Abstract
Gloiopeltis tenax (G. tenax) is widely distributed along the Chinese coastal areas and is commonly used in the treatment of diarrhea and colitis. This study aimed at investigating the bioactivities of the volatile constituents in G. tenax. We extracted the essential constituents of G. tenax by supercritical carbon dioxide extraction (CO2-SFE), then identified and analyzed the constituents by gas chromatography-mass spectrometry (GC-MS). In total, 30 components were identified in the G. tenax extract. The components showed remarkable antioxidant activity (radical scavenging activity of 2,2-diphenyl-1-picrylhydrazyl (DPPH)), lipid peroxidation inhibition capacity (in a β-carotene/linoleic acid-coupled oxidation reaction), and hydroxyl radical-scavenging activity (by deoxyribose degradation by iron-dependent hydroxyl radical), compared to butylated hydroxytoluene. In microdilution assays, G. tenax extracts showed a moderate inhibitory effects on Staphyloccocus aureus (minimum inhibitory concentration (MIC) = 3.9 mg/mL), Enterococcus faecalis (7.8 mg/mL), Pseudomonas aeruginosa (15.6 mg/mL), and Escherichia coli (3.9 mg/mL). Antioxidant and antimicrobial activities of G. tenax were related to the active chemical composition. These results suggest that the CO2-SFE extract from G. tenax has potential to be used as a natural antioxidant and antimicrobial agent in food processing.
1. Introduction
Gloiopeltis tenax is an annual red alga that belongs to the Rhodophyta phylum, the Florideophyeeae class, the Cryptonemiales order, and the Endocladiaceae GloioPeltis J. Agardh family. Gloiopeltis consist of seven species, distributed in the temperate waters of the North Pacific coast or slightly to the north-south extension [1]. In China, there are only Gloiopeltis tenax and Gloiopeltis furcata, edible species that have been used for medicinal purposes, and also as a precious resource of marine algae [2].
Antioxidants have multiple functions in biological systems, including defense against oxidative damage and participation in the major cell signaling pathways. One principal cellular function of antioxidants is to prevent damage caused by the action of reactive oxygen species (ROS) [3]. ROS are responsible for aging [4], and excessive ROS have been implicated in the cause of various human diseases, such as diabetes [5], neurodegenerative disease [6] and cancer [7]. Different studies show that antioxidant substances that scavenge free radicals play an important role in the prevention of free radical-induced diseases. Several synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butyhydroquinone (TBHQ), are commercially available and currently used. However, concerns about their safety and toxicity are hindering their use by the food industry [8,9]. In addition, due to the occurrence of resistance to antimicrobials and the incidence of infectious diseases, there is a need to search for new antimicrobial compounds that may inhibit microorganisms by different mechanisms than those in current use [10,11]. Therefore, research on alternative antioxidants and antimicrobials from natural origins has drawn increasing attention. Particularly in recent years consumer awareness of food quality and safety issues has significantly improved.
Great effort has been focused on, e.g., medicinal plants for the extraction of natural and low-cost antioxidants and antimicrobials that can replace synthetic additives. Oxidative stress results from an imbalance between excess prooxidants and depletion of antioxidants. Algae are exposed to large amounts of light and high concentrations of oxygen during their life cycle. This combination favors the generation of free radicals, as well as other powerful oxidizers [12]. It is suggested that the absence of oxidative damage in the structural components of the algae and their stability against adverse conditions are due to the presence of antioxidants. Therefore, algae can be expected to serve as a rich source of antioxidants. Research on Gloipeltis algae has mainly concentrated on G. furcata, while G. tenax has remained little characterized. The reason is that G. tenax is further offshore and distributed in lower mid-tidal area, whereas G. furcata is distributed in the mid-tidal area. Therefore G. tenax has been less accessible than G. furcata, and biological yield has been very limited due to difficulty in acquisition.
Many reports have been published highlighting the variety of biological activities of Gloiopeltis. For instance, Fang et al. [2] identified 18 compounds with anticholinesterase activity from G. furcata extracts. With IC50 values ranging from 1.14 to 15.89 μg/mL, these compounds exhibited mild AChE/BchE inhibitory activities. Bae et al. [13] found that methanol extracts of G. furcata markedly induced G2/M arrest and reduced the viability of HepG2 Cells in a concentration-dependent manner (0–100 μg/mL). Ethyl acetate extracts of G. furcata inhibit production of pro-inflammatory mediators with 100 μg/mL [14], and funoran at 0.1% concentration from G. furcata strongly inhibited both bacteria causing dental caries and Streptococcus sobrinus adsorption to saliva-coated hydroxyapatite more than 50% [15,16]. Polysaccharides from G. tenax have been shown to have anti-tumor effects through augmentation of T-helper, T-cytotoxic and NK cells, inhibited the growth of tumors by 36.4%–65.6% at doses of 10 and 50 mg/kg, and prolonged the survival time by 33.3%–79.2% [17]. These results indicate that Gloiopeltis extracts play different roles through different mechanisms. We demonstrate that these biological activities are not just related to the water-soluble polysaccharide, but may be connected with fat-soluble ingredients such as essential oils. To the best of our knowledge, there are no previous reports on chemical composition and biological activity of essential oils of G. tenax. The aims of this study were to characterize the composition of the G. tenax by gas chromatography-mass spectrometry (GC-MS) and physiological activity. The goal was also to test the antioxidant and antibacterial activities of the analyzed the G. tenax extract as a potentially new source of biologically active natural products.
2. Results and Discussion
2.1. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of CO2-SFE Extracts from G. tenax
The ingredient of G. tenax were extracted by CO2-SFE and analyzed by GC-MS. Chemical composition was separated by programmed temperature gas chromatography, and then individual MS fragments were analyzed. The percentage composition of the extract was computed by normalization to the GC peak areas without using correction factors. Identification of constituents was based on comparison of their Kovats Index (KI) and the MS fragmentation pattern with reference compositions in the database of the NIST Mass Spectral Search Program. The GC-MS total ion chromatogram (TIC) of the G. tenax extraction is shown in Figure 1.
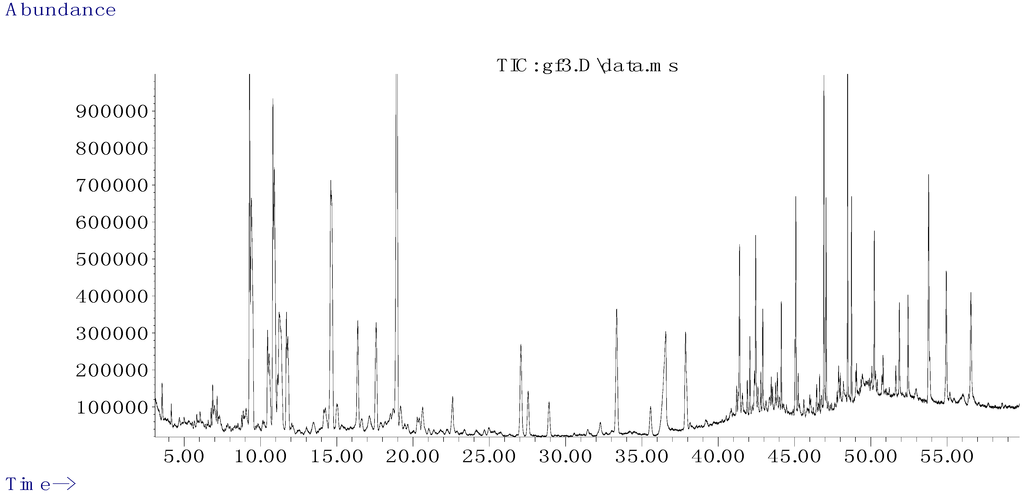
Figure 1.
The gas chromatography-mass spectrometry (GC-MS) total icon chromatogram (TIC) of volatile constituents from G. tenax.
Thirty compounds were identified in all of the oils analyzed, twenty-three of which are listed in Table 1. The compounds that are not listed in Table 1 are alkanes. 78% were identified by GC-MS combined with KI. The others were not identified in the database of the NIST Mass Spectral Search Program, possibly because the comparison of the obtained mass spectra with those of reference compounds was too low to enable identification. The identified components include six sesquiterpenes (14.39%), three ketones (5.02%), seven fatty acids and their esters (29.1%), two phenols (1.71%), and three sterols (12.81%) (Table 1).

Table 1.
The chemical composition of the extract identified by GC-MS and Kovats Index (KI).
| NO. | RT a | Constituents | KI b | % c |
|---|---|---|---|---|
| 1 | 7.890 | p-hydroxybenzaldehyde | 1374.4 | 0.57 |
| 2 | 9.302 | (−)-thujopsene | 1437.1 | 4.68 |
| 3 | 10.484 | α-curcumene | 1484.0 | 1.54 |
| 4 | 10.833 | α-zingiberene | 1497.8 | 2.98 |
| 5 | 11.285 | (+)-cuparene | 1511.4 | 0.28 |
| 6 | 11.322 | (−)-β-bisabolene | 1512.5 | 1.00 |
| 7 | 14.703 | cedrol | 1607.3 | 3.91 |
| 8 | 16.431 | vanillylacetone | 1644.8 | 1.92 |
| 9 | 19.016 | n-heptadecane | 1700.7 | 10.30 |
| 10 | 23.014 | myristic acid | 1769.8 | 2.85 |
| 11 | 27.614 | fitone | 1842.1 | 2.53 |
| 12 | 33.382 | methhyl hexadecanoate | 1927.6 | 1.32 |
| 13 | 37.460 | palmitic acid | 1987.3 | 21.21 |
| 14 | 41.236 | linoleic acid | 2092.9 | 0.23 |
| 15 | 41.354 | hexadeca-1,4-lactone | 2096.5 | 0.57 |
| 16 | 42.602 | cis-9-octadecenoic acid | 2153.2 | 0.73 |
| 17 | 43.002 | stearic acid | 2172.0 | 0.93 |
| 18 | 46.128 | oleamide | 2361.5 | 0.24 |
| 19 | 46.948 | 2,2′-methylenebis(6-tert-butyl-4-methylphenol) | 2422.4 | 1.14 |
| 20 | 48.040 | 2-monopalmitin | 2511.2 | 1.83 |
| 21 | 52.476 | cholesta-4,6-dien-3β-ol | 2894.9 | 6.62 |
| 22 | 56.619 | cholesterol | 3122.3 | 5.74 |
| 23 | 58.639 | cholesta-3,5-dien-7-one | 3196.6 | 0.45 |
a Retention times (min); b Kovats Indexes were calculated from our analyses with respect to a series of n-alkenes; c Percentage of relative amount to total.
Extraction of volatile components using conventional processes, such as solvent extraction, is conducted at high pressure and temperature and is time-consuming. Supercritical fluid extraction (SFE) is a rapid, selective, effective and convenient technique for sample preparation before the analysis of compounds. Supercritical fluids have been used as solvents for a wide variety of applications, such as essential oil extraction, and more than 90% of all analytical SFE is performed with CO2. Under conditions of low pressure and low temperature, and no influence of solvent, use of CO2-SFE is a good way to extract the volatile constituents of G. tenax. Some compounds were identified by GC-MS (Table 1). These compounds have physiological activity, including antioxidant, anti-inflammatory, and antimicrobial activities. Sesquiterpenes are formed from a 15-carbon skeleton of terpenoids. Widely distributed in nature, the structure of the skeleton is different, and the diverse biological activity of sesquiterpenes has been shown to include antitumor, antibacterial, antiviral, and antimalarial activities [18,19]. Sesquiterpenes can also protect against alcohol-induced gastric mucosal lesions and oxidative damage [20]. (−)-Thujopsene, cedrol, (+)-cuparene, α-curcumene, (−)-β-bisabolene, α-zingiberene are sesquiterpenes were identified. Thujopsene has shown potent antibacterial activity, for example against Phytophthora ramorum at 2.0–3.0 ppm [21]. Inhaling vaporized Cedrol ((64.0 ± 7.7) × 10−9 M) can affect autonomic nervous functions in humans, such as induce sedative effects, decrease heart rate and blood pressure [22], and (+)-cuparene, α-curcumene and (−)-β-bisabolene mostly exhibit a strong physiological activity [23,24]. Vanillylacetone and fitone both have some physical activity, especially vanillylacetone. For instance, zingerone acts mainly by increasing systemic superoxide dismutase activity to protect the effect of zingerone against 6-hydroxydopamine-induced dopamine reduction, (65 nmol/kg, ip) [25]. Zingerone (10 μg/mL) has also been shown to mitigate radiation-induced mortality and cytogenetic damage, which may be attributed to inhibition of the radiation-induced decline in endogenous antioxidant levels and scavenging of radiation-induced free radicals [26]. Zingerone exerts its potent anti-inflammatory action by increasing HNF-4 and PPAR activities, while suppressing NF-kappaB activity at a dose of either 2 or 8 mg/kg/day for 10 days. [27].
2.2. Antioxidant Activity Assays
Due to the complex nature of phytochemicals and determination of antioxidant activity according to the reaction mechanism, we used three methods to measure the antioxidant properties of G. tenax extracts: a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, a β-carotene/linoleic acid-coupled oxidation reaction, and a deoxyribose degradation assay. All three methods detected increasing antioxidant activity with increasing dose. Butylated hydroxytoluene (BHT), a strong antioxidant, was used as a positive control and exhibited the highest antioxidant activity. The G. tenax extract showed dose-dependent scavenging activity (Figure 2).
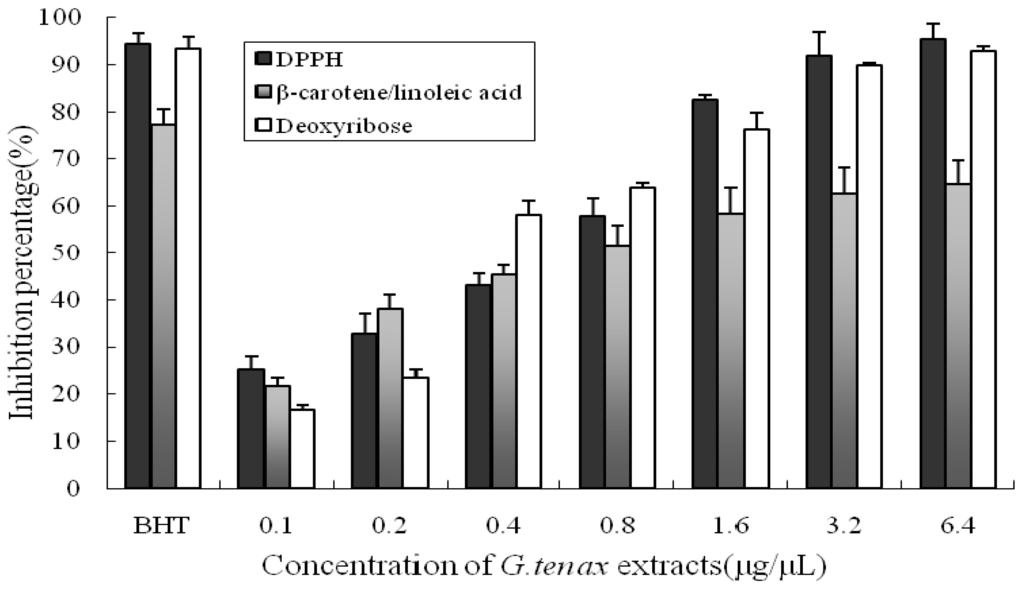
Figure 2.
Antioxidant activities of G. tenax extract and the positive control as detected by different methods.
Reactive oxygen species (ROS), including hydrogen peroxide (H2O2), superoxide anion (O2•−), and hydroxyl radical (OH•), are linked to cell damage [28]. Previous literature has demonstrated that the interaction of a potential antioxidant with DPPH depends on its structural conformation. The number of DPPH molecules that are reduced seems to be correlated with the number of electron-donating hydroxyl groups in the antioxidant molecule [29]. This structural requirement could be linked to the presence of phenolic compounds, which are known to be widely distributed in natural herb and spice extracts. Phenolic compounds have a high reducing ability to eliminate free radicals because of both their alcoholic hydroxyl group and conjugated π electrons of the benzene ring [30]. The percentage of inhibition of DPPH by G. tenax extracts was evaluated at different extract volumes (Table 2 and Figure 2). In the β-carotene/linoleic acid-coupled oxidation assay, antioxidants are capable of reducing the rate of chain reaction initiated during lipid peroxidation mainly by scavenging the intermediate peroxyl free radicals formed when linoleic acid is oxidized. This also depends on the hydrogen-donating ability of antioxidants [12]. Although hydrogen peroxide itself is not the most reactive, its in vivo toxicity can be partly attributed to hydroxyl radical formation in the cells. Addition of hydrogen peroxide to cells in culture can lead to transition metal ion-dependent OH• mediated oxidative DNA damage [31], as well as other detrimental effects on nucleic acids [32], protein [33], and lipids [34]. Deoxyribose on exposure to hydroxyl radicals, generated by Fenton reaction, degrades into fragments and generates a pink chromophore on heating with thiobarbituric acid (TBA) at low pH [35]. We measured the ability of G. tenax extracts to scavenge hydroxyl radicals by studying the competition between deoxyribose and G. tenax extract for hydroxyl radicals (Table 2 and Figure 2). Phenolic compounds we identified in G. tenax extracts, such as p-hydroxybenzaldehyde and 2,2′-methylenebis(6-tert-btyl-4-methylphenol), acted as effective antioxidants and free radical terminators in the deoxyribose degradation assay. Vanillylacetone and fatty acids, the active compounds we identified, may also be related to antioxidant activity of G. tenax. Accumulated evidence demonstrates the antioxidant activity of essential fatty acid components extracted from various plants. Therefore, tetradecanoic acid, n-hexadecanoic acid, linoleic acid and oleic acid present in G. tenax may contribute to the antioxidant activity [36].

Table 2.
Absorbance of the G. tenax extract and the positive control by three different methods: 2,2-diphenyl-1-picrylhydrazyl (DPPH), β-carotene/linoleic acid, and deoxyribose assays.
| Sample | DPPH | ∆S β-Carotene/Linoleic Acid | Deoxyribose |
|---|---|---|---|
| 0.1 μg/μL | 0.2206 ± 0.0079 * | 0.0154 ± 0.0003 * | 0.7544 ± 0.0091 * |
| 0.2 μg/μL | 0.1979 ± 0.0124 * | 0.0121 ± 0.0006 * | 0.6925 ± 0.0159 * |
| 0.4 μg/μL | 0.1680 ± 0.0078 * | 0.0107 ± 0.0004 * | 0.3792 ± 0.0277 * |
| 0.8 μg/μL | 0.1237 ± 0.0106 * | 0.0095 ± 0.0009 * | 0.3255 ± 0.0091 * |
| 1.6 μg/μL | 0.0514 ± 0.0030 * | 0.0082 ± 0.0011 * | 0.2136 ± 0.0299 * |
| 3.2 μg/μL | 0.0235 ± 0.0147 * | 0.0073 ± 0.0011 * | 0.0911 ± 0.0049 * |
| 6.4 μg/μL | 0.0134 ± 0.0097 * | 0.0069 ± 0.0010 * | 0.0628 ± 0.0070 * |
| BHT (0.05 mM) | 0.0162 ± 0.0175 * | 0.0044 ± 0.0037 * | 0.0623 ± 0.0103 * |
| Blank | 0.2947 ± 0.0074 | 0.0196 ± 0.0053 | 0.9365 ± 0.0325 |
∆S: The difference value of absorbance at 0 min and 120 min in β-carotene/linoleic acid-coupled oxidation reaction; * P < 0.05, compared with blank control; The data are expressed as the mean ± SD (n = 3).
2.3. Antimicrobial Susceptibility Testing
The antimicrobial activity of G. tenax was assessed both qualitatively and quantitatively by disc diffusion. G. tenax extracts exhibited moderate broad-spectrum antimicrobial action. Zones of inhibition exhibited a dose-dependent relationship with increasing concentrations of extract. Minimum inhibitory concentration (MIC) values for inhibition of Staphyloccocus aureus, Enterococcus faecalis, Pseudomonas aeruginosa and Escherichia coli were 3.9, 7.8, 15.6, and 3.9 mg/mL, respectively (Table 3).

Table 3.
The antibacterial activity of the G. tenax extracts by the diameters of the inhibition zones (mm) using the disc diffusion method (minimum inhibitory concentration (MIC) in mg/mL).
| Bacterial strains | G. tenax extracts dose (mg) | MIC (mg/mL) | ||||
|---|---|---|---|---|---|---|
| 0.3 | 0.6 | 1.2 | 2.5 | 5.0 | ||
| Staphyloccocus aureus | 24.3 | 25.1 | 26.4 | 27.7 | 28.9 | 3.9 |
| Enterococcus faecalis | 11.2 | 15.6 | 20.3 | 24.5 | 29.3 | 7.8 |
| Pseudomonas aeruginosa | 10.2 | 15.5 | 23.1 | 24.2 | 26.6 | 15.6 |
| Escherichia coli | 15.4 | 18.9 | 20.3 | 21.6 | 23.2 | 3.9 |
Many diseases are caused by microbial infection, indicating the need for more antimicrobial agents [37]. Gloiopeltis is often used to treat intestinal diarrhea. Although much is known about the biological activities of Gloiopeltis extracts, little is known about the anti-diarrheal mechanisms. Gloiopeltis extracts display broad biological effects, such as the ability to regulate the immune system, inhibit bacterial growth, and prevent adhesion of bacteria to target surfaces. We demonstrate that G. tenax extracts inhibit bacterial growth, suggesting that Gloiopeltis extracts exert their antibacterial activity directly, rather than indirectly by enhancing the immune system.
GC-MS analysis identified compounds, within G. tenax extracts, with known antibacterial activity. Sesquiterpenes, such as thujopsene, (+)-cuparene and cedrol, all have antibacterial activity [21,38,39]. Zingerone (vanillylacetone) and its derivatives can inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice [40]. Studies have shown that zingerone might exert beneficial therapeutic effects on hypermotility-induced diarrhea by abrogating excessive gastrointestinal motility [41]. Therefore, vanillylacetone is the likely active constituent responsible for the antidiarrheal efficacy of G. tenax. A number of free fatty acids are known to possess antibacterial activity against Gram-positive bacteria, as well as their esters [42,43]. Some synthetic fatty acid analogs of cholesterol show excellent antibacterial activity in vitro [44]. The antimicrobial activity and therapeutic efficacy of oleic acid in a liposomal formulation against methicillin-resistant S. aureus is known [45]. The presence of a hydroxyl group in compounds, especially phenolic components, such as 4-hydroxybenzaldehde found in G. tenax extract, can destabilize the bacterial membrane to increase the activity of antimicrobials. Such components at low concentrations might be involved in some type of synergism with the other active compounds.
3. Experimental Section
3.1. Plant Materials
G. tenax was provided and authenticated by the Nan Ao Marine Biological Research Station of Shantou University in Guangdong Province. It was collected at Nan Ao Island. The study area is on the Tropic of Cancer, located 116°56′–117°09′E and 23°23′–23°29′N off the city of Shantou, eastern Guangdong Province, neighboring the Fujian Province, between Hong Kong and Taiwan. This region is at the northern edge of algae distribution in East Asia.
3.2. Chemicals and Reagents
Alkane standard solutions of C8–C20 (mixture No. 04070) and C21–C40 (mixture No.04071) were from Fluka Chemika (Buchs, Switzerland). 2,2-Diphenyl-1-picrylhydazyl (DPPH), β-carotene, butylated hydroxytoluene (BHT), ascorbic acid, and linoleic acid were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). 2-Deoxy-D-ribose was purchased from Amresco. 2-Thiobarbituric acid (TBA) and EDTA were obtained from Aladdin (Shanghai, China). All other chemicals and solvents (ethanol, H2O2, tween-40) were of analytical grade. Ultrapure water was used for the experiments.
3.3. Supercritical Fluid Extraction with Carbon Dioxide
CO2-SFE involved use of an HA221-50-06 extractor (Huaan Supercritical Equipment Co., Nantong, Jiangsu, China). The instrument was run with CO2 for both the extraction and cooling gases. The extraction pressure and temperature were 300 bars and 45 °C, respectively. G. tenax powder was placed into 5 L extraction thimbles. The samples were extracted with pure CO2, with ethanol used as an entrainer. The amount of ethanol, 1 L, was approximately equal to the sample volume. Fractions were collected in both separators into two separate containers. The addition of a polar co-solvent (ethanol 95%) led to increased efficiency of extraction.
3.4. Gas Chromatography-Mass Spectrometry Analysis
GC-MS analysis involved an Agilent 7890 GC equipped with a quadrupole 5975 mass spectrometer (Agilent Technologies, CA, USA) and an HP-5MS column (i.d., 0.25 mm; length, 30 m; film thickness, 0.25 μm). Oven temperature was maintained at 100 °C for 2 min initially and then sequentially raised to 140 °C at a rate of 10 °C/min, to 170 °C at a rate of 1 °C/min, and to 280 °C/min at a rate of 8 °C/min where the temperature was finally held for 10 min. The MS conditions were: ionization voltage, 70 eV; emission current, 10 mAmp; scan rate, 1 scan/s; mass range, 45–450 M/Z; trap temp., 150 °C; transfer line temp., 280 °C. Operating conditions were: injector temp., 280 °C; FID temp., 280 °C.; carrier(He) flow rate, 0.8 mL/min. Samples were injected splitless. Two microliters of sample, dissolved in ethyl acetate was injected.
Identification of the constituents was based on comparison of their Kovats Index (KI) and the MS fragmentation pattern with reference compositions in the database of the NIST Mass Spectral Search Program (NIST 08 mass spectral database, National Institute of Standards and Technology, Washington, DC, USA). To determine the KI value of the components, a commercial aliphatic hydrocarbon mixture (Sigma-Aldrich) was added to the essential oil before injecting it into the GC/MS equipment and analyzed under the same conditions as above. The following quasi-linear equation for the temperature-programmed Kovats Index was used:
where KI(x) is the temperature-programmed Kovats Index of interest and t(z), t(z + 1), and t(x) were the retention times in minutes of the two standard n-alkanes containing z and z + 1 carbons and index of interest, respectively: t(z) < t(x) < t(z + 1).
{KI(x) = 100 × Z + 100 × [t(x) − t(z)]/[t(z + 1) − t(z)]}
3.5. Antioxidant Assays of Extracts
3.5.1. DPPH Radical-Scavenging System
The DPPH radical scavenging capacity of each herbal extract was evaluated according to Blois, with minor modifications [46]. DPPH radical was prepared in ethanol to a final concentration of 2 × 10−4 mol/L. Different amounts of samples at a concentration of 10 mg/mL (solid extract dissolved in 70% ethanol) were added to 150 μL of freshly prepared DPPH radical solution, then 70% ethanol was added to a final volume of 200 μL and the mixture was kept in the dark for 30 min. The absorbance of the reaction mixture was measured at 517 nm. A control was measured in the same way except that the extract was replaced by 70% ethanol. All experiments were performed in triplicate. Scavenging activity (I%) was calculated by the equation:
where Asample is the absorbance of the sample and Acontrol is the absorbance of the control. BHT was used as positive control.
{I% = [Acontrol − (Asample − Ablank)]/Acontrol × 100}
3.5.2. β-Carotene/Linoleic Acid-Coupled Oxidation Reaction
Antioxidant activity in the musts was assessed with the β-carotene/linoleate model system [47]. For this purpose, a solution of β-carotene was prepared by dissolving 2 mg in 10 mL of chloroform. An amount of 0.02 mL of linoleic acid and 0.2 mL of tween 40 was subsequently added, and the mixture was left standing at 20 °C for 15 min. After evaporation of the chloroform in a rotary evaporator at 40 °C, 50 mL of oxygen-saturated distilled water at 25 °C was added and the mixture was vortexed vigorously (1 min) to form an emulsion (β-carotene/linoleic acid emulsion). The necessary wells of a 96-well microtiter plate were charged with each different volume of sample and 100 μL of emulsion per well. The microplate was placed on a horizontal shaker and shaken at 100 rpm (during 1 min). A control sample was also prepared in parallel. Absorbance measurements (470 nm) were made at t = 0 min and after incubation at 50 °C for 120 min. All experiments were performed in triplicate. Antioxidant activity was expressed as the percent of inhibition with respect to the control sample and calculated as follows:
where SA0 and CA0 are the absorbance values of the sample and the control determined at 0 min; the SAt and CAt were the absorbance values of test sample and control measured after 120 min. BHT was used as positive control.
{AA% = [1 − (SA0 − SA1)/(CA0 − CAt)] × 100}
3.5.3. Deoxyribose Degradation by Iron-Dependent Hydroxyl Radical
The method of Gutteridge (1987) was employed with minor modifications [48]. Briefly, 1 mL of the reaction solution consisted of the corresponding volume sample, 0.1 mL 1 mM FeCl3, 0.1 mL 1.04 mM EDTA, 0.1 mL 20 mM H2O2, 0.1 mL 2mM L-ascorbic acid and 0.1 mL 60 mM deoxyribose in potassium phosphate buffer (50 mM, pH 7.4). Then, the reaction mixture was incubated for 1 h at 37 °C, after addition of 1 mL of 2.8% (w/v) trichloroacetic acid and 1 mL of 1% (w/w) TBA (1% in 50 mM NaOH). Deoxyribose degradation was measured by the TBA reaction. Further it was heated in a boiling water bath for 15 min and absorbance was measured at 532 nm against a blank. All experiments were carried out in triplicate.
where A0, A1, A2 represent the absorbance of control, samples, and blank, respectively. BHT was used as positive control.
{OH•% = [A0 − (A1 − A2)]/A0 × 100}
3.6. Antimicrobial Susceptibility Testing and Determination of Minimum Inhibitory Concentration (MIC)
Antimicrobial susceptibility testing for G. tenax involved the Kirby-Bauer (KB) disk diffusion method against Gram-positive and -negative bacteria. Microorganisms included Staphylococcus aureus (American Type Culture Collection (ATCC) No. 25923), Enterococcus faecalis (ATCC 29212), Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853). Briefly, a suspension of the tested microorganism (0.1 mL of 108 cells/mL) was spread on solid media plates. Filter paper discs (6 mm in diameter) were impregnated with 10 µL of different concentrations of G. tenax extract and placed on the inoculated plates, then incubated at 37 °C for 24 h.
A broth microdilution method was used to determine the MIC against the susceptible microorganisms using the KB method according to the National Committee for Clinical Laboratory Standards. A serial doubling dilution of G. tenax extract from 0.24 to 500 μg/μL was prepared in 50 μL Mueller-Hinton broth (MHB) in each well of a 96-well microtiter plate. Freshly grown microbial suspensions in MHB were standardized to a cell density of 1.5 × 108 (McFarland No. 0.5) and added to the wells (50 μL). After incubation at 37 °C for 24 h, the MIC was defined as the lowest concentration of G. tenax extract at which the microorganism did not demonstrate visible growth.
3.7. Statistical Analyses
Assays were carried out in triplicate, and each analysis of all samples was performed in duplicate; results displayed are averages. Absorbance values are expressed as means ± SD. Differences between variables were tested by one-way ANOVA with SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.
4. Conclusions
This study extracted constituents of G. tenax through CO2-SFE. The results of GC-MS analysis demonstrated that sesquiterpenes, ketones, fatty acids and their esters, phenols, and sterols were the main components of the G. tenax extract. Extracts showed remarkable antioxidant activity and moderate broad-spectrum antimicrobial action. Analytical experiments and limited biological assessments indicate that the antioxidant and antimicrobial activities of G. tenax might be due to the identified chemical composition. These results suggest that G. tenax has potential to be used as a source of natural antioxidants and antimicrobial agents in food processing.
Acknowledgments
This study was undertaken and supported within the teamwork projects funded by the Guangdong Natural Science Foundation (9351503102000001) and the 211 project of Guangdong province.
References
- Lim, B.L.; Ryu, I.H. Purification, structural characterization, and antioxidant activity of antioxidant substance from the red seaweed Gloiopeltis tenax. J. Med. Food 2009, 12, 442–451. [Google Scholar] [CrossRef]
- Fang, Z.; Jeong, S.Y.; Jung, H.A.; Choi, J.S.; Min, B.S.; Woo, M.H. Anticholinesterase and antioxidant constituents from Gloiopeltis furcata. Chem. Pharm. Bull. 2010, 58, 1236–1239. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ganesan, K.; Rao, P. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty—An edible seaweed. Food Chem. 2008, 107, 289–295. [Google Scholar] [CrossRef]
- Barja, G. Free radicals and aging. Trends Neurosci. 2004, 27, 595–600. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Paolisso, G. Oxidative stress and diabetic vascular complications. Diabetes Care 1996, 19, 257–267. [Google Scholar]
- Jenner, P. Oxidative damage in neurodegenerative disease. Lancet 1994, 344, 796–798. [Google Scholar] [CrossRef]
- Loft, S.; Poulsen, H. Cancer risk and oxidative DNA damage in man. J. Mol. Med. 1996, 74, 297–312. [Google Scholar] [CrossRef]
- Takahashi, O.; Hiraga, K. Dose-response study of hemorrhagic death by dietary butylated hydroxytoluene (BHT) in male rats. Toxicol. Appl. Pharmacol. 1978, 43, 399–406. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tayama, K. Nephrotoxicity of butylated hydroxytoluene in phenobarbital-pretreated male rats. Arch. Toxicol. 1988, 61, 359–365. [Google Scholar] [CrossRef]
- Eloff, J. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- Rojas, R.; Bustamante, B.; Bauer, J.; Fernández, I.; Albán, J.; Lock, O. Antimicrobial activity of selected Peruvian medicinal plants. J. Ethnopharmacol. 2003, 88, 199–204. [Google Scholar] [CrossRef]
- Souza, B.W.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.; Ferreira, A.C.; Teixeira, J.A.; Vicente, A.A. Antioxidant potential of two red seaweeds from the Brazilian coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar]
- Bae, S.J.; Choi, Y.H. Methanol extract of the seaweed Gloiopeltis furcata induces G2/M arrest and inhibits cyclooxygenase-2 activity in human hepatocarcinoma HepG2 cells. Phytother. Res. 2007, 21, 52–57. [Google Scholar] [CrossRef]
- Yang, E.J.; Moon, J.Y.; Kim, M.J.; Kim, D.S.; Kim, C.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Inhibitory effect of Jeju endemic seaweeds on the production of pro-inflammatory mediators in mouse macrophage cell line RAW 264.7. J. Zhejiang Univ. Sci. B 2010, 11, 315–322. [Google Scholar]
- Kurihara, H.; Goto, Y.; Aida, M.; Hosokawa, M.; Takahashi, K. Antibacterial activity against cariogenic bacteria and inhibition of insoluble glucan production by free fatty acids obtained from dried Gloiopeltis furcata. Fish. Sci. 1999, 65, 129–132. [Google Scholar] [CrossRef]
- Saeki, Y.; Kato, T.; Naito, Y.; Takazoe, I.; Okuda, K. Inhibitory effects of funoran on the adherence and colonization of mutans streptococci. Caries Res. 1996, 30, 119–125. [Google Scholar] [CrossRef]
- Ren, D.L.; Wang, J.Z.; Noda, H.; Amano, H.; Ogawa, S. The effects of an algal polysaccharide from Gloiopeltis tenax on transplantable tumors and immune activities in mice. Planta Med. 1995, 61, 120–125. [Google Scholar] [CrossRef]
- Modzelewska, A.; Sur, S.; Kumar, S.K.; Khan, S.R. Sesquiterpenes: Natural products that decrease cancer growth. Curr. Med. Chem. Anticancer Agents 2005, 5, 477–499. [Google Scholar] [CrossRef]
- Abraham, W.R. Bioactive sesquiterpenes produced by fungi: Are they useful for humans as well? Curr. Med. Chem. 2001, 8, 583–606. [Google Scholar] [CrossRef]
- Repetto, M.G.; Boveris, A. Bioactivity of sesquiterpenes: Compounds that protect from alcohol-induced gastric mucosal lesions and oxidative damage. Mini Rev. Med. Chem. 2010, 10, 615–623. [Google Scholar] [CrossRef]
- Manter, D.K.; Kelsey, R.G.; Karchesy, J.J. Antimicrobial activity of extractable conifer heartwood compounds toward Phytophthora ramorum. J. Chem. Ecol. 2007, 33, 2133–2147. [Google Scholar] [CrossRef]
- Umeno, K.; Hori, E.; Tsubota, M.; Shojaku, H.; Miwa, T.; Nagashima, Y.; Yada, Y.; Suzuki, T.; Ono, T.; Nishijo, H. Effects of direct cedrol inhalation into the lower airway on autonomic nervous activity in totally laryngectomized subjects. Br. J. Clin. Pharmacol. 2008, 65, 188–196. [Google Scholar] [CrossRef]
- Lenfeld, J.; Motl, O.; Trka, A. Anti-inflammatory activity of extracts from Conyza canadensis. Pharmazie 1986, 41, 268–269. [Google Scholar]
- Nishikawa, K.; Aburai, N.; Yamada, K.; Koshino, H.; Tsuchiya, E.; Kimura, K. The bisabolane sesquiterpenoid endoperoxide, 3,6-epidioxy-1,10-bisaboladiene, isolated from Cacalia delphiniifolia inhibits the growth of human cancer cells and induces apoptosis. Biosci. Biotechnol. Biochem. 2008, 72, 2463–2466. [Google Scholar] [CrossRef]
- Kabuto, H.; Nishizawa, M.; Tada, M.; Higashio, C.; Shishibori, T.; Kohno, M. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] prevents 6-hydroxydopamine-induced dopamine depression in mouse striatum and increases superoxide scavenging activity in serum. Neurochem. Res. 2005, 30, 325–332. [Google Scholar] [CrossRef]
- Rao, B.N.; Archana, P.R.; Aithal, B.K.; Rao, B.S. Protective effect of zingerone, a dietary compound against radiation induced genetic damage and apoptosis in human lymphocytes. Eur. J. Pharmacol. 2011, 657, 59–66. [Google Scholar] [CrossRef]
- Kim, M.K.; Chung, S.W.; Kim, D.H.; Kim, J.M.; Lee, E.K.; Kim, J.Y.; Ha, Y.M.; Kim, Y.H.; No, J.K.; Chung, H.S.; et al. Modulation of age-related NF-kappaB activation by dietary zingerone via MAPK pathway. Exp. Gerontol. 2010, 45, 419–426. [Google Scholar] [CrossRef]
- Farrugia, G.; Balzan, R. Oxidative stress and programmed cell death in yeast. Front. Oncol. 2012, 2, 64. [Google Scholar]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Lietão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS One 2011, 6, e26012. [Google Scholar]
- 31. Niu, Y.; Wang, H.; Xie, Z.; Whent, M.; Gao, X.; Zhang, X.; Zou, S.; Yao, W.; Yu, L. Structural analysis and bioactivity of a polysaccharide from the roots of Astragalus membranaceus (Fisch) Bge. var. mongolicus (Bge.) Hsiao. Food Chem. 2011, 128, 620–626. [Google Scholar]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef]
- Cabiscol, E.; Piulats, E.; Echave, P.; Herrero, E.; Ros, J. Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 27393–27398. [Google Scholar]
- Bilinski, T.; Litwinska, J.; Blszczynski, M.; Bajus, A. Superoxide dismutase deficiency and the toxicity of the products of autooxidation of polyunsaturated fatty acids in yeast. Biochim. Biophys. Acta 1001, 102–106. [Google Scholar]
- Halliwell, B.; Gutteridge, J.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar]
- Pal, M.; Ghosh, M. Prophylactic effect of alpha-linolenic acid and alpha-eleostearic acid against MeHg induced oxidative stress, DNA damage and structural changes in RBC membrane. Food Chem. Toxicol. 2012, 50, 2811–2818. [Google Scholar] [CrossRef]
- Mantey, I.; Hill, R.; Foster, A.; Wilson, S.; Wade, J.; Edmonds, M. Infection of foot ulcers with Staphylococcus aureus associated with increased mortality in diabetic patients. Commun. Dis. Public Health 2000, 3, 288–290. [Google Scholar]
- Ishikawa, N.K.; Fukushi, Y.; Yamaji, K.; Tahara, S.; Takahashi, K. Antimicrobial cuparene-type sesquiterpenes, enokipodins C and D, from a mycelial culture of Flammulina velutipes. J. Nat. Prod. 2001, 64, 932–934. [Google Scholar] [CrossRef]
- Johnston, W.H.; Karchesy, J.J.; Constantine, G.H.; Craig, A.M. Antimicrobial activity of some Pacific Northwest woods against anaerobic bacteria and yeast. Phytother. Res. 2001, 15, 586–588. [Google Scholar] [CrossRef]
- Chen, J.C.; Huang, L.J.; Wu, S.L.; Kuo, S.C.; Ho, T.Y.; Hsiang, C.Y. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J. Agric. Food Chem. 2007, 55, 8390–8397. [Google Scholar] [CrossRef]
- Iwami, M.; Shiina, T.; Hirayama, H.; Shima, T.; Takewaki, T.; Shimizu, Y. Inhibitory effects of zingerone, a pungent component of Zingiber officinale Roscoe, on colonic motility in rats. J. Nat. Med. 2011, 65, 89–94. [Google Scholar] [CrossRef]
- Georgel, P.; Crozat, K.; Lauth, X.; Makrantonaki, E.; Seltmann, H.; Sovath, S.; Hoebe, K.; Du, X.; Rutschmann, S.; Jiang, Z.F.; et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect. Immun. 2005, 73, 4512–4521. [Google Scholar] [CrossRef]
- Skrivanova, E.; Marounek, M.; Dlouha, G.; Kanka, J. Susceptibility of Clostridium perfringens to CC fatty acids. Lett. Appl. Microbiol. 2005, 41, 77–81. [Google Scholar] [CrossRef]
- Farshori, N.N.; Banday, M.R.; Ahmad, A.; Khan, A.U.; Rauf, A. Synthesis, characterization, and in vitro antimicrobial activities of 5-alkenyl/hydroxyalkenyl-2-phenylamine-1,3,4-oxadiazoles and thiadiazoles. Bioorg. Med. Chem. Lett. 2010, 20, 1933–1938. [Google Scholar] [CrossRef]
- Huang, C.M.; Chen, C.H.; Pornpattananangkul, D.; Zhang, L.; Chan, M.; Hsieh, M.F. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 2011, 32, 214–221. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Shon, M.Y.; Kim, T.H.; Sung, N.J. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003, 82, 593–597. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem. J. 1987, 243, 709–714. [Google Scholar]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).