The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies
Abstract
:1. Introduction
2. Materials and Methods
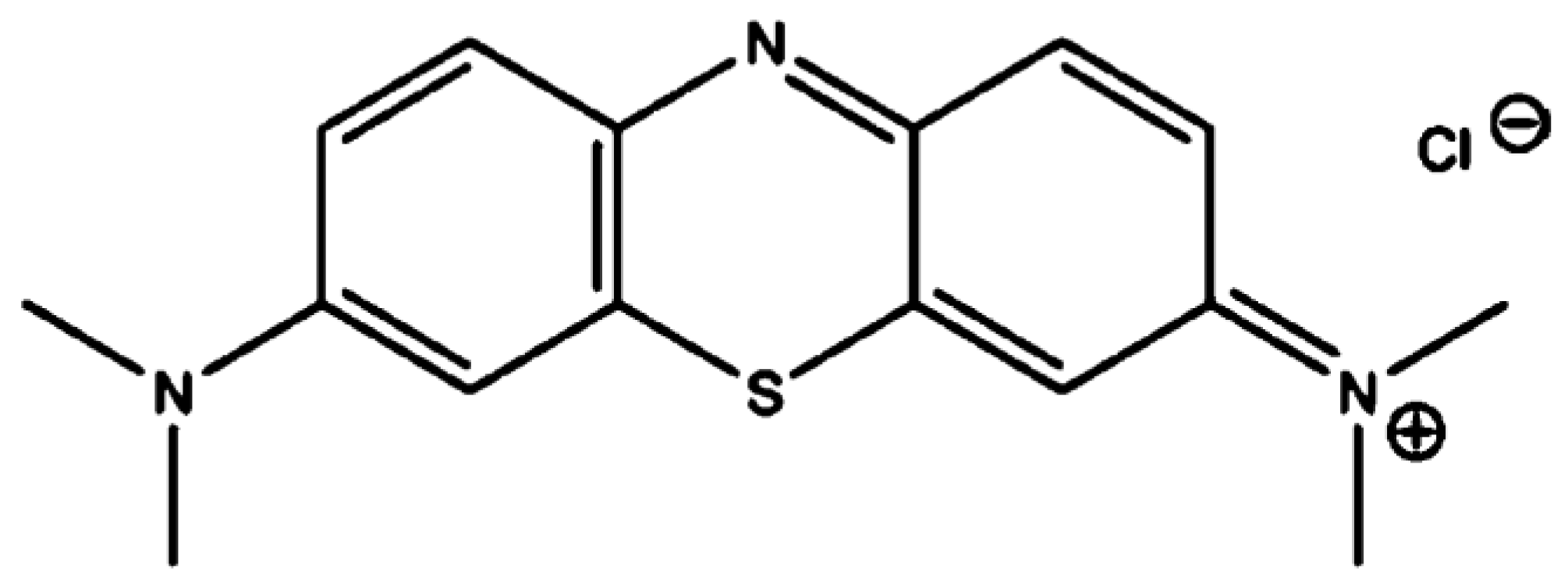
2.1. Chemicals and Preparation of Dye Solution
2.2. Adsorbent Nanoparticles (A. platensis) Preparation
2.3. Characterization of the Adsorbent (A. platensis Nanoparticle)
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. BET Analysis
2.3.3. FTIR Spectral Analysis
2.3.4. Ultraviolet Spectra (UV)
2.4. Batch Experiments of the Adsorption Process
2.5. Dyes Removal Efficiency
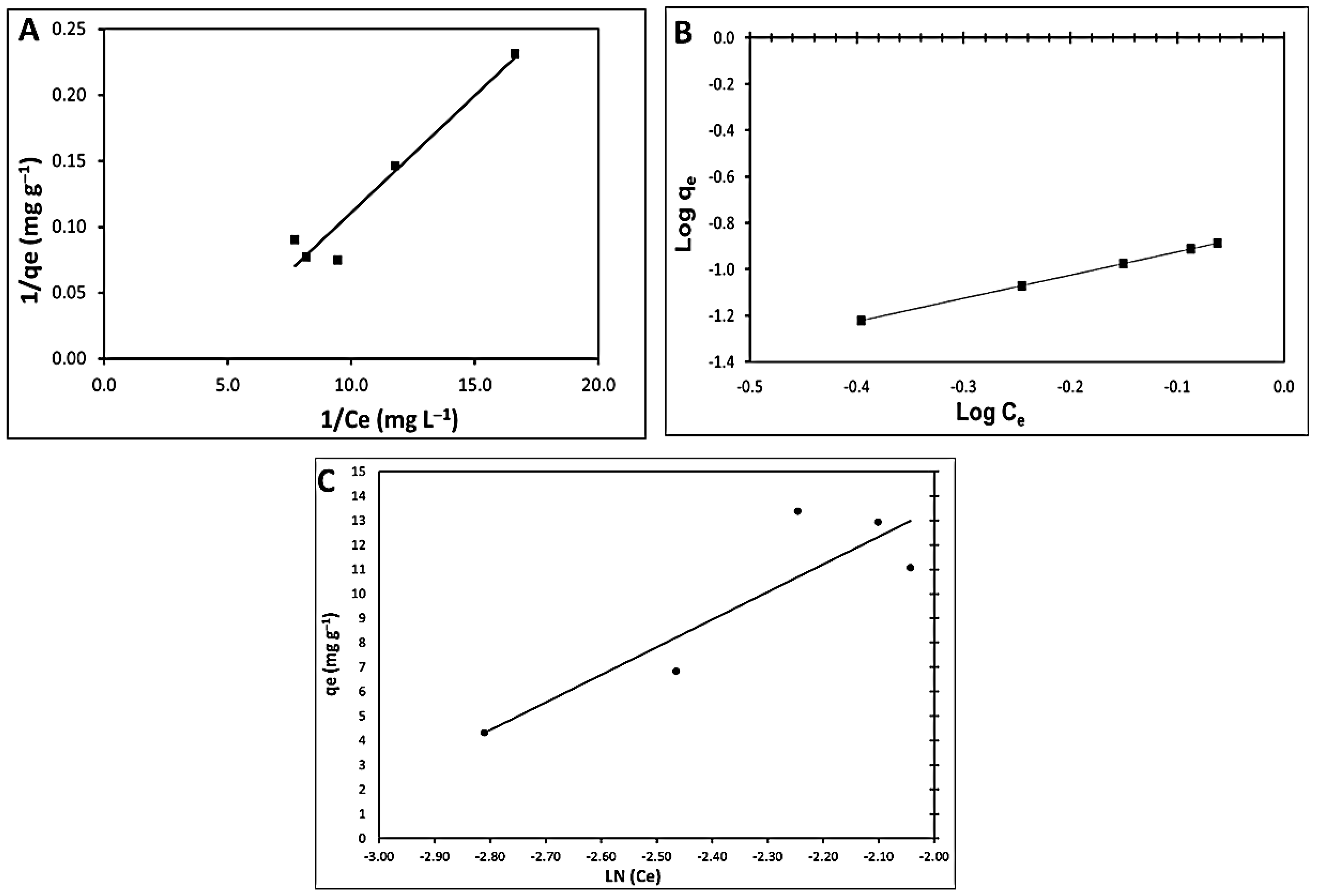
2.6. Mathematical Models (Isotherm Study)
2.7. Adsorption Kinetics
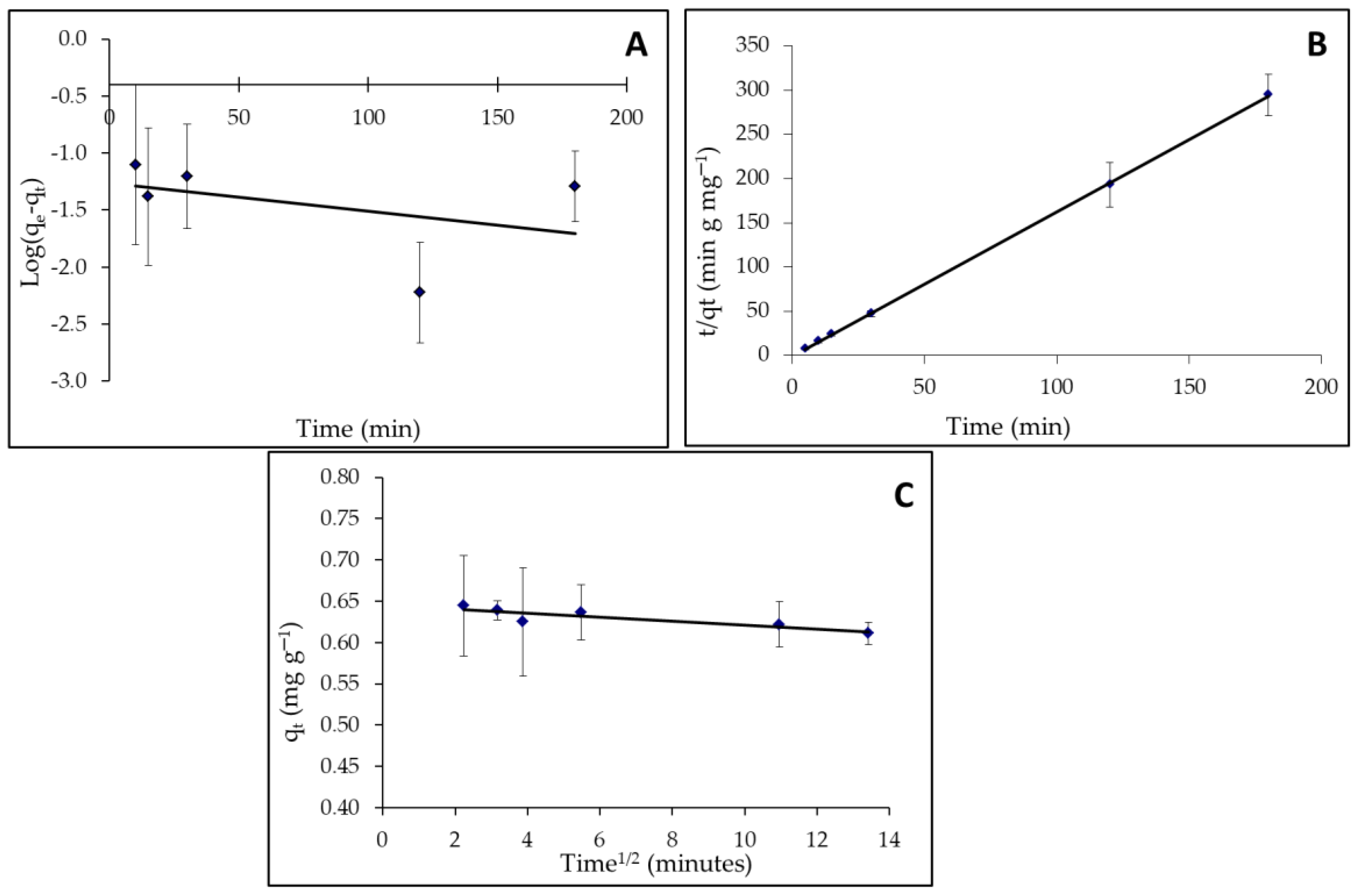
2.8. Theoretic Background of Adsorption Kinetics
2.8.1. Kinetic Model of Pseudo-First-Order
2.8.2. Pseudo-Second-Order Kinetic Model
2.8.3. The Intraparticle Diffusion Model
3. Results and Discussion
3.1. Characterization of the Adsorbent Material (Binding Mechanism)
3.1.1. FTIR Analysis
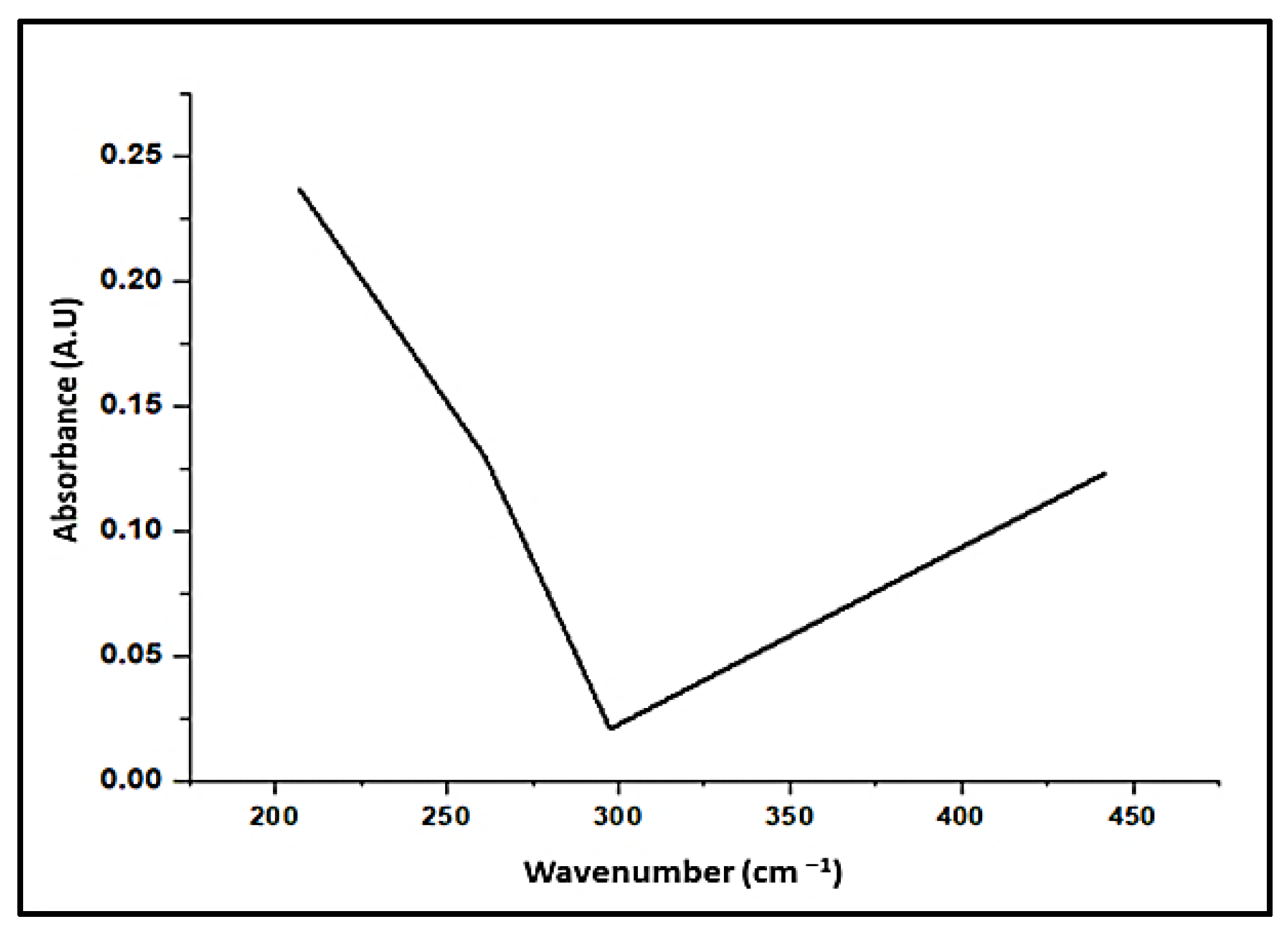
3.1.2. UV Examination
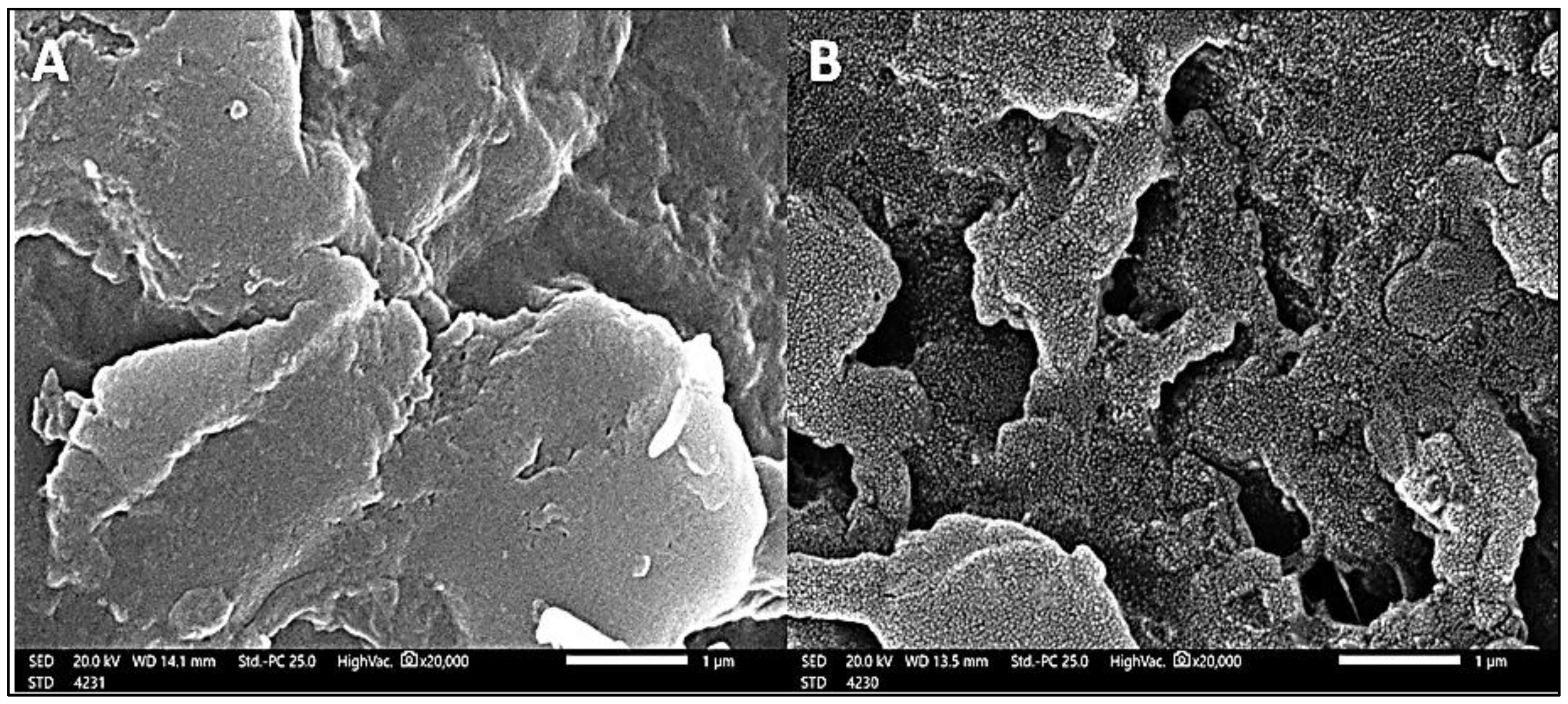
3.1.3. SEM
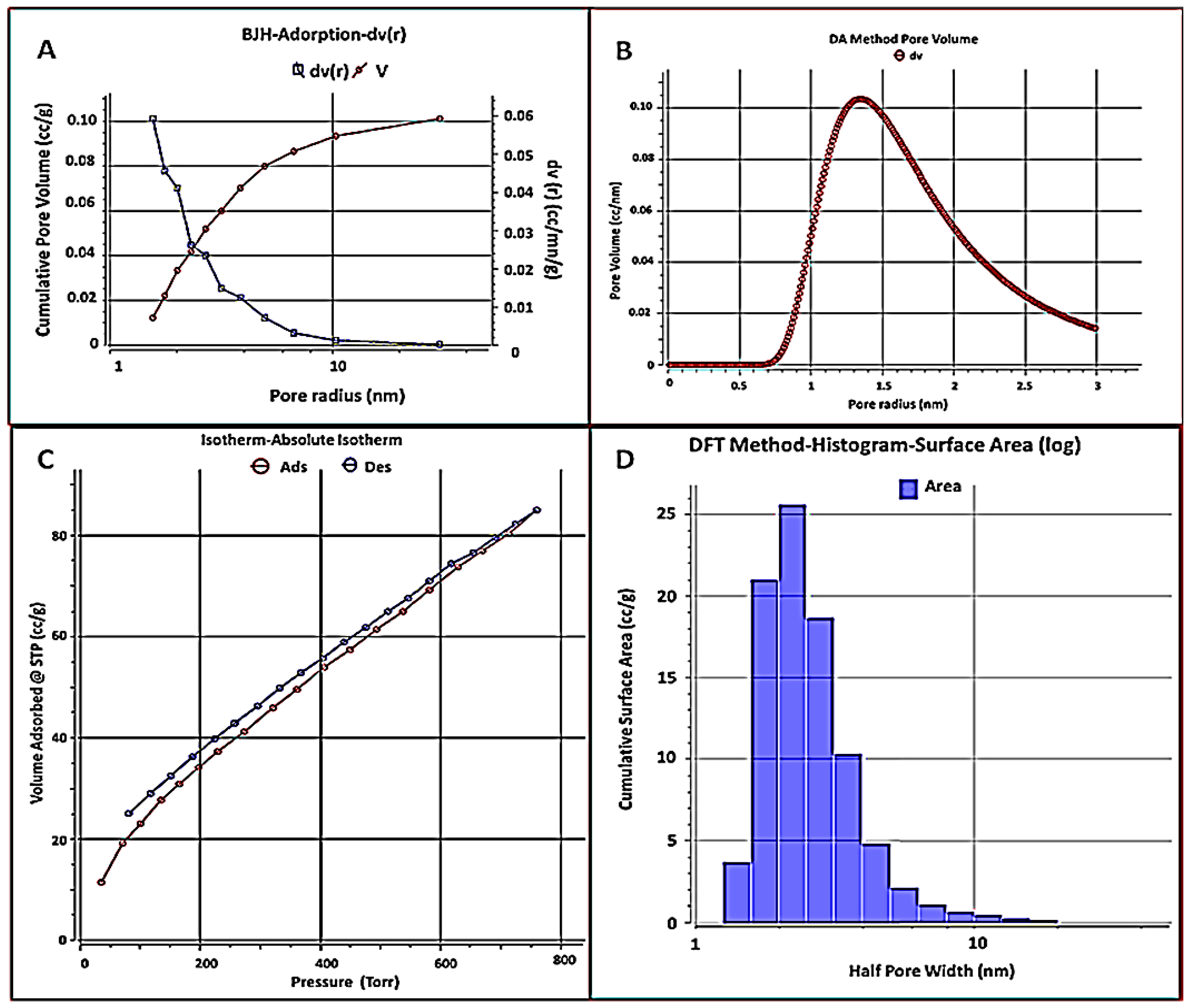
3.1.4. BET Analysis
3.2. Adsorbent Parameters Optimizations
3.2.1. Effect of Initial MBD Concentration
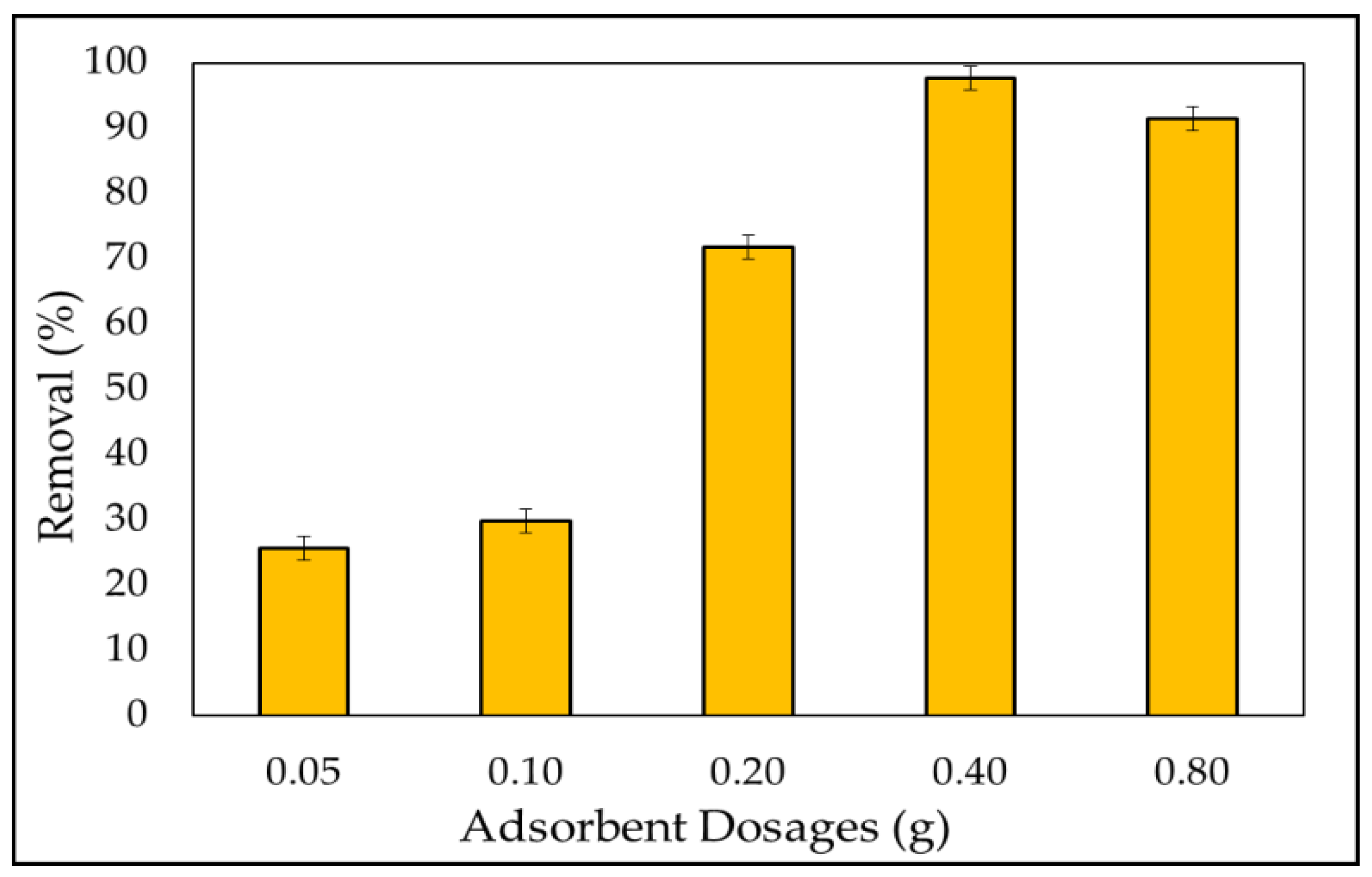
3.2.2. Adsorbent Dosage
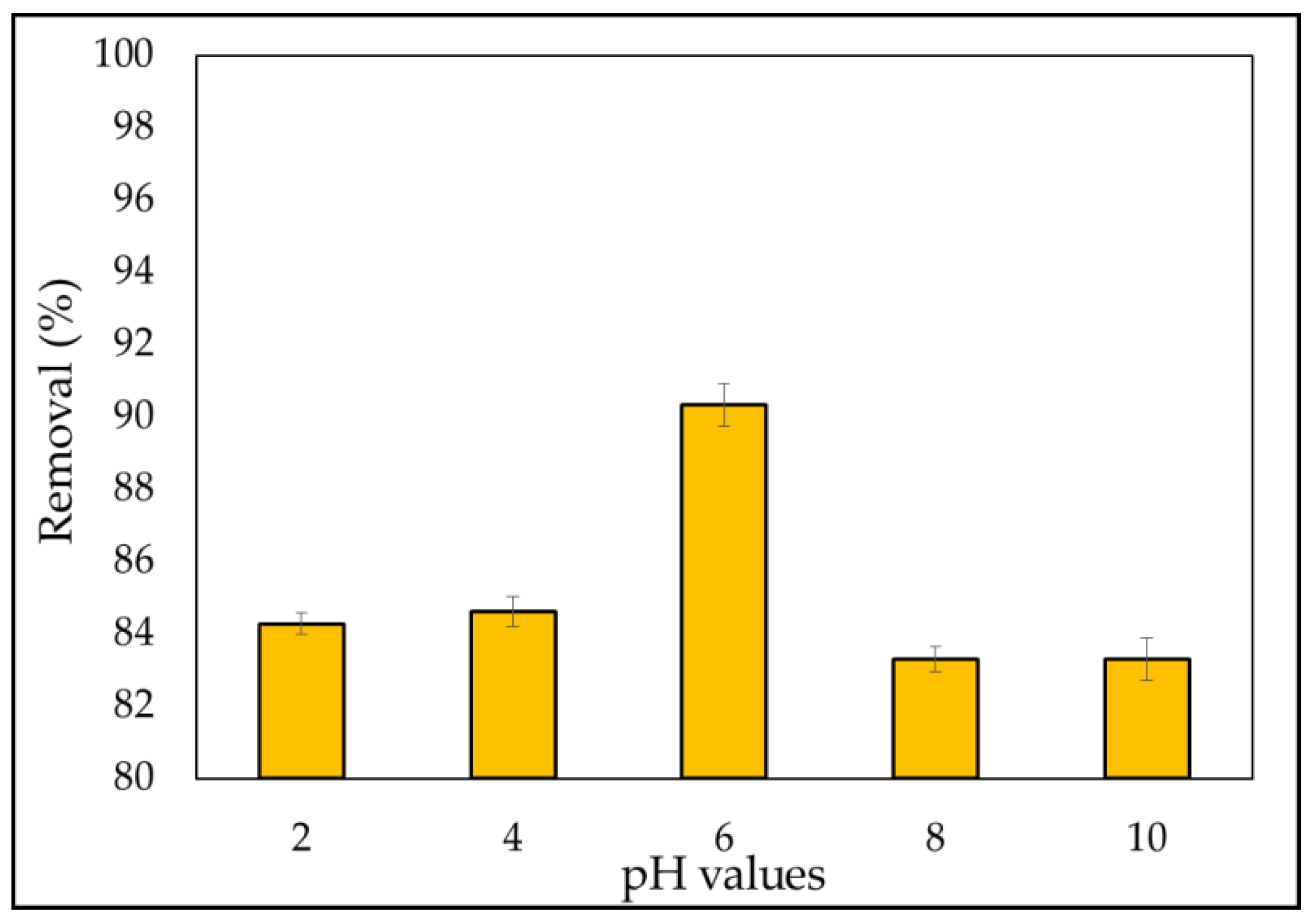
3.2.3. Effect of pH
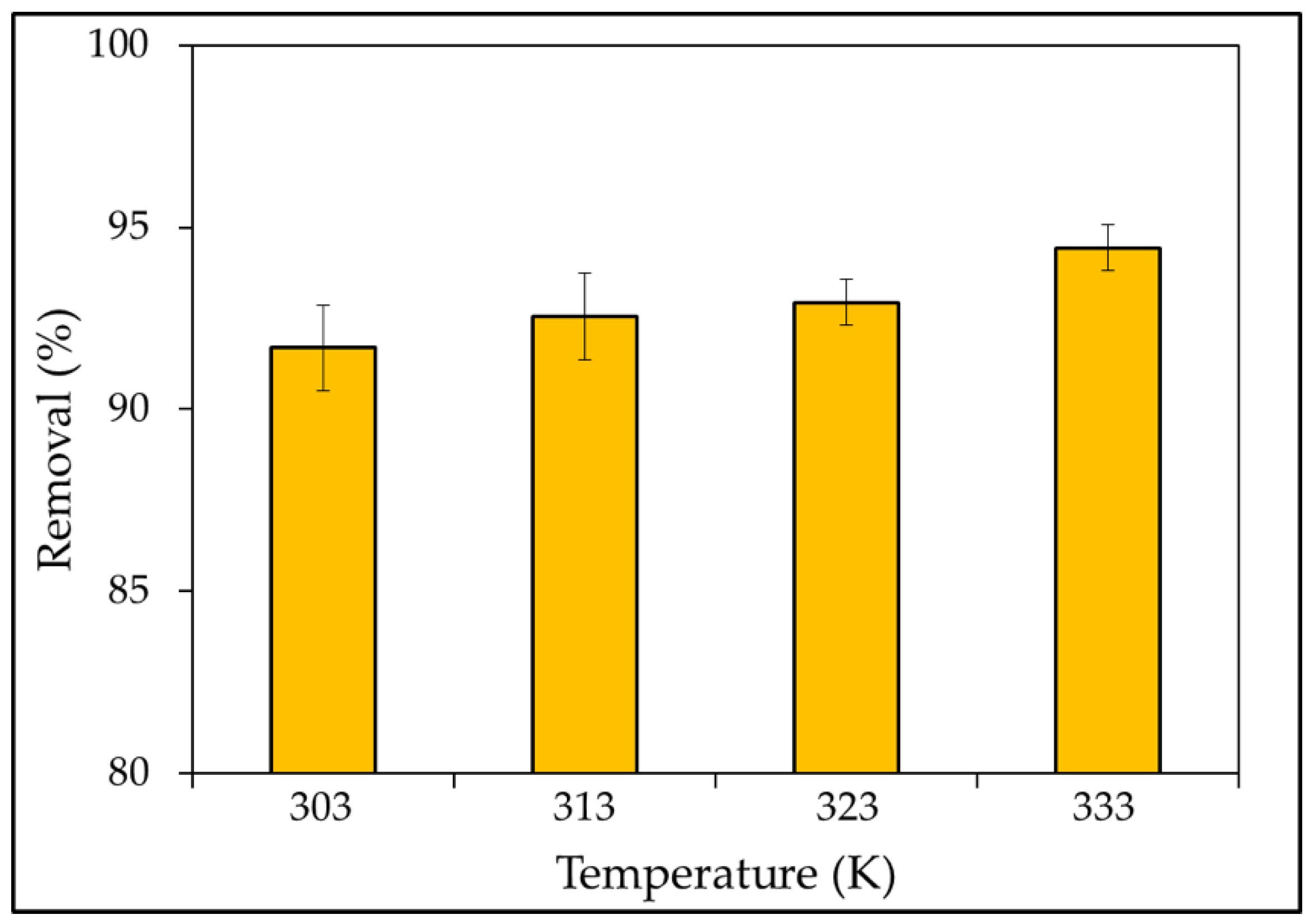
3.2.4. Effect of Temperature
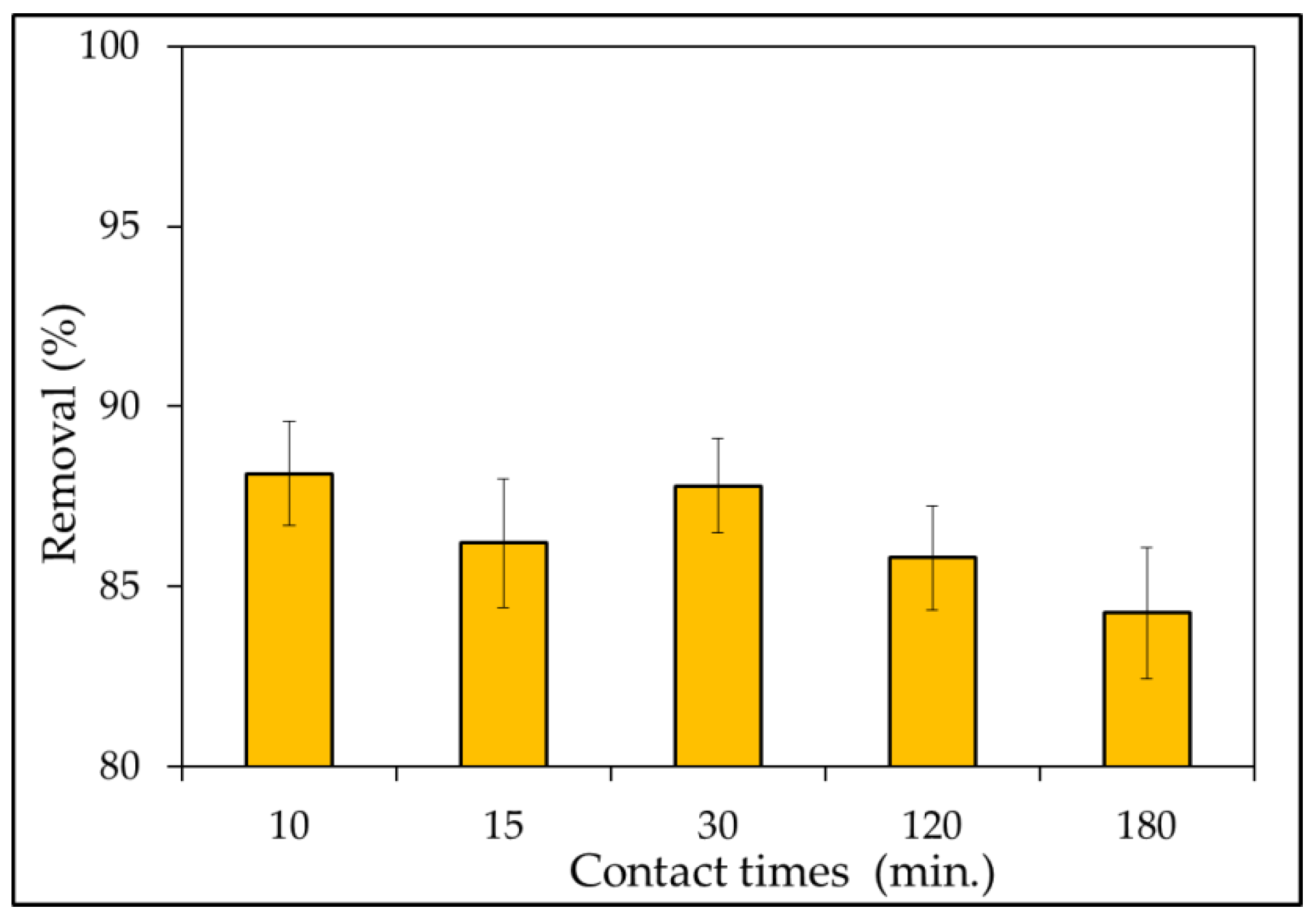
3.2.5. Effect of Contact Times
3.3. Mechanism of Adsorption
3.4. Adsorption Dynamics
3.4.1. Kinetic Study
3.4.2. Adsorption Isotherm Modeling
3.5. Goodness of Model Fit
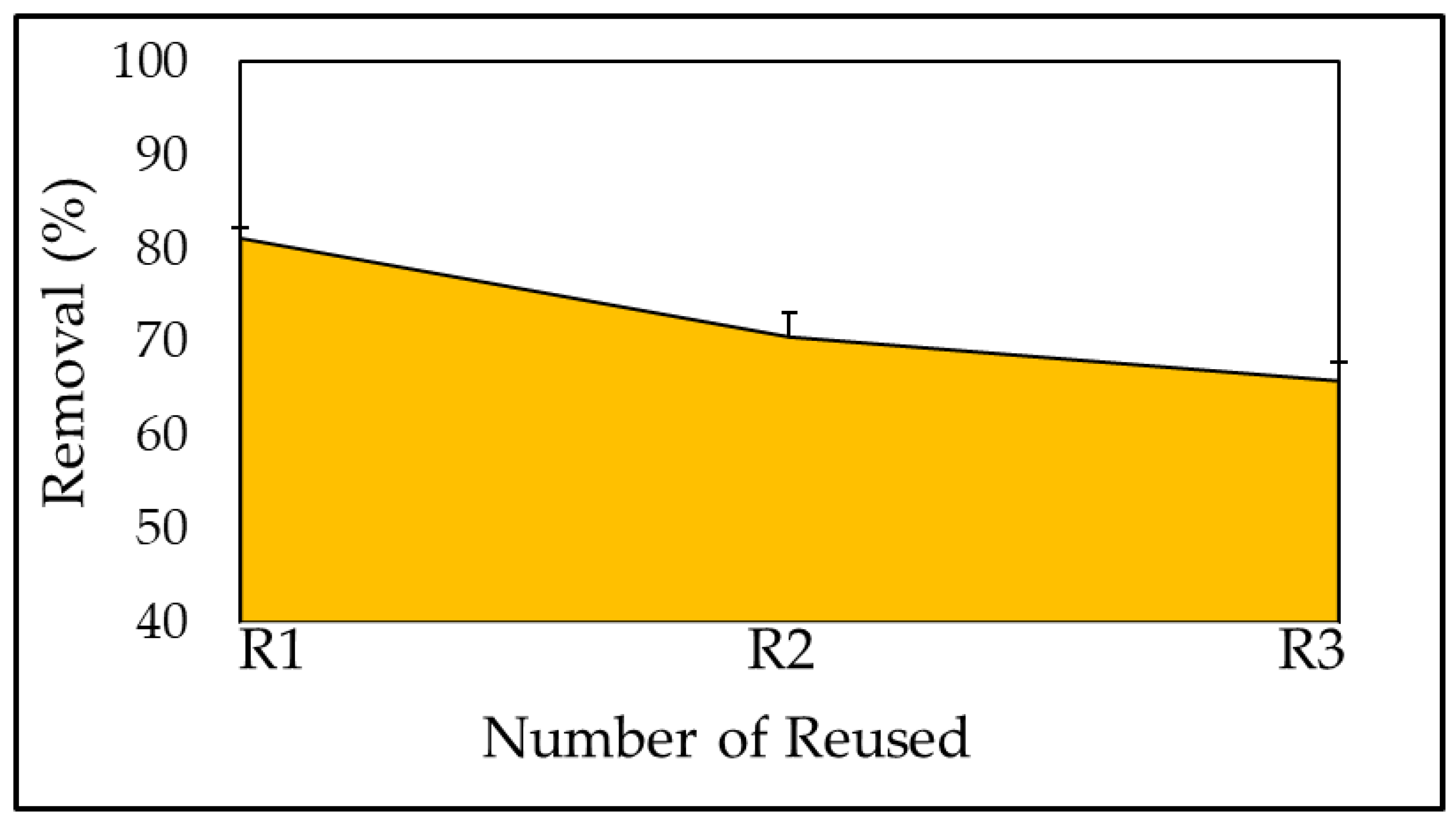
3.6. Reuse of the Adsorbent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, A.; Bhaskar, S.; Battampara, P.; Reddy, N.; Ramamurthy, S.S. Integrated Photo-Plasmonic coupling of bioinspired Sharp-Edged silver Nano-particles with Nano-films in extended cavity functional interface for Cellphone-aided femtomolar sensing. Mater. Lett. 2022, 316, 132025. [Google Scholar] [CrossRef]
- Daija, L.; Selberg, A.; Rikmann, E.; Zekker, I.; Tenno, T.; Tenno, T. The influence of lower temperature, influent fluctuations and long retention time on the performance of an upflow mode laboratory-scale septic tank. Desalination Water Treat. 2016, 57, 18679–18687. [Google Scholar] [CrossRef]
- Eddebbagh, M.; Abourriche, A.; Berrada, M.; Zina, M.B.; Bennamara, A. Adsorbent material from pomegranate (Punica granatum) leaves: Optimization on removal of methylene blue using response surface methodology. J. Mater. Environ. Sci. 2016, 7, 2021–2033. [Google Scholar]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Abualnaja, K.M.; Alprol, A.E.; Ashour, M.; Mansour, A.T. Influencing Multi-Walled Carbon Nanotubes for the Removal of Ismate Violet 2R Dye from Wastewater: Isotherm, Kinetics, and Thermodynamic Studies. Appl. Sci. 2021, 11, 4786. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.; Ashour, M. Dried Brown Seaweed’s Phytoremediation Potential for Methylene Blue Dye Removal from Aquatic Environments. Polymers 2022, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Viraraghavan, T. Fungal decolorization of dye wastewaters: A review. Bioresour. Technol. 2001, 79, 251–262. [Google Scholar] [CrossRef]
- Rahman, I.; Saad, B. Utilization of guava seeds as a source of activated carbon for removal of methylene blue from aqueous solution. Malays. J. Chem. 2003, 5, 8–14. [Google Scholar]
- Subburam, V. Activated parthenium carbon as an adsorbent for the removal of dyes and heavy metal ions from aqueous solution. Bioresour. Technol. 2002, 85, 205–206. [Google Scholar]
- Banat, F.; Al-Asheh, S.; Al-Makhadmeh, L. Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochem. 2003, 39, 193–202. [Google Scholar] [CrossRef]
- Swaminathan, S.; Muthumanickkam, A.; Imayathamizhan, N. An effective removal of methylene blue dye using polyacrylonitrile yarn waste/graphene oxide nanofibrous composite. Int. J. Environ. Sci. Technol. 2015, 12, 3499–3508. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, S.; Ramamurthy, S.S. Synergistic coupling of titanium carbonitride nanocubes and graphene oxide for 800-fold fluorescence enhancements on smartphone based surface plasmon-coupled emission platform. Mater. Lett. 2021, 298, 130008. [Google Scholar] [CrossRef]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- López-Maldonado, E.A.; Oropeza-Guzmán, M.T. Nejayote biopolyelectrolytes multifunctionality (glucurono ferulauted arabinoxylans) in the separation of hazardous metal ions from industrial wastewater. Chem. Eng. J. 2021, 423, 130210. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A review. J. Environ. Manag. 2010, 91, 1915–1929. [Google Scholar] [CrossRef]
- Song, Z.; Williams, C.; Edyvean, R. Treatment of tannery wastewater by chemical coagulation. Desalination 2004, 164, 249–259. [Google Scholar] [CrossRef]
- Semerjian, L.; Ayoub, G. High-pH–magnesium coagulation–flocculation in wastewater treatment. Adv. Environ. Res. 2003, 7, 389–403. [Google Scholar] [CrossRef]
- Harper, T.R.; Kingham, N.W. Removal of arsenic from wastewater using chemical precipitation methods. Water Environ. Res. 1992, 64, 200–203. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Guida, S.; Van Peteghem, L.; Luqmani, B.; Sakarika, M.; McLeod, A.; McAdam, E.J.; Jefferson, B.; Rabaey, K.; Soares, A. Ammonia recovery from brines originating from a municipal wastewater ion exchange process and valorization of recovered nitrogen into microbial protein. Chem. Eng. J. 2022, 427, 130896. [Google Scholar] [CrossRef]
- Schwermer, C.U.; Krzeminski, P.; Wennberg, A.C.; Vogelsang, C.; Uhl, W. Removal of antibiotic resistant E. coli in two Norwegian wastewater treatment plants and by nano-and ultra-filtration processes. Water Sci. Technol. 2018, 77, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Bartels, C.R.; Wilf, M.; Andes, K.; Iong, J. Design considerations for wastewater treatment by reverse osmosis. Water Sci. Technol. 2005, 51, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Ismail, G.A.; Allam, N.G.; El-Gemizy, W.M.; Salem, M.A.-S. Potential role of Spirulina (Arthrospira) platensis biomass for removal of TiO2 NPs-MG hybrid nanocomposite produced after wastewater treatment by TiO2 nanoparticles. An. Acad. Bras. Ciências 2021, 93, e20201669. [Google Scholar] [CrossRef] [PubMed]
- Ismail, G.A.; Allam, N.G.; El-Gemizy, W.M.; Salem, M.A. The role of silver nanoparticles biosynthesized by Anabaena variabilis and Spirulina platensis cyanobacteria for malachite green removal from wastewater. Environ. Technol. 2021, 42, 4475–4489. [Google Scholar] [CrossRef]
- Aziz, K.; Mamouni, R.; Azrrar, A.; Kjidaa, B.; Saffaj, N.; Aziz, F. Enhanced biosorption of bisphenol A from wastewater using hydroxyapatite elaborated from fish scales and camel bone meal: A RSM@ BBD optimization approach. Ceram. Int. 2022, 48, 15811–15823. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.; Nada, A.A.; O’donovan, A.; Thakur, V.K.; Elkelish, A. Mycogenic silver nanoparticles from endophytic Trichoderma atroviride with antimicrobial activity. J. Renew. Mater. 2020, 8, 171. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; El-Desoky, H.S.; El-Moselhy, K.M.; Amer, A.; Abou El-Naga, E.H.; Mohamedein, L.I.; Al-Prol, A.E. Removal of cadmium from aqueous solution using marine green algae, Ulva lactuca. Egypt. J. Aquat. Res. 2014, 40, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Aziz, K.; Aziz, F.; Mamouni, R.; Aziz, L.; Anfar, Z.; Azrrar, A.; Kjidaa, B.; Saffaj, N.; Laknifli, A. High thiabendazole fungicide uptake using Cellana tramoserica shells modified by copper: Characterization, adsorption mechanism, and optimization using CCD-RSM approach. Environ. Sci. Pollut. Res. 2021, 1–16. [Google Scholar] [CrossRef]
- Amer, A.; Abdel-Moneim, M. Bioremediation of Reactive Blue 19 and Reactive Black 5 from Aqueous Solution by using Fungi Aspergillus niger. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1676–1686. [Google Scholar]
- Alprol, A.E.; El-Metwally, M.; Amer, A. Sargassum latifolium as eco-friendly materials for treatment of toxic nickel (II) and lead (II) ions from aqueous solution. Egypt. J. Aquat. Biol. Fish. 2019, 23, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Alprol, A.E.; Heneash, A.M.M.; Ashour, M.; Abualnaja, K.M.; Alhashmialameer, D.; Mansour, A.T.; Sharawy, Z.Z.; Abu-Saied, M.A.; Abomohra, A.E. Potential Applications of Arthrospira platensis Lipid-Free Biomass in Bioremediation of Organic Dye from Industrial Textile Effluents and Its Influence on Marine Rotifer (Brachionus plicatilis). Materials 2021, 14, 4446. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Alprol, A.E.; Heneash, A.M.M.; Saleh, H.; Abualnaja, K.M.; Alhashmialameer, D.; Mansour, A.T. Ammonia Bioremediation from Aquaculture Wastewater Effluents Using Arthrospira platensis NIOF17/003: Impact of Biodiesel Residue and Potential of Ammonia-Loaded Biomass as Rotifer Feed. Materials 2021, 14, 5460. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Bhawana, P.; Fulekar, M. Microbial decolorization and degradation of synthetic dyes: A review. Rev. Environ. Sci. Bio/Technol. 2013, 12, 75–97. [Google Scholar] [CrossRef]
- Mishra, S.; Cheng, L.; Maiti, A. The utilization of agro-biomass/byproducts for effective bio-removal of dyes from dyeing wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104901. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Danish, M.; Singh, R.S.; Rafatullah, M.; HPS, A.K. Exploiting microbial biomass in treating azo dyes contaminated wastewater: Mechanism of degradation and factors affecting microbial efficiency. J. Water Process Eng. 2021, 43, 102255. [Google Scholar] [CrossRef]
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as Support for Microbial Biomass-Based Adsorbents with Applications in Removal of Heavy Metals and Dyes. Polymers 2021, 13, 2893. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Gharieb, M.; Abou-El-Souod, G. Biodegradation of dyes by some green algae and cyanobacteria. Int. Biodeterior. Biodegrad. 2009, 63, 699–704. [Google Scholar] [CrossRef]
- Mansour, A.T.; Ashour, M.; Alprol, A.E.; Alsaqufi, A.S. Aquatic Plants and Aquatic Animals in the Context of Sustainability: Cultivation Techniques, Integration, and Blue Revolution. Sustainability 2022, 14, 3257. [Google Scholar] [CrossRef]
- Sharawy, Z.Z.; Ashour, M.; Abbas, E.; Ashry, O.; Helal, M.; Nazmi, H.; Kelany, M.; Kamel, A.; Hassaan, M.; Rossi, W.; et al. Effects of dietary marine microalgae, Tetraselmis suecica, on production, gene expression, protein markers and bacterial count of Pacific white shrimp Litopenaeus vannamei. Aquac. Res. 2020, 51, 2216–2228. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Soliman, A.A.F.; Hassanien, H.A.; Alsanie, W.F.; Gaber, A.; Elshobary, M.E. The Potential of a New Commercial Seaweed Extract in Stimulating Morpho-Agronomic and Bioactive Properties of Eruca vesicaria (L.) Cav. Sustainability 2021, 13, 4485. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.; Hassanien, H.A.; Gaber, A.; Ammar, G. Impact of Seaweed Liquid Extract Biostimulant on Growth, Yield, and Chemical Composition of Cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- Ashour, M.; Mabrouk, M.M.; Abo-Taleb, H.A.; Sharawy, Z.Z.; Ayoub, H.F.; Van Doan, H.; Davies, S.J.; El-Haroun, E.; Goda, A.M.S.A. A liquid seaweed extract (TAM®) improves aqueous rearing environment, diversity of zooplankton community, whilst enhancing growth and immune response of Nile tilapia, Oreochromis niloticus, challenged by Aeromonas hydrophila. Aquaculture 2021, 543, 736915. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alsaqufi, A.S.; Alkhamis, Y.A.; Al-Gazar, F.F.; Zaki, M.A.; Nour, A.A.M.; Ramadan, K.M. The Evaluation of Arthrospira platensis Bioactivity and their Dietary Supplementation to Nile Tilapia Vegetarian Diet on Growth Performance, Feed Utilization, Body Composition and Hemato-Biochemical Parameters. Ann. Anim. Sci. 2021, 21, 1061–1080. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Almutairi, A.W. A close-loop integrated approach for microalgae cultivation and efficient utilization of agar-free seaweed residues for enhanced biofuel recovery. Bioresour. Technol. 2020, 317, 124027. [Google Scholar] [CrossRef]
- Ashour, M.; Elshobary, M.E.; El-Shenody, R.; Kamil, A.-W.; Abomohra, A.E.-F. Evaluation of a native oleaginous marine microalga Nannochloropsis oceanica for dual use in biodiesel production and aquaculture feed. Biomass Bioenergy 2019, 120, 439–447. [Google Scholar] [CrossRef]
- Zaki, M.A.; Ashour, M.; Heneash, A.M.M.; Mabrouk, M.M.; Alprol, A.E.; Khairy, H.M.; Nour, A.M.; Mansour, A.T.; Hassanien, H.A.; Gaber, A.; et al. Potential Applications of Native Cyanobacterium Isolate (Arthrospira platensis NIOF17/003) for Biodiesel Production and Utilization of Its Byproduct in Marine Rotifer (Brachionus plicatilis) Production. Sustainability 2021, 13, 1769. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Elshobary, M. Biodiesel, bioethanol, and biobutanol production from microalgae. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Singapore, 2019; pp. 293–321. [Google Scholar]
- El-Shenody, R.A.; Ashour, M.; Ghobara, M.M.E. Evaluating the chemical composition and antioxidant activity of three Egyptian seaweeds: Dictyota dichotoma, Turbinaria decurrens, and Laurencia obtusa. Braz. J. Food Technol. 2019, 22, e2018203. [Google Scholar] [CrossRef] [Green Version]
- Elshobary, M.E.; El-Shenody, R.A.; Ashour, M.; Zabed, H.M.; Qi, X. Antimicrobial and antioxidant characterization of bioactive components from Chlorococcum minutum. Food Biosci. 2020, 35, 100567. [Google Scholar] [CrossRef]
- Vega, J.; Álvarez-Gómez, F.; Güenaga, L.; Figueroa, F.L.; Gómez-Pinchetti, J.L. Antioxidant activity of extracts from marine macroalgae, wild-collected and cultivated, in an integrated multi-trophic aquaculture system. Aquaculture 2020, 522, 735088. [Google Scholar] [CrossRef]
- Shao, W.; Ebaid, R.; El-Sheekh, M.; Abomohra, A.; Eladel, H. Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): An overview. Grasas Aceites 2019, 70, e292. [Google Scholar] [CrossRef] [Green Version]
- Garza-Gonzalez, M.; Alcalá-Rodríguez, M.; Pérez-Elizondo, R.; Cerino-Córdova, F.; Garcia-Reyes, R.; Loredo-Medrano, J.; Soto-Regalado, E. Artificial Neural Network for predicting biosorption of methylene blue by Spirulina sp. Water Sci. Technol. 2011, 63, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Essa, D.; Abo-Shady, A.; Khairy, H.; Abomohra, A.E.-F.; Elshobary, M. Potential cultivation of halophilic oleaginous microalgae on industrial wastewater. Egypt. J. Bot. 2018, 58, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Tadda, M.A.; Li, C.; Gouda, M.; Abomohra, A.E.-F.; Shitu, A.; Ahsan, A.; Zhu, S.; Liu, D. Enhancement of nitrite/ammonia removal from saline recirculating aquaculture wastewater system using moving bed bioreactor. J. Environ. Chem. Eng. 2021, 9, 105947. [Google Scholar] [CrossRef]
- Tu, R.; Jin, W.; Xi, T.; Yang, Q.; Han, S.-F.; Abomohra, A.E.-F. Effect of static magnetic field on the oxygen production of Scenedesmus obliquus cultivated in municipal wastewater. Water Res. 2015, 86, 132–138. [Google Scholar] [CrossRef]
- Aly, M.; Gad, A. Chemical composition and potential application of Spirulina platensis biomass. J. Am. Sci. 2010, 6, 819–826. [Google Scholar]
- Madkour, F.F.; Kamil, A.E.-W.; Nasr, H.S. Production and nutritive value of Spirulina platensis in reduced cost media. Egypt. J. Aquat. Res. 2012, 38, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Mabrouk, M.M.; Ashour, M.; Labena, A.; Zaki, M.A.A.; Abdelhamid, A.F.; Gewaily, M.S.; Dawood, M.A.O.; Abualnaja, K.M.; Ayoub, H.F. Nanoparticles of Arthrospira platensis improves growth, antioxidative and immunological responses of Nile tilapia (Oreochromis niloticus) and its resistance to Aeromonas hydrophila. Aquac. Res. 2022, 53, 125–135. [Google Scholar] [CrossRef]
- Sharawy, Z.Z.; Ashour, M.; Labena, A.; Alsaqufi, A.S.; Mansour, A.T.; Abbas, E.M. Effects of dietary Arthrospira platensis nanoparticles on growth performance, feed utilization, and growth-related gene expression of Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2022, 551, 737905. [Google Scholar] [CrossRef]
- Dotto, G.; Lima, E.; Pinto, L. Biosorption of food dyes onto Spirulina platensis nanoparticles: Equilibrium isotherm and thermodynamic analysis. Bioresour. Technol. 2012, 103, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Ellatif, S.; El-Sheekh, M.M.; Senousy, H.H. Role of microalgal ligninolytic enzymes in industrial dye decolorization. Int. J. Phytoremediat. 2021, 23, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Şeker, A.; Shahwan, T.; Eroğlu, A.E.; Yılmaz, S.; Demirel, Z.; Dalay, M.C. Equilibrium, thermodynamic and kinetic studies for the biosorption of aqueous lead (II), cadmium (II) and nickel (II) ions on Spirulina platensis. J. Hazard. Mater. 2008, 154, 973–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaman, H.G.; Baloo, L.; Aziz, F.; Kutty, S.R.; Ashraf, A. COD adsorption and optimization from produced water using chitosan–ZnO nanocomposite. Appl. Nanosci. 2022, 12, 1885–1898. [Google Scholar] [CrossRef]
- Cheung, W.; Szeto, Y.; McKay, G. Enhancing the adsorption capacities of acid dyes by chitosan nano particles. Bioresour. Technol. 2009, 100, 1143–1148. [Google Scholar] [CrossRef]
- Moorthy, A.K.; Rathi, B.G.; Shukla, S.P.; Kumar, K.; Bharti, V.S. Acute toxicity of textile dye Methylene blue on growth and metabolism of selected freshwater microalgae. Environ. Toxicol. Pharmacol. 2021, 82, 103552. [Google Scholar] [CrossRef]
- Ravikumar, K.; Deebika, B.; Balu, K. Decolourization of aqueous dye solutions by a novel adsorbent: Application of statistical designs and surface plots for the optimization and regression analysis. J. Hazard. Mater. 2005, 122, 75–83. [Google Scholar] [CrossRef]
- Abualnaja, K.M.; Alprol, A.E.; Abu-Saied, M.A.; Mansour, A.T.; Ashour, M. Studying the Adsorptive Behavior of Poly(Acrylonitrile-co-Styrene) and Carbon Nanotubes (Nanocomposites) Impregnated with Adsorbent Materials towards Methyl Orange Dye. Nanomaterials 2021, 11, 1144. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Temkin, M.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physiochim. URSS 1940, 12, 217–225. [Google Scholar]
- Hood, M.M.; Taggart, R.K.; Smith, R.C.; Hsu-Kim, H.; Henke, K.R.; Graham, U.; Groppo, J.G.; Unrine, J.M.; Hower, J.C. Rare earth element distribution in fly ash derived from the Fire Clay coal, Kentucky. Coal Combust. Gasif. Prod. 2017, 9, 22–33. [Google Scholar] [CrossRef]
- Levankumar, L.; Muthukumaran, V.; Gobinath, M. Batch adsorption and kinetics of chromium (VI) removal from aqueous solutions by Ocimum americanum L. seed pods. J. Hazard. Mater. 2009, 161, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; McKay, G.; Wase, D.; Forster, C. Study of the sorption of divalent metal ions on to peat. Adsorpt. Sci. Technol. 2000, 18, 639–650. [Google Scholar] [CrossRef]
- Michalak, I.; Mironiuk, M.; Godlewska, K.; Trynda, J.; Marycz, K. Arthrospira (Spirulina) platensis: An effective biosorbent for nutrients. Process Biochem. 2020, 88, 129–137. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Royer, B.; Bach, M.V.; Dotto, G.L.; Pinto, L.A.; Calvete, T. Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of Reactive Red 120 dye from aqueous effluents. J. Hazard. Mater. 2012, 241, 146–153. [Google Scholar] [CrossRef]
- Maurya, R.; Ghosh, T.; Paliwal, C.; Shrivastav, A.; Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Biosorption of methylene blue by de-oiled algal biomass: Equilibrium, kinetics and artificial neural network modelling. PLoS ONE 2014, 9, e109545. [Google Scholar] [CrossRef] [Green Version]
- Șuteu, D.; Zaharia, C.; Rusu, G. Reactive dye removal from aqueous solution by sorption on modified ash. Cercet. Agron. Mold. 2010, 43, 59–65. [Google Scholar]
- Zhang, L.; Luo, J.; Menkhaus, T.J.; Varadaraju, H.; Sun, Y.; Fong, H. Antimicrobial nano-fibrous membranes developed from electrospun polyacrylonitrile nanofibers. J. Membr. Sci. 2011, 369, 499–505. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Pinto, R.B.; Lima, E.C.; Calvete, T.; Amavisca, C.V.; Royer, B.; Cunha, M.L.; Fernandes, T.H.; Pinto, I.S. Removal of remazol black B textile dye from aqueous solution by adsorption. Desalination 2011, 269, 92–103. [Google Scholar] [CrossRef]
- Celekli, A.; Yavuzatmaca, M.; Bozkurt, H. An eco-friendly process: Predictive modelling of copper adsorption from aqueous solution on Spirulina platensis. J. Hazard. Mater. 2010, 173, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Abdulwahid, K.E.; Dwaish, A.S.; Dakhil, O.A. Green synthesis and characterization of zinc oxide nanoparticles from Cladophora glomerata and its antifungal activity against some fungal isolates. Plant Arch. 2019, 19, 3527–3532. [Google Scholar]
- Rajeswari, R.; Jeyaprakash, K. Bioactive potential analysis of brown seaweed Sargassum wightii using UV-VIS and FT-IR. J. Drug Deliv. Ther. 2019, 9, 150–153. [Google Scholar] [CrossRef] [Green Version]
- Adeyi, A.A.; Jamil, S.N.A.M.; Abdullah, L.C.; Choong, T.S.Y.; Lau, K.L.; Abdullah, M. Adsorptive removal of methylene blue from aquatic environments using thiourea-modified poly (acrylonitrile-co-acrylic acid). Materials 2019, 12, 1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dmytryk, A.; Saeid, A.; Chojnacka, K. Biosorption of microelements by Spirulina: Towards technology of mineral feed supplements. Sci. World J. 2014, 2014, 356328. [Google Scholar] [CrossRef] [Green Version]
- Ara, N.J. Development of Dyes Removal Method from Textile Waste Water; University of Dhaka: Dhaka, Bangladesh, 2015. [Google Scholar]
- Module 3: Characteristics of Particles-Particle Size Categories; US Environmental Protection Agency: Washington, DC, USA, 2010.
- D’silva, A. Adsorption of antioxidants by carbon blacks. Carbon 1998, 36, 1317–1325. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Abate, G.Y.; Alene, A.N.; Habte, A.T.; Getahun, D.M. Adsorptive removal of malachite green dye from aqueous solution onto activated carbon of Catha edulis stem as a low cost bio-adsorbent. Environ. Syst. Res. 2020, 9, 29. [Google Scholar] [CrossRef]
- Almeida, C.; Debacher, N.; Downs, A.; Cottet, L.; Mello, C. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloïd Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem. 2005, 40, 1347–1361. [Google Scholar] [CrossRef]
- Amrhar, O.; Nassali, H.; Elyoubi, M. Modeling of adsorption isotherms of methylene blue onto natural illitic clay: Nonlinear regression analysis. Moroc. J. Chem. 2015, 3, 582–593. [Google Scholar]
- El-Moselhy, K.; Abdel-Azzem, M.; Amer, A.; Al-Prol, A. Adsorption of Cu (II) and Cd (II) from aqueous solution by using rice husk adsorbent. Phys. Chem. Indian J. 2017, 12, 109. [Google Scholar]
- Idris, M.N.; Ahmad, Z.A.; Ahmad, M.A. Adsorption equilibrium of malachite green dye onto rubber seed coat based activated carbon. Int. J. Basic Appl. Sci. 2011, 11, 38–43. [Google Scholar]
- Amuda, O.S.; Edewor, T.I.; Afolabi, T.; Hung, Y.-T. Steam-activated carbon prepared from Chrysophyllum albidum seed shell for the adsorption of cadmium in wastewater: Kinetics, equilibrium and thermodynamic studies. Int. J. Environ. Waste Manag. 2013, 12, 213–229. [Google Scholar] [CrossRef]
- Amuda, O.S.; Olayiwola, A.O.; Alade, A.O.; Farombi, A.G.; Adebisi, S.A. Adsorption of methylene blue from aqueous solution using steam-activated carbon produced from Lantana camara stem. J. Environ. Prot. 2014, 5, 1352. [Google Scholar] [CrossRef] [Green Version]
- Calvete, T.; Lima, E.C.; Cardoso, N.F.; Vaghetti, J.C.; Dias, S.L.; Pavan, F.A. Application of carbon adsorbents prepared from Brazilian-pine fruit shell for the removal of reactive orange 16 from aqueous solution: Kinetic, equilibrium, and thermodynamic studies. J. Environ. Manag. 2010, 91, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Rubín, E.; Rodríguez, P.; Herrero, R.; Sastre de Vicente, M.E. Biosorption of phenolic compounds by the brown alga Sargassum muticum. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2006, 81, 1093–1099. [Google Scholar]
- Navarro, A.E.; Lazo, J.C.; Cuizano, N.A.; Sun-Kou, M.R.; Llanos, B.P. Insights into Removal of phenol from aqueous solutions by low cost adsorbents: Clays versus algae. Sep. Sci. Technol. 2009, 44, 2491–2509. [Google Scholar] [CrossRef]
- Shi, H.; Li, W.; Zhong, L.; Xu, C. Methylene blue adsorption from aqueous solution by magnetic cellulose/graphene oxide composite: Equilibrium, kinetics, and thermodynamics. Ind. Eng. Chem. Res. 2014, 53, 1108–1118. [Google Scholar] [CrossRef]
- Hema, M.; Arivoli, S. Comparative study on the adsorption kinetics and thermodynamics of dyes onto acid activated low cost carbon. Int. J. Phys. Sci. 2007, 2, 10–17. [Google Scholar]
- Martini, S.; Afroze, S.; Roni, K.A. Modified eucalyptus bark as a sorbent for simultaneous removal of COD, oil, and Cr (III) from industrial wastewater. Alex. Eng. J. 2020, 59, 1637–1648. [Google Scholar] [CrossRef]
- Dakhil, I.H. Acomparative Study for Removal of Dyes from Textile Effluents by Low Cost Adsorbents. Mesop. Environ. J. 2016, 1–9. [Google Scholar]
- AL-Aoh, H.A.; Yahya, R.; Jamil Maah, M.; Radzi Bin Abas, M. Adsorption of methylene blue on activated carbon fiber prepared from coconut husk: Isotherm, kinetics and thermodynamics studies. Desalination Water Treat. 2014, 52, 6720–6732. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Ghiasvand, A.; Noorimotlagh, Z. Removal of methylene blue and acid orange 7 from aqueous solutions by activated carbon coated with zinc oxide (ZnO) nanoparticles: Equilibrium, kinetic, and thermodynamic study. Desalination Water Treat. 2015, 55, 252–262. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, Z.; Wang, M.; Wu, Y. Adsorption of reactive dyes on activated carbon developed from Enteromorpha prolifera. Am. J. Anal. Chem. 2013, 04, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Abedi, S.; Zavvar Mousavi, H.; Asghari, A. Investigation of heavy metal ions adsorption by magnetically modified aloe vera leaves ash based on equilibrium, kinetic and thermodynamic studies. Desalination Water Treat. 2016, 57, 13747–13759. [Google Scholar] [CrossRef]
- Lopez, J.A.; González, F.; Bonilla, F.A.; Zambrano, G.; Gómez, M.E. Synthesis and characterization of Fe3O4 magnetic nanofluid. Rev. Latinoam. Metal. Mater. 2010, 30, 60–66. [Google Scholar]
- Peng, X.; Li, Y.; Luan, Z.; Di, Z.; Wang, H.; Tian, B.; Jia, Z. Adsorption of 1, 2-dichlorobenzene from water to carbon nanotubes. Chem. Phys. Lett. 2003, 376, 154–158. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto White Pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Singh, I.; Singh, S. Study of algal mediated biosynthesis of nanoparticle: Future of green nanotechnology. Curr. Life Sci. 2019, 5, 7–14. [Google Scholar]
- Volesky, B. Biosorption and me. Water Res. 2007, 41, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Tezer, S. Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopus arrhizus in a batch system: Effect of temperature. Process Biochem. 2000, 36, 431–439. [Google Scholar] [CrossRef]
- Sen, T.K.; Sarzali, M.V. Removal of cadmium metal ion (Cd2+) from its aqueous solution by aluminium oxide (Al2O3): A kinetic and equilibrium study. Chem. Eng. J. 2008, 142, 256–262. [Google Scholar] [CrossRef]
- Hossain, M.; Ngo, H.H.; Guo, W.; Nguyen, T. Removal of copper from water by adsorption onto banana peel as bioadsorbent. Geomate J. 2012, 2, 227–234. [Google Scholar] [CrossRef]
- Ong, P.S. Utilization of Mango Leaf as Low-Cost Adsorbent for the Removal of Cu (II) Ion from Aqueous Solution; UTAR: Kampar, Malaya, 2011. [Google Scholar]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Malachite green “a cationic dye” and its removal from aqueous solution by adsorption. Appl. Water Sci. 2017, 7, 3407–3445. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, S.; Mohammadi, L.; Rahdar, A.; Rahdar, S.; Dehghani, R.; Adaobi Igwegbe, C.; Kyzas, G.Z. Acid dye removal from aqueous solution by using neodymium (III) oxide nanoadsorbents. Nanomaterials 2020, 10, 556. [Google Scholar] [CrossRef] [Green Version]
- Vijayaraghavan, K.; Balasubramanian, R. Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J. Environ. Manag. 2015, 160, 283–296. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Gorecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue–green algae Spirulina sp.: Kinetics, equilibrium and the mechanism of the process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef]
- Berenjian, A.; Maleknia, L.; Fard, G.C.; Almasian, A. Mesoporous carboxylated Mn2O3 nanofibers: Synthesis, characterization and dye removal property. J. Taiwan Inst. Chem. Eng. 2018, 86, 57–72. [Google Scholar] [CrossRef]
- Abdelwahab, O.; Amin, N. Adsorption of phenol from aqueous solutions by Luffa cylindrica fibers: Kinetics, isotherm and thermodynamic studies. Egypt. J. Aquat. Res. 2013, 39, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Venkataraghavan, R.; Thiruchelvi, R.; Sharmila, D. Statistical optimization of textile dye effluent adsorption by Gracilaria edulis using Plackett-Burman design and response surface methodology. Heliyon 2020, 6, e05219. [Google Scholar] [CrossRef] [PubMed]
- Robati, D.; Mirza, B.; Ghazisaeidi, R.; Rajabi, M.; Moradi, O.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Adsorption behavior of methylene blue dye on nanocomposite multi-walled carbon nanotube functionalized thiol (MWCNT-SH) as new adsorbent. J. Mol. Liq. 2016, 216, 830–835. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Khanday, W.; Marrakchi, F.; Asif, M.; Hameed, B. Mesoporous zeolite–activated carbon composite from oil palm ash as an effective adsorbent for methylene blue. J. Taiwan Inst. Chem. Eng. 2017, 70, 32–41. [Google Scholar] [CrossRef]
- Caparkaya, D.; Cavas, L. Biosorption of Methylene Blue by a Brown Alga Cystoseira barbatula Kützing. Acta Chim. Slov. 2008, 55, 55144880. [Google Scholar]
- Zou, W.; Bai, H.; Gao, S.; Li, K. Characterization of modified sawdust, kinetic and equilibrium study about methylene blue adsorption in batch mode. Korean J. Chem. Eng. 2013, 30, 111–122. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Kim, I.; Han, J.-I. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohydr. Polym. 2012, 90, 1314–1322. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.-S.; Lee, D.-J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Chieng, H.I.; Lim, L.B.; Priyantha, N. Sorption characteristics of peat from Brunei Darussalam for the removal of rhodamine B dye from aqueous solution: Adsorption isotherms, thermodynamics, kinetics and regeneration studies. Desalination Water Treat. 2015, 55, 664–677. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Zhang, Y.; Tang, S.; Guo, C.; Wu, J.; Lau, R. Anionic and cationic dyes adsorption on porous poly-melamine-formaldehyde polymer. Chem. Eng. Res. Des. 2016, 114, 258–267. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Mohmmadi, L.; Ahmadi, S.; Rahdar, A.; Khadkhodaiy, D.; Dehghani, R.; Rahdar, S. Modeling of adsorption of methylene blue dye on Ho-CaWO4 nanoparticles using response surface methodology (RSM) and artificial neural network (ANN) techniques. MethodsX 2019, 6, 1779–1797. [Google Scholar] [CrossRef] [PubMed]
- Kareem, A.; Abd Alrazak, N.; Aljebori, K.H.; Aljebori, A.; Algboory, H.L.; Alkaim, A.F. Removal of methylene blue dye from aqueous solutions by using activated carbon/ureaformaldehyde composite resin as an adsorbent. Int. J. Chem. Sci. 2016, 14, 635–648. [Google Scholar]
- Mohammadi, A.; Veisi, P. High adsorption performance of β-cyclodextrin-functionalized multi-walled carbon nanotubes for the removal of organic dyes from water and industrial wastewater. J. Environ. Chem. Eng. 2018, 6, 4634–4643. [Google Scholar] [CrossRef]
- Batzias, F.; Sidiras, D.; Schroeder, E.; Weber, C. Simulation of dye adsorption on hydrolyzed wheat straw in batch and fixed-bed systems. Chem. Eng. J. 2009, 148, 459–472. [Google Scholar] [CrossRef]
- Lata, S.; Singh, P.; Samadder, S. Regeneration of adsorbents and recovery of heavy metals: A review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef] [Green Version]


| Before Adsorption | After Adsorption | |||
|---|---|---|---|---|
| Wave Number (cm−1) | Annotations | Wave Number (cm−1) | Difference (cm−1) | Annotations |
| 3279.90 | O–H group | 3729.92 | 450 | O–H group and –NH groups |
| 3111.91 | new peaks | |||
| 2959.97 & 2923.44 | CH2 group | 2923.44 | 36.5 | Aliphatic C–H group |
| 2854.48 | Asymmetric CH3 & symmetric CH2 stretching | 2853.86 | 0.61 | Asymmetric CH3 and symmetric CH2 stretching |
| 2143.56 | C=O of the carboxylic groups or ester groups | disappears | C=O of the carboxylic groups or ester groups | |
| 1656.51 | C=O, C=N, N–H or C=C groups | 1654.92 | 1.58 | C=O, C=N, N–H or C=C groups |
| 1546.33 | C=C stretching | 1542.98 | 3.34 | C=C stretching |
| 1456.35 | new peaks | C=C stretch aromatic | ||
| 1409.30 | –C=O stretches | 1403.10 | 6.19 | Sp3 C–H bend |
| 1313.08 | C–O | 1317.83 | 4.75 | |
| 1242.68 | –O–C links of the organic phosphate groups | 1242.26 | 0.41 | C–N stretch of amide or amine groups |
| 1106.10 | new peaks | |||
| 1079.08 | C–O stretching of ether groups | disappears | ||
| 864.69 | P–O, S–O, and aromatic C–H stretching | 876.95 | 12.258 | P–O, S–O, and aromatic C–H stretching |
| 701.65 | new peaks | |||
| 661.77 | –P–O, –S–O, and aromatic –CH stretching or Silicate | 659.96 | 45.33 | Aromatic sp2 C–H bend or Alkene sp2 C–H bend |
| 621.32 | 616.44 | |||
| 522.32 | 519.50 | 2.815 | ||
| 474.68 | 464.72 | 9.96 | ||
| 420.83 | ||||
| Peak No. | Wavelength (nm) | Optical Density (O.D) |
|---|---|---|
| 1 | 441.5 | 0.123 |
| 2 | 297.5 | 0.021 |
| 3 | 269.5 | 0.105 |
| 4 | 261 | 0.13 |
| 5 | 207 | 0.237 |
| Characteristics | Data | Unit |
|---|---|---|
| Specific surface area (Multipoint) | 139.837 | m2 g−1 |
| Langmuir method | 261.836 | m2 g−1 |
| BJH adsorption | 71.0792 | m2 g−1 |
| BJH desorption | 60.5881 | m2 g−1 |
| Total pore volume (Vp) | 0.131752 | cc g−1 |
| Mean pore size | 1.88438 | nm |
| Average particle radius | 9.751 | nm |
| Model | Parameter | Value |
|---|---|---|
| Pseudo-First-Order Kinetic | qe (exp.) | 0.626 |
| qe (calc.) (mg g−1) | 19.45 | |
| k1 × 103 (min−1) | 5.76 | |
| R2 | 0.169 | |
| Pseudo-Second-Order Kinetic | qe (calc.) | 0.61 |
| k2 × 103 (g mg−1 min−1) | 2283.97 | |
| R2 | 0.999 | |
| Intra-Particle Diffusion | C (mg g−1) | 0.0024 |
| Kdif (mg g−1 min1/2) | 0.645 | |
| R2 | 0.775 |
| Isotherm Model | Isotherm Parameter | Value | X2 |
|---|---|---|---|
| Langmuir | Qmax (mg g−1) | 58.82 | 11.13 |
| RL | 0.127 | ||
| b | 3.8 | ||
| R2 | 0.936 | ||
| Freundlich | n | 0.595 | 822.1 |
| 1/n | 1.682 | ||
| KF (mg1−1/nL1/ng−1) | 6.66 | ||
| R2 | 1 | ||
| Tempkin | A (L g−1) | 24.337 | 233.45 |
| B (mg L−1) | 36.091 | ||
| R2 | 0.790 |
| Adsorbent | Capacity (mg g−1) | Conditions | Ref. |
|---|---|---|---|
| Eugenia umbelliflora | 157.2 | pH 10, sorbent dosage 6 g L−1, and time 35 min, respectively | [126] |
| MWCNT–SH | 166.7 | 60 (min) 298 K pH 6 | [127] |
| MWCNT | 100 | 60 (min) 298 K pH 6 | [127] |
| Carbon nanotubes | 35.4 | 45 (min) 273 K pH 7 | [128] |
| Composite of graphene–CNT | 65.79 | 30 (min) 283 K pH 7 | [129] |
| The brown alga | 38.61 | [130] | |
| Modified saw dust | 111.46 | [131] | |
| Spent rice biomass | 8.3 | [132] | |
| Banana peel | 20.8 | pH 7.2 | [133] |
| Artocarpus odoratissimus skin | 184.6 | pH 4.6 | [134] |
| Polydopamine microspheres | 161.29 | 298 K | [135] |
| Poly-melamine-formaldehyde polymer | 80.8 | 298 K | [136] |
| Ho-CaWO4 nanoparticles | 103.09 | pH 2.03, time 15.16 min, adsorbent dosage 1.91 g | [137] |
| Steam-activated carbon produced from lantana camara Stem | 19.84 | 20 °C, 60 min, 2 g, 50 mg L−1, pH 8. | [98] |
| Activated carbon/ureaformaldehyde composite | 1.414 | T = 298 K; MB Conc. = 5 mg L−1; Shaking speed = 100 rpm; Natural pH = 6.4 | [138] |
| Activated carbon coated with zinc oxide (ZnO nanoparticles) | 66.66 | MB Conc. = 5 mg L−1, contact time = 120 min and AC–ZnO conc. = 1.5 g/L | [107] |
| MWCNTs/Gly/β-CD | 90.90 | 20 min; MB Conc. = 5 mg L−1 | [139] |
| Sargassum latifolium | 7.8 | MB Conc. = 5 mg L−1; 120 min; temperature of 50 °C | [6] |
| Hydrolyzed wheat straw | 5.8 | MB Conc. = 5 mg L−1; 5 to 15 min | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies. Materials 2022, 15, 3922. https://doi.org/10.3390/ma15113922
Mansour AT, Alprol AE, Abualnaja KM, El-Beltagi HS, Ramadan KMA, Ashour M. The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies. Materials. 2022; 15(11):3922. https://doi.org/10.3390/ma15113922
Chicago/Turabian StyleMansour, Abdallah Tageldein, Ahmed E. Alprol, Khamael M. Abualnaja, Hossam S. El-Beltagi, Khaled M. A. Ramadan, and Mohamed Ashour. 2022. "The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies" Materials 15, no. 11: 3922. https://doi.org/10.3390/ma15113922
APA StyleMansour, A. T., Alprol, A. E., Abualnaja, K. M., El-Beltagi, H. S., Ramadan, K. M. A., & Ashour, M. (2022). The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies. Materials, 15(11), 3922. https://doi.org/10.3390/ma15113922










