Xyloketal B Exhibits Its Antioxidant Activity through Induction of HO-1 in Vascular Endothelial Cells and Zebrafish
Abstract
:1. Introduction
2. Results
2.1. Involvement of HO-1 in the Protective Effects of Xyloketal B on AngII-Induced Apoptosis in HUVECs
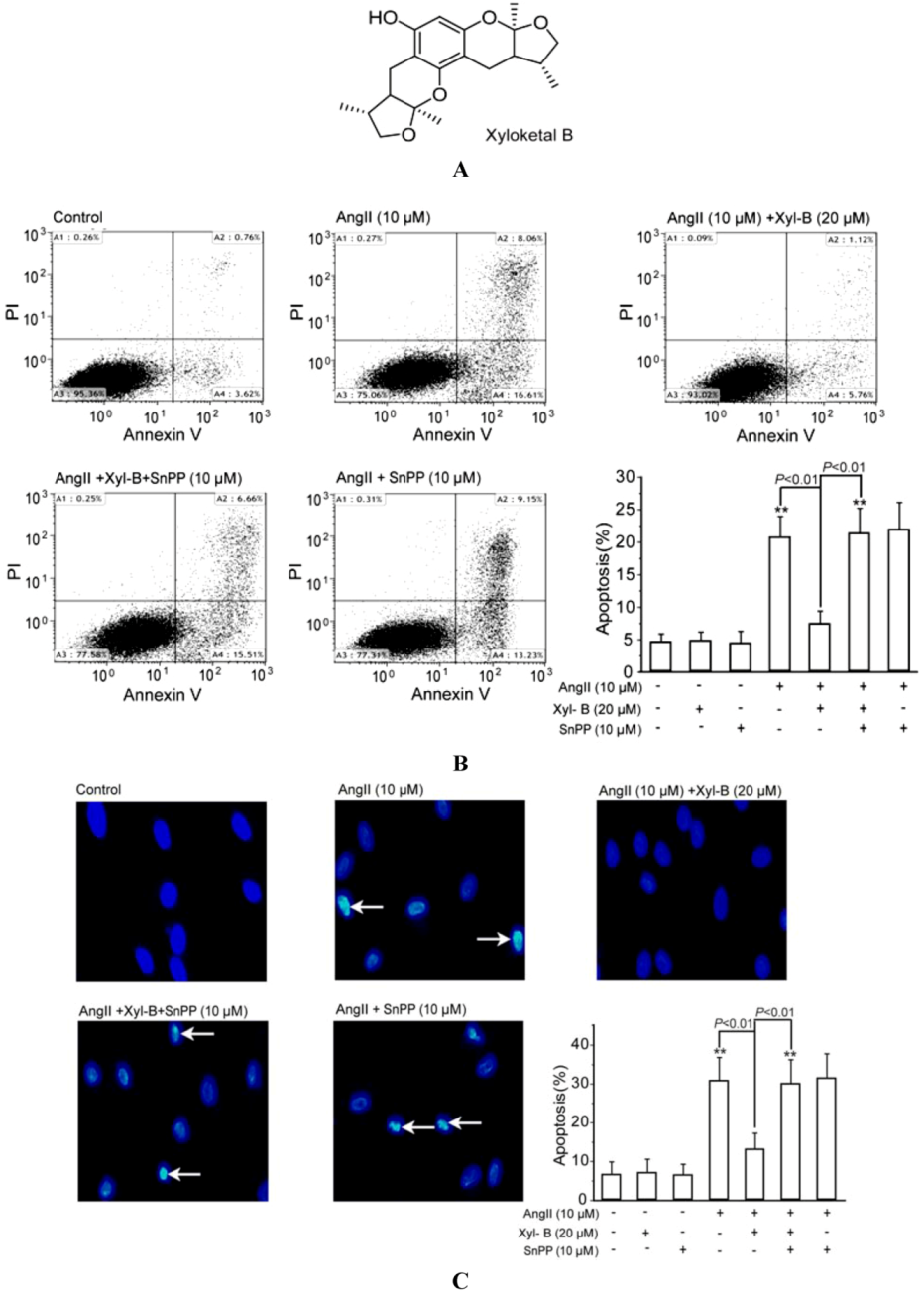
2.2. Involvement of HO-1 in the Inhibitory Effect of Xyloketal B on Ang II-Induced ROS Overproduction in HUVECs and PMA-Induced Respiratory Burst of Zebrafish Embryos

2.3. Xyloketal B Induced HO-1 Expression in HUVECs
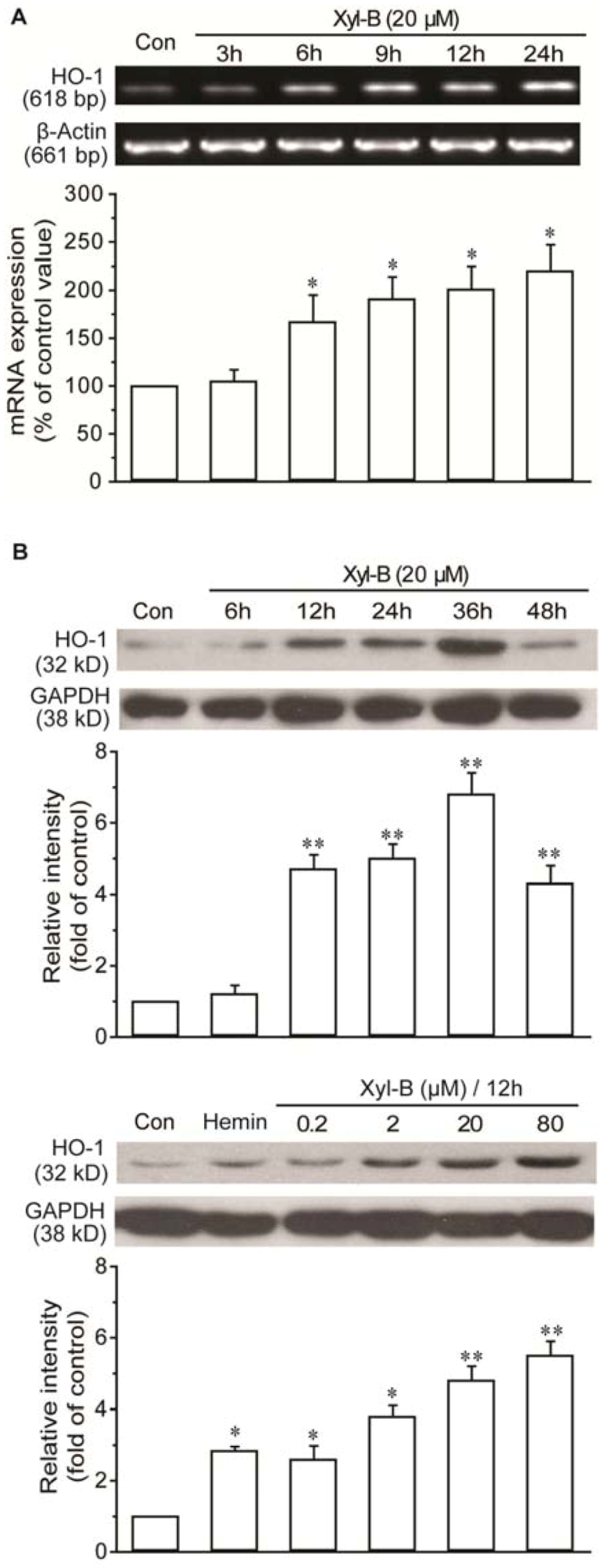
2.4. Xyloketal B Induced Activation of Translocation of Nrf-2 and Antioxidant Response Element (ARE) Binding in HUVECs
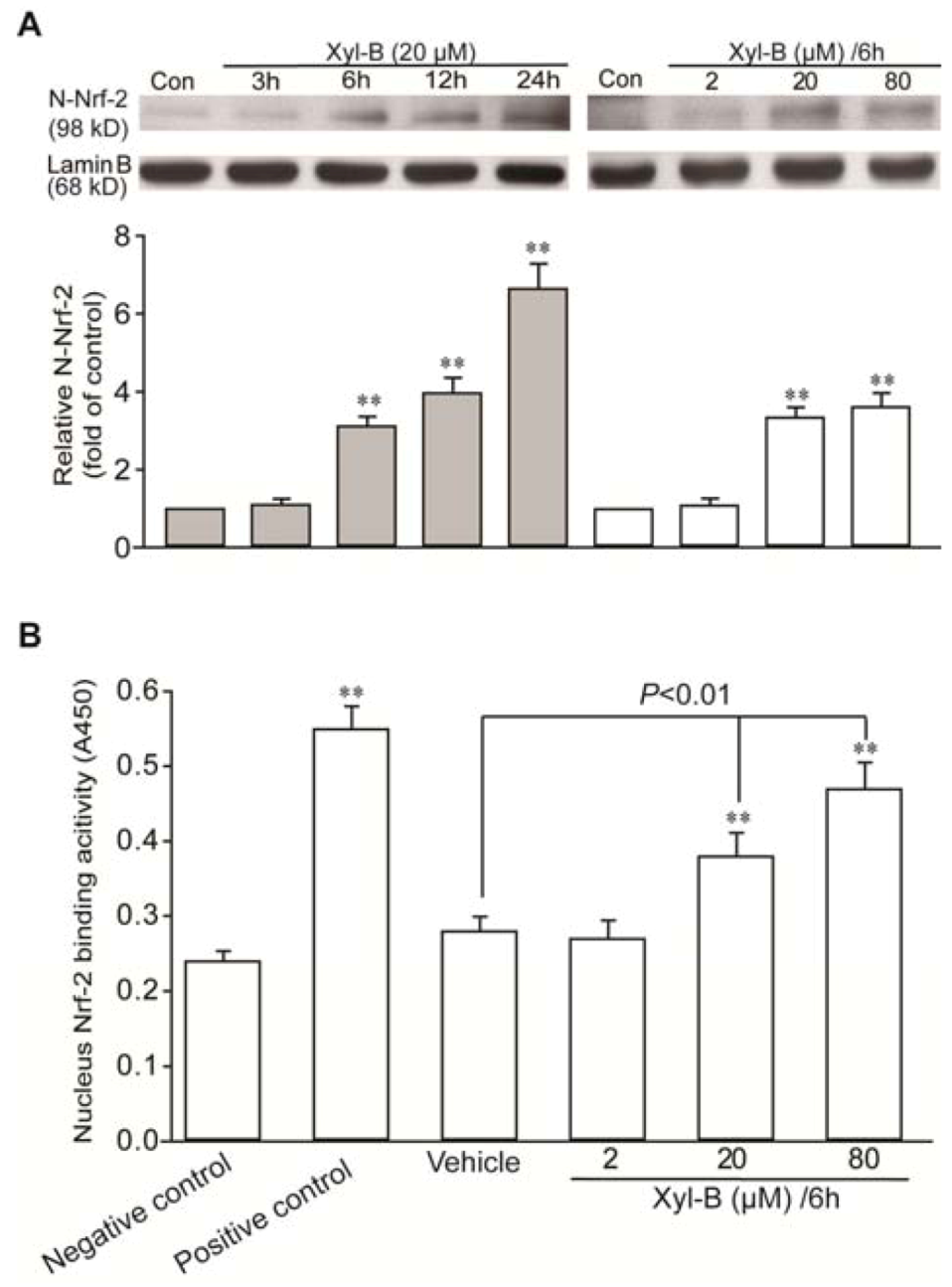
2.5. Effects of Xyloketal B on PI3K/Akt and ERK Signaling in HUVECs
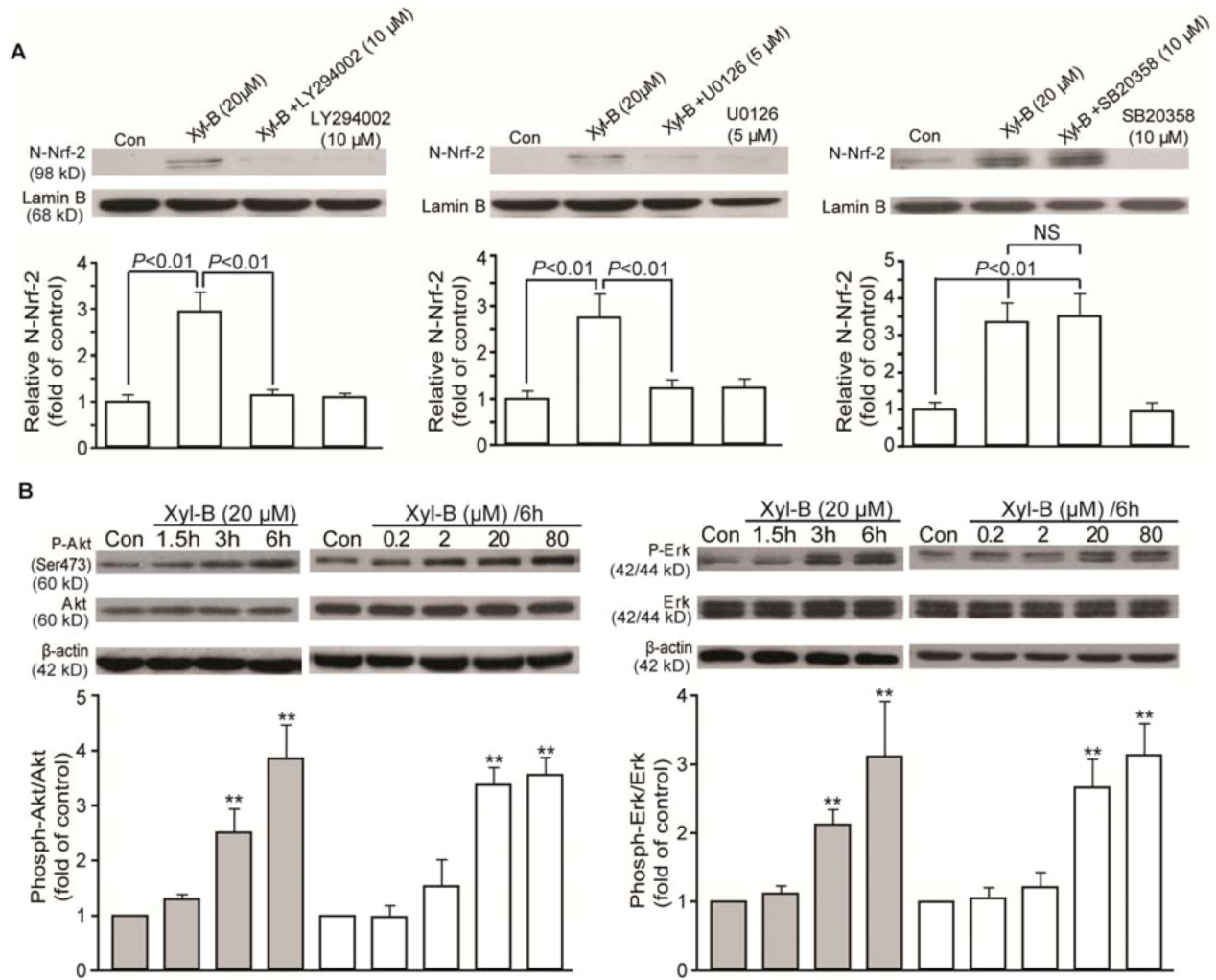
3. Experimental Section
3.1. Chemicals
3.2. Zebrafish Husbandry
3.3. Cell Culture
3.4. Cell Apoptosis Assay by Annexin V-FITC (Fluorescein Isothiocyanate)/Propidium Iodide (PI) Staining and DAPI Staining
3.4.1. Annexin V-FITC/PI Staining
3.4.2. DAPI Staining
3.5. ROS Measurement
3.6. Respiratory Burst Assay in Zebrafish
3.7. RT-PCR Analysis
| Gene | PCR primer sequences | PCR protocol |
|---|---|---|
| HO-1 | Forward: GCT CAA CAT CCA GCT CTT TGA GG | 95 °C/30 s |
| (284 bp) | Reverse: GAC AAA GTT CAT GGC CCTGGG A | 62 °C/30 s |
| 72 °C/1 min | ||
| GAPDH | Forward: TATCGTGGAAGGACTCATGACC | 95 °C/30 s |
| (625 bp) | Reverse: TACATGGCAACTGTGAGGGG | 55 °C/30 s |
| 72 °C/1 min |
3.8. Nuclear Protein Extraction
3.9. Western Blotting Analysis
3.10. Analysis of Binding Activity of Nrf-2 to Antioxidant Response Element (ARE)
3.11. Statistics
4. Discussion
5. Conclusions
Acknowledgments
References
- Wu, Q.; Huang, K. Protective effect of ebselen on cytotoxicity induced by cholestane-3 beta, 5 alpha, 6 beta-triol in ECV-304 cells. Biochim. Biophys. Acta 2006, 1761, 350–359. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sano, K.; Takakura, K.; Saito, I.; Shinohara, Y.; Asano, T.; Yasuhara, H. Ebselen in acute ischemic stroke: A placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke 1998, 29, 12–17. [Google Scholar]
- Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Luo, J.; Zhou, S.; Vrijmoed, L.L.; Jones, E.B.; Krohn, K.; Steingrover, K.; Zsila, F. Five unique compounds: Xyloketals from mangrove fungus Xylaria sp. from the South China Sea coast. J. Org. Chem. 2001, 66, 6252–6256. [Google Scholar]
- Chen, W.L.; Qian, Y.; Meng, W.F.; Pang, J.Y.; Lin, Y.C.; Guan, Y.Y.; Chen, S.P.; Liu, J.; Pei, Z.; Wang, G.L. A novel marine compound xyloketal B protects against oxidized LDL-induced cell injury in vitro. Biochem. Pharmacol. 2009, 78, 941–950. [Google Scholar]
- Lu, X.L.; Yao, X.L.; Liu, Z.; Zhang, H.; Li, W.; Li, Z.; Wang, G.L.; Pang, J.; Lin, Y.; Xu, Z.; et al. Protective effects of xyloketal B against MPP+-induced neurotoxicity in Caenorhabditis elegans and PC12 cells. Brain Res. 2010, 1332, 110–119. [Google Scholar]
- Zhao, J.; Li, L.; Ling, C.; Li, J.; Pang, J.Y.; Lin, Y.C.; Liu, J.; Huang, R.; Wang, G.L.; Pei, Z.; Zeng, J. Marine compound Xyloketal B protects PC12 cells against OGD-induced cell damage. Brain Res. 2009, 1302, 240–247. [Google Scholar]
- Duckers, H.J.; Boehm, M.; True, A.L.; Yet, S.F.; San, H.; Park, J.L.; Clinton Webb, R.; Lee, M.E.; Nabel, G.J.; Nabel, E.G. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat. Med. 2001, 7, 693–698. [Google Scholar]
- Buckley, B.J.; Marshall, Z.M.; Whorton, A.R. Nitric oxide stimulates Nrf-2 nuclear translocation in vascular endothelium. Biochem. Biophys. Res. Commun. 2003, 307, 973–979. [Google Scholar] [CrossRef]
- Chen, J.S.; Huang, P.H.; Wang, C.H.; Lin, F.Y.; Tsai, H.Y.; Wu, T.C.; Lin, S.J.; Chen, J.W. Nrf-2 mediated heme oxygenase-1 expression, an antioxidant-independent mechanism, contributes to anti-atherogenesis and vascular protective effects of Ginkgo biloba extract. Atherosclerosis 2011, 214, 301–309. [Google Scholar] [CrossRef]
- Kim, Y.M.; Pae, H.O.; Park, J.E.; Lee, Y.C.; Woo, J.M.; Kim, N.H.; Choi, Y.K.; Lee, B.S.; Kim, S.R.; Chung, H.T. Heme oxygenase in the regulation of vascular biology: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 14, 137–167. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef]
- Hosick, P.A.; Stec, D.E. Heme oxygenase, a novel target for the treatment of hypertension and obesity? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R207–R214. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Jazwa, A.; Cuadrado, A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr. Drug Targets 2010, 11, 1517–1531. [Google Scholar] [CrossRef]
- Ferrandiz, M.L.; Devesa, I. Inducers of heme oxygenase-1. Curr. Pharm. Des. 2008, 14, 473–486. [Google Scholar] [CrossRef]
- Meijer, A.H.; Spaink, H.P. Host-pathogen interactions made transparent with the zebrafish model. Curr. Drug Targets 2011, 12, 1000–1017. [Google Scholar] [CrossRef]
- Nayak, A.S.; Lage, C.R.; Kim, C.H. Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio). Toxicol. Sci. 2007, 98, 118–124. [Google Scholar] [CrossRef]
- Phennicie, R.T.; Sullivan, M.J.; Singer, J.T.; Yoder, J.A.; Kim, C.H. Specific resistance to Pseudomonas aeruginosa infection in zebrafish is mediated by the cystic fibrosis transmembrane conductance regulator. Infect. Immun. 2010, 78, 4542–4550. [Google Scholar] [CrossRef]
- Hermann, A.C.; Millard, P.J.; Blake, S.L.; Kim, C.H. Development of a respiratory burst assay using zebrafish kidneys and embryos. J. Immunol. Methods 2004, 292, 119–129. [Google Scholar] [CrossRef]
- Datla, S.R.; Dusting, G.J.; Mori, T.A.; Taylor, C.J.; Croft, K.D.; Jiang, F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension 2007, 50, 636–642. [Google Scholar] [CrossRef]
- Mazza, F.; Goodman, A.; Lombardo, G.; Vanella, A.; Abraham, N.G. Heme oxygenase-1 gene expression attenuates angiotensin II-mediated DNA damage in endothelial cells. Exp. Biol. Med. (Maywood) 2003, 228, 576–583. [Google Scholar]
- Morita, T.; Imai, T.; Sugiyama, T.; Katayama, S.; Yoshino, G. Heme oxygenase-1 in vascular smooth muscle cells counteracts cardiovascular damage induced by angiotensin II. Curr. Neurovasc. Res. 2005, 2, 113–120. [Google Scholar] [CrossRef]
- Kang, K.W.; Lee, S.J.; Kim, S.G. Molecular mechanism of Nrf-2 activation by oxidative stress. Antioxid. Redox Signal. 2005, 7, 1664–1673. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, C.; Han, A.L.; Youn, M.J.; Lee, J.H.; Kim, Y.; Kim, E.S.; Kim, H.J.; Kim, J.K.; Lee, H.K.; et al. Ebselen attenuates cisplatin-induced ROS generation through Nrf-2 activation in auditory cells. Hear. Res. 2009, 251, 70–82. [Google Scholar] [CrossRef]
- Berger, S.P.; Hunger, M.; Yard, B.A.; Schnuelle, P.; van der Woude, F.J. Dopamine induces the expression of heme oxygenase-1 by human endothelial cells in vitro. Kidney Int. 2000, 58, 2314–2319. [Google Scholar] [CrossRef]
- Yang, L.; Quan, S.; Nasjletti, A.; Laniado-Schwartzman, M.; Abraham, N.G. Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure. Hypertension 2004, 43, 1221–1226. [Google Scholar] [CrossRef]
- Schuhmacher, S.; Wenzel, P.; Schulz, E.; Oelze, M.; Mang, C.; Kamuf, J.; Gori, T.; Jansen, T.; Knorr, M.; Karbach, S.; et al. Pentaerythritol tetranitrate improves angiotensin II-induced vascular dysfunction via induction of heme oxygenase-1. Hypertension 2010, 55, 897–904. [Google Scholar] [CrossRef]
- Aizawa, T.; Ishizaka, N.; Taguchi, J.; Nagai, R.; Mori, I.; Tang, S.S.; Ingelfinger, J.R.; Ohno, M. Heme oxygenase-1 is upregulated in the kidney of angiotensin II-induced hypertensive rats: Possible role in renoprotection. Hypertension 2000, 35, 800–806. [Google Scholar] [CrossRef]
- Liu, X.M.; Peyton, K.J.; Shebib, A.R.; Wang, H.; Durante, W. Compound C stimulates heme oxygenase-1 gene expression via the Nrf-2-ARE pathway to preserve human endothelial cell survival. Biochem. Pharmacol. 2011, 82, 371–379. [Google Scholar]
- Dikalova, A.; Clempus, R.; Lassegue, B.; Cheng, G.; McCoy, J.; Dikalov, S.; San Martin, A.; Lyle, A.; Weber, D.S.; Weiss, D.; et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 2005, 112, 2668–2676. [Google Scholar]
- Lee, I.T.; Luo, S.F.; Lee, C.W.; Wang, S.W.; Lin, C.C.; Chang, C.C.; Chen, Y.L.; Chau, L.Y.; Yang, C.M. Overexpression of HO-1 protects against TNF-alpha-mediated airway inflammation by down-regulation of TNFR1-dependent oxidative stress. Am. J. Pathol. 2009, 175, 519–532. [Google Scholar] [CrossRef]
- Chen, Y.; Junger, W.G. Measurement of oxidative burst in neutrophils. Methods Mol. Biol. 2012, 844, 115–124. [Google Scholar] [CrossRef]
- Beliaeva, N.F.; Kashirtseva, V.N.; Medvedeva, N.V.; Khudoklinova, I.; Ipatova, O.M.; Archakov, A.I. Zebrafish as a model organism for biomedical studies (in Russian). Biomed. Khim. 2010, 56, 120–131. [Google Scholar]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Xiang, Q.; Pei, Z.; Liu, X.; Lu, B.; Chen, L.; Wang, G.; Pang, J.; Lin, Y. Design and synthesis of novel xyloketal derivatives and their vasorelaxing activities in rat thoracic aorta and angiogenic activities in zebrafish angiogenesis screen. J. Med. Chem. 2010, 53, 4642–4653. [Google Scholar] [CrossRef]
- Bouton, C.; Demple, B. Nitric oxide-inducible expression of heme oxygenase-1 in human cells. Translation-independent stabilization of the mRNA and evidence for direct action of nitric oxide. J. Biol. Chem. 2000, 275, 32688–32693. [Google Scholar]
- Morita, T. Heme oxygenase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1786–1795. [Google Scholar] [CrossRef]
- Prestera, T.; Talalay, P.; Alam, J.; Ahn, Y.I.; Lee, P.J.; Choi, A.M. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: Regulation by upstream antioxidant-responsive elements (ARE). Mol. Med. 1995, 1, 827–837. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Z.-X.; Chen, J.-W.; Yuan, F.; Huang, Y.-Y.; Zhao, L.-Y.; Li, J.; Su, H.-X.; Liu, J.; Pang, J.-Y.; Lin, Y.-C.; et al. Xyloketal B Exhibits Its Antioxidant Activity through Induction of HO-1 in Vascular Endothelial Cells and Zebrafish. Mar. Drugs 2013, 11, 504-522. https://doi.org/10.3390/md11020504
Li Z-X, Chen J-W, Yuan F, Huang Y-Y, Zhao L-Y, Li J, Su H-X, Liu J, Pang J-Y, Lin Y-C, et al. Xyloketal B Exhibits Its Antioxidant Activity through Induction of HO-1 in Vascular Endothelial Cells and Zebrafish. Marine Drugs. 2013; 11(2):504-522. https://doi.org/10.3390/md11020504
Chicago/Turabian StyleLi, Zhen-Xing, Jian-Wen Chen, Feng Yuan, Yun-Ying Huang, Li-Yan Zhao, Jie Li, Huan-Xing Su, Jie Liu, Ji-Yan Pang, Yong-Cheng Lin, and et al. 2013. "Xyloketal B Exhibits Its Antioxidant Activity through Induction of HO-1 in Vascular Endothelial Cells and Zebrafish" Marine Drugs 11, no. 2: 504-522. https://doi.org/10.3390/md11020504
APA StyleLi, Z.-X., Chen, J.-W., Yuan, F., Huang, Y.-Y., Zhao, L.-Y., Li, J., Su, H.-X., Liu, J., Pang, J.-Y., Lin, Y.-C., Lu, X.-L., Pei, Z., Wang, G.-L., & Guan, Y.-Y. (2013). Xyloketal B Exhibits Its Antioxidant Activity through Induction of HO-1 in Vascular Endothelial Cells and Zebrafish. Marine Drugs, 11(2), 504-522. https://doi.org/10.3390/md11020504




