Bioactive Compounds from a Gorgonian Coral Echinomuricea sp. (Plexauridae)
Abstract
:1. Introduction
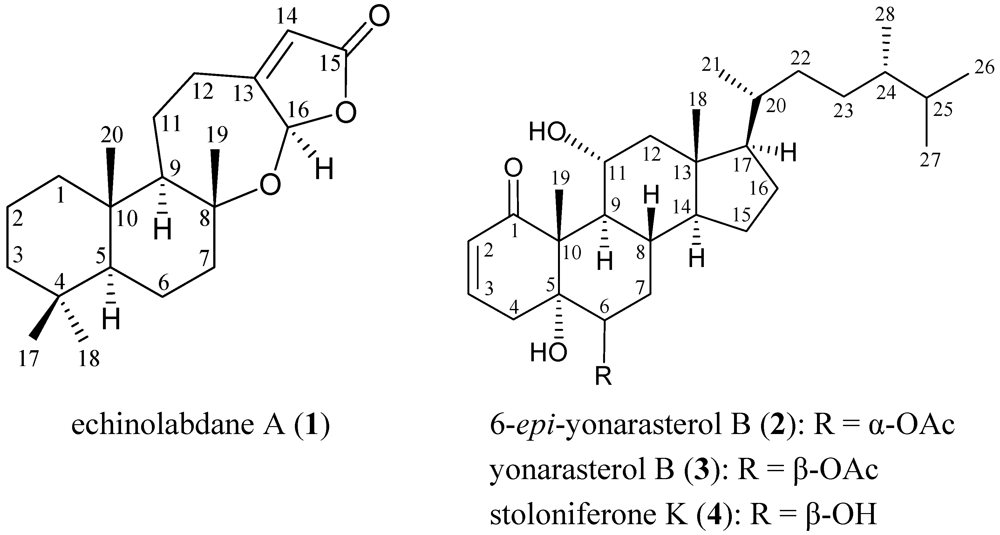
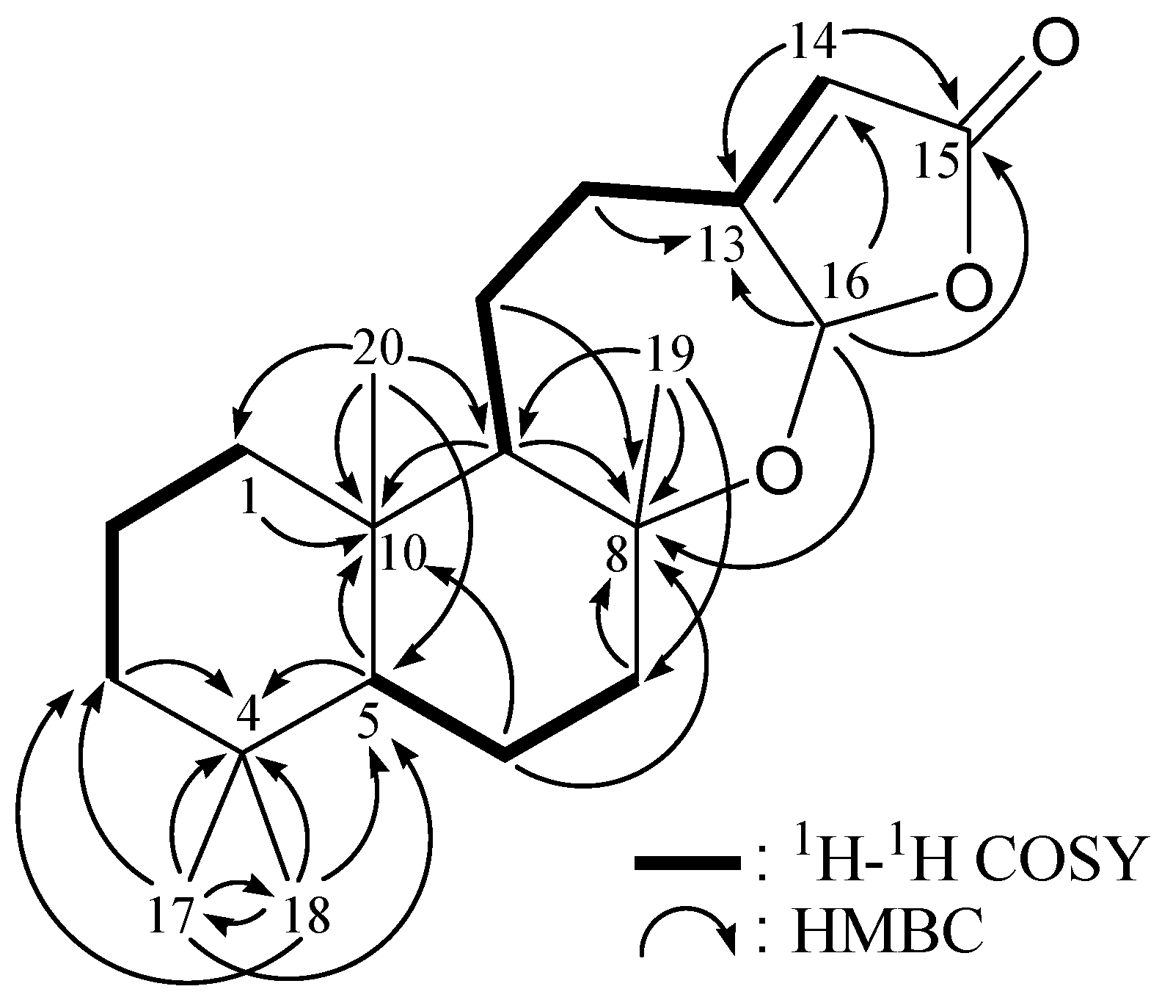
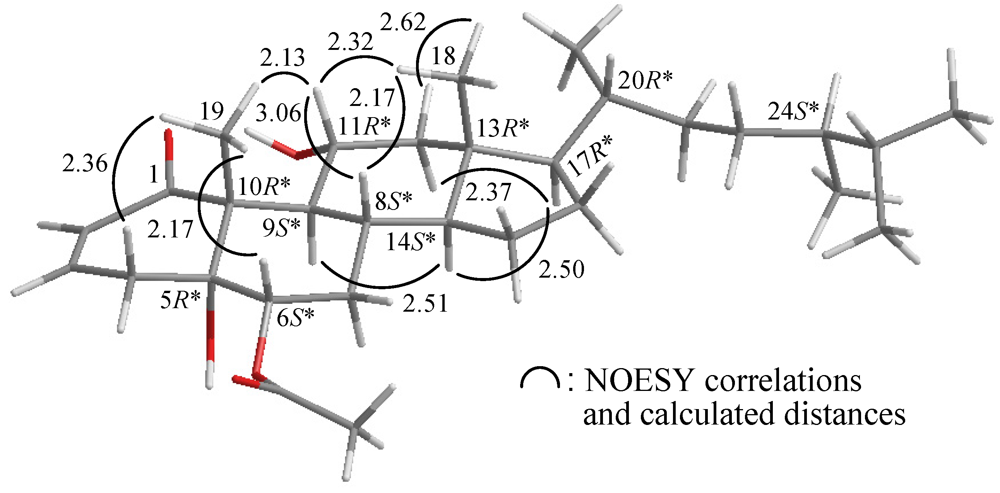
2. Results and Discussion
| Position | δΗ (J in Hz) | δC, Mult. | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1a | 1.69 m | 40.6, CH2 | H-1b, H2-2 | C-10 |
| 1b | 1.91 m | H-1a, H2-2 | C-9 | |
| 2a | 1.45 m | 18.6, CH2 | H2-1, H-2b, H2-3 | n.o. |
| 2b | 1.63 m | H2-1, H-2a, H2-3 | n.o. | |
| 3a | 1.16 dd(13.6, 4.0) | 41.7, CH2 | H2-2, H-3b | C-4, -17 |
| 3b | 1.39 m | H2-2, H-3a | n.o. | |
| 4 | 33.4, C | |||
| 5 | 0.92 dd (9.6, 2.0) | 56.1, CH | H2-6 | C-4, -6, -10 |
| 6a | 1.29 m | 19.9, CH2 | H-5, H-6b, H2-7 | C-8, -10 |
| 6b | 1.72 m | H-5, H-6a, H2-7 | C-5, -8, -10 | |
| 7a | 0.94 m | 39.8, CH2 | H2-6, H-7b | C-6 |
| 7b | 1.78 br d (11.6) | H2-6, H-7a | C-8 | |
| 8 | 82.5, C | |||
| 9 | 1.38 m | 60.6, CH | H2-11 | C-8, -10, -11, -20 |
| 10 | 39.2, C | |||
| 11a | 1.51 m | 22.1, CH2 | H-9, H-11b, H2-12 | C-9 |
| 11b | 1.94 m | H-9, H-11a, H2-12 | C-8, -9 | |
| 12a | 2.22 m | 29.3, CH2 | H2-11, H-12b, H-14 | C-13, -14 |
| 12b | 2.91 ddd (13.6, 3.2, 2.4) | H2-11, H-12a | n.o. | |
| 13 | 169.3, C | |||
| 14 | 5.82 br s | 117.0, CH | H-12a | C-12, -13, -15, -16 |
| 15 | 170.6, C | |||
| 16 | 6.07 s | 100.9, CH | C-8, -13, -14, -15 | |
| 17 | 0.89 s | 33.4, CH3 | C-3, -4, -5, -18 | |
| 18 | 0.80 s | 21.4, CH3 | C-3, -4, -5, -17 | |
| 19 | 1.25 s | 22.3, CH3 | C-7, -8, -9 | |
| 20 | 0.78 s | 15.6, CH3 | C-1, -5, -9, -10 |
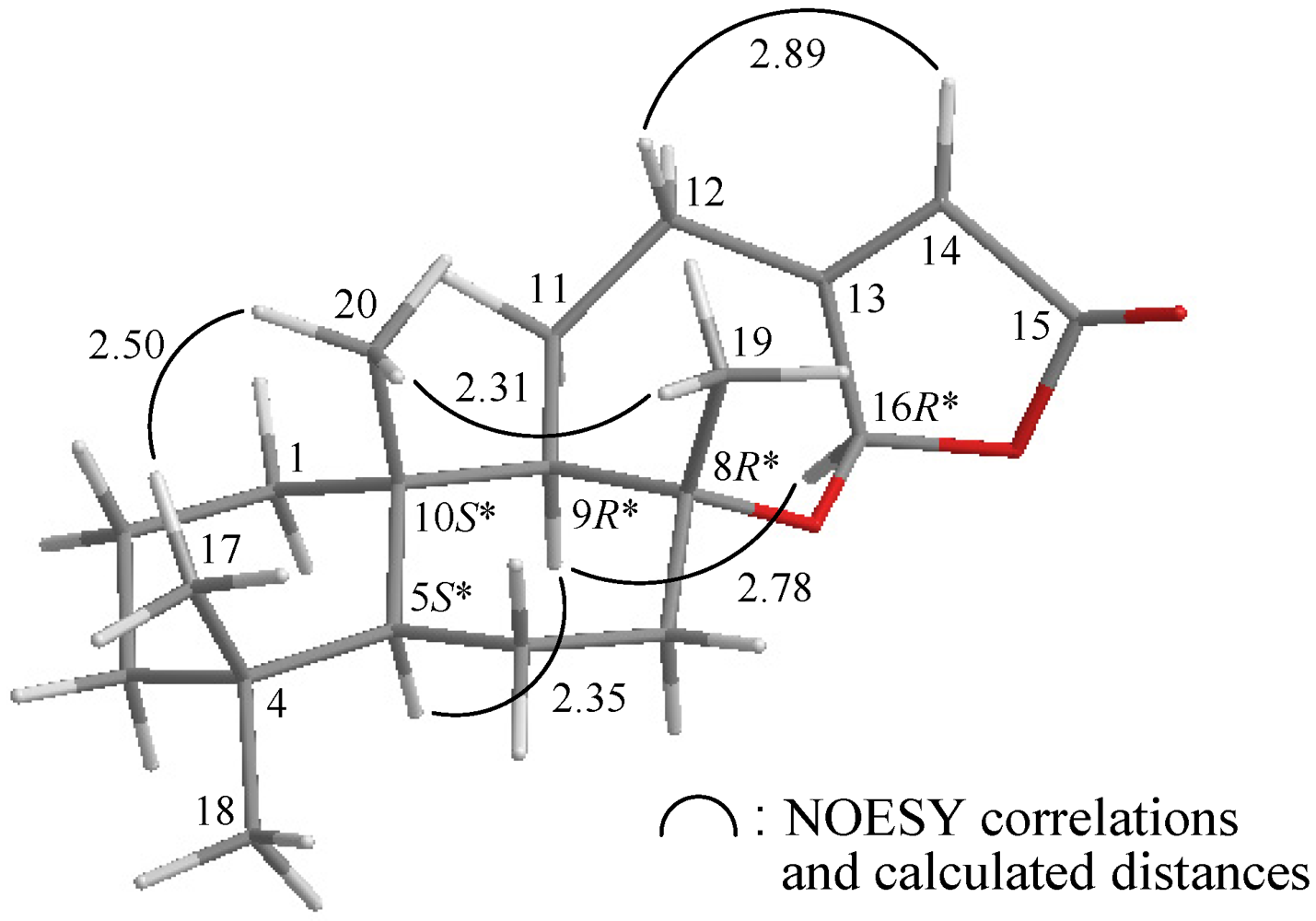
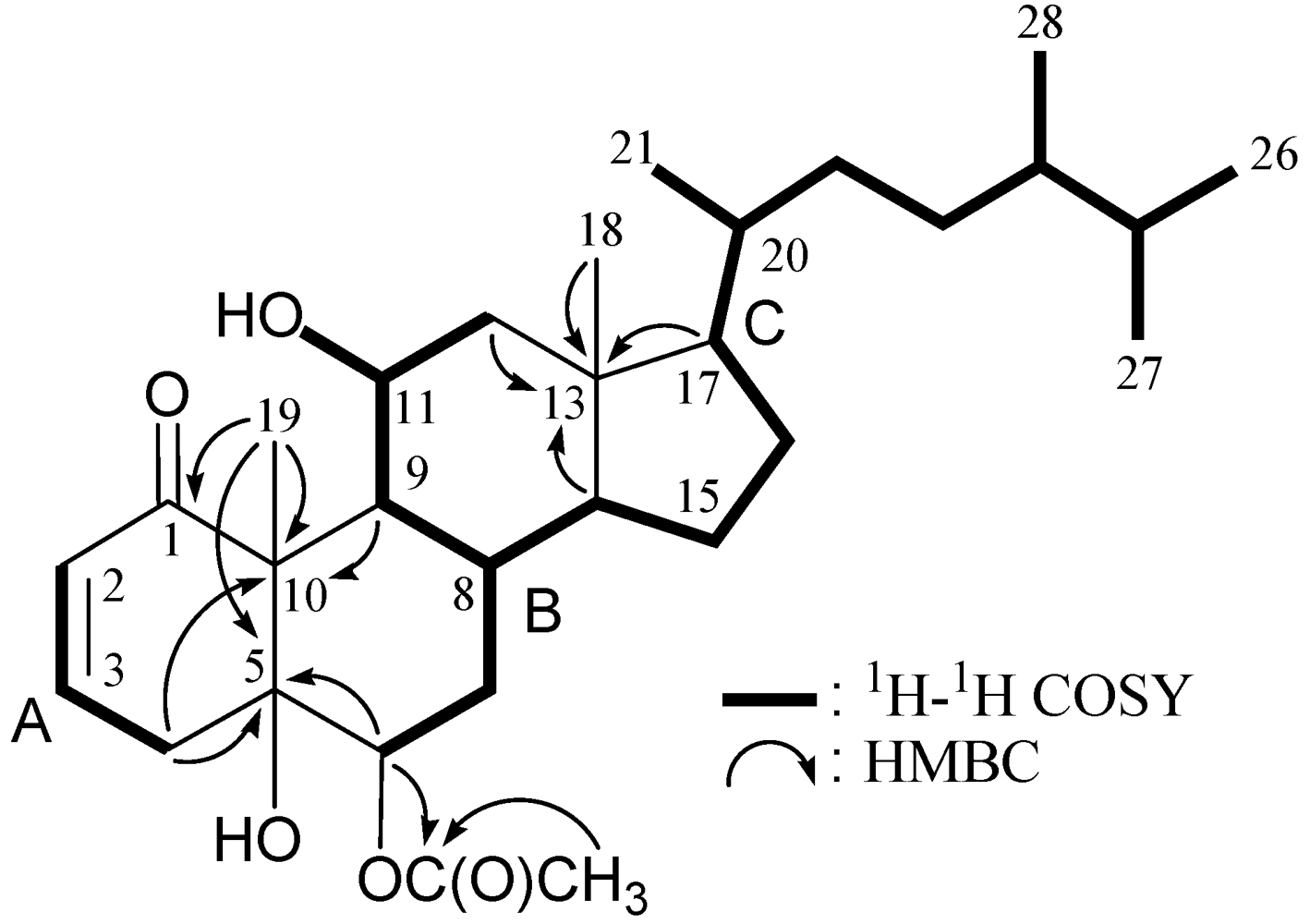
| Position | δΗ (J in Hz) | δC,Mult. | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 205.8, C | |||
| 2 | 6.15 dd (10.5, 2.5) | 128.8, CH | H-3 | n.o. |
| 3 | 6.68 ddd (10.5, 5.5, 2.5) | 140.7, CH | H-2, H2-4 | n.o. |
| 4a | 2.48 dd (20.5, 5.5) | 31.1, CH2 | H-3, H-4β | C-2, -3, -5, -10 |
| 4b | 2.91 br d (20.5) | H-3, H-4α | n.o. | |
| 5 | 78.4, C | |||
| 6 | 5.06 dd (12.0, 5.5) | 75.1, CH | H2-7 | C-5, -8, acetate carbonyl |
| 7a | 1.29 m | 33.8, CH2 | H-6, H-7b, H-8 | C-6, -8, -9 |
| 7b | 2.03 m | H-6, H-7a, H-8 | C-9 | |
| 8 | 1.27 m | 29.2, CH | H2-7, H-9, H-14 | n.o. |
| 9 | 1.62 m | 54.3, CH | H-8, H-11 | C-10 |
| 10 | 54.3, C | |||
| 11 | 3.91 br s | 66.9, CH | H-9, H2-12, OH-11 | n.o. |
| 12a | 1.13, m | 48.9, CH2 | H-11, H-12β | C-11, -13, -14, -17, -18 |
| 12b | 2.24 dd (12.5, 5.0) | H-11, H-12α | C-11, -13, -14, -17, -18 | |
| 13 | 43.1, C | |||
| 14 | 1.19 m | 54.9, CH | H-8, H2-15 | C-13, -18 |
| 15 | 1.57 m | 23.9, CH2 | H-14, H2-16 | n.o. |
| 16 | 1.31 m; 1.89 m | 28.1, CH2 | H2-15, H-17 | n.o. |
| 17 | 1.15 m | 55.9, CH | H2-16, H-20 | C-13, -18 |
| 18 | 0.67 s | 13.0, CH3 | C-12, -13, -14, -17 | |
| 19 | 1.34 s | 9.8, CH3 | C-1, -5, -9, -10 | |
| 20 | 1.32 m | 36.0, CH | H-17, H3-21, H2-22 | C-22 |
| 21 | 0.89 d (6.5) | 18.7, CH3 | H-20 | C-17, -20, -22 |
| 22a | 0.91 m | 33.5, CH2 | H-20, H-22b, H2-23 | C-20, -23, -24 |
| 22b | 1.37 m | H-20, H-22a, H2-23 | n.o. | |
| 23a | 0.93 m | 30.6, CH2 | H2-22, H-23b, H-24 | C-20, -22, -24 |
| 23b | 1.36 m | H2-22, H-23a, H-24 | C-22 | |
| 24 | 1.20 m | 39.0, CH | H2-23, H-25, H3-28 | C-22 |
| 25 | 1.56 m | 31.4, CH | H-24, H3-26, H3-27 | C-24, -26, -27, -28 |
| 26 | 0.85 d (7.0) | 20.5, CH3 | H-25 | C-24, -25, -27 |
| 27 | 0.78 d (6.5) | 17.6, CH3 | H-25 | C-24, -25, -26 |
| 28 | 0.77 d (6.5) | 15.4, CH3 | H-24 | C-23, -24, -25 |
| OH-11 | 1.74 d (4.0) | H-11 | n.o. | |
| 6-OAc | 171.5, C | |||
| 2.11 s | 21.2, CH3 | Acetate carbonyl |
| Compounds | Superoxide Anions | Elastase Release | |||
|---|---|---|---|---|---|
| IC50 (µg/mL) | Inh % a | IC50 (μg/mL) | Inh % a | ||
| 1 | >10.0 | 2.52 ± 3.02 | >10.0 | 1.83 ± 3.46 | |
| 2 | 2.98 ± 0.29 | 89.76 ± 5.63 | 1.13 ± 0.55 | 95.54 ± 6.17 | |
| DPI b | 0.82 ± 0.31 | ||||
| Elastatinal b | 31.82 ± 5.92 | ||||
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Molecular Mechanics Calculations
3.5. Cytotoxicity Testing
3.6. Superoxide Anion Generation and Elastase Release by Human Neutrophils
4. Conclusions
Acknowledgments
- Samples Availability: Not available.
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef]
- Berrue, F.; Kerr, R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef]
- Chung, H.-M.; Hwang, T.-L.; Wang, W.-H.; Fang, L.-S.; Sung, P.-J. Curcuphenol derivatives from the gorgonian Echinomuricea sp. Heterocycles 2009, 78, 2595–2600. [Google Scholar] [CrossRef]
- Allinger, N.L. Conformation analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Iwashima, M.; Nara, K.; Iguchi, K. New marine steroids, yonarasterols, isolated from the Okinawan soft coral, Clavularia virids. Steroids 2000, 65, 130–137. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Lo, I.-W.; Wang, S.-K.; Dai, C.-F. New cytotoxic steroids from the soft coral Clavularia virids. Steroids 2007, 72, 573–579. [Google Scholar] [CrossRef]
- Wright, J.L.C.; McInnes, A.G.; Shimizu, S.; Smith, D.G.; Walter, J.A.; Idler, D.; Khalil, W. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonances spectroscopy. Can. J. Chem. 1978, 56, 1898–1903. [Google Scholar] [CrossRef]
- Bayer, F.M. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed; Australian Institute of Marine Science: Queensland, Australia, 2001; pp. 59-60, 194-195. [Google Scholar]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenz-amido) benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Wang, C.-C.; Kuo, Y.-H.; Huang, H.-C.; Wu, Y.-C.; Kuo, L.-M.; Wu, Y.-H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2011, 28, 1755–1772. [Google Scholar] [CrossRef]
- Sims, J.J.; Lin, G.H.Y.; Wing, R.M.; Fenical, W. Marine natural products. Concinndiol, a bromo-diterpene alcohol from the red alga, Laurencia concinna. J. Chem. Soc. Chem. Commum. 1973. [Google Scholar]
- Howard, B.M.; Fenical, W.; Finer, J.; Hirotsu, K.; Clardy, J. Neoconcinndiol hydroperoxide, a novel marine diterpenoid from the red alga Laurencia. J. Am. Chem. Soc. 1977, 99, 6440–6441. [Google Scholar]
- Howard, B.M.; Fenical, W. Isoconcinndiol, a brominated diterpenoid from Laurencia snyderae var. guadalupensis. Phytochemistry 1980, 19, 2774–2776. [Google Scholar] [CrossRef]
- Fukuzawa, A.; Miyamoto, M.; Kumagai, Y.; Abiko, A.; Takaya, Y.; Masamune, T. Structure of new bromoditerpenes, pinnatols, from the marine red alga Laurencia pinnata Yamada. Chem. Lett. 1985, 14, 1259–1262. [Google Scholar]
- Iliopoulou, D.; Mihopoulos, N.; Roussis, V.; Vagias, C. New brominated labdane diterpenes from the red alga Laurencia obtusa. J. Nat. Prod. 2003, 66, 1225–1228. [Google Scholar] [CrossRef]
- Rudi, A.; Kashman, Y. Chelodane, barekoxide, and zaatirin-three new diterpenoids from the marine sponge Chelonaplysilla erecta. J. Nat. Prod. 1992, 55, 1408–1414. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Trivellone, E.; Cimino, G.; Uriz, M.J. Chemical diversity in the Mediterranean sponge Raspaciona aculeata: Structure and absolute stereochemistry of blanesin. Tetrahedron Lett. 1994, 35, 7871–7874. [Google Scholar]
- Nakamura, H.; Wu, H.; Ohizumi, Y.; Hirata, Y. Agelasine-A, -B, -C and -D, novel bicyclic diterpenoids with a 9-methyladeninium unit possessing inhibitory effects on Na,K-ATPase from the Okinawan sea sponge Agelas sp. Tetrahedron Lett. 1984, 25, 2989–2992. [Google Scholar]
- Davies-Coleman, M.T.; Faulkner, D.J. New diterpenoic acid glycerides from the Antarctic nudibranch Austrodoris kerguelensis. Tetrahedron 1991, 47, 9743–9750. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chung, H.-M.; Hong, P.-H.; Su, J.-H.; Hwang, T.-L.; Lu, M.-C.; Fang, L.-S.; Wu, Y.-C.; Li, J.-J.; Chen, J.-J.; Wang, W.-H.; et al. Bioactive Compounds from a Gorgonian Coral Echinomuricea sp. (Plexauridae). Mar. Drugs 2012, 10, 1169-1179. https://doi.org/10.3390/md10051169
Chung H-M, Hong P-H, Su J-H, Hwang T-L, Lu M-C, Fang L-S, Wu Y-C, Li J-J, Chen J-J, Wang W-H, et al. Bioactive Compounds from a Gorgonian Coral Echinomuricea sp. (Plexauridae). Marine Drugs. 2012; 10(5):1169-1179. https://doi.org/10.3390/md10051169
Chicago/Turabian StyleChung, Hsu-Ming, Pei-Han Hong, Jui-Hsin Su, Tsong-Long Hwang, Mei-Chin Lu, Lee-Shing Fang, Yang-Chang Wu, Jan-Jung Li, Jih-Jung Chen, Wei-Hsien Wang, and et al. 2012. "Bioactive Compounds from a Gorgonian Coral Echinomuricea sp. (Plexauridae)" Marine Drugs 10, no. 5: 1169-1179. https://doi.org/10.3390/md10051169
APA StyleChung, H.-M., Hong, P.-H., Su, J.-H., Hwang, T.-L., Lu, M.-C., Fang, L.-S., Wu, Y.-C., Li, J.-J., Chen, J.-J., Wang, W.-H., & Sung, P.-J. (2012). Bioactive Compounds from a Gorgonian Coral Echinomuricea sp. (Plexauridae). Marine Drugs, 10(5), 1169-1179. https://doi.org/10.3390/md10051169







