Sources of Secondary Metabolite Variation in Dysidea avara (Porifera: Demospongiae): The Importance of Having Good Neighbors
Abstract
1. Introduction
2. Results
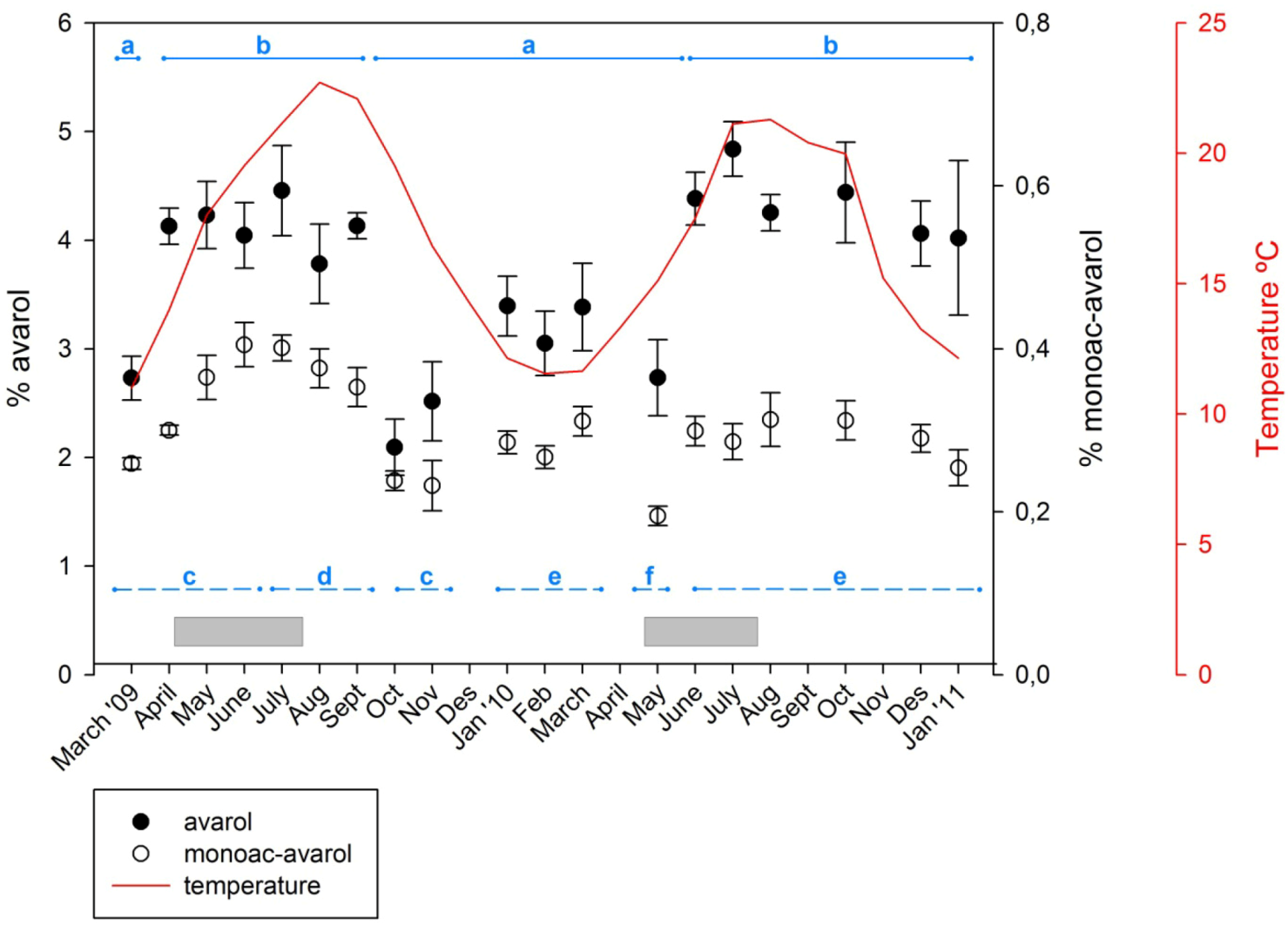
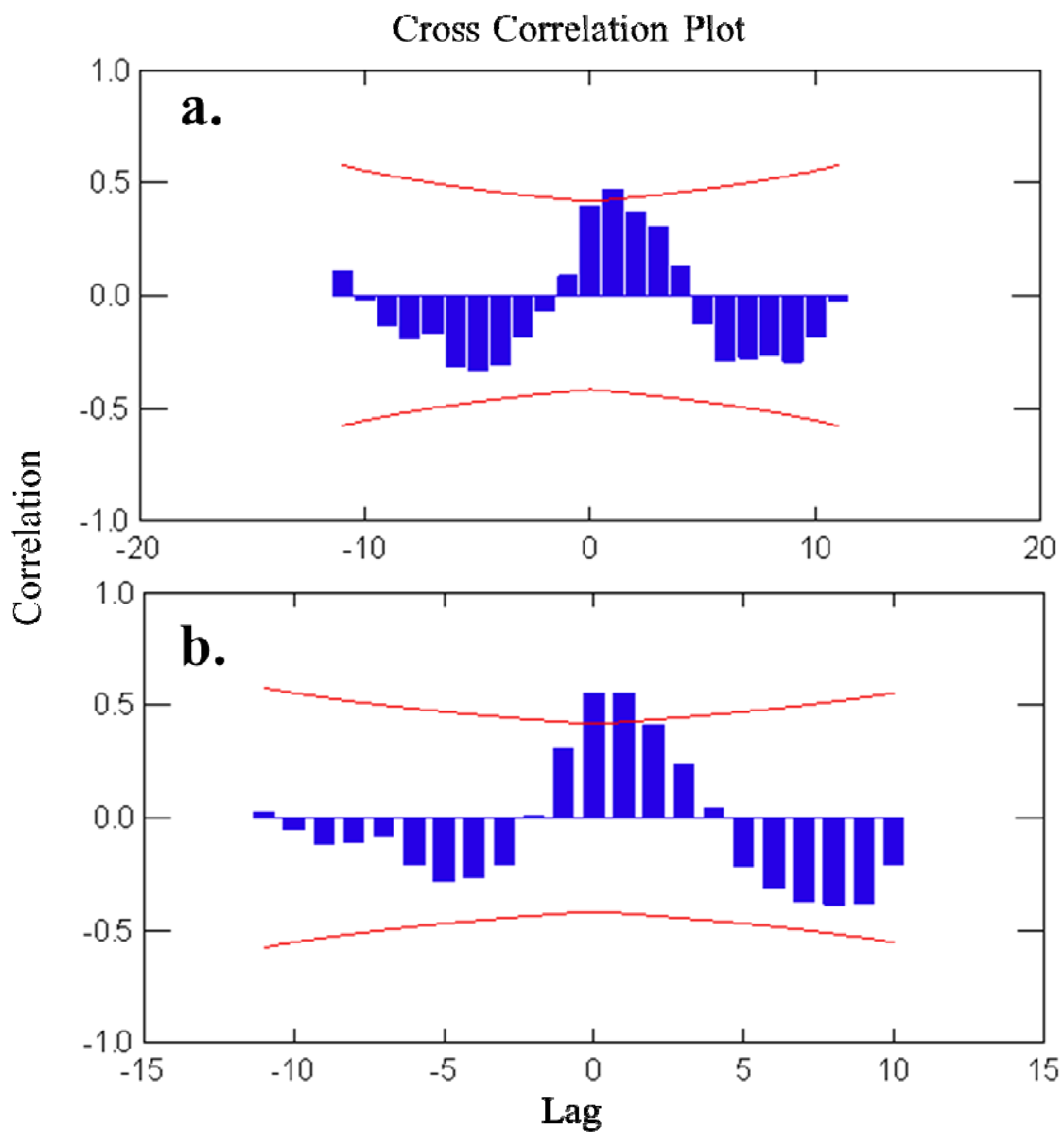
2.1. Temporal Variation of Metabolites
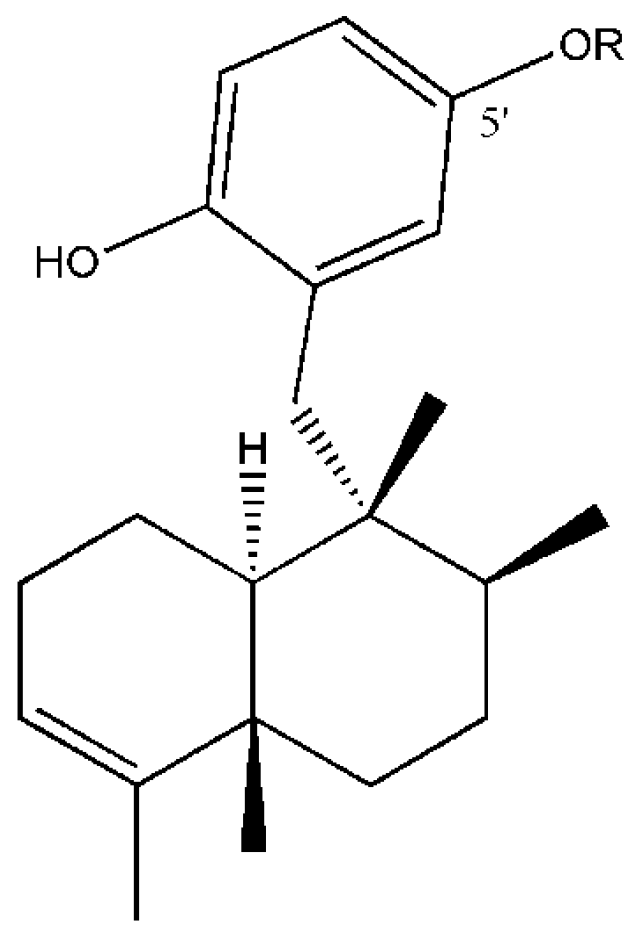
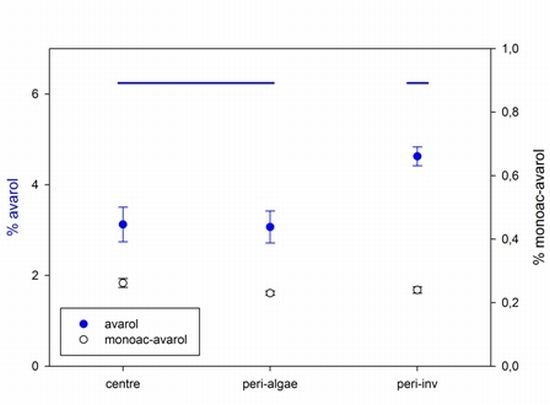
2.2. Intra-Individual Variation of Metabolites
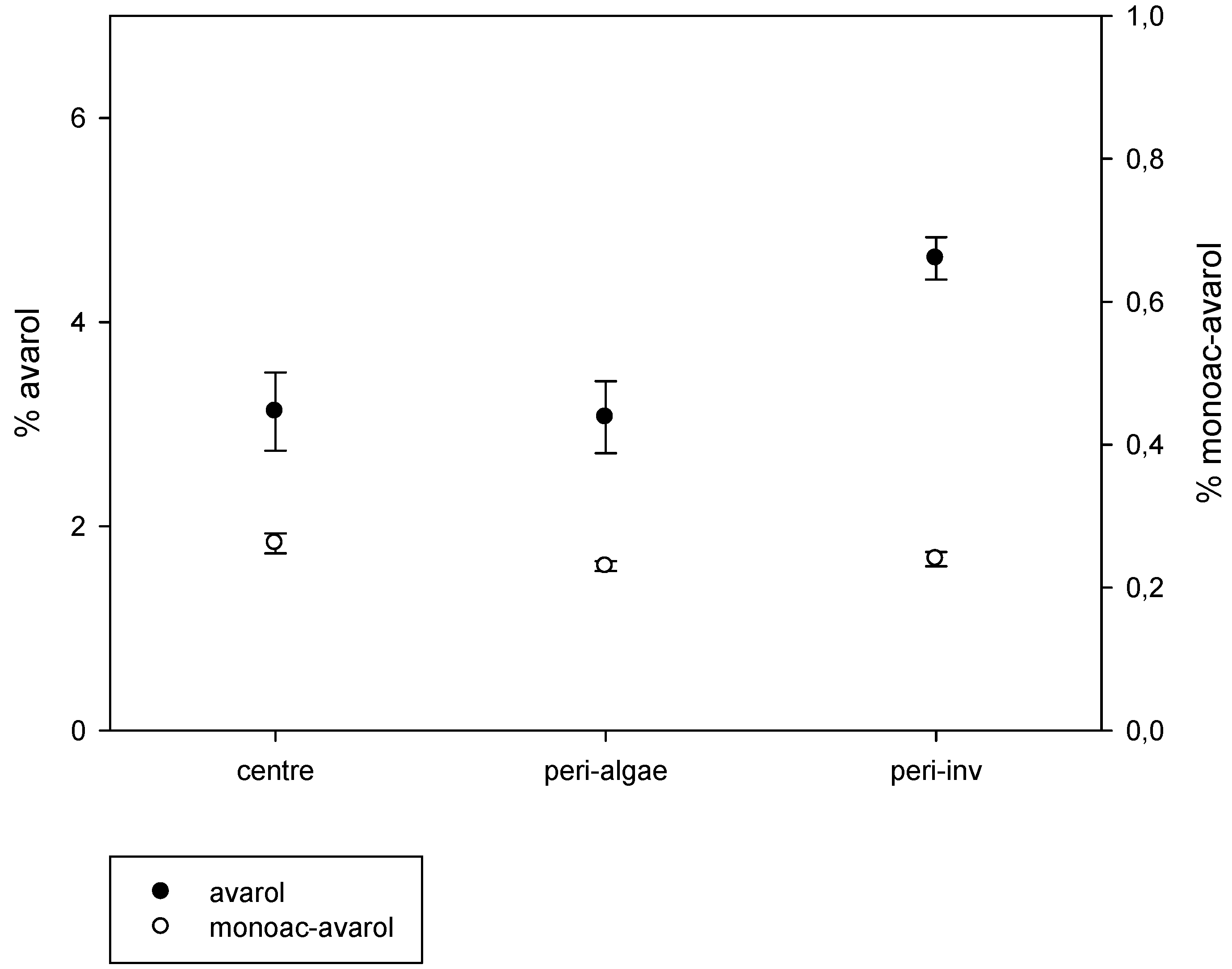
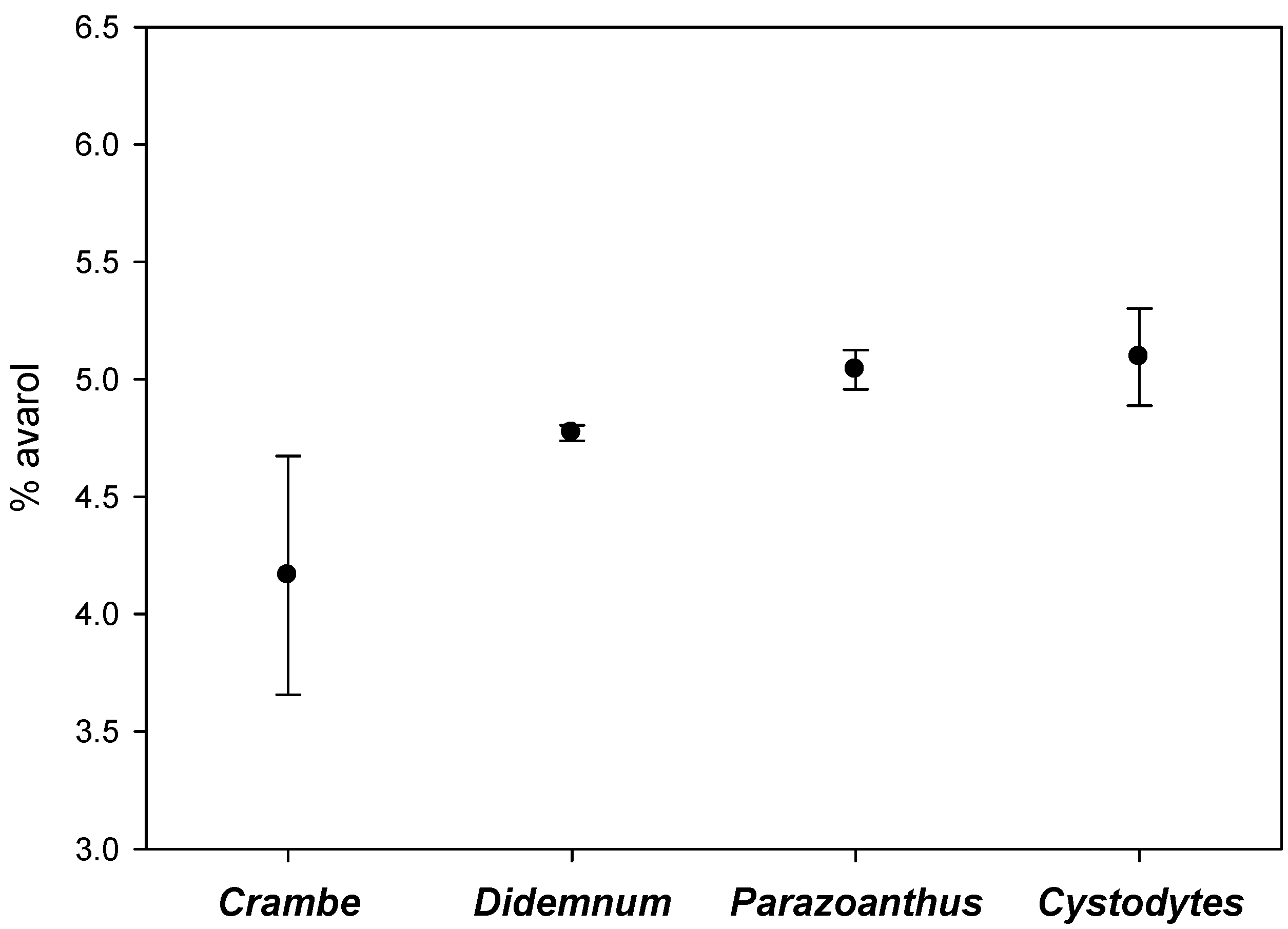
3. Discussion
4. Experimental Section
4.1. Sampling
4.2. Preparation of the Analytical Standards
4.3. Chemical Extraction Procedure
4.4. HPLC Analysis and Quantification
4.5. Data Analysis
5. Conclusions
Acknowledgments
References
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinicals trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef]
- Sipkema, D.; Franssen, M.C.R.; Osinga, R.; Tramper, J.; Wijffels, R.H. Marine sponges as pharmacy. Mar. Biotechnol. 2005, 7, 142–162. [Google Scholar] [CrossRef]
- Dayton, P.K.; Robilliard, G.A.; Paine, R.T.; Dayton, L.B. Biological accommodation in the benthic community at McMurdoSound, Antarctica. Ecolog. Monogr. 1974, 44, 105–128. [Google Scholar] [CrossRef]
- Ruzicka, R.; Gleason, D.F. Latitudinal variation in spongivorous fishes and the effectiveness of sponge chemical defenses. Oecologia 2008, 154, 785–794. [Google Scholar] [CrossRef]
- Paul, V.J.; Puglisi, M.P. Chemical mediation of interactions among marine organisms. Nat. Prod. Rep. 2004, 21, 189–209. [Google Scholar] [CrossRef]
- Wulff, J.L. Ecological interactions of marine sponges. Can. J. Zool. 2006, 84, 146–166. [Google Scholar] [CrossRef]
- Thoms, C.; Schupp, P.J. Chemical defense strategies in sponges: A review. In Porifera Research: Biodiversity, Innovation and Sustainability; Custódio, M.R., Lôbo-Hajdu, G., Hajdu, E., Muricy, G., Eds.; Série Livros 28; Museu Nacional: Rio de Janeiro, Brazil, 2007; pp. 627–637. [Google Scholar]
- Abdo, D.A.; Motti, C.A.; Battershill, C.N.; Harvey, E.S. Temperature and spatiotemporal variability of salicylihalamide A in the sponge Haliclona sp. J. Chem. Ecol. 2007, 33, 1635–1645. [Google Scholar] [CrossRef]
- Koopmans, M.; Martens, D.; Wijffels, R.H. Towards commercial production of sponge medicines. Mar. Drugs 2009, 7, 787–802. [Google Scholar] [CrossRef]
- Noyer, C.; Thomas, O.P.; Becerro, M.A. Patterns of chemical diversity in the Mediterranean Sponge Spongia lamella. PLoS One 2011, 6, 1–11. [Google Scholar]
- Sacristan-Soriano, O.; Banaigs, B.; Becerro, M.A. Relevant spatial scales of Chemicals variation in Aplysina aerophoba. Mar. Drugs 2011, 9, 2499–2513. [Google Scholar] [CrossRef]
- Hay, M.E. Marine chemical ecology: What’s known and what’s next? J. Exp. Mar. Biol. Ecol. 1996, 200, 103–134. [Google Scholar] [CrossRef]
- Becerro, M.A.; Thacker, R.W.; Turon, X.; Uriz, M.J.; Paul, V.J. Biogeography of sponge chemical ecology: Comparisons of tropical and temperate defenses. Oecologia 2003, 135, 91–101. [Google Scholar]
- Martí, R.; Uriz, M.J.; Turon, X. Seasonal and spatial variation of species toxicity in Mediterranean seaweed communities: Correlation to biotic and abiotic factors. Mar. Ecol. Prog. Ser. 2004, 282, 73–85. [Google Scholar] [CrossRef]
- Becerro, M.A.; Turon, X.; Uriz, M.J. Natural variation of toxicity in encrusting sponge Crambe crambe (Schmidt) in relation to size and environment. J. Chem. Ecol. 1995, 21, 1931–1946. [Google Scholar] [CrossRef]
- Paul, V.J.; Ritson-Williams, R.; Sharp, K. Marine Chemical ecology in benthic environments. Nat. Prod. Rep. 2011, 28, 345–387. [Google Scholar] [CrossRef]
- Swearing, D.C.; Pawlik, J.R. Variablity in the chemical defense of the sponge Chondrilla nucula against predatory reef fishes. Mar. Biol. 1998, 131, 619–627. [Google Scholar] [CrossRef]
- Duckworth, A.R.; Batershill, C.N. Population dynamics and chemical ecology of New Zealand Demospongiae Latrunculia sp. nov. and Polymastia croceus (Poecilosclerida: Latrunculiidae: Polymastiidae). N. Z. J. Mar. Freshw. Res. 2001, 35, 935–949. [Google Scholar]
- Sacristan-Soriano, O.; Banaigs, B.; Becerro, M.A. Temporal trend in the secondary metabolite production of the sponge Aplysina aerophoba. Mar. Drugs 2012, 10, 677–693. [Google Scholar] [CrossRef]
- Turon, X.; Becerro, M.A.; Uriz, M.J. Seasonal patterns of toxicity in benthic invertebrates: The encrusting sponge Crambe crambe (Poecilosclerida). Oikos 1996, 75, 33–40. [Google Scholar]
- Ivanisevic, J.; Thomas, O.P.; Pedel, L.; Pénez, N.; Ereskovsky, A.V.; Culioli, G.; Pérez, T. Biochemical trade-offs: Evidence for ecologically linked secondary metabolism of the sponge Oscarella balibaloi. PLoS One 2011, 6, 1–11. [Google Scholar]
- Ivanisevic, J.; Pérez, T.; Ereskovsky, A.V.; Barnathan, G.; Thomas, O.P. Lysophospholipids in the Mediterranean sponge Oscarella tuberculata: Seasonal variability and putative biological role. J. Chem. Ecol. 2011, 37, 537–545. [Google Scholar] [CrossRef]
- Page, M.; West, L.; Northcote, P.; Battershill, C.; Kelly, M. Spatial and temporal variability of cytotoxic metabolites in populations of the New Zealand sponge Mycale hentscheli. J. Chem. Ecol. 2005, 31, 1161–1173. [Google Scholar] [CrossRef]
- Thompson, J.E.; Barrow, K.D.; Faulkner, D.J. Localization of two brominated metabolites, aerothionin and homoaerothionin, in spherulous cells of the marine sponge Aplysina fistularis (=Verongia thiona). Acta Zool. 1983, 64, 199–210. [Google Scholar] [CrossRef]
- Uriz, M.J.; Turon, X.; Galera, J.; Tur, J.M. New light on the cell location of avarol within the sponge Dysidea avara (Dendroceratida). Cell Tissue Res. 1996, 285, 519–527. [Google Scholar] [CrossRef]
- Uriz, M.J.; Becerro, M.A.; Tur, J.M.; Turon, X. Location of toxicity within the Mediterranean sponge Crambe crambe (Demospongiae, Poecilosclerida). Mar. Biol. 1996, 124, 583–590. [Google Scholar] [CrossRef]
- Turon, X.; Becerro, M.A.; Uriz, M.J. Distribution of brominated compounds within the sponge Aplysina aerophoba: Coupling of X-ray microanalysis with cryofixation techniques. Cell Tissue Res. 2000, 301, 311–322. [Google Scholar] [CrossRef]
- Salomon, C.E.; Deerinck, T.; Ellisman, M.H.; Faulkner, D.J. The cellular localization of dercitamide in the Palauan sponge Oceanapia sagittaria. Mar. Biol. 2001, 139, 313–319. [Google Scholar] [CrossRef]
- Becerro, M.A.; Paul, V.J.; Starmer, J. Intracolonial variation in Chemicals defenses of the sponge Cacospongia sp. and its consequences on generalist fish predators and the specialist nudibranch predator Glossodoris pallida. Mar. Ecol. Prog. Ser. 1998, 168, 187–196. [Google Scholar] [CrossRef]
- Schupp, P.; Eder, C.; Paul, V.; Proksch, P. Distribution of secondary metabolites in the sponge Oceanapia sp. and its ecological implications. Mar. Biol. 1999, 135, 573–580. [Google Scholar] [CrossRef]
- Furrow, F.B.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Surface sequestration of chemical feeding deterrents in the Antartic sponge Latrinculia apicalis as an optimal defense against sea star spongivory. Mar. Biol. 2003, 143, 443–449. [Google Scholar] [CrossRef]
- Rhode, S.; Schupp, P.J. Allocation of chemical and structural defences in the sponge Melophlus sarasinorum. J. Exp. Mar. Biol. Ecol. 2011, 399, 76–83. [Google Scholar] [CrossRef]
- Sirirak, T.; Intaraksa, N.; Kaewsuwan, S.; Yuenyongsawad, S.; Suwanborirux, K.; Plubrukarn, A. Intracolonial allocation of Trisoxazole Macrolides in the sponge Pachastrissa nux. Chem. Biodivers. 2011, 8, 2238–2246. [Google Scholar] [CrossRef]
- Uriz, M.J.; Turon, X.; Becerro, M.A.; Galera, J. Feeding deterrence in sponges. The role of toxicity, physical defenses, energetic contents, and life-history stage. J. Exp. Mar. Biol. Ecol. 1996, 205, 187–204. [Google Scholar]
- Turon, X.; Becerro, M.A.; Uriz, M.J.; Llopis, J. Small-Scale association measures in epibenthic communities as a clue for allelochemical enteractions. Oecologia 1996, 108, 351–360. [Google Scholar]
- Engel, S.; Pawlik, J.R. Allelopathic activities of sponge extracts. Mar. Ecol. Prog. Ser. 2000, 207, 273–281. [Google Scholar] [CrossRef]
- Chaves-Fonnegra, A.; Castellanos, L.; Zea, S.; Duque, C.; Rodríguez, J.; Jiménez, C. Clionapyrrolidine A-A Metabolite from the Encrusting and Excavating Sponge Cliona tenuis that Kills Coral Tissue upon Contact. J. Chem. Ecol. 2008, 34, 1565–1574. [Google Scholar] [CrossRef]
- Sipkema, D.; Osinga, R.; Schatton, W.; Mendola, D.; Tramper, J.; Wijffels, R.H. Large-Scale production of pharmaceuticals by marine sponges: Sea, cell or synthesis? Biotechnol. Bioeng. 2005, 90, 202–222. [Google Scholar]
- Minale, L.; Riccio, R.; Sodno, G. Avarol, a novel sesquiterpenoid hydroquinone with a rearranged drimane skeleton from the sponge Disidea avara. Tetrahedron Lett. 1974, 38, 3401–3404. [Google Scholar] [CrossRef]
- Crispino, A.; de Giulio, A.; de Rosa, S.; Strazzulio, G. Anew bioactive derivate of avarol from the marine sponge Dysidea avara. J. Nat. Prod. 1989, 52, 646–648. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Maidhof, A.; Zahn, R.K.; Schröder, H.C.; Gasic, M.J.; Heidemann, D.; Bernd, A.; Kurelec, B.; Eich, E.; Sibert, G. Potent antileukemic activity of the novel cytostatic agent avarone and its analogues in vitro and in vivo. Cancer Res. 1985, 45, 4822–4827. [Google Scholar]
- Amigo, M.; Paya, M.; Braza-Boils, A.; de Rosa, S.; Terencio, M.C. Avarol inhibits TNF-alpha generation and NF-kappaB activation in human cells and in animal models. Life Sci. 2008, 82, 256–264. [Google Scholar] [CrossRef]
- Pietschmann, R.; Shatton, M.; Schatton, W. Process for preparation of compositions with a high content in avarol and their use. Eur. Pat. EP1391197, 2004. [Google Scholar]
- Gordaliza, M. Cytotoxic terpene quinones from marine sponges. Mar. Drugs 2010, 8, 2849–2870. [Google Scholar] [CrossRef]
- Sakurai, J.; Oguchi, T.; Watanabe, K.; Abe, H.; Kanno, S.; Ishikawa, M.; Katoh, T. Highly efficient total synthesis of the marine natural products (+)-Avarone, (+)-Avarol, (−)-Neovarone, (−)-Neoavarol and (+)-Aureol. Chem. Eur. J. 2008, 14, 829–837. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Böhm, M.; Batel, R.; de Rosa, S.; Tommonaro, G.; Müller, I.M.; Schröder, H.C. Application of cell culture for the production of bioactive compounds from sponges: Synthesis of avarol by primmorphs from Dysidea avara. J. Nat. Prod. 2000, 63, 1077–1081. [Google Scholar] [CrossRef]
- Sipkema, D.; Heilig, H.G.H.J.; Akkermans, A.D.L.; Osinga, R.; Wijffels, R.H. Sponge-Cell culture? A molecular identification method for sponge cells. Mar. Biotechnol. 2003, 5, 443–449. [Google Scholar] [CrossRef]
- De Caralt, S.; Sánchez-Fontenla, J.; Uriz, M.J.; Wijffels, R.H. In Situ aquaculture methods for Dysidea avara (Demospongiae, Porifera) in the Northwestern Mediterranean. Mar. Drugs 2010, 8, 1731–1742. [Google Scholar] [CrossRef]
- Tsoukatou, M.; Maréchal, J.P.; Hellio, C.; Novakovic, I.; Tufegdzic, S.; Sladic, D.; Gasić, M.J.; Clare, A.S.; Vagias, C.; Roussis, V. Evaluation of the activity of the sponge metabolites avarol and avarone and their synthetic derivatives against fouling micro- and macroorganisms. Mar. Drugs 2007, 12, 1022–1034. [Google Scholar]
- Fagerström, T.; Larsson, S.; Tenow, O. On optimal defence in plants. Funct. Ecol. 1987, 1, 73–81. [Google Scholar] [CrossRef]
- Lopez-legentil, S.; Bontemps-Subielos, N.; Turon, X.; Banaigs, B. Secondary metabolite and inorganic contents in Cystodytes sp. (Ascidiacea): Temporal patterns and association with reproduction and growth. Mar. Biol. 2007, 151, 293–299. [Google Scholar]
- Cronin, G. Resource allocation in seaweeds and marine invertebrates: Chemical defense patterns in relation to defence theories. In Marine Chemical Ecology; McClintock, J.B., Baker, B.J., Eds.; CRC Press: New York, NY, USA, 2001; pp. 325–353. [Google Scholar]
- Coma, R.; Ribes, M.; Gili, J.M.; Zabala, M. Seasonality in coastal benthic ecosystems. Trends Ecol. Evol. 2000, 11, 448–453. [Google Scholar]
- Turon, X.; Galera, J.; de Caralt, S.; Tarjuelo, I.; Uriz, M.J. Pautas de crecimiento en esponjas incrustantes del Mediterráneo: Seguimiento a largo plazo de Crambe crambe (Poecilosclerida) y Dysidea avara (Dendroceratida). In Proceedings of XI Simposium Ibérico: Estudios del Bentos Marino, Málaga, Spain; 2000. [Google Scholar]
- Ebel, R.; Brenzinger, M.; Kunze, A.; Gross, H.J.; Proksch, P. Wound activation of protoxins in marine sponge Aplysina aerophoba. J. Chem. Ecol. 1997, 23, 1451–1462. [Google Scholar] [CrossRef]
- Webster, N.S.; Xavier, J.R.; Freckelton, M.; Motti, C.A.; Cobb, R. Shifts in microbial and chemical patterns within the marine sponge Aplysina aerophoba during a disease outbreak. Environ. Microbiol. 2008, 10, 3366–3376. [Google Scholar] [CrossRef]
- Thacker, R.W.; Becerro, M.A.; Lumbang, W.A.; Paul, V.J. Allelopathic interactions between sponges on tropical reef. Ecology 1998, 79, 1740–1750. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Buss, L. Allelopathy and spatial competition among coral reef invertebrates. Proc. Natl. Acad. Sci.USA 1975, 72, 5160–5163. [Google Scholar] [CrossRef]
- Uriz, M.J.; Martin, D.; Turon, X.; Ballesteros, E.; Hughes, R.; Acebal, C. An approach to the ecological significance of chemically mediated bioactivity in Mediterranean benthic communities. Mar. Ecol. Prog. Ser. 1991, 70, 175–188. [Google Scholar] [CrossRef]
- Becerro, M.A.; Uriz, M.J.; Turon, X. Chemically-Mediated interactions in benthic organisms: The chemical ecology of Crambe crambe (Porifera, Poecilosclerida). Hydrobiologia 1997, 356, 77–89. [Google Scholar] [CrossRef]
- Zabala, M.; Ballesteros, E. Surface dependent strategies and energy flux in benthic marine communities, or why corals do not exist in the Mediterranean. Sci. Mar. 1989, 53, 3–17. [Google Scholar]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. Annu. Rev. 2006, 44, 123–195. [Google Scholar]
- Dias, G.M.; Delboni, C.G.M.; Duarte, L.F.L. Effects of competition on sexual and clonal reproduction of a tunicate: The importance of competitor identity. Mar. Ecol. Prog. Ser. 2008, 362, 149–156. [Google Scholar] [CrossRef]
- Adler, F.R.; Harvell, C.D. Inducible defences, phenotypic variability and biotic environments. Trends Ecol. Evol. 1990, 5, 407–410. [Google Scholar] [CrossRef]
- Ballesteros, E. Structure and dynamics of north-western Mediterranean phytobenthic communities: A conceptual model. Oecol. Aquat. 1990, 10, 223–242. [Google Scholar]
- Sales, M.; Ballesteros, E. Seasonal dynamics and annual production of Cystoseira crinite (Fucales: Ochrophyta)-dominated assemblages from the northwestern Mediterranean. Sci. Mar. 2012, 76, 391–401. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Narum, S.R. Beyond Bonferroni: Less conservative analyses for conservation genetics. Conserv. Genetics 2006, 7, 783–787. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Caralt, S.; Bry, D.; Bontemps, N.; Turon, X.; Uriz, M.-J.; Banaigs, B. Sources of Secondary Metabolite Variation in Dysidea avara (Porifera: Demospongiae): The Importance of Having Good Neighbors. Mar. Drugs 2013, 11, 489-503. https://doi.org/10.3390/md11020489
De Caralt S, Bry D, Bontemps N, Turon X, Uriz M-J, Banaigs B. Sources of Secondary Metabolite Variation in Dysidea avara (Porifera: Demospongiae): The Importance of Having Good Neighbors. Marine Drugs. 2013; 11(2):489-503. https://doi.org/10.3390/md11020489
Chicago/Turabian StyleDe Caralt, Sonia, Delphine Bry, Nataly Bontemps, Xavier Turon, Maria-Jesus Uriz, and Bernard Banaigs. 2013. "Sources of Secondary Metabolite Variation in Dysidea avara (Porifera: Demospongiae): The Importance of Having Good Neighbors" Marine Drugs 11, no. 2: 489-503. https://doi.org/10.3390/md11020489
APA StyleDe Caralt, S., Bry, D., Bontemps, N., Turon, X., Uriz, M.-J., & Banaigs, B. (2013). Sources of Secondary Metabolite Variation in Dysidea avara (Porifera: Demospongiae): The Importance of Having Good Neighbors. Marine Drugs, 11(2), 489-503. https://doi.org/10.3390/md11020489