Inhibition of Bladder Tumor Growth by Chitooligosaccharides in an Experimental Carcinogenesis Model
Abstract
:1. Introduction
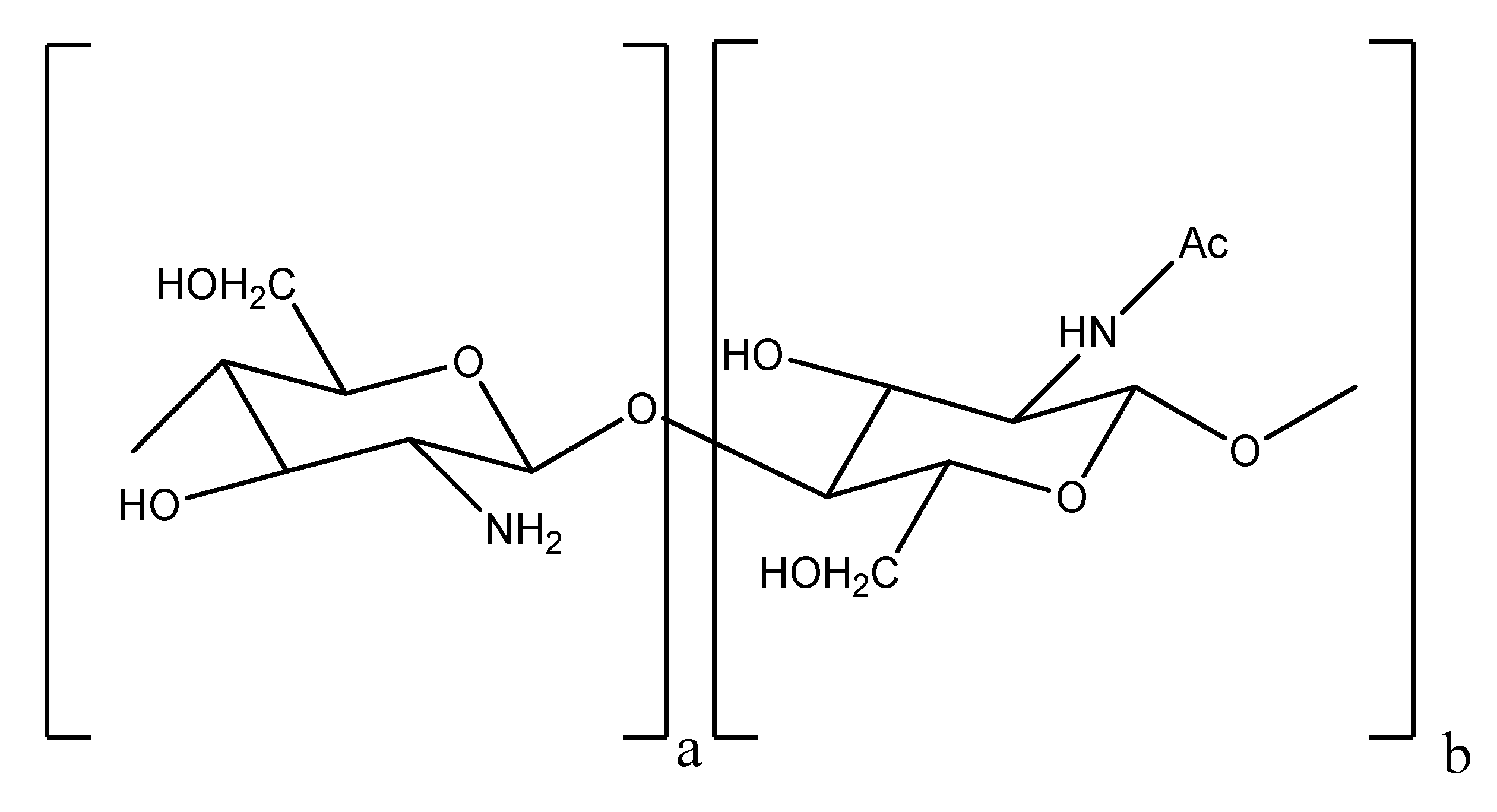
2. Results and Discussion
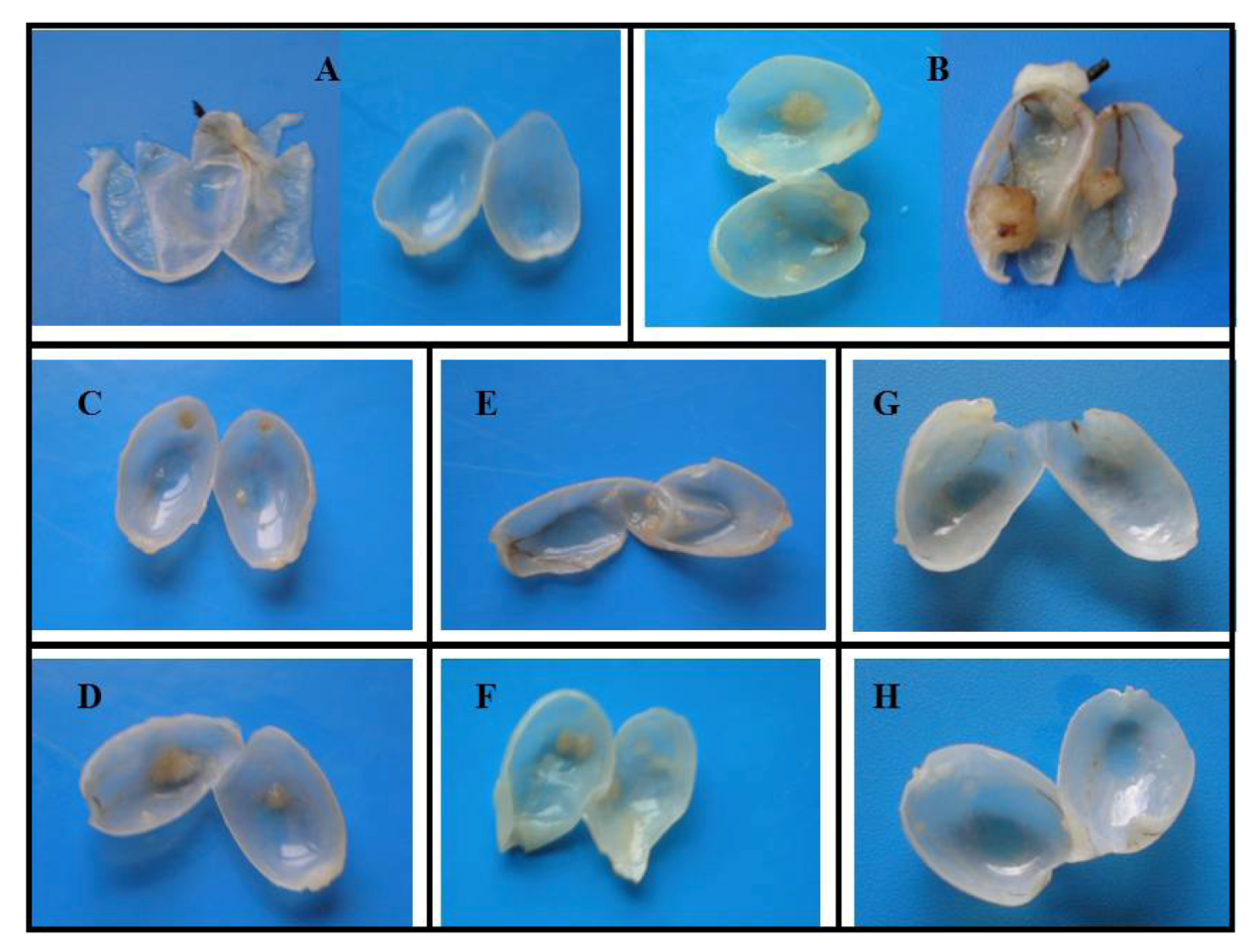
| Rat Groups (n = 5 each) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Macroscopic analysis | ||||||||||
| Number of tumors | ||||||||||
| % of tumors/group | 0 | 40 | 100 | 0 | 20 | 80 | 0 | 20 | 25 | 80 |
| Total no. of tumors | 0 | 2 | 9 | 0 | 1 | 5 | 0 | 1 | 2 | 9 |
| No. of tumors/rat | 0 | 0.4 | 1.8 | 0 | 0.2 | 1 | 0 | 0.2 | 0.4 | 1.8 |
| Tumor volume | ||||||||||
| Per tumor (mm3) | 0 | 2.6 | 3.22 | 0 | 1.04 | 3.25 | 0 | 1.04 | 1.3 | 4,49 |
| Microscopic (histological) analysis | ||||||||||
| Pre-neoplastic lesions | ||||||||||
| Hyperplasia | 0 | 1/2 | 6/9 | 0 | 0 | 2/5 | 0 | 0 | 0 | 5/9 |
| High-grade dysplasia | 0 | 1/2 | 3/9 | 0 | 1/1 | 2/5 | 0 | 1/1 | 0 | 4/9 |
| Malignant lesions, tumor | ||||||||||
| Papillary | 0 | 1/2 | 9/9 | 0 | 1/1 | 4/4 | 0 | 1/1 | 1/2 | 7/9 |
| Infiltrative | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2/9 |
| Tumor grading | ||||||||||
| Low grade (G1) | 0 | 1/2 | 5/9 | 0 | 0 | 3/4 | 0 | 0 | 1/2 | 3/9 |
| High grade (G2/G3) | 0 | 1/2 | 4/9 | 0 | 1/1 | 1/4 | 0 | 1/1 | 1/2 | 6/9 |
| Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Markers | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Urea # | 15.45 | 14.62 | 17.22 | 19.82 | 16 (±1.21) | 16.47 | 15.5 | 15.63 | 15.73 | 15.55 | |
| (±0.95) | (±0.82) | (±1.39) | (±1.26) | (±0.37) | (±0.99) | (±1.59) | (±0.67) | (±0.76) | |||
| Creat * | 0.42 | 0.39 | 0.38 | 0.5 | 0.46 | 0.4 | 0.40 | 0.39 | 0.40 | 0.45 | |
| (0.46–0.38) | (0.46–0.32) | (0.41–0.35) | (0.52–0.49) | (0.52–0.41) | (0.43–0.39) | (0.43–0.34) | (0.42–0.33) | (0.44–0.37) | (0.47–0.43) | ||
| Uric Acid * | 0.4 | 0.35 | 0.6 | 1 | 1 | 0.5 | 0.58 | 0.5 | 0.58 | 0.5 | |
| (0.52–0.3) | (0.47–0.27) | (0.7–0.5) | (1.05–0.9) | (1.35–0.75) | (0.55–0.45) | (0.63–0.5) | (0.55–0.45) | (0.63–0.55) | (0.55–0.48) | ||
| G.P.T. * | 42 | 37.5 | 47 | 51 | 45 | 132.5 | 30.75 | 24.25 | 29 | 49.5 | |
| (43.5–40.5) | (39.3–36.8) | (49.5–46) | (53.8–49.5) | (46–42.5) | (141.8–115) | (36.25–25) | (25.5–21.8) | (29.8–28.3) | (54–45.25) | ||
| G.O.T. * | 64 | 66 | 67 | 75.5 | 67 | 213 | 69 | 72 | 69.75 | 82 | |
| (68–60.5) | (72.8–63.8) | (69–65) | (82.5–73.5) | (69.5–64.5) | (237.8–193) | (71.8–66.8) | (80.8–64.8) | (75–60.75) | (84.3–79.3) | ||
| α-Amylase # | 547.5 | 500.75 | 510.5 | 544.5 | 453.75 | 459.75 | 531.75 | 552 | 551.5 | 545 | |
| (±106.96) | (±79.78) | (±25.44) | (±23.9) | (±34.97) | (±39.79) | (±15.84) | (±31.51) | (±115.23) | (±77.18) | ||
| Cholesterol * | 47.5 | 42.5 | 45 | 46 | 43.5 | 39.5 | 62.25 | 57.25 | 63 | 42 | |
| (50–42.25) | (43.5–41) | (46–44) | (47.3–44.3) | (47–39.5) | (41.5–38.3) | (69.5–55.3) | (63.25–51) | (69–57.5) | (43.3–40.5) | ||
| HDL * | 27.5 | 22 | 27 | 28 | 24 | 23 | 36.75 | 33 | 35.5 | 24 | |
| (29–25.5) | (23.3–21.8) | (27.25–27) | (28.8–27.3) | (25.3–22) | (24–23) | (40.3–33) | (35.5–31) | (38.8–31.8) | (24.5–23.5) | ||
| LDL * | 13 | 14.5 | 12 | 11.5 | 13.5 | 12.5 | 1.68 | 1.73 | 1.8 | 13.5 | |
| (15–11.75) | (16–12.75) | (13.5–11) | (12–11) | (15–12.75) | (13–12) | (1.7–1.68) | (1.8–1.68) | (1.83–1.78) | (14.5–12.8) | ||
| Atherogenic * | 1.8 | 1.85 | 1.65 | 1.65 | 1.9 | 1.7 | 20.75 | 19 | 17.5 | 1.75 | |
| (1.9–1.7) | (1.93–1.78) | (1.7–1.6) | (1.7–1.6) | (1.9–1.875) | (1.7–1.67) | (24–18.25) | (20–17.5) | (18.8–15.8) | (1.8–1.7) | ||
| Trigly * | 75 | 74.5 | 48 | 59.5 | 55.5 | 34.5 | 134 | 122.5 | 128.75 | 50 | |
| (86–67) | (78.75–67) | (52.3–44.5) | (65.7–51.8) | (59.8–49.7) | (41–28.25) | (136–130.8) | (123.5–115) | (134.3–126) | (53.5–40.8) | ||
3. Experimental Section
3.1. COS Characterization (MW and DD)

3.2. Animals and Treatment
3.3. Blood and Organs Collection
3.4. Blood Analysis
3.5. Macroscopic Tumor Analysis
3.6. Microscopic Tumor Analysis
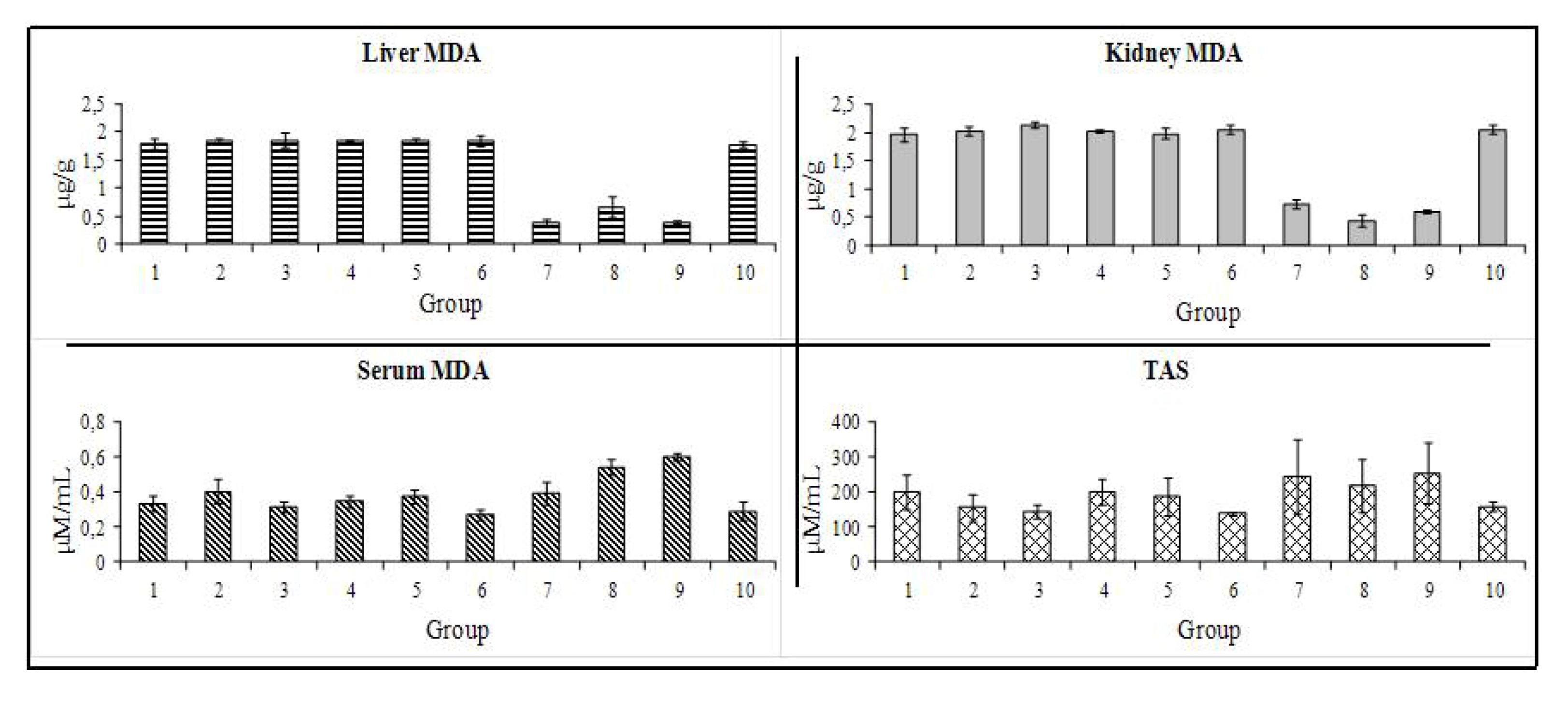
3.7. Antioxidant Capacity
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Lin, C.W.; Chen, L.J.; Lee, P.L.; Lee, C.I.; Lin, C.J.; Chiu, J.J. The inhibition of TNF-α-induced E-selectin expression in endothelial cells via the JNK/NF-κB pathways by highly N-acetylated chitooligosaccharides. Biomaterials 2007, 28, 1355–1366. [Google Scholar] [CrossRef]
- Vernazza, C.L.; Gibson, G.R.; Rastall, R.A. In vitro fermentation of chitosan derivatives by mixed cultures of human faecal bacteria. Carbohydr. Polym. 2005, 60, 539–545. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Morrissey, M.T. Marine Nutraceuticals and Functional Foods; Shahidi, F., Barrow, C., Eds.; CRC Press: New York, NY, USA, 2008; pp. 155–182. [Google Scholar]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 66–77. [Google Scholar]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Water-solubility of partially N-acetylated chotosans as a function of pH, effect of chemical composition and depolymerisation. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Kim, S.K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Chae, S.Y.; Jang, M.K.; Nah, J.W. Influence of molecular weight on oral absorption of water soluble chitosans. J. Control Release 2005, 102, 383–394. [Google Scholar] [CrossRef]
- Reddy, B.S.; Hamid, R.; Rao, C.V. Effect of dietary oligofructose and inulin on colonic preneoplastic aberrant crypt foci inhibition. Carcinogenesis 1997, 18, 1371–1374. [Google Scholar] [CrossRef]
- Wijnands, M.V.W.; Appel, M.J.; Hollanders, V.M.H.; Woutersen, R.A.A. Comparison of the effects of dietary cellulose and fermentable galacto-oligosaccharide, in a rat model of colorectal carcinogenesis, fermentable fibre confers greater protection than non-fermentable fibre in both high and low fat backgrounds. Carcinogenesis 1999, 20, 651–656. [Google Scholar] [CrossRef]
- Wijnands, M.V.W.; Schoterman, H.C.; Bruijntjes, J.P.; Hollanders, V.M.H.; Wouterse, R.A. Effect of dietary galacto-oligosaccharides on azoxymethane-induced aberrant crypt foci and colorectal cancer in Fischer 344 rats. Carcinogenesis 2001, 22, 127–132. [Google Scholar] [CrossRef]
- Maeda, Y.; Kimura, Y. Antitumor effects of various low molecular-weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice. Nutr. Cancer 2004, 134, 945–950. [Google Scholar]
- Grasso, M. Bladder cancer: A major public health issue. Eur. Urol. Suppl. 2008, 7, 510–515. [Google Scholar] [CrossRef]
- Ferlay, J.; Autier, P.; Boniol, M.; Heanue, M.; Colombet, M.; Boyle, P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann. Oncol. 2007, 18, 581–592. [Google Scholar]
- Pisani, P.; Bray, F.; Parkin, D.M. Estimates of world-wide prevalence of cancer for 25 sites in the adult prevalence. Int. J. Cancer 2002, 97, 72–81. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables, a combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder cancer: Epidemiology, staging, grading and diagnosis. Urology 2005, 66, 4–34. [Google Scholar]
- Sangar, V.K.; Ragavan, N.; Matanheleia, S.S.; Watson, M.W.; Blades, R.A. The economic consequences of prostate and bladder cancer in UK. BJU Int. 2005, 95, 59–63. [Google Scholar]
- Malkowicz, S.B.; van Poppel, H.; Mickisch, G.; Pansadoro, V.; Thüroff, J.; Soloway, M.S.; Chang, S.; Benson, M.; Fukui, I. Muscle-invasive urothelial carcinoma of the bladder. Urology 2007, 69, 3–16. [Google Scholar]
- Evans, C.; Debruyne, F.; Payne, H.; Solsona, E.; Teillac, P.; Tubaro, A. Bladder cancer, management and future directions. Eur. Urol. Suppl. 2007, 6, 365–373. [Google Scholar] [CrossRef]
- Fukushima, S.; Hirose, M.; Tsuda, H.; Shirai, T.; Hirao, K. Histological classification of urinary bladder cancers in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Gann 1976, 67, 81–90. [Google Scholar]
- Montironi, R.; Mazzucchelli, R. Preneoplastic Lesions and Conditions of the urinary bladder. EAU Update Ser. 1976, 1, 53–63. [Google Scholar] [CrossRef]
- Sauter, G.; Algaba, F.; Amin, M.; Busch, C.; Cheville, J.; Gasser, T.; Grignon, D.J.; Hofstadter, F.; Lopez-Beltran, A.; Epstein, J.I. WHO Classification of Tumors of the Urinary System and Male Genital Organs; Eble, J.N., Sauter, G., Epstein, J.L., Sesterhenn, I., Eds.; IARCC Press: Lyon, France, 2004; pp. 29–34. [Google Scholar]
- Gofrit, O.N.; Birman, T.; Ayesh, S.; Ohana, P.; Hochberg, A. Chemically induced bladder cancer—A sonographic and norphologic description. Urology 2006, 68, 231–235. [Google Scholar]
- Hameed, D.A.; el-Metwally, T.H. The effectiveness of retinoic acid treatment in bladder cancer, impact on recurrence, survival and TGF-alpha and VEGF as end-point biomarkers. Cancer Biol. Ther. 2008, 7, 92–100. [Google Scholar] [CrossRef]
- Leppert, J.T.; Shvarts, O.; Kawaoka, K.; Lieberman, R.; Belldegrun, A.S.; Pantuck, A.J. Prevention of bladder cancer: A review. Eur. Urol. 2006, 49, 226–234. [Google Scholar] [CrossRef]
- Parada, B.; Sereno, J.; Reis, F.; Teixeira-Lemos, E.; Garrido, P.; Pinto, A.F.; Cunha, M.F.; Pinto, R.; Mota, A.; Figueiredo, A.; Teixeira, F. Anti-inflammatory, anti-proliferative and antioxidant profiles of selective cyclooxygenase-2 inhibition as chemoprevention for rat bladder carcinogenesis. Cancer Biol. Ther. 2009, 8, 1615–1622. [Google Scholar] [CrossRef]
- Hattori, K.; Iida, K.; Joraku, A.; Tsukamoto, S.; Akaza, H.; Oyasu, R. Chemopreventive effects of cyclooxygenase-2 inhibitor and epidermal growth factor-receptor kinase inhibitor on rat urinary bladder carcinogenesis. BJU Int. 2006, 97, 640–643. [Google Scholar] [CrossRef]
- Nam, K.S.; Shon, Y.H. Suppression of metastasis of human breast cancer cells by chitosan oligosaccharides. J. Microbiol. Biotechnol. 2009, 19, 629–633. [Google Scholar]
- Suzuki, K.; Mikami, T.; Okawa, Y.; Tokoro, A.; Suzuki, S.; Suzuki, M. Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose. Carbohydr. Res. 1986, 151, 403–408. [Google Scholar] [CrossRef]
- Tokoro, A.; Tatewaki, N.; Suzuki, K.; Mikami, T.; Suzuki, S.; Suzuki, M. Growth-inhibitory effect of hexa-N-acetylchitohexaose and chitohexaose against Meth-A solid tumor. Chem. Pharm. Bull. 1988, 36, 784–790. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Ding, Y.; Shi, G.; Wan, C. Research of the degradation products of chitosan’s angiogenic function. Appl. Surf. Sci. 2008, 255, 260–262. [Google Scholar]
- Kam, P.C.; See, A.U. Cyclo-oxygenase isoenzymes, physiological and pharmacological role. Anaesthesia 2000, 55, 442–449. [Google Scholar] [CrossRef]
- Harris, R.E. Cyclooxygenase-2 (COX-2) and the inflammogenesis of cancer. Subcell. Biochem. 2007, 42, 93–126. [Google Scholar] [CrossRef]
- DeGortari, A.E.; Han, C.; Glickman, L.T. Cisplatin versus cisplatin combined with piroxicam in a canine model of human invasive urinary bladder cancer. Cancer Chemother. Pharmacol. 2000, 46, 221–226. [Google Scholar] [CrossRef]
- Grubbs, C.J.; Lubet, R.A.; Koki, A.T.; Leahy, K.M.; Masferrer, J.L.; Steele, V.E.; Kelloff, G.J.; Hill, D.L.; Seibert, K. Celecoxib inhibits N-Butyl-N-(4-hydroxybutyl) nitrosamine induced urinary bladder cancers in Male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000, 60, 5599–5602. [Google Scholar]
- Okajima, E.; Denda, A.; Ozono, S.; Takahama, M.; Akai, H.; Sasaki, Y.; Kitayama, W.; Wakabayashi, K.; Konishi, Y. Chemopreventive effects of nimesulide, a selective cyclooxygenase-2 inhibitor, on the development of rat urinary bladder carcinomas initiated by N-butyl-N-(4-hydroxybutyl) nitrosamine. Cancer Res. 1998, 58, 3028–3031. [Google Scholar]
- De Heer, P.; Sandel, M.H.; Guertens, G.; de Boeck, G.; Koudijs, M.M.; Nagelkerke, J.F.; Junggeburt, J.M.; de Bruijn, E.A.; van de Velde, C.J.; Kuppen, P.J. Celecoxib inhibits growth of tumors in a syngeneic rat liver metastases model for colorectal cancer. Cancer Chemother. Pharmacol. 2008, 62, 811–819. [Google Scholar] [CrossRef]
- Keller, J.J.; Giardiello, F.M. Chemoprevention strategies using NSAIDs and COX-2 inhibitors. Cancer Biol. Ther. 2003, 2, 140–149. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Nargi, D.; Horton, L.; Reddy, B.S.; Bosland, M.C.; Narayanan, B.A. Inflammatory processes of prostate tissue microenvironment drive rat prostate carcinogenesis, preventive effects of celecoxib. Prostate 2009, 69, 133–141. [Google Scholar] [CrossRef]
- Ristimäki, A.; Nieminen, O.; Saukkonen, K.; Hotakainen, K.; Nordiling, S.; Haglund, C. Expression of cyclooxygenase-2 in human transitional cell carcinoma of the urinary bladder. Am. J. Pathol. 2001, 158, 849–853. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Spindola, H.; de Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef]
- Lee, S.H.; Senevirathne, M.; Ahn, C.B.; Kim, S.K.; Je, J.Y. Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264.7 macrophage cells. Bioorg. Med. Chem. Lett. 2009, 19, 6655–6658. [Google Scholar] [CrossRef]
- Baxter, A.; Dillon, M.; Taylor, K.D.A.; Robert, G.A.F. Improved method for i.r. determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1992, 14, 166–169. [Google Scholar] [CrossRef]
- El-kott, A.F. Flow cytometry and KI67 expression in rat’s urinary bladder carcinogenesis treated with Allium sativum. Cancer Ther. 2007, 5, 185–192. [Google Scholar]
- Estepa, V.; Ródenas, S.; Martín, M.C. Optimización de un método para la determinación de la peroxidación lipídica en suero humano. Anal. Real Acad. Nac. Farm. 2001, 67, 1–10. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fernandes, J.C.; Sereno, J.; Garrido, P.; Parada, B.; Cunha, M.F.X.; Reis, F.; Pintado, M.E.; Santos-Silva, A. Inhibition of Bladder Tumor Growth by Chitooligosaccharides in an Experimental Carcinogenesis Model. Mar. Drugs 2012, 10, 2661-2675. https://doi.org/10.3390/md10122661
Fernandes JC, Sereno J, Garrido P, Parada B, Cunha MFX, Reis F, Pintado ME, Santos-Silva A. Inhibition of Bladder Tumor Growth by Chitooligosaccharides in an Experimental Carcinogenesis Model. Marine Drugs. 2012; 10(12):2661-2675. https://doi.org/10.3390/md10122661
Chicago/Turabian StyleFernandes, João C., José Sereno, Patricia Garrido, Belmiro Parada, Maria F. X. Cunha, Flávio Reis, Manuela E. Pintado, and Alice Santos-Silva. 2012. "Inhibition of Bladder Tumor Growth by Chitooligosaccharides in an Experimental Carcinogenesis Model" Marine Drugs 10, no. 12: 2661-2675. https://doi.org/10.3390/md10122661
APA StyleFernandes, J. C., Sereno, J., Garrido, P., Parada, B., Cunha, M. F. X., Reis, F., Pintado, M. E., & Santos-Silva, A. (2012). Inhibition of Bladder Tumor Growth by Chitooligosaccharides in an Experimental Carcinogenesis Model. Marine Drugs, 10(12), 2661-2675. https://doi.org/10.3390/md10122661







