometer with KBr disks. NMR spectra were measured on a Bruker AM-400 spectrometer at 25 °C. ESIMS and HRESIMS were carried out on a Finnigan LC QDECA instrument. A Shimadzu LC-20 AT equipped with an SPD-M20A PDA detector was used for HPLC, a YMC-pack ODS-A column (250 × 10 mm, 5 µm, 12 nm) and a chiral column (Phenomenex Lux, cellulose-2, 250 × 10 mm, 5 µm) was used for semipreparative HPLC separation. Silica gel (300–400 mesh, Qingdao Marine Chemical Co., Ltd., Qingdao, Shandong, China), C18 reversed-phase (Rp-C18) silica gel (12 nm, 50 µm, YMC Co., Ltd., Kyoto, Japan), Sephadex LH-20 gel (Amersham Biosciences, Piscataway, NJ, USA), used for column chromatography (CC). All solvents used were of analytical grade (Guangzhou Chemical Reagents Company, Ltd., Guangzhou, Guangdong, China).
3.2. Animal Material
The gorgonian E. pseudosassapo was collected at a depth of 18–25 m in 29 July 2012 in Daya Bay of Guangdong Province, China and frozen immediately after collection, and were identified by one of the authors (Cheng-Qi Fan). A voucher specimen (accession number: LSH201207) has been deposited at the School of Pharmaceutical Sciences, Sun Yat-sen University, China.
3.3. Extraction and Isolation
Specimens of E. pseudosassapo (550 g, wet weight) were extracted with CH2Cl2/MeOH (1:1, 3 × 1 L) at room temperature (rt) to give 13.7 g of crude extract. The crude extract was subjected to silica gel column chromatography eluted with a CH2Cl2/MeOH gradient (9:1→1:9) to afford five fractions (Fr. I–V). Fr. III (1.4 g) was chromatographed over Sephadex LH-20 (CH2Cl2/MeOH, v/v, 1:1), followed by Rp-C18 silica gel eluted with a CH3CN/H2O gradient (5:5→10:0) to obtain four sub-fractions (Fr. IIIa–IIId). Fr. IIIb was further separated by HPLC equipped with a chiral column (CH3CN, 3 mL/min) to afford 1 (17 mg, tR 17 min) and 2 (4.9 mg, tR 20 min). Fr. IIId was purified by repeating the HPLC conditions described above to yield 4 (11 mg, tR 23 min) and 5 (3.7 mg, tR 27 min). Fr. IIIc was chromatographed by HPLC equipped with an ODS-A column (CH3CN/H2O, 90:10, 3 mL/min) to afford 3 (5.2 mg, tR 15 min) and 6 (5.1 mg, tR 20min).
Malonganenone L (1): colorless oil; UV (MeOH) λmax (log ε) 211 (4.42), 225 (4.38), 253 (4.31) nm; IR νmax 3145, 1709, 1610, 1464, 1250, 1128, 1060 cm−1; 1H and 13C NMR, see Table 1 and Table 2; HRESIMS [M + Na]+ m/z 459.2721 (calcd. for C26H36N4O2Na, 459.2736).
Malonganenone M (2): colorless oil; UV (MeOH) λmax (log ε) 209 (4.22), 225 (4.17), 254 (4.06) nm; IR νmax 3011, 1714, 1607, 1458, 1240, 1123, 1046 cm−1; 1H and 13C NMR, see Table 1 and Table 2; HRESIMS [M + Na]+ m/z 459.2725 (calcd. for C26H36N4O2Na, 459.2736).
Malonganenone N (3): colorless oil; UV (MeOH) λmax (log ε) 208 (3.68), 223 (3.60), 254 (3.16) nm; IR νmax 2937, 1732, 1627, 1439, 1379, 1215, 1136, 1039 cm−1; 1H and 13C NMR, see Table 1 and Table 2; HRESIMS [M + Na]+ m/z 459.2725 (calcd. for C26H36N4O2Na, 459.2736).
Malonganenone O (4): colorless oil; UV (MeOH) λmax (log ε) 211 (4.39), 249 (4.22) nm; IR νmax 2932, 1744, 1654, 1514, 1445, 1218, 1127, 1033 cm−1; 1H and 13C NMR, see Table 1 and Table 2; HRESIMS [M − H]− m/z 467.3021 (calcd. for C27H39N4O3, 467.3022).
4. Conclusions
In our continuing investigation on the chemical constituents of marine invertebrates collected from the South China Sea, six new tetraprenylated alkaloids, designated as malonganenones L–Q (1–6), were isolated from the gorgonian Echinogorgia pseudossapo. The structures of 1–6 featuring a methyl group at N-3 and a tetraprenyl chain at N-7 in the hypoxanthine core were established by extensive spectroscopic analyses. Compounds 1–6 were tested for their inhibitory activity against the phosphodiesterases (PDEs)-4D, 5A, and 9A, and compounds 1 and 6 exhibited moderate inhibitory activity against PDE4D with IC50 values of 8.5 and 20.3 µM, respectively. Phosphodiesterase-4 (PDE4), which specifically catalyzes the hydrolysis of cyclic adenosine monophosphate (cAMP), is a therapeutic target of high interest for central nervous system (CNS), inflammatory, and respiratory diseases [24]. Natural PDE4 inhibitors are very rare. To the best of our knowledge, this is the first investigation of this group of compounds on the inhibitory activity of the phosphodiesterases.
Supplementary Files
Acknowledgments
The authors thank the National Natural Science Foundation of China (No. 81102339) and the Key Laboratory of East China Sea & Oceanic Fishery Resources Exploitation and Utilization, Ministry of Agriculture, China (No. K201203) for providing financial support to this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berrue, F.; Kerr, R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef]
- Wang, L.H.; Sheu, J.H.; Kao, S.Y.; Su, J.H.; Chen, Y.H.; Chen, Y.H.; Su, Y.D.; Chang, Y.C.; Fang, L.S.; Wang, W.H.; et al. Natural product chemistry of gorgonian corals of the family Plexauridae distributed in the Indo-Pacific Ocean. Mar. Drugs 2012, 10, 2415–2434. [Google Scholar] [CrossRef]
- Keyzers, R.A.; Gray, C.A.; Schleyer, M.H.; Whibley, C.E.; Hendricks, D.T.; Davies-Coleman, M.T. Malonganenones A–C, novel tetraprenylated alkaloids from the Mozambique gorgonian Leptogorgia gilchristi. Tetrahedron 2006, 62, 2200–2206. [Google Scholar] [CrossRef]
- Sorek, H.; Rudi, A.; Benayahu, Y.; Ben-Califa, N.; Neumann, D.; Kashman, Y. Nuttingins A–F and malonganenones D–H, tetraprenylated alkaloids from the Tanzanian gorgonian Euplexaura nuttingi. J. Nat. Prod. 2007, 70, 1104–1109. [Google Scholar] [CrossRef]
- Zhang, J.R.; Li, P.L.; Tang, X.L.; Qi, X.; Li, G.Q. Cytotoxic tetraprenylated alkaloids from the South China Sea gorgonian Euplexaura robusta. Chem. Biodivers. 2012, 9, 2218–2224. [Google Scholar] [CrossRef]
- Gao, C.; Qi, S.; Luo, X.; Wang, P.; Zhang, S. Sterols from the South China Sea gorgonian Echinogorgia pseudossapo. Tianran Chanwu Yanjiu Yu Kaifa 2011, 23, 1006–1010. (in Chinese). [Google Scholar]
- Xu, W.; Liao, X.; Xu, S.; Liao, L. Isolation and identification of two polyhydroxylated sterols. Guangdong Huagong 2012, 39, 305–306. (in Chinese). [Google Scholar]
- Qou, Y.; Qi, S.; Zhang, S.; Yang, J.; Xiao, Z. New polyoxygenated steroids from the South China Sea gorgonian Echinogorgia aurantiaca. Pharmazie 2006, 61, 645–647. [Google Scholar]
- Zeng, L.; Wu, J. Structure determination of echifloristerol. Zhongshan Daxue Xuebao Ziran Kexueban 1989, 28, 28–31. (in Chinese). [Google Scholar]
- Li, R.; Long, K.; Zhou, Y.; Chen, F. Studies on the Chinese gorgonian. Zhongshan Daxue Xuebao Ziran Kexueban 1982, 4, 65–69. (in Chinese). [Google Scholar]
- Guo, Q.; Wei, Y.; Wang, C.; Shao, C.; Li, L.; Liu, X.; Guo, Q. Study on chemical constituents of Echinogorgia sassapo reticulata (esper). Zhongguo Haiyang Yaowu 2010, 29, 32–35. [Google Scholar]
- Su, J.; Long, K.; Jian, Z. Chemical constituents of Chinese gorgonia. (V). A new marine C29-sterol from Echinogorgia pseudossapo (Koelliker). Zhongshan Daxue Xuebao Ziran Kexueban 1984, 1, 97–101. (in Chinese). [Google Scholar]
- Liao, L.; Liao, X.J.; Xu, S.H. Compounds with nitrogen in coral Echinogorgia sp. Tianran Chanwu Yanjiu Yu Kaifa 2010, 22, 392–394. [Google Scholar]
- Gao, C.H.; Wang, Y.F.; Li, S.; Qian, P.Y.; Qi, S.H. Alkaloids and sesquiterpenes from the South China Sea gorgonian Echinogorgia pseudossapo. Mar. Drugs 2011, 9, 2479–2487. [Google Scholar]
- Tanaka, J.; Miki, H.; Higa, T. Echinofuran, a new furanosesquiterpene from the gorgonian Echinogorgia praelonga. J. Nat. Prod. 1992, 55, 1522–1524. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Lopez, G.M.P.; Gavagnin, M.; Villani, G.; Naik, C.G.; Cimino, G. New bioactive hydrogenated linderazulene-derivatives from the gorgonian Echinogorgia complexa. Tetrahedron Lett. 2007, 48, 2569–2571. [Google Scholar]
- Liao, L.; Wang, N.; Liang, Q.; Liao, X.J.; Xu, S.H. Three ceramides from gorgonian Echinogorgia sp. Zhongcaoyao 2010, 41, 851–854. (in Chinese). [Google Scholar]
- Tillekeratne, L.M.V.; de Silva, E.D.; Mahindaratne, M.P.D.; Schmitz, F.J.; Gunasekera, S.P.; Alderslade, P. Xanthyletin and xanthoxyletin from a gorgonian, Echinogorgia sp. J. Nat. Prod. 1989, 52, 1303–1304. [Google Scholar] [CrossRef]
- Han, Q.H.; Fan, C.Q.; Lu, Y.N.; Wu, J.P.; Liu, X.; Yin, S. Chemical constituents from the ascidian Aplidium constellatum. Biochem. Syst. Ecol. 2013, 48, 6–8. [Google Scholar] [CrossRef]
- Chen, S.K.; Zhao, P.; Shao, Y.X.; Li, Z.; Zhang, C.; Liu, P.; He, X.; Luo, H.B.; Hu, X. Moracin M from Morus alba L. is a natural phosphodiesterase-4 inhibitor. Bioorg. Med. Chem. Lett. 2012, 22, 3261–3264. [Google Scholar] [CrossRef]
- Li, Z.; Cai, Y.H.; Cheng, Y.K.; Lu, X.; Shao, Y.X.; Li, X.; Liu, M.; Liu, P.; Luo, H.B. Identification of novel phosphodiesterase-4D inhibitors prescreened by molecular dynamics-augmented modeling and validated by bioassay. J. Chem. Inf. Model. 2013, 53, 972–981. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, S.K.; Cai, Y.H.; Lu, X.; Li, Z.; Cheng, Y.K.; Zhang, C.; Hu, X.; He, X.; Luo, H.B. The molecular basis for the inhibition of phosphodiesterase-4D by three natural resveratrol analogs. Isolation, molecular docking, molecular dynamics simulations, binding free energy, and bioassay. Biochim. Biophys. Acta 2013, 1834, 2089–2096. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, Y.; Ding, W.; Wu, X.; Wan, J.; Luo, H. Chemical constituents of Aloe barbadensis Miller and their inhibitory effects on phosphodiesterase-4D. Fitoterapia 2013, 91, 159–165. [Google Scholar] [CrossRef]
- Burgin, A.B.; Magnusson, O.T.; Singh, J.; Witte, P.; Staker, B.L.; Bjornsson, J.M.; Thorsteinsdottir, M.; Hrafnsdottir, S.; Hagen, T.; Kiselyov, A.S.; et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat. Biotechnol. 2010, 28, 63–72. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).