Adjuvant Activity of Sargassum pallidum Polysaccharides against Combined Newcastle Disease, Infectious Bronchitis and Avian Influenza Inactivated Vaccines
Abstract
:1. Introduction
2. Results
2.1. Changes in the Antibody Titer
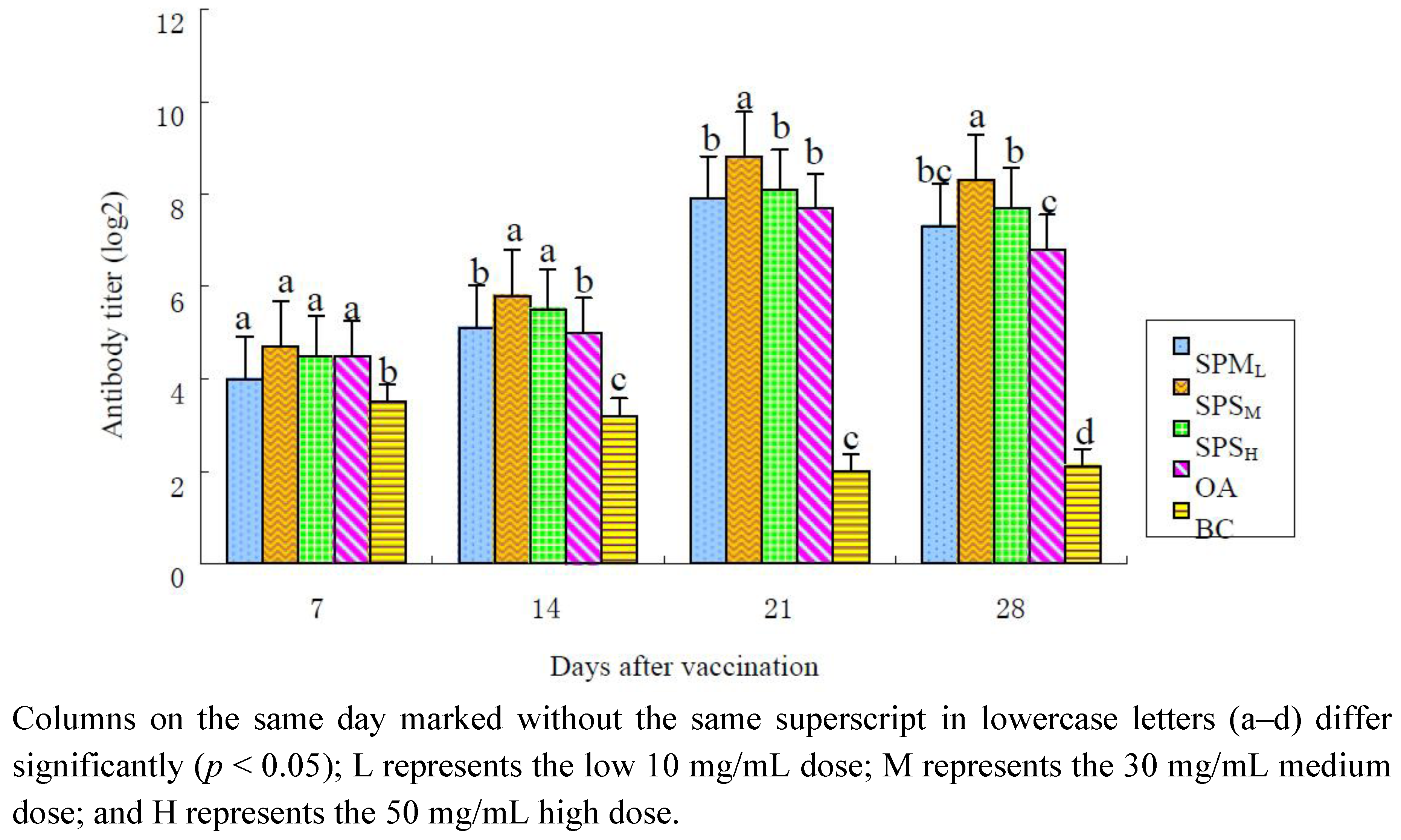
2.1.1. Changes in the ND Antibody Titer
| Group | Days after the Immunization | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| SPPL | 4.1 ± 0.40 a, b | 5.0 ± 0.27 b | 7.8 ± 0.25 b | 7.0 ± 0.30 b, c |
| SPPM | 4.9 ± 0.48 a | 6.1 ± 0.40 a | 8.8 ± 0.25 a | 8.6 ± 0.32 a |
| SPPH | 4.5 ± 0.19 a | 6.0 ± 0.19 a | 7.9 ± 0.23 b | 7.6 ± 0.38 b |
| OA | 4.6 ± 0.38 a | 4.9 ± 0.23 b | 7.6 ± 0.26 b | 6.5 ± 0.33 c |
| BC | 3.5 ± 0.19 b | 3.3 ± 0.41 c | 2.0 ± 0.27 c | 2.1 ± 0.13 d |
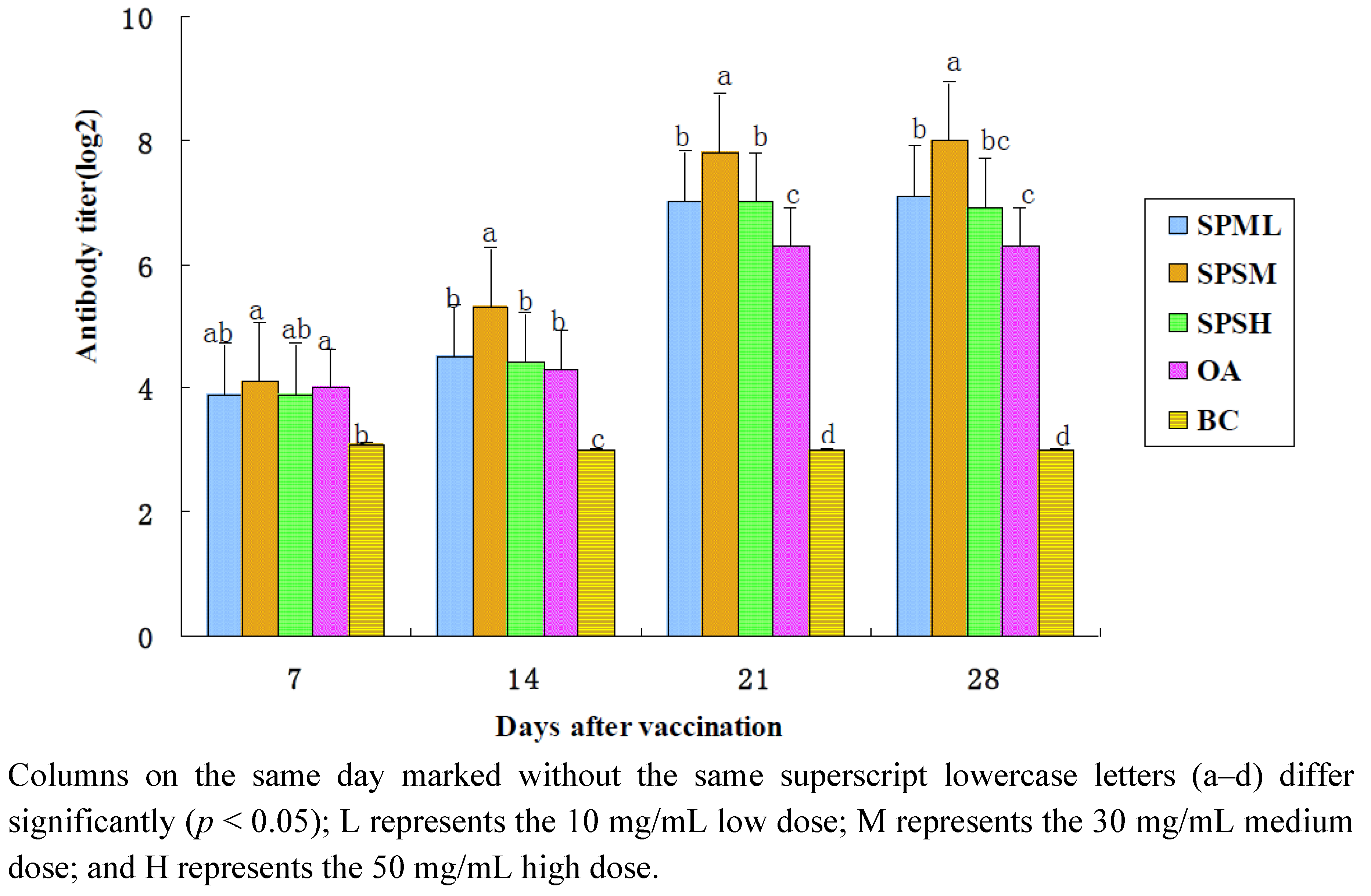
2.1.2. Changes in the IB Antibody Titer
| Group | Days after the Immunization | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| SPPL | 3.9 ± 0.30 a, b | 4.5 ± 0.27 b | 7.0 ± 0.27 b | 7.1 ± 0.23 b |
| SPPM | 4.1 ± 0.23 a | 5.3 ± 0.25 a | 7.8 ± 0.25 a | 8.0 ± 0.27 a |
| SPPH | 3.9 ± 0.30 a, b | 4.4 ± 0.26 b | 7.0 ± 0.33 b | 6.9 ± 0.30 b, c |
| OA | 4.0 ± 0.27 a | 4.3 ± 0.25 b | 6.3 ± 0.25 c | 6.3 ± 0.25 c |
| BC | 3.1 ± 0.23 b | 3.0 ± 0.19 c | 3.0 ± 0.19 d | 3.0 ± 0.30 d |
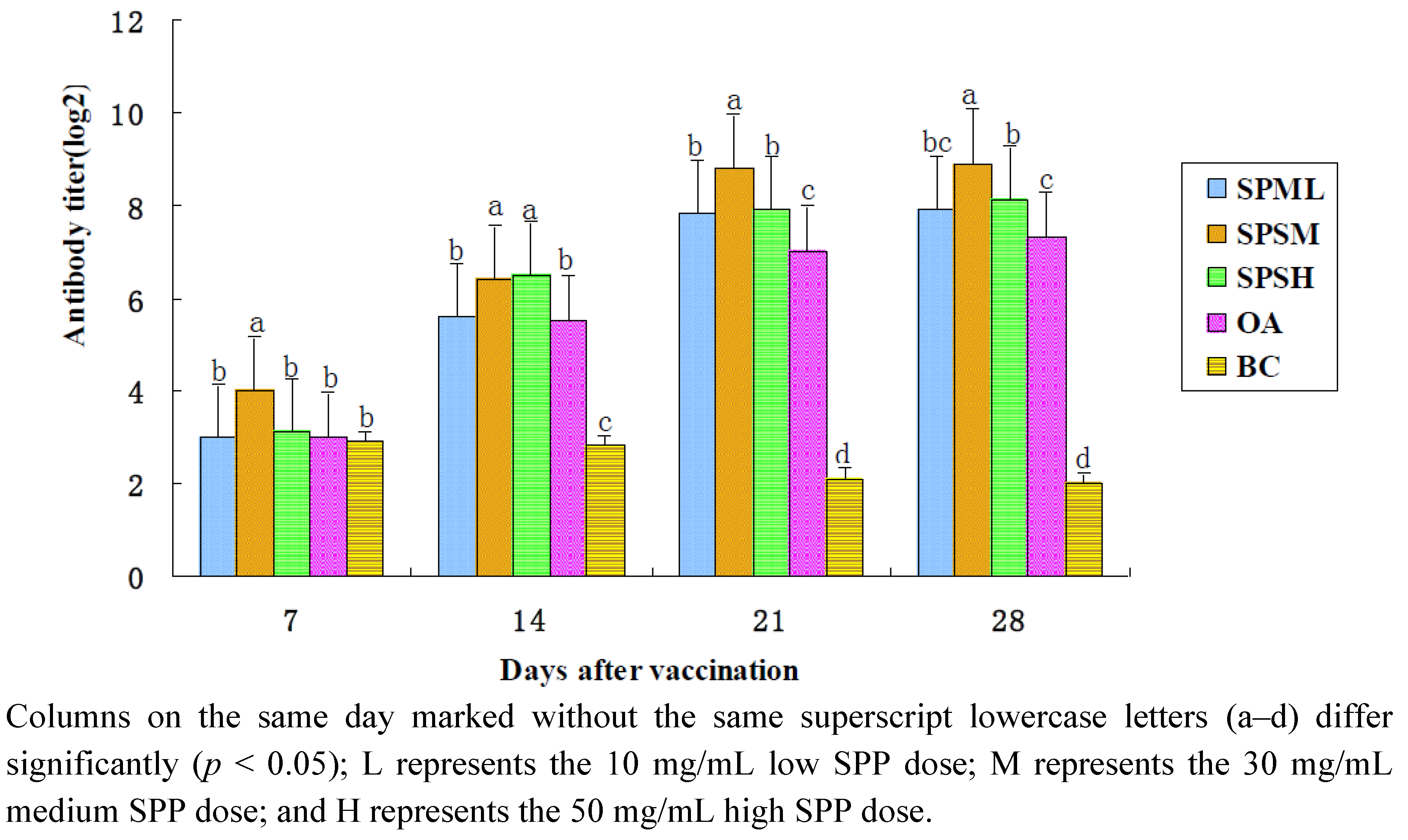
2.1.3. Changes in the AI Antibody Titer
| Group | Days after the Immunization | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| SPPL | 3.0 ± 0.27 b | 5.6 ± 0.18 b | 7.8 ± 0.25 b | 7.9 ± 0.30 b, c |
| SPPM | 4.0 ± 0.27 a | 6.4 ± 0.32 a | 8.8 ± 0.16 a | 8.9 ± 0.23 a |
| SPPH | 3.1 ± 0.30 b | 6.5 ± 0.19 a | 7.9 ± 0.30 b | 8.1 ± 0.23 b |
| OA | 3.0 ± 0.76 b | 5.5 ± 0.27 b | 7.0 ± 0.27 c | 7.3 ± 0.25 c |
| BC | 2.9 ± 0.23 b | 2.8 ± 0.16 c | 2.1 ± 0.30 d | 2.0 ± 0.27 d |
2.2. Changes in the Lymphocyte Proliferation Assay
| Group | Days after the Immunization | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| SPPL | 0.241 ± 0.088 b | 0.279 ± 0.025 b | 0.402 ± 0.049 c | 0.395 ± 0.073 c |
| SPPM | 0.260 ± 0.026 a | 0.295 ± 0.013 a | 0.449 ± 0.042 a | 0.526 ± 0.017 a |
| SPPH | 0.257 ± 0.041 a | 0.282 ± 0.015 b | 0.417 ± 0.062 b | 0.477 ± 0.047 b |
| OA | 0.234 ± 0.035 b | 0.257 ± 0.017 c | 0.354 ± 0.029 d | 0.382 ± 0.026 d |
| BC | 0.213 ± 0.010 c | 0.188 ± 0.009 d | 0.201 ± 0.024 e | 0.158 ± 0.086 e |
2.3. Changes in the Lymphocyte Subsets
2.3.1. Changes in CD4+ Lymphocyte Subsets
| Group | Days after the Immunization | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| SPPL | 34.9 ± 3.84 c | 36.4 ± 3.71 b, c | 36.9 ± 3.74 c | 39.8 ± 3.70 b |
| SPPM | 39.3 ± 4.55 a | 40.2 ± 4.23 a | 40.3 ± 3.90 a | 42.5 ± 4.18 a |
| SPPH | 36.9 ± 4.11 b | 37.7 ± 4.02 b | 38.3 ± 3.79 b | 39.0 ± 4.05 b |
| OA | 34.6 ± 3.95 c | 35.0 ± 3.86 c | 36.7 ± 4.32 c | 37.5 ± 3.93 c |
| BC | 29.2 ± 4.56 d | 27.4 ± 3.80 d | 28.0 ± 3.54 d | 26.1 ± 3.61 d |
2.3.2. Changes in CD8+ Lymphocyte Subsets
| Group | Days after the Immunization | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| SPPL | 18.5 ± 2.15 | 20.8 ± 2.44 | 23.9 ± 1.41 | 22.8 ± 2.35 |
| SPPM | 19.7 ± 3.47 | 22.9 ± 2.27 | 23.6 ± 1.39 | 22.4 ± 2.12 |
| SPPH | 20.7 ± 2.64 | 21.2 ± 3.57 | 22.4 ± 2.89 | 21.8 ± 1.23 |
| OA | 19.4 ± 2.08 | 21.5 ± 1.62 | 21.9 ± 3.02 | 22.6 ± 3.27 |
| BC | 21.6 ± 1.48 | 21.5 ± 3.82 | 22.0 ± 3.31 | 22.8 ± 2.46 |
2.3.3. The Changes in CD4+/CD8+ Values
| Group | Days after the Immunization | |||
|---|---|---|---|---|
| 7 | 14 | 21 | 28 | |
| SPPL | 1.80 ± 0.40 c | 1.75 ± 0.33 a | 1.57 ± 0.17 b, c | 1.73 ± 0.18 b, c |
| SPPM | 2.01 ± 0.35 a | 1.75 ± 0.12 a | 1.73 ± 0.29 a | 1.85 ± 0.37 a |
| SPPH | 1.91 ± 0.28 b | 1.74 ± 0.17 a | 1.74 ± 0.34 a | 1.79 ± 0.16 b |
| OA | 1.73 ± 0.14 c, d | 1.68 ± 0.09 b | 1.64 ± 0.50 b | 1.66 ± 0.45 c |
| BC | 1.34 ± 0.15 e | 1.29 ± 0.26 c | 1.25 ± 0.41 d | 1.12 ± 0.22 d |
3. Experimental
3.1. Preparation of Polysaccharides
3.2. Vaccine and Virus
3.3. Reagents
3.4. Animals and Immunization
3.5. Serum Antibody Titer Assay [23,24]
3.5.1. Serum ND Antibody Titer Assay
3.5.2. Serum IB Antibody Titer Assay
3.5.3. Serum AI Antibody Titer Assay
3.6. Peripheral Blood Lymphocyte Proliferation Assay
3.7. Flow Cytometry Analysis
3.8. Statistical Analysis
4. Discussion
5. Conclusion
- The results of this study show that the appropriate dose of polysaccharide significantly enhances the specific immune response in chickens and improves vaccine effectiveness, promoting an earlier peak that increases rapidly and lasts for a long time;
- The lymphocyte transformation state corresponds to the A570 values measured using the MTT method. The data indicate that the SPP induce T lymphocytes to multiply and exhibit certain effectiveness;
- In this study, the CD4+ T lymphocyte content and CD4+/CD8+ values in all the test groups were higher than those in the control group, and the medium SPP dose groups were significantly higher than the control group, which indicates that the appropriate dose of polysaccharide can promote the proliferation of peripheral CD4+ T lymphocytes in chickens, thereby enhancing cellular immunity;
- Co-administration of SPP induced an increase in the proliferation rate and antibody production in lymphocytes, and an appropriate dose of SPP up-regulated both the cellular and humoral immune responses when used as an adjuvant for the combined ND-IB-AI vaccines.
Acknowledgements
Conflict of Interest
References
- 1. Fan, Y.; Hu, Y.; Wang, D.; Guo, Z.; Guo, L.; Zhao, B.; Zhang, J.; Wang, Y.; Nguyen, T.L. Epimedium polysaccharide and propolis flavone can synergistically stimulate lymphocyte proliferation in vitro and enhance the immune responses to ND vaccine in chickens. Int. J. Biol. Macromol. 2010, 47, 87–92. [Google Scholar] [CrossRef]
- Tseng, L.P.; Chiou, C.J.; Chen, C.C.; Deng, M.C.; Chung, T.W.; Huang, Y.Y.; Liu, D.Z. Effect of lipopolysaccharide on intranasal administration of liposomal Newcastle disease virus vaccine to SPF chickens. Vet. Immunol. Immunopathol. 2009, 131, 285–289. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, Y.; Wang, D.; Ma, X.; Zhao, X.; Zhao, B.; Wang, J.; Liu, P. Sulfated modification can enhance the adjuvanticity of lentinan and improve the immune effect of ND vaccine. Vaccine 2009, 27, 660–665. [Google Scholar] [CrossRef]
- Layton, R.C.; Petrovsky, N.; Gigliotti, A.P.; Pollock, Z.; Knight, J.; Donart, N.; Pyles, J.; Harrod, K.S.; Gao, P.; Koster, F. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine 2011, 29, 6242–6251. [Google Scholar] [CrossRef]
- Le, T.; Le, T.; Doan, T.H.; Quyan, D.; Le, K.X.; Pham, V.; Nagataki, M.; Nomura, H.; Ikeue, Y.; Watanabe, Y.; Agatsuma, T. The adjuvant effect of Sophy β-glucan to the antibody response in poultry immunized by the avian influenza A H5N1 and H5N2 vaccines. J. Microbiol. Biotechnol. 2011, 21, 405–411. [Google Scholar]
- De Vries, J.J.; Bungener, L.; Ter Veer, W.; van Alphen, L.; van der Ley, P.; Wilschut, J.; Huckriede, A. Incorporation of LpxL1, a detoxified lipopolysaccharide adjuvant, in influenza H5N1 virosomes increases vaccine immunogenicity. Vaccine 2009, 27, 947–955. [Google Scholar]
- Liu, H.-J.; Jiang, N.; Li, J.-J.; Lian, Y.-I.; Zhang, P. Study on the extraction and purification of polysaccharides from Cordyceps Militaris. Acta Agric. Jiangxi 2007, 19, 80–82. [Google Scholar]
- Cao, J.H.; Li, H. Progress in study on the immunoregulation effect of polysaccharides. Chin. J. Biochem. Pharm. 1999, 20, 104–107. [Google Scholar]
- Zhang, H.B.; Ge, L.J.; Yang, H.J.; Yang, S.H.; Wang, C.F.; Gao, Y.D.; Zhong, J.F. In vitro endometritis pathogenic bacteria of inhibition research of the function by single type polysaccharide traditional Chinese herb. Southwest China J. Agric. Sci. 2009, 22, 798–801. [Google Scholar]
- Chen, Z.W.; Gao, J.F.; Hu, T.J.; He, Y.; Liu, W.; Hu, S.; Zhao, W.; Li, B.; Liang, J.X.; Guan, Z.Y. Experimental study of Sargassum polysaccharide’s immune modulatory effect in mice. China Anim. Husb. Vet. Med. 2009, 1, 57–59. [Google Scholar]
- He, Y.; Chen, Z.W.; Gao, J.F.; Hu, T.J.; Liu, W.; Zhao, W.; Hu, S.; Zhao, W.; Guan, Z.Y.; Li, B.; Liang, J.X. Immunoregulation effect of Sargassum polysaccharides on white-leg shrimp. J. Anhui Agric. Sci. 2008, 36, 13664–13665. [Google Scholar]
- Liu, Q.Y.; Meng, Q.Y. Effects of Sargassum confusum polysaccharide on the expression of p53 and Rb genes in mouse sarcoma S180 cells. J. South. Med. Univ. 2008, 28, 1378–1381. [Google Scholar]
- Ouyang, J.; Hu, Y.Z.; Qian, Z.G. TCM research progress of immune polysaccharide as a vaccine adjuvant (in Chinese). J. Yunnan Coll. Tradit. Chin. Med. 2002, 25, 14–17. [Google Scholar]
- Lu, X.T.; Dai, J.H.; Liao, M. Advances of studies on immunoregulation activities of polysaccharide. Prog. Vet. Med. 2003, 24, 10–12. [Google Scholar]
- Ung, C.Y.; Li, H.; Kong, C.Y.; Wang, J.F.; Chen, Y.Z. Usefulness of traditionally defined herbal properties for distinguishing prescriptions of traditional Chinese medicine from non-prescription recipes. J. Ethnopharmacol. 2007, 109, 21–28. [Google Scholar] [CrossRef]
- Kong, X.F.; Hu, Y.L.; Rui, R.; Wang, D.; Li, X. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int. Immunopharmacol. 2004, 4, 975–982. [Google Scholar] [CrossRef]
- Kong, X.F.; Hu, Y.L.; Yin, Y.L.; Wu, G.Y.; Rui, R.; Wang, D.Y.; Yang, C.B. Chinese herbal ingredients are effective immune stimulators for chickens infected with the Newcastle disease virus. Poult. Sci. 2006, 85, 2169–2175. [Google Scholar]
- Liu, S.S.; Hu, T.J.; Zeng, Y.; Wei, Y.Y.; Li, Y.H. Research Progress of Chemical Structure and Pharmacological Effect of Sargassum Polysaccharides (in Chinese). In Proceedings of 2009 Academic Annual Meeting of China Institute of Animal Husbandry and Veterinary Medicine Veterinary Medicine Branch, Nanchang, China, 11-14 August 2009.
- Ye, H. Purification, Bioactivity and Structure of Polysaccharides from the Brown Seaweed Sargassum Pallidum. Ph.D. Thesis, Nanjing Agriculture University, Nanjing, China, June 2008. [Google Scholar]
- Meng, Q.Y.; Liu, Z.H.; Xu, M.Y.; Higashino, G. Extraction and analysis of the Sargassum hemiphyllum (Turner) C. Ag. Polysaccharide (SHP) (in Chinese). Spectrosc. Spectr. Anal. 2004, 24, 1560–1562. [Google Scholar]
- Veterinary Pharmacopoeia Commission of the People’s Republic of China, Veterinary Pharmacopoeia of the People’s Republic of China; Chemical Industrial Press: Beijing, China, 2000; pp. 72–73.
- Liu, B.Q. Veterinary Biological Product; China Agriculture Press: Beijing, China, 1995. [Google Scholar]
- Guo, L.W.; Wang, D.Y.; Hu, Y.L.; Zhao, X.; Wang, Y.; Yang, S.; Wang, J.; Fan, Y.; Han, G.; Gao, H. Adjuvanticity of compound polysaccharides on chickens against Newcastle disease and avian influenza vaccine. Int. J. Biol. Macromol. 2012, 50, 512–517. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, M.; Li, M. Protective efficacy of commercial Newcastle disease vaccines against challenge of goose origin virulent Newcastle disease virus in geese. Avian Dis. 2008, 52, 467–471. [Google Scholar] [CrossRef]
- Mohanty, S.B.; Dutta, S.K. Veterinary Virology; Lea and Febiger Philadelphia: London, UK, 1981. [Google Scholar]
- National Standardization Management Committee, Diagnostic Technique of Highly Pathogenic Avian Influenza; Standard Press of China: Beijing, China, 2003.
- Li, X.R.; Jin, H.; Wang, X.; Lu, J. Measurement of chicken splenic lymphocyte activation by MTT colorimetric assay. Anim. Husb. Vet. Med. 1996, 28, 3–5. [Google Scholar]
- Ashwood, P.; Wakefield, A.J. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J. Neuroimmunol. 2006, 173, 126–134. [Google Scholar] [CrossRef]
- Wang, D.; Hu, Y.; Sun, J.; Kong, X.; Zhang, B.; Liu, L. Comparative study on adjuvanticity of compound Chinese herbal medicinal ingredients. Vaccine 2005, 23, 3704–3708. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, X.; Tong, G. The fuction of CD4+/CD8+ in celluar immunization and its relationship with PRRS. Chin. J. Prev. Vet. Med. 2002, 22, 151–152. [Google Scholar]
- Wang, D.Y.; Hu, Y.L.; Zhang, B.K.; Liu, J.G.; Wang, X.T. Comparison on immune synergism of several Chinese herbal medicinal ingredients with interleukin-2. J. Nanjing Agric. Univ. 2005, 28, 140–142. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, L.-J.; Li, M.-Y.; Li, Y.-T.; Feng, J.-J.; Hao, F.-Q.; Zhang, L. Adjuvant Activity of Sargassum pallidum Polysaccharides against Combined Newcastle Disease, Infectious Bronchitis and Avian Influenza Inactivated Vaccines. Mar. Drugs 2012, 10, 2648-2660. https://doi.org/10.3390/md10122648
Li L-J, Li M-Y, Li Y-T, Feng J-J, Hao F-Q, Zhang L. Adjuvant Activity of Sargassum pallidum Polysaccharides against Combined Newcastle Disease, Infectious Bronchitis and Avian Influenza Inactivated Vaccines. Marine Drugs. 2012; 10(12):2648-2660. https://doi.org/10.3390/md10122648
Chicago/Turabian StyleLi, Li-Jie, Ming-Yi Li, Yan-Tuan Li, Jing-Jing Feng, Feng-Qiang Hao, and Lun Zhang. 2012. "Adjuvant Activity of Sargassum pallidum Polysaccharides against Combined Newcastle Disease, Infectious Bronchitis and Avian Influenza Inactivated Vaccines" Marine Drugs 10, no. 12: 2648-2660. https://doi.org/10.3390/md10122648
APA StyleLi, L.-J., Li, M.-Y., Li, Y.-T., Feng, J.-J., Hao, F.-Q., & Zhang, L. (2012). Adjuvant Activity of Sargassum pallidum Polysaccharides against Combined Newcastle Disease, Infectious Bronchitis and Avian Influenza Inactivated Vaccines. Marine Drugs, 10(12), 2648-2660. https://doi.org/10.3390/md10122648




