Recent Progress in the Removal of Legacy and Emerging Organic Contaminants from Wastewater Using Metal–Organic Frameworks: An Overview on Adsorption and Catalysis Processes
Abstract
:1. Introduction
2. Types of Environmentally Relevant MOFs
2.1. Nomenclature of MOFs
2.2. Stability of MOFs in Aquatic Environment
3. Removal of Priority and Emerging Organic Contaminants from Wastewater by Adsorption Processes Using MOFs
4. Removal of Priority and Emerging Organic Contaminants from Wastewater by Catalysis Processes Using MOFs
4.1. Fenton Processes Involving MOFs
4.2. Electrocatalysis Processes Involving MOFs
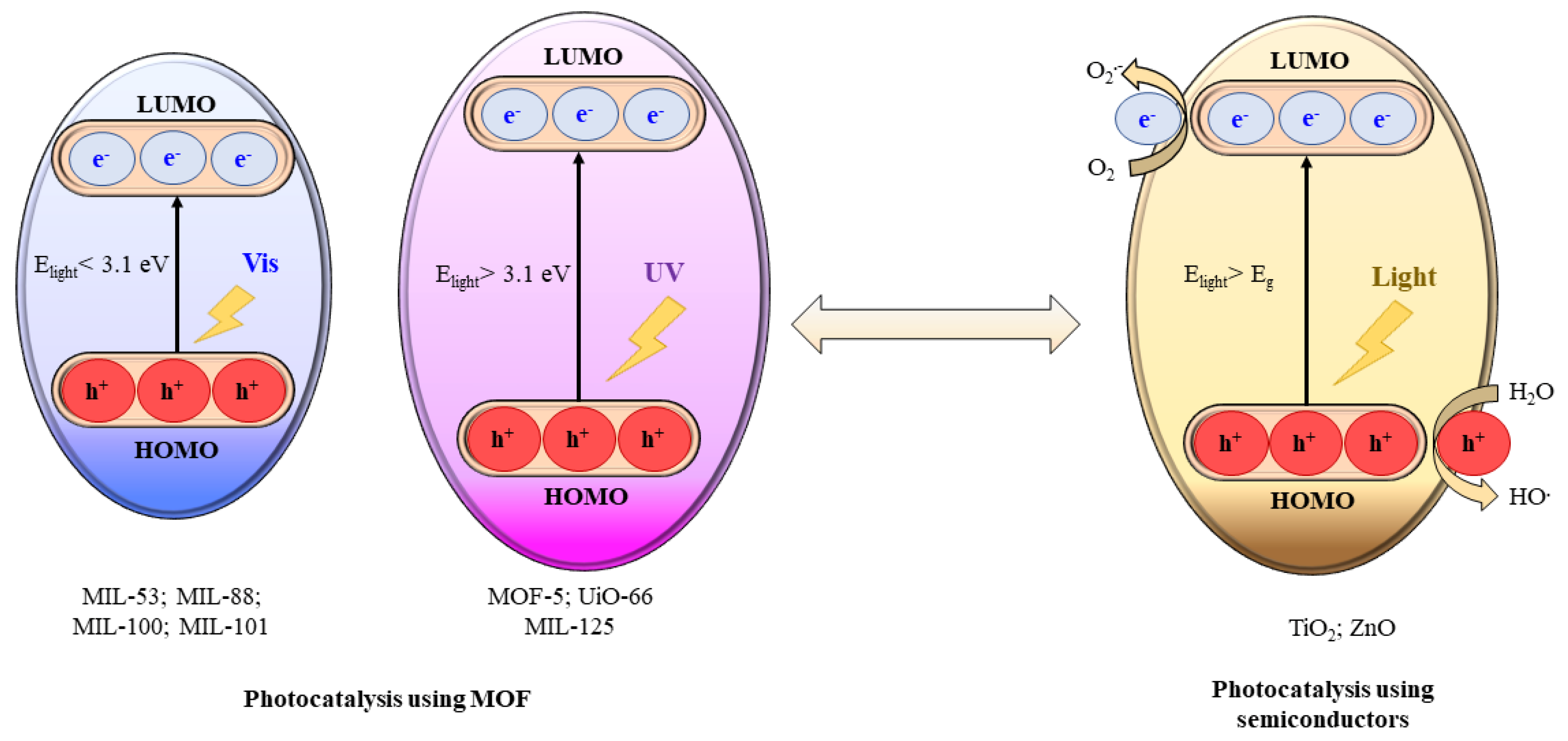
4.3. Photocatalysis Processes Involving MOFs
5. Challenges in Using MOFs for the Removal of Priority and Emerging Organic Contaminants from Wastewater
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, L.; Jun, B.-M.; Jang, M.; Park, C.M.; Muñoz-Senmache, J.C.; Hernández-Maldonado, A.J.; Heyden, A.; Yu, M.; Yoon, Y. Removal of Contaminants of Emerging Concern by Metal-Organic Framework Nanoadsorbents: A Review. Chem. Eng. J. 2019, 369, 928–946. [Google Scholar] [CrossRef]
- Sengupta, A.; Jebur, M.; Kamaz, M.; Wickramasinghe, S.R. Removal of Emerging Contaminants from Wastewater Streams Using Membrane Bioreactors: A Review. Membranes 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and Removal of Organic Micropollutants: An Overview of the Watch List of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Henneberg, A.; Köhler, H.-R.; Rault, M.; Richter, D.; Scheurer, M.; Suchail, S.; Triebskorn, R. Does Wastewater Treatment Plant Upgrading with Activated Carbon Result in an Improvement of Fish Health? Aquat. Toxicol. 2017, 192, 184–197. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of Iron-Free Fenton-like Systems for Activating H2O2 in Advanced Oxidation Processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Lee, B.C.; Lim, F.Y.; Loh, W.H.; Ong, S.L.; Hu, J. Emerging Contaminants: An Overview of Recent Trends for Their Treatment and Management Using Light-Driven Processes. Water 2021, 13, 2340. [Google Scholar] [CrossRef]
- Abbas, T.; Wadhawan, T.; Khan, A.; McEvoy, J.; Khan, E. Iron Turning Waste: Low Cost and Sustainable Permeable Reactive Barrier Media for Remediating Dieldrin, Endrin, DDT and Lindane in Groundwater. Environ. Pollut. 2021, 289, 117825. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Stevens, G.W.; Mumford, K.A. Hydrocarbon Adsorption Performance and Regeneration Stability of Diphenyldichlorosilane Coated Zeolite and Its Application in Permeable Reactive Barriers: Column Studies. Microporous Mesoporous Mater. 2020, 294, 109843. [Google Scholar] [CrossRef]
- Gholami, F.; Mosmeri, H.; Shavandi, M.; Dastgheib, S.M.M.; Amoozegar, M.A. Application of Encapsulated Magnesium Peroxide (MgO2) Nanoparticles in Permeable Reactive Barrier (PRB) for Naphthalene and Toluene Bioremediation from Groundwater. Sci. Total Environ. 2019, 655, 633–640. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Park, S.S.; Hendon, C.H.; Fielding, A.J.; Walsh, A.; O’Keeffe, M.; Dincă, M. The Organic Secondary Building Unit: Strong Intermolecular π Interactions Define Topology in MIT-25, a Mesoporous MOF with Proton-Replete Channels. J. Am. Chem. Soc. 2017, 139, 3619–3622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Pang, H.; Huang, S.; Tang, H.; Wang, S.; Qiu, M.; Chen, Z.; Yang, H.; Song, G.; Fu, D.; et al. Recent Advances in Metal-Organic Framework Membranes for Water Treatment: A Review. Sci. Total Environ. 2021, 800, 149662. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, P.; Sharma, V.K.; Ma, X.; Zhou, H.-C. Metal-Organic Frameworks for Environmental Applications. Cell Rep. Phys. Sci. 2021, 2, 100348. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A Review on Metal-Organic Frameworks: Synthesis and Applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Rezakazemi, M.; Tajahmadi, S.; Bahi, A.; Ko, F.; Aminabhavi, T.M.; Li, J.-R.; Arjmand, M. UiO-66 Metal–Organic Frameworks in Water Treatment: A Critical Review. Prog. Mater. Sci. 2022, 125, 100904. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Adegboyega, S.A.; Giwa, A.-R.A. Remediation Potentials of Composite Metal-Organic Frameworks (MOFs) for Dyes as Water Contaminants: A Comprehensive Review of Recent Literatures. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100568. [Google Scholar] [CrossRef]
- Rojas, S.; Horcajada, P. Metal–Organic Frameworks for the Removal of Emerging Organic Contaminants in Water. Chem. Rev. 2020, 120, 8378–8415. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Yuksel, N.; Kose, A.; Fellah, M.F. A DFT Investigation of Hydrogen Adsorption and Storage Properties of Mg Decorated IRMOF-16 Structure. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128510. [Google Scholar] [CrossRef]
- Xiang, H.; Ameen, A.; Shang, J.; Jiao, Y.; Gorgojo, P.; Siperstein, F.R.; Fan, X. Synthesis and Modification of Moisture-Stable Coordination Pillared-Layer Metal-Organic Framework (CPL-MOF) CPL-2 for Ethylene/Ethane Separation. Microporous Mesoporous Mater. 2020, 293, 109784. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Omary, M.A. Fluorous Metal−Organic Frameworks for High-Density Gas Adsorption. J. Am. Chem. Soc. 2007, 129, 15454–15455. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, V.; Kim, D.; Eubank, J.F.; Luebke, R.; Liu, X.; Adil, K.; Lah, M.S.; Eddaoudi, M. A Supermolecular Building Approach for the Design and Construction of Metal–Organic Frameworks. Chem. Soc. Rev. 2014, 43, 6141–6172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room Temperature Synthesis of Metal-Organic Frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Venkata Sravani, V.; Sengupta, S.; Sreenivasulu, B.; Gopakumar, G.; Tripathi, S.; Chandra, M.; Brahmmananda Rao, C.V.S.; Suresh, A.; Nagarajan, S. Highly Efficient Functionalized MOF-LIC-1 for Extraction of U(vi) and Th(Iv) from Aqueous Solution: Experimental and Theoretical Studies. Dalton Trans. 2022, 51, 3557–3571. [Google Scholar] [CrossRef]
- Bergaoui, M.; Khalfaoui, M.; Awadallah-F, A.; Al-Muhtaseb, S. A Review of the Features and Applications of ZIF-8 and Its Derivatives for Separating CO2 and Isomers of C3- and C4- Hydrocarbons. J. Nat. Gas Sci. Eng. 2021, 96, 104289. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Q.; Huang, A. A Superhydrophobic Zeolitic Imidazolate Framework (ZIF-90) with High Steam Stability for Efficient Recovery of Bioalcohols. Chem. Commun. 2016, 52, 3400–3402. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Cu2[o-Br-C6H3(CO2)2]2(H2O)2·(DMF)8(H2O)2: A Framework Deliberately Designed to Have the NbO Structure Type. J. Am. Chem. Soc. 2002, 124, 376–377. [Google Scholar] [CrossRef]
- Li, Y.; Hou, G.; Yang, J.; Xie, J.; Yuan, X.; Yang, H.; Wang, M. Facile Synthesis of MOF 235 and Its Superior Photocatalytic Capability under Visible Light Irradiation. RSC Adv. 2016, 6, 16395–16403. [Google Scholar] [CrossRef]
- Valvekens, P.; Bloch, E.D.; Long, J.R.; Ameloot, R.; De Vos, D.E. Counteranion Effects on the Catalytic Activity of Copper Salts Immobilized on the 2,2′-Bipyridine-Functionalized Metal–Organic Framework MOF-253. Catal. Today 2015, 246, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Kaur, G.; Øien-Ødegaard, S.; Lazzarini, A.; Chavan, S.M.; Bordiga, S.; Lillerud, K.P.; Olsbye, U. Controlling the Synthesis of Metal–Organic Framework UiO-67 by Tuning Its Kinetic Driving Force. Cryst. Growth Des. 2019, 19, 4246–4251. [Google Scholar] [CrossRef]
- Ye, X.; Liu, D. Metal–Organic Framework UiO-68 and Its Derivatives with Sufficiently Good Properties and Performance Show Promising Prospects in Potential Industrial Applications. Cryst. Growth Des. 2021, 21, 4780–4804. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Le, H.T.N.; Do, T.S.; Pham, V.T.; Lam Tran, D.; Ho, V.T.T.; Tran, T.V.; Nguyen, D.C.; Nguyen, T.D.; Bach, L.G.; et al. Metal-Organic Framework MIL-53(Fe) as an Adsorbent for Ibuprofen Drug Removal from Aqueous Solutions: Response Surface Modeling and Optimization. J. Chem. 2019, 2019, 5602957. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Yang, Z.; Li, X.; Hou, L.; Alhassan, S.I.; Wang, H. Synthesis of Hierarchical Hollow MIL-53(Al)-NH2 as an Adsorbent for Removing Fluoride: Experimental and Theoretical Perspective. Environ. Sci. Pollut. Res. 2021, 28, 6886–6897. [Google Scholar] [CrossRef]
- Andrew Lin, K.-Y.; Chang, H.-A.; Hsu, C.-J. Iron-Based Metal Organic Framework, MIL-88A, as a Heterogeneous Persulfate Catalyst for Decolorization of Rhodamine B in Water. RSC Adv. 2015, 5, 32520–32530. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Hanif Abu Bakar, N.H.; Fathihah Abdullah, N.A.; Basheer, C.; Saad, B. Removal of Anthracene in Water by MIL-88(Fe), NH2-MIL-88(Fe), and Mixed-MIL-88(Fe) Metal–Organic Frameworks. RSC Adv. 2019, 9, 41490–41501. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, P.; Salles, F.; Wuttke, S.; Devic, T.; Heurtaux, D.; Maurin, G.; Vimont, A.; Daturi, M.; David, O.; Magnier, E.; et al. How Linker’s Modification Controls Swelling Properties of Highly Flexible Iron(III) Dicarboxylates MIL-88. J. Am. Chem. Soc. 2011, 133, 17839–17847. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Alinia Asli, M.; Vossoughi, M. Metal-Organic Framework (MIL-100 (Fe)): Synthesis, Detailed Photocatalytic Dye Degradation Ability in Colored Textile Wastewater and Recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Du, P.D.; Thanh, H.T.M.; To, T.C.; Thang, H.S.; Tinh, M.X.; Tuyen, T.N.; Hoa, T.T.; Khieu, D.Q. Metal-Organic Framework MIL-101: Synthesis and Photocatalytic Degradation of Remazol Black B Dye. J. Nanomater. 2019, 2019, 6061275. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF Stability: Approaches and Applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef] [Green Version]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal–Organic Framework (MOF) Compounds: Photocatalysts for Redox Reactions and Solar Fuel Production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molavi, H.; Hakimian, A.; Shojaei, A.; Raeiszadeh, M. Selective Dye Adsorption by Highly Water Stable Metal-Organic Framework: Long Term Stability Analysis in Aqueous Media. Appl. Surf. Sci. 2018, 445, 424–436. [Google Scholar] [CrossRef]
- Li, Z.-G.; Li, K.; Dong, L.-Y.; Guo, T.-M.; Azeem, M.; Li, W.; Bu, X.-H. Acoustic Properties of Metal-Organic Frameworks. Research 2021, 2021, 9850151. [Google Scholar] [CrossRef] [PubMed]
- Zeleňák, V.; Saldan, I. Factors Affecting Hydrogen Adsorption in Metal–Organic Frameworks: A Short Review. Nanomaterials 2021, 11, 1638. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Jia, X.; Li, G.; An, T.; Gao, Y. Novel Approach for Removing Brominated Flame Retardant from Aquatic Environments Using Cu/Fe-Based Metal-Organic Frameworks: A Case of Hexabromocyclododecane (HBCD). Sci. Total Environ. 2018, 621, 1533–1541. [Google Scholar] [CrossRef]
- Su, H.; Lv, J.; Yang, L.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Rapid and Selective Adsorption of a Typical Aromatic Organophosphorus Flame Retardant on MIL-101-Based Metal–Organic Frameworks. RSC Adv. 2020, 10, 2198–2208. [Google Scholar] [CrossRef] [Green Version]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Abu Bakar, N.H.H.; Saad, B. Removal of Pyrene from Aqueous Solution Using Fe-Based Metal-Organic Frameworks. IOP Conf. Ser. Earth Environ. Sci. 2020, 549, 12061. [Google Scholar] [CrossRef]
- Mohd Azmi, L.H.; Williams, D.; Ladewig, B.P. Can Metal Organic Frameworks Outperform Adsorptive Removal of Harmful Phenolic Compound 2-Chlorophenol by Activated Carbon? Chem. Eng. Res. Des. 2020, 158, 102–113. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, H.; Cao, T.; Qian, L.; Wang, Y.; Zhao, G. Efficient Degradation of High Concentration Azo-Dye Wastewater by Heterogeneous Fenton Process with Iron-Based Metal-Organic Framework. J. Mol. Catal. A Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, M.; Li, K.; Zuo, Y.; Han, Y.; Wang, J.; Song, C.; Zhang, G.; Guo, X. Facile Synthesis of Fe-Containing Metal–Organic Frameworks as Highly Efficient Catalysts for Degradation of Phenol at Neutral PH and Ambient Temperature. CrystEngComm 2015, 17, 7160–7168. [Google Scholar] [CrossRef]
- Gao, C.; Chen, S.; Quan, X.; Yu, H.; Zhang, Y. Enhanced Fenton-like Catalysis by Iron-Based Metal Organic Frameworks for Degradation of Organic Pollutants. J. Catal. 2017, 356, 125–132. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, D.; Chen, Y.; Hu, Y. Enhanced Degradation of Triclosan in Heterogeneous E-Fenton Process with MOF-Derived Hierarchical Mn/Fe@PC Modified Cathode. Chem. Eng. J. 2020, 384, 123324. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Zeng, T.; Shang, Q.; Zhou, H.; Pan, Z.; Cheng, Q. Two Pure MOF-Photocatalysts Readily Prepared for the Degradation of Methylene Blue Dye under Visible Light. Dalton Trans. 2018, 47, 4251–4258. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, Y.-G.; Ma, Y.-L. Highly Stable Mn-Doped Metal–Organic Framework Fenton-Like Catalyst for the Removal of Wastewater Organic Pollutants at All Light Levels. ACS Omega 2021, 6, 2949–2955. [Google Scholar] [CrossRef]
- Xu, J.; Shimakoshi, H.; Hisaeda, Y. Development of Metal-Organic Framework (MOF)-B12 System as New Bio-Inspired Heterogeneous Catalyst. J. Organomet. Chem. 2015, 782, 89–95. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Ramli, A.; Abu Bakar, N.H.; Saad, B.; Rozaini, M.N.; Isiyaka, H.A.; Jagaba, A.H.; Aldaghri, O.; et al. A Critical Review on Metal-Organic Frameworks and Their Composites as Advanced Materials for Adsorption and Photocatalytic Degradation of Emerging Organic Pollutants from Wastewater. Polymers 2020, 12, 2648. [Google Scholar] [CrossRef]
- Zhu, D.; Hyun, S.; Pignatello, J.J.; Lee, L.S. Evidence for Π−π Electron Donor−Acceptor Interactions between π-Donor Aromatic Compounds and π-Acceptor Sites in Soil Organic Matter through PH Effects on Sorption. Environ. Sci. Technol. 2004, 38, 4361–4368. [Google Scholar] [CrossRef]
- Badea, S.-L.; Mustafa, M.; Lundstedt, S.; Tysklind, M. Leachability and Desorption of PCBs from Soil and Their Dependency on pH and Dissolved Organic Matter. Sci. Total Environ. 2014, 499, 220–227. [Google Scholar] [CrossRef]
- Wang, B.; Wang, P.; Xie, L.-H.; Lin, R.-B.; Lv, J.; Li, J.-R.; Chen, B. A Stable Zirconium Based Metal-Organic Framework for Specific Recognition of Representative Polychlorinated Dibenzo-p-Dioxin Molecules. Nat. Commun. 2019, 10, 3861. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, G.; Shen, J.; Wang, M.; Yang, Q.; Zheng, M. Environmental Characteristics and Formations of Polybrominated Dibenzo-p-Dioxins and Dibenzofurans. Environ. Int. 2021, 152, 106450. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Feng, M. Water Depollution Using Metal-Organic Frameworks-Catalyzed Advanced Oxidation Processes: A Review. J. Hazard. Mater. 2019, 372, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, S.; Yu, Z.; Zhang, L.; Wang, D.; Pang, B.; Sun, W. Application of Fe-MOFs in Advanced Oxidation Processes. Res. Chem. Intermed. 2019, 45, 3777–3793. [Google Scholar] [CrossRef]
- Martínez, F.; Leo, P.; Orcajo, G.; Díaz-García, M.; Sanchez-Sanchez, M.; Calleja, G. Sustainable Fe-BTC Catalyst for Efficient Removal of Mehylene Blue by Advanced Fenton Oxidation. Catal. Today 2018, 313, 6–11. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Matin, M. Hydroxylation of Phenol Catalyzed by Iron Metal-Organic Framework (Fe-BTC) with Hydrogen Peroxide. J. Mater. Sci. Chem. Eng. 2020, 8, 55–64. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Liu, Z.; Wang, R.; Liu, H. Fe-Based MOFs for Efficient Adsorption and Degradation of Acid Orange 7 in Aqueous Solution via Persulfate Activation. Appl. Surf. Sci. 2016, 369, 130–136. [Google Scholar] [CrossRef]
- Xue, W.; Zhou, Q.; Li, F. The Feasibility of Typical Metal–Organic Framework Derived Fe, Co, N Co-Doped Carbon as a Robust Electrocatalyst for Oxygen Reduction Reaction in Microbial Fuel Cell. Electrochim. Acta 2020, 355, 136775. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Wang, S.; Liu, T.; Jia, B.; Liu, W.; Dong, P. Metal-Organic Framework-Derived Iron Oxide Modified Carbon Cloth as a High-Power Density Microbial Fuel Cell Anode. J. Clean. Prod. 2022, 341, 130725. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent Advances in MOF-Based Photocatalysis: Environmental Remediation under Visible Light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Salazar-Aguilar, A.D.; Vega, G.; Casas, J.A.; Vega-Díaz, S.M.; Tristan, F.; Meneses-Rodríguez, D.; Belmonte, M.; Quintanilla, A. Direct Hydroxylation of Phenol to Dihydroxybenzenes by H2O2 and Fe-Based Metal-Organic Framework Catalyst at Room Temperature. Catalysts 2020, 10, 172. [Google Scholar] [CrossRef] [Green Version]
- Acero, J.L.; Gunten, U. von Influence of Carbonate on the Ozone/Hydrogen Peroxide Based Advanced Oxidation Process for Drinking Water Treatment. Ozone Sci. Eng. 2000, 22, 305–328. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, H. A Comprehensive Kinetic Model for Phenol Oxidation in Seven Advanced Oxidation Processes and Considering the Effects of Halides and Carbonate. Water Res. X 2022, 14, 100129. [Google Scholar] [CrossRef] [PubMed]
- Tufail, A.; Price, W.E.; Hai, F.I. A Critical Review on Advanced Oxidation Processes for the Removal of Trace Organic Contaminants: A Voyage from Individual to Integrated Processes. Chemosphere 2020, 260, 127460. [Google Scholar] [CrossRef] [PubMed]
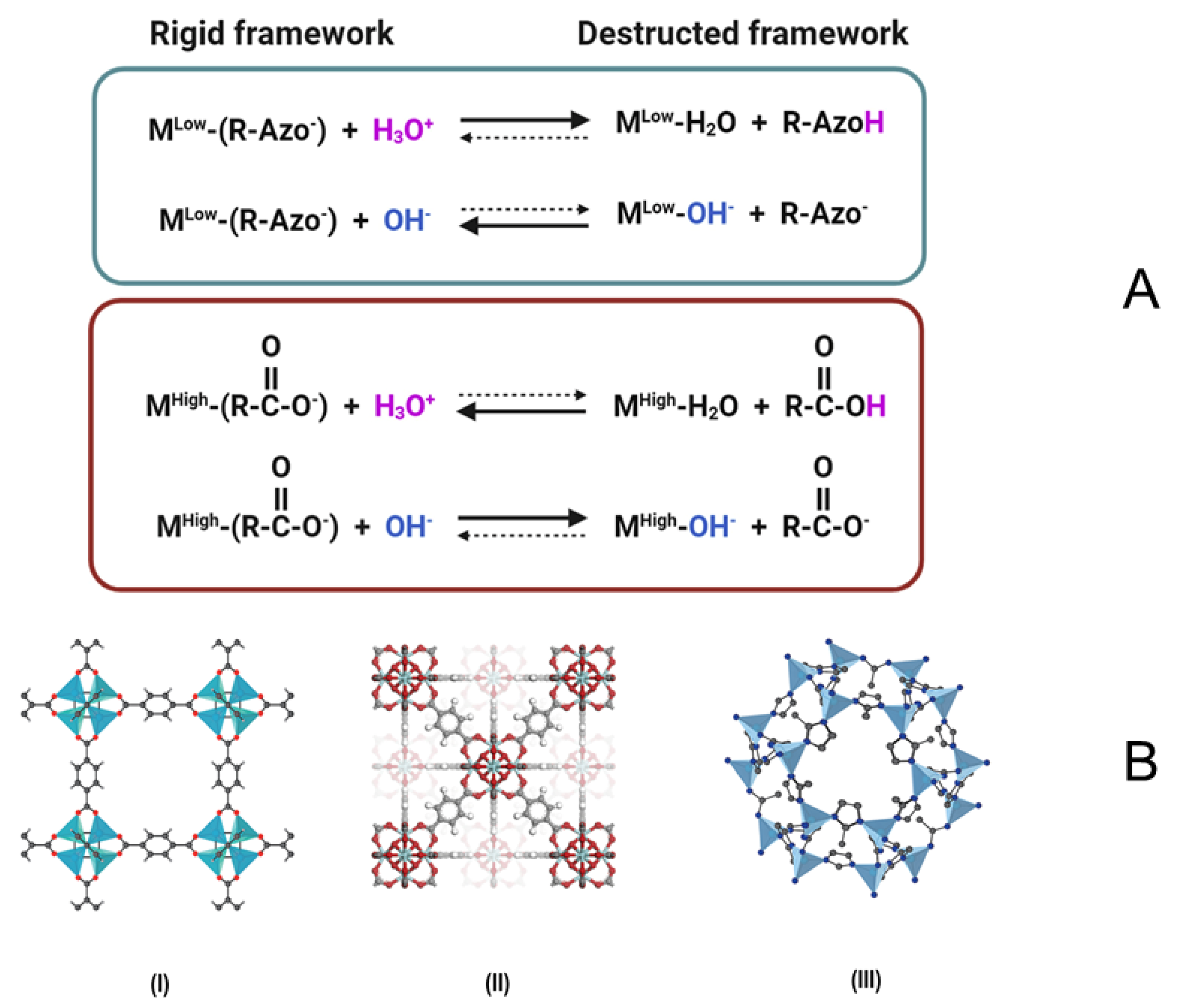
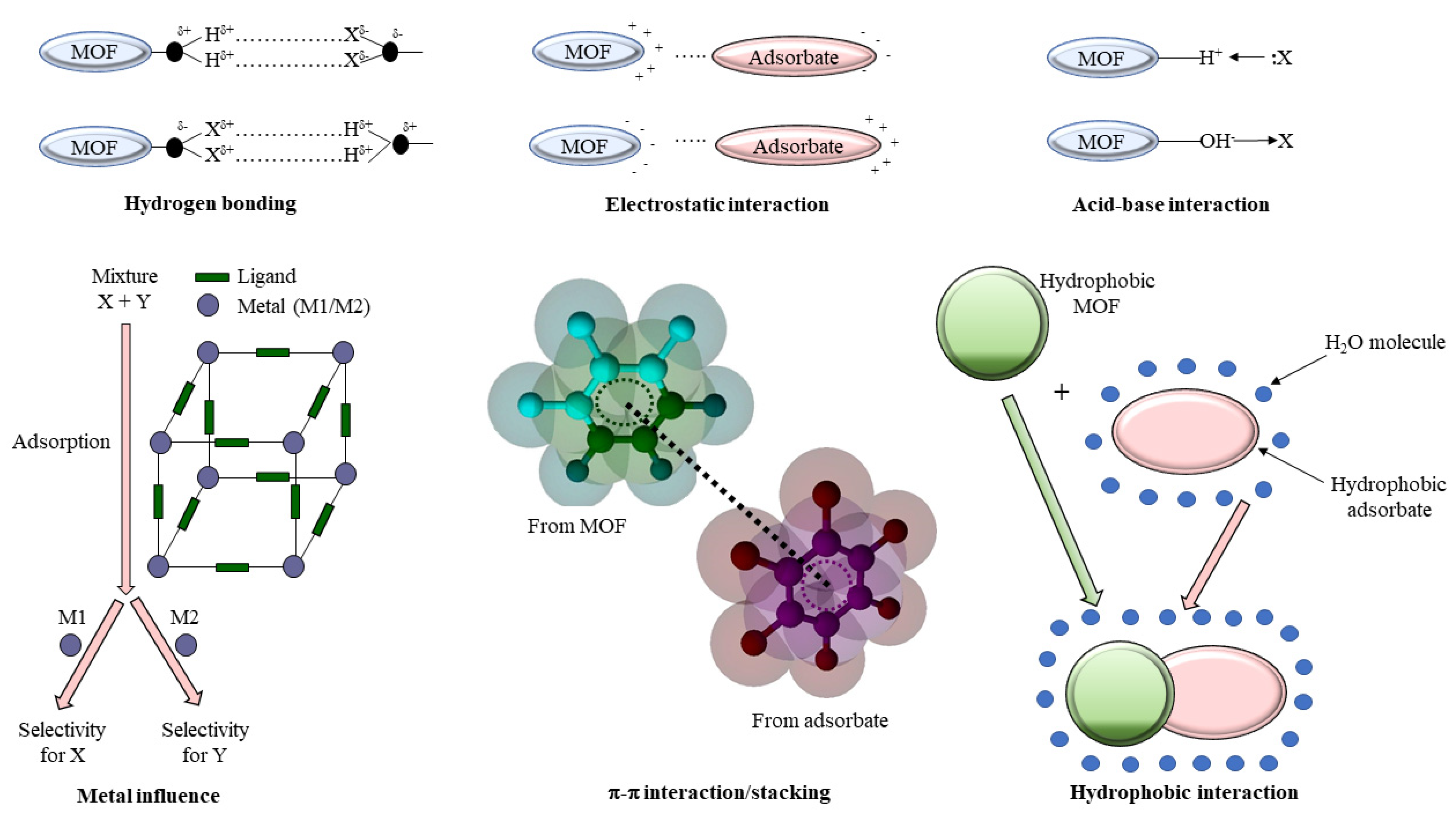
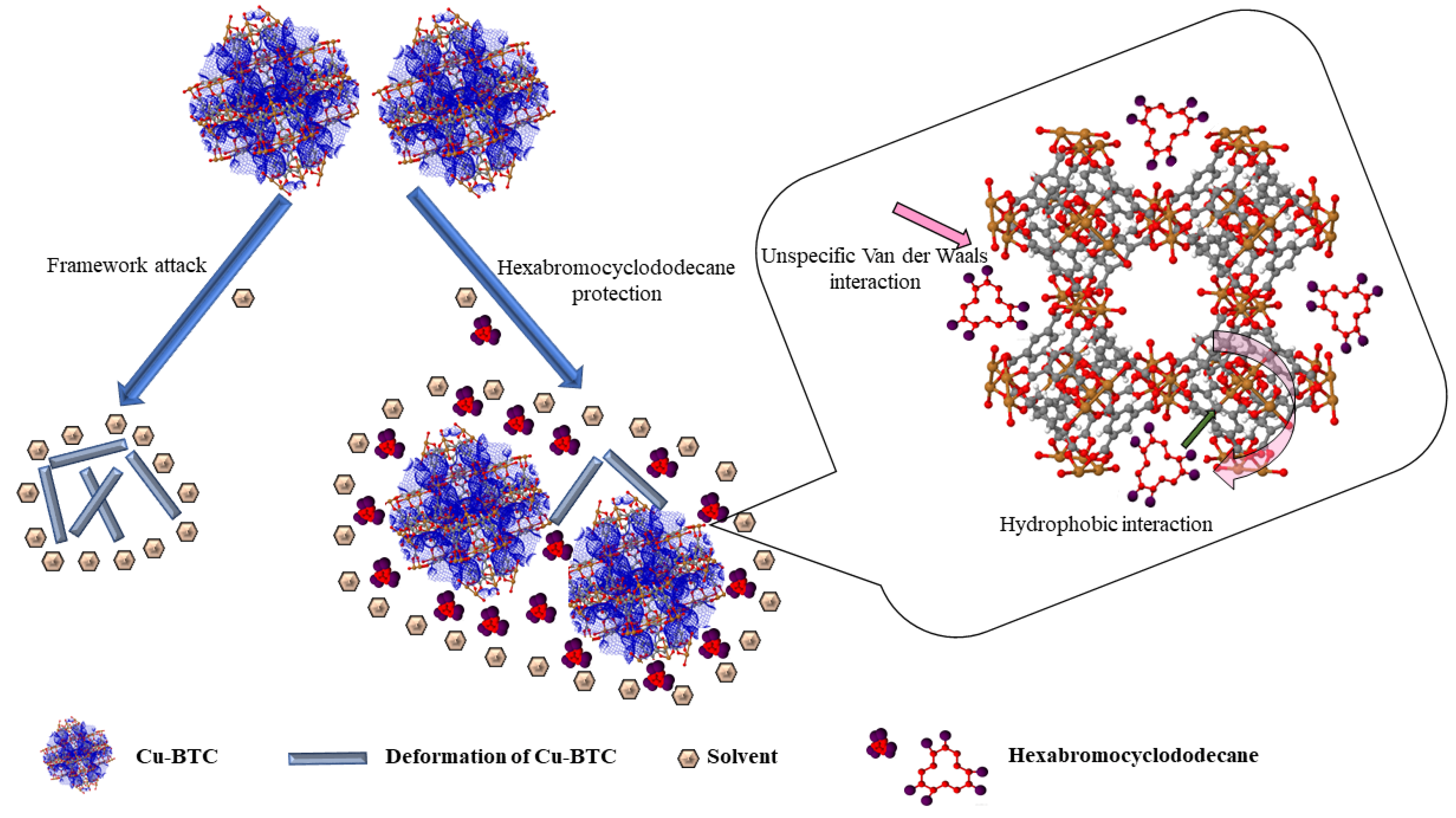
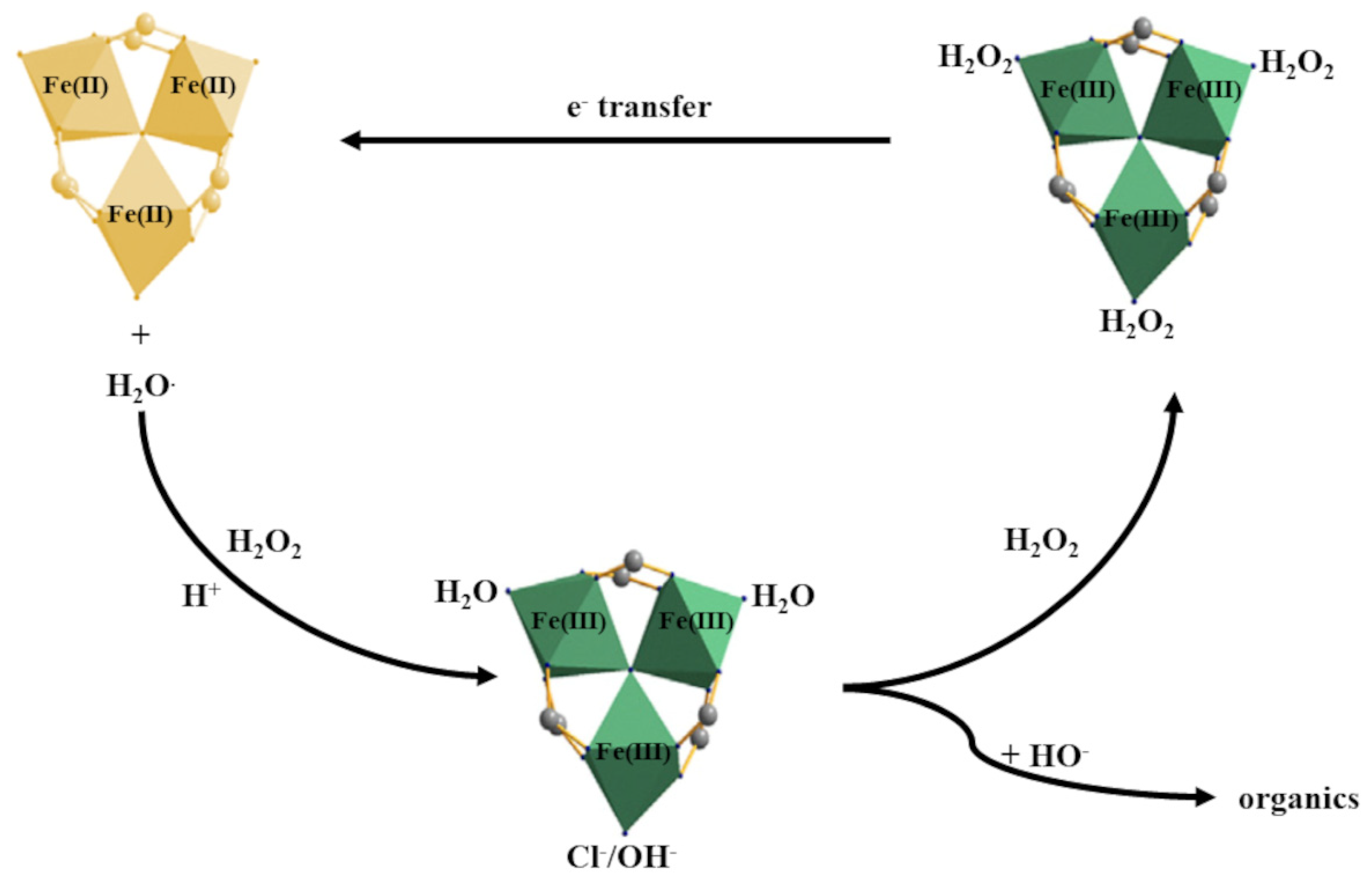
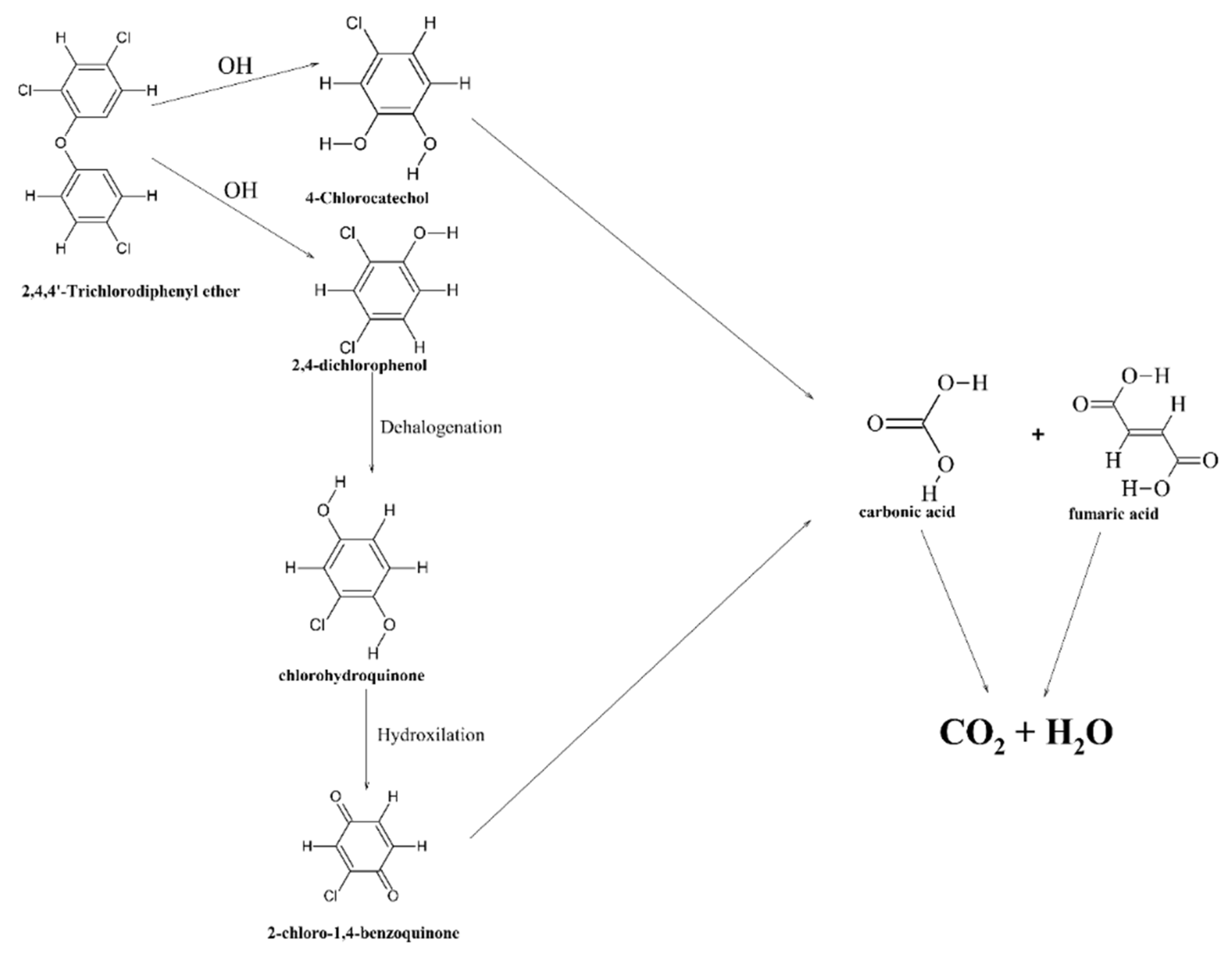
| No. | Name | Molecular Formula | Reference | Abbreviation Legend |
|---|---|---|---|---|
| 1 | IRMOF-1 or MOF-5 | Zn4O(BDC)3. 7DEF.3H2O | [18] | IsoReticular metal–organic frameworks |
| 2 | IRMOF-16 | Zn4O(TPDC)3. 17DEF.2H2O | [19] | |
| 3 | CPL-2 | Cu2(PZDC)2(4,4′-BPY) | [20] | Coordination polymers with a pillared layer structure |
| 4 | F-MOF-1 | [Cu(HFBBA)(phen)2](H2HFBBA)2(H2O)(HCO2) | [21] | Fluorinated metal–organic framework |
| 5 | MOP-1 | Cu24(m-BDC)24(DMF)14(H2O)10 | [22] | Metal–organic polyhedra |
| 6 | HKUST-1 (MOF-199) | Cu3(BTC)2 | [23] | Hong Kong University of Science and Technology |
| 7 | LIC-1 | Gd2(BDC-NH2)3(DMF)4 | [24] | Leiden Institute of Chemistry |
| 8 | ZIF-8 | Zn(MIM)2 | [25] | Zeolite imidazolate framework |
| 9 | ZIF-90 | Zn(FIM)2 | [26] | |
| 10 | MOF-74 | Zn2DOT | [23] | Metal–organic frameworks |
| 11 | MOF-101 | Cu2(BDC-Br)2(H2O)2 | [27] | |
| 12 | MOF-177 | Zn4O(BTB)2 | [23] | |
| 13 | MOF-235 | [Fe3O(BDC)3(DMF)3][FeCl4].(DMF)3 | [28] | |
| 14 | MOF-253 | Al(OH)(BPYDC) | [29] | |
| 15 | UiO-66 | Zr6O6(BDC)6 | [30] | Universitetet i Oslo |
| 16 | UiO-67 | Zr6O6(BPDC)6 | [31] | |
| 17 | UiO-68 | Zr6O6(TPDC)6 | [32] | |
| 18 | MIL-53 | Al(OH)(BDC) | [33] | Materials of Institut Lavoisier |
| 19 | MIL-53(Al)-NH2 | Al(OH)(BDC-NH2) | [34] | |
| 20 | MIL-88A | Fe3O(MeOH)3(O2CCH=CHCO2)3.MeCO2.nH2O | [35] | |
| 21 | MIL-88-Fe | Fe3O(MeOH)3(O2C(CH2)2CO2)3. AcO.(MeOH)4.5 | [36] | |
| 22 | MIL-88B-4CH3 | 2Fe3O(OH)(H2O)2(BDC-Me2)3 | [37] | |
| 23 | MIL-100-Fe | FeIII3 O(H2O)2F.(BTC)2. nH2O | [38] | |
| 24 | MIL-101 | Cr3O(H2O)2F.(BDC)3. nH2O | [39] |
| No. | Name of MOF | Target Contaminant | Reference | Type of Process | Performance of Adsorption/Catalysis Processes |
|---|---|---|---|---|---|
| 1 | Cu-BTC and Fe-BTC | HBCD | [46] | Adsorption | Over 80% of HBCD removed by Cu-BTC |
| 2 | Cr-MIL-101 and Fe-MIL-101-NH2 | TPhP | [47] | Adsorption | Removal efficency of 90.2% by Cr-MIL-101 |
| 3 | MIL-88(Fe) and NH2-MIL-88(Fe) | Pyrine | [48] | Adsorption | Removal efficency of 96.0% for NH2-MIL-88(Fe) and 99.7% for MIL-88(Fe)) |
| 4 | MIL-101 and MIL-101-NH2, | 2-chlorophenol (2-CP) | [49] | Adsorption | Removal efficiency of 60% on MIL-101 |
| 5 | MIL-100(Fe) and FeII@MIL-100(Fe) | Methylene blue | [50] | Fenton | Removal efficiency of around 96% -for MIL-100(Fe) and and 90% FeII@MIL-100(Fe) |
| 6 | MIL-53(Fe) | Phenol | [51] | Fenton | 90% degradation |
| 7 | MIL-88B-Fe | Phenol | [52] | Fenton | 99% degradation |
| 8 | Mn-doped MIL-53(Fe) | TCS | [53] | Electrocatalysis | Removal efficency of 99.9 ± 0.1% |
| 9 | Cu(4,4′-bipy)Cl]n (1) and [Co(4,4′-bipy)·(HCOO)2]n (2) | Methylene blue | [54] | Photocatalysis | Removal efficiency of 83.18% for Cu(4,4′-bipy)Cl]n (1) and of 67.83% [Co(4,4′-bipy)·(HCOO)2]n (2) |
| 10 | Mn-doped MIL-88-Fe | Phenol | [55] | Photocatalysis | Removal efficency of 96% |
| 11 | B12–Ru@[Zn4Ru2(bpdc)4·4C2NH8·9DMF]n | DDT | [56] | Photocatalysis | Transformation yield of 99% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badea, S.-L.; Niculescu, V.-C. Recent Progress in the Removal of Legacy and Emerging Organic Contaminants from Wastewater Using Metal–Organic Frameworks: An Overview on Adsorption and Catalysis Processes. Materials 2022, 15, 3850. https://doi.org/10.3390/ma15113850
Badea S-L, Niculescu V-C. Recent Progress in the Removal of Legacy and Emerging Organic Contaminants from Wastewater Using Metal–Organic Frameworks: An Overview on Adsorption and Catalysis Processes. Materials. 2022; 15(11):3850. https://doi.org/10.3390/ma15113850
Chicago/Turabian StyleBadea, Silviu-Laurentiu, and Violeta-Carolina Niculescu. 2022. "Recent Progress in the Removal of Legacy and Emerging Organic Contaminants from Wastewater Using Metal–Organic Frameworks: An Overview on Adsorption and Catalysis Processes" Materials 15, no. 11: 3850. https://doi.org/10.3390/ma15113850
APA StyleBadea, S.-L., & Niculescu, V.-C. (2022). Recent Progress in the Removal of Legacy and Emerging Organic Contaminants from Wastewater Using Metal–Organic Frameworks: An Overview on Adsorption and Catalysis Processes. Materials, 15(11), 3850. https://doi.org/10.3390/ma15113850







