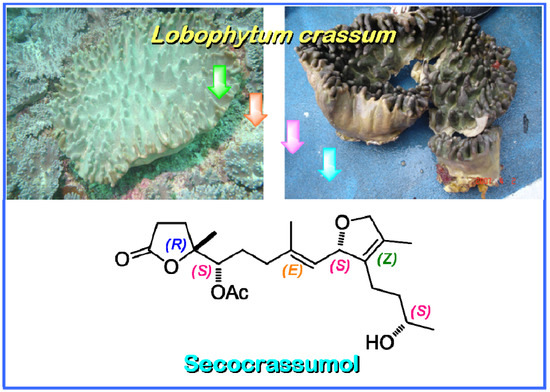
Secocrassumol, a seco-Cembranoid from the Dongsha Atoll Soft Coral Lobophytum crassum
Abstract
:1. Introduction
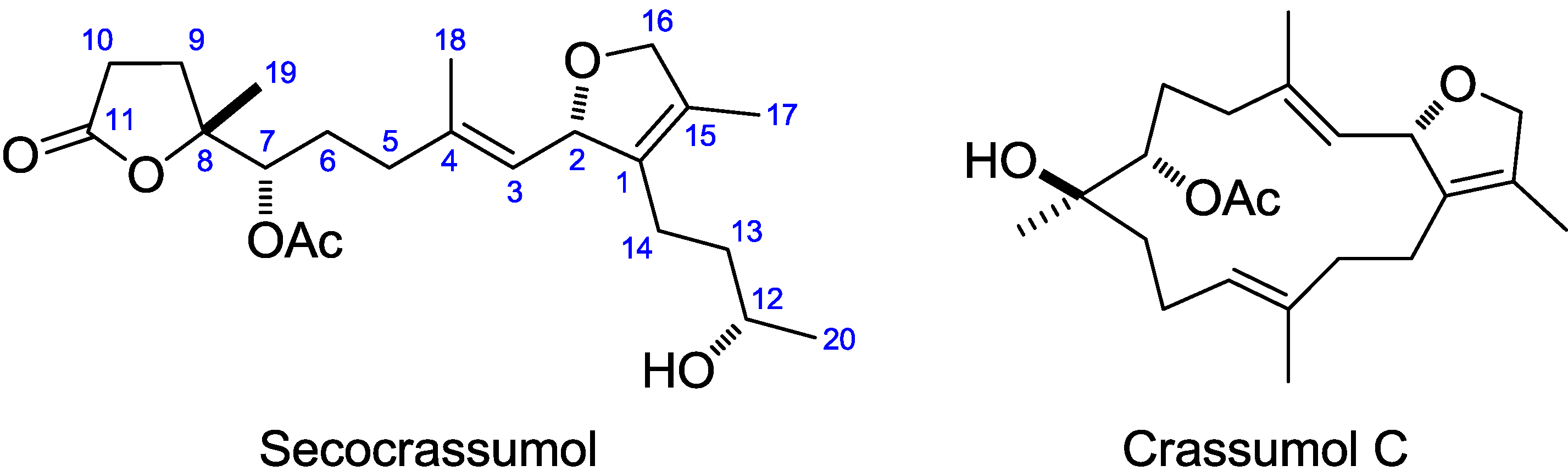

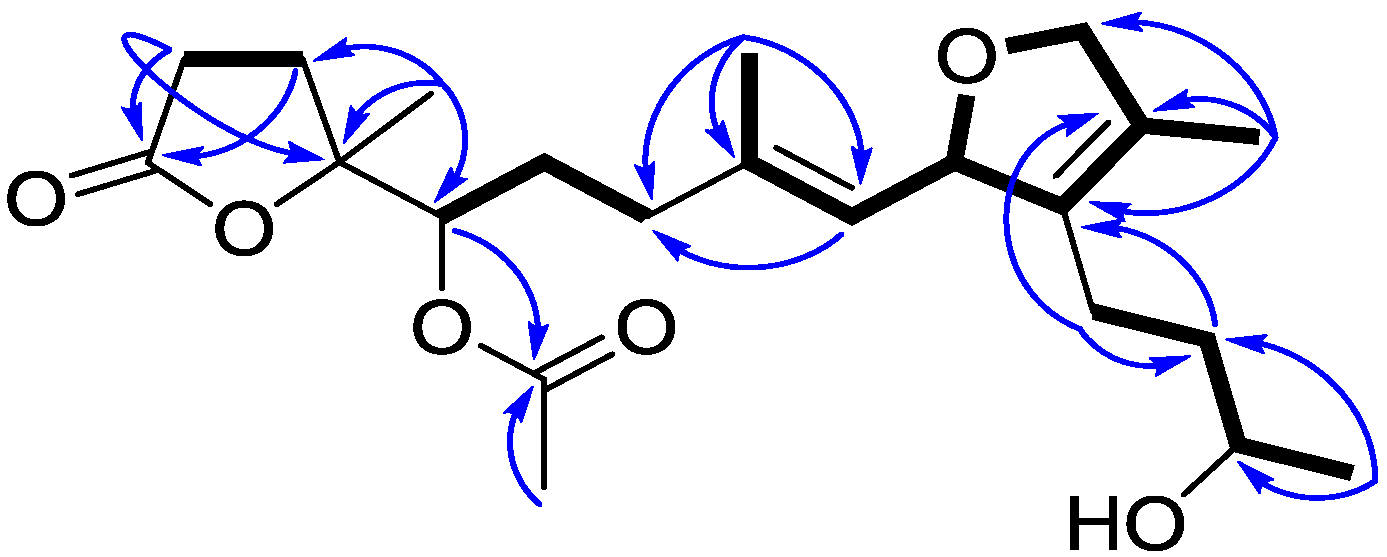
2. Results and Discussion
| # | 13C | 1H | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 133.0 (qC) b | |||
| 2 | 84.4 (CH) | 5.42 m | H-3, H-16, H-17 | |
| 3 | 126.0 (CH) | 5.09 br·d (9.2, 0.8) c | H-2, H-18 | C-5, C-18 |
| 4 | 138.2 (qC) | |||
| 5 | 35.8 (CH2) | 2.04 m | H-6 | |
| 6 | 27.6 (CH2) | a: 1.94 m | H-5, H-7 | |
| 7 | 75.9 (CH) | 5.01 dd (10.4, 2.4) | H-6 | C-8, C-9, 7-OAc |
| 8 | 82.6 (qC) | |||
| 9 | 29.9 (CH2) | a: 2.22 m | H-10 | C-10, C-11, C-19 |
| 10 | 28.7 (CH2) | 2.61 m | H-9 | C-8, C-9, C-11 |
| 11 | 176.2 (qC) | |||
| 12 | 68.0 (CH) | 3.76 m | H-13, H-20 | |
| 13 | 37.5 (CH2) | 1.49 m | H-12, H-14 | C-1, C-14, C-20 |
| 14 | 21.1 (CH2) | a: 2.24 m | H-13 | C-1, C-2, C-13 |
| 15 | 128.4 (qC) | |||
| 16 | 78.1 (CH2) | a: 4.54 dd (11.6, 5.6) | H-2, H-17 | C-1 |
| 17 | 10.0 (CH3) | 1.66 s | H-2, H-16 | C-1, C-15, C-16 |
| 18 | 16.4 (CH3) | 1.76 s | H-3 | C-3, C-4, C-5 |
| 19 | 22.5 (CH3) | 1.40 s | C-7, C-8, C-9 | |
| 20 | 23.3 (CH3) | 1.19 d (6.0) | H-12 | C-12, C-13 |
| 7-OAc | 20.9 (CH3) | 2.09 s | 7-OAc | |


3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Preparation of (R)- and (S)-MTPA Esters of Secocrassumol
3.5. Cytotoxicity Assay
3.6. Anti-HCMV Assay
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coll, J.C. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 1992, 92, 613–631. [Google Scholar] [CrossRef]
- Haagen-Smit, A.J.; Wang, T.H.; Mirov, N.T. Composition of gum turpentines of pines. XIII. A report on Pinus albicaulis. J. Am. Pharm. Assoc. Sci. Ed. 1951, 40, 557–559. [Google Scholar] [CrossRef]
- Ciereszko, L.S.; Sifford, D.H.; Weinheimer, A.J. Chemistry of coelenterates. I. Occurrence of terpenoid compounds in gorgonians. Ann. N.Y. Acad. Sci. 1960, 90, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323, and references cited therein. [Google Scholar] [CrossRef] [PubMed]
- Kashman, Y.; Groweiss, A. Lobolide: A new epoxy cembranolide from marine origin. Tetrahedron Lett. 1977, 13, 1159–1160. [Google Scholar] [CrossRef]
- Kashman, Y.; Carmely, S.; Groweiss, A. Further cembranoid derivatives from the Red Sea soft corals Alcyonium flaccidum and Lobophytum crassum. J. Org. Chem. 1981, 46, 3592–3596. [Google Scholar] [CrossRef]
- Kinamoni, Z.; Groweiss, A.; Carmely, S.; Kashman, Y. Several new cembranoid diterpenes from three soft corals of the Red Sea. Tetrahedron 1983, 39, 1643–1648. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Tapiolas, D.M. Studies of Australian soft corals. XXXIII. New cembranoid diterpenes from a Lobophytum species. Aust. J. Chem. 1983, 36, 2289–2295. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Decosta, M.S.L.; Desilva, E.D.; Mackay, M.F.; Mahendran, M.; Willis, R.H. The structure determination of a new cembranolide diterpene from the soft coral Lobophytum cristigalli (Coelenterata, Octocorallia, Alcyonacea). Aust. J. Chem. 1984, 34, 545–552. [Google Scholar] [CrossRef]
- Bowdon, B.F.; Coll, J.C.; Heaton, A.; Konig, G.; Bruck, M.A.; Cramer, R.E.; Klein, D.M.; Scheuer, P.J. The structures of four isomeric dihydrofuran-containing cembranoid diterpenes from several species of soft coral. J. Nat. Prod. 1987, 50, 650–659. [Google Scholar] [CrossRef]
- Wang, S.-K.; Duh, C.-Y.; Wu, Y.-C.; Wang, Y.; Cheng, M.-C.; Soong, K.; Fang, L.-S. Cytotoxic cembranolides from the soft coral Lobophytum michaelae. J. Nat. Prod. 1992, 55, 1430–1435. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Rao, C.V.; Anjaneyulu, V.; Satyanarayana, P.; Rao, P.V.S. New diterpenes from a new species of Lobophytum soft coral of the South Andaman coast. Tetrahedron 1992, 48, 3111–3120. [Google Scholar] [CrossRef]
- Coval, S.J.; Patton, R.W.; Petrin, J.M.; James, L.; Rothofsky, M.L.; Lin, S.L.; Patel, M.; Reed, J.K.; McPhil, A.T.; Bishop, W.R. A cembranolide diterpene farnesyl protein transferase inhibitor from the marine soft coral Lobophytum cristagalli. Bioorg. Med. Chem. Lett. 1996, 6, 909–912. [Google Scholar] [CrossRef]
- Yamada, K.; Ryu, K.; Miyamoto, T.; Higuchi, R. Bioactive terpenoids from octocorallia. 4. Three new cembrane-type diterpenoids from the soft coral Lobophytum schoedei. J. Nat. Prod. 1997, 60, 798–801. [Google Scholar] [CrossRef]
- Higuchi, R.; Miyamoto, T.; Yamada, K.; Komori, T. Cytotoxic and ichthyotoxic compounds from marine opisthobranchia and soft coral. Toxicon 1998, 36, 1703–1705. [Google Scholar] [CrossRef]
- Matthee, G.F.; Konig, G.M.; Wright, A.D. Three new diterpenes from the marine soft coral Lobophytum crassum. J. Nat. Prod. 1998, 61, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Gustafson, K.R.; Boyd, M.R. HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species. J. Nat. Prod. 2000, 63, 531–533. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Huang, B.-T.; Dai, C.-F. Cytotoxic cembrenolide diterpenes from the Formosan soft coral Lobophytum crassum. J. Nat. Prod. 2000, 63, 884–885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Krohn, K.; Ding, J.; Miao, Z.-H.; Zhou, X.-H.; Chen, S.-H.; Pescitelli, G.; Salvadori, P.; Kurtan, T.; Guo, Y.-W. Structural and stereochemical studies of α-methylene-γ-lactone-bearing cembrane diterpenoids from a South China sea soft coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Wen, Z.-H.; Chiou, S.-F.; Wang, S.-K.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Durumolides A–E, Anti-inflammatory and antibacterial cembranolides from the soft coral Lobophytum durum. Tetrahedron 2008, 64, 9698–9704. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wen, Z.-H.; Wang, S.-K.; Chiou, S.-F.; Hsu, C.-H.; Dai, C.-F.; Duh, C.-Y. Anti-inflammatory cembranolides from the soft coral Lobophytum durum. Bioorg. Med. Chem. 2009, 17, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Wen, Z.-H.; Chiou, S.-F.; Wang, S.-K.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod. 2009, 72, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-T.; Wang, S.-K.; Cheng, S.-Y.; Duh, C.-Y. Lobocrasol, a new diterpenoid from the soft coral Lobophytum crassum. Org. Lett. 2009, 14, 3012–3014. [Google Scholar] [CrossRef]
- Lin, S.-T.; Wang, S.-K.; Duh, C.-Y. Cembranoids from the Dongsha Atoll soft coral Lobophytum crassum. Mar. Drugs. 2011, 9, 2705–2716. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Lin, S.-T.; Wang, S.-K.; Duh, C.-Y. α-Tocopherols from the Formosan soft coral Lobophytum crassum. Bull. Chem. Soc. Jpn. 2011, 7, 783–787. [Google Scholar] [CrossRef]
- Wahlberg, I.; Arndt, R.; Nishida, T.; Enzell, C.R. Tobacco chemistry. 63. Syntheses and stereostructures of six tobacco seco-cembranoids. Acta. Chem. Scand. 1986, 40B, 123–134. [Google Scholar] [CrossRef]
- Iwashima, M.; Matsumoto, Y.; Takenaka, Y.; Iguchi, K.; Yamori, T. New marine diterpenoids from the Okinawan soft coral Clavularia koellikeri. J. Nat. Prod. 2002, 65, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Kobayashi, M. Marine terpenes and terpenoids. IV. Isolation of new cembranoid and secocembranoid lactones from the soft coral Sinularia mayi. Chem. Pharm. Bull. 1988, 36, 488–494. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Acosta, A.L. New cembranoid diterpenes and a geranylgeraniol derivative from the common Caribbean sea whip Eunicea succinea. J. Nat. Prod. 1997, 60, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa Reddy, N.; Venkateshwar Goud, T.; Venkateswarlu, Y. Seco-sethukarailin, a novel diterpenoid from the soft coral Sinularia dissecta. J. Nat. Prod. 2002, 65, 1059–1060. [Google Scholar] [CrossRef] [PubMed]
- Ospina, C.A.; Rodríguez, A.D.; Sánchez, J.A.; Ortega-Barria, E.; Capson, T.L.; Mayer, A.M.S. Caucanolides A‒F, unusual antiplasmodial constituents from a Colombian collection of the gorgonian coral Pseudopterogorgia bipinnata. J. Nat. Prod. 2005, 68, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; Kehraus, S.; Nett, M.; König, G.M.; Beil, W.; Wright, A.D. New cytotoxic cembrane based diterpenes from the soft corals Sarcophyton cherbonnieri and Nephthea sp. Org. Biomol. Chem. 2003, 1, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-J.; Tseng, Y.-J.; Huang, C.-Y.; Wen, Z.-H.; Dai, C.-F.; Sheu, J.-H. Cytotoxic and anti-inflammatory diterpenoids from the Dongsha Atoll soft coral Sinularia flexibilis. Tetrahedron 2012, 68, 244–249. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; MacDonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological syatems. Cancer Chemother. Rep. 1972, 3, 1–91. [Google Scholar]
- Hou, R.-S.; Duh, C.-Y.; Chiang, M.Y.; Lin, C.-N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995, 58, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Balzarini, J.; Tabarrini, O.; Andrei, G.; Snoeck, R.; Cecchetti, V.; Fravolini, A.; de Clercq, E.; Pannecouque, C. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother. 2005, 56, 847–855. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.-Y.; Wang, S.-K.; Duh, C.-Y. Secocrassumol, a seco-Cembranoid from the Dongsha Atoll Soft Coral Lobophytum crassum. Mar. Drugs 2014, 12, 6028-6037. https://doi.org/10.3390/md12126028
Cheng S-Y, Wang S-K, Duh C-Y. Secocrassumol, a seco-Cembranoid from the Dongsha Atoll Soft Coral Lobophytum crassum. Marine Drugs. 2014; 12(12):6028-6037. https://doi.org/10.3390/md12126028
Chicago/Turabian StyleCheng, Shi-Yie, Shang-Kwei Wang, and Chang-Yih Duh. 2014. "Secocrassumol, a seco-Cembranoid from the Dongsha Atoll Soft Coral Lobophytum crassum" Marine Drugs 12, no. 12: 6028-6037. https://doi.org/10.3390/md12126028