CdS-Decorated Porous Anodic SnOx Photoanodes with Enhanced Performance under Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nanoporous SnOx Layers
2.2. Deposition of Cadmium Sulfide
2.3. Characterization of the Obtained Materials
2.4. Photoelectrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2012, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Babu, V.J.; Ramakrishna, S. Photoelectrode nanomaterials for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2017, 42, 11078–11109. [Google Scholar] [CrossRef]
- Zhao, Y.; Hoivik, N.; Wang, K. Recent advance on engineering titanium dioxide nanotubes for photochemical and photoelectrochemical water splitting. Nano Energy 2016, 30, 728–744. [Google Scholar] [CrossRef]
- Sołtys-Mróz, M.; Syrek, K.; Wiercigroch, E.; Małek, K.; Rokosz, K.; Raaen, S.; Sulka, G.D. Enhanced visible light photoelectrochemical water splitting using nanotubular FeOx-TiO2 annealed at different temperatures. J. Power Sources 2021, 507, 230274. [Google Scholar] [CrossRef]
- Huang, M.-C.; Wang, T.; Wu, B.-J.; Lin, J.-C.; Wu, C.-C. Anodized ZnO nanostructures for photoelectrochemical water splitting. Appl. Surf. Sci. 2016, 360, 442–450. [Google Scholar] [CrossRef]
- Mika, K.; Syrek, K.; Uchacz, T.; Sulka, G.D.; Zaraska, L. Dark nanostructured ZnO films formed by anodic oxidation as photoanodes in photoelectrochemical water splitting. Electrochim. Acta 2022, 414, 140176. [Google Scholar] [CrossRef]
- Fernández-Domene, R.; Sánchez-Tovar, R.; Lucas-Granados, B.; Roselló-Márquez, G.; Garcia-Anton, J. A simple method to fabricate high-performance nanostructured WO3 photocatalysts with adjusted morphology in the presence of complexing agents. Mater. Des. 2017, 116, 160–170. [Google Scholar] [CrossRef]
- Zych, M.; Syrek, K.; Pisarek, M.; Sulka, G.D. Synthesis and characterization of anodic WO3 layers in situ doped with C, N during anodization. Electrochim. Acta 2022, 411, 140061. [Google Scholar] [CrossRef]
- Deng, H.; Huang, M.-C.; Weng, W.-H.; Lin, J.-C. Iron oxide nanotube film formed on carbon steel for photoelectrochemical water splitting: Effect of annealing temperature. Surf. Interface Anal. 2016, 48, 1278–1284. [Google Scholar] [CrossRef]
- Syrek, K.; Kemona, S.; Czopor, J.; Zaraska, L.; Sulka, G.D. Photoelectrochemical properties of anodic iron oxide layers. J. Electroanal. Chem. 2022, 909, 116143. [Google Scholar] [CrossRef]
- Palacios-Padrós, A.; Altomare, M.; Lee, K.; Díez-Pérez, I.; Sanz, F.; Schmuki, P. Controlled thermal annealing tunes the photoelectrochemical properties of nanochanneled tin-oxide structures. ChemElectroChem 2014, 1, 1133–1137. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Gurgul, M.; Chlebda, D.K.; Socha, R.; Sulka, G.D. Controlled synthesis of nanoporous tin oxide layers with various pore diameters and their photoelectrochemical properties. Electrochim. Acta 2017, 254, 238–245. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Wiercigroch, E.; Malek, K.; Kozieł, M.; Andrzejczuk, M.; Marzec, M.M.; Jarosz, M.; Brzozka, A.; Sulka, G.D. The effect of anodizing potential and annealing conditions on the morphology, composition and photoelectrochemical activity of porous anodic tin oxide films. Electrochim. Acta 2019, 319, 18–30. [Google Scholar] [CrossRef]
- Zaraska, L.; Gilek, D.; Gawlak, K.; Jaskuła, M.; Sulka, G.D. Formation of crack-free nanoporous tin oxide layers via simple one-step anodic oxidation in NaOH at low applied voltages. Appl. Surf. Sci. 2016, 390, 31–37. [Google Scholar] [CrossRef]
- Gurgul, M.; Gawlak, K.; Syrek, K.; Kozieł, M.; Sulka, G.D.; Zaraska, L. The influence of water-induced crystallization on the photoelectrochemical properties of porous anodic tin oxide films. J. Ind. Eng. Chem. 2020, 90, 159–165. [Google Scholar] [CrossRef]
- Gurgul, M.; Kozieł, M.; Zaraska, L. Tuning the photoelectrochemical properties of narrow band gap nanoporous anodic SnOx films by simple soaking in water. Materials 2021, 14, 1777. [Google Scholar] [CrossRef]
- Wang, L.; Palacios-Padros, A.; Kirchgeorg, R.; Tighineanu, A.; Schmuki, P. Enhanced photoelectrochemical water splitting efficiency of a hematite–ordered Sb:SnO2 host–guest system. ChemSusChem 2014, 7, 421–424. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Mane, R.S.; Joo, O.-S. Spraying distance and titanium chloride surface treatment effects on DSSC performance of electrosprayed SnO2 photoanodes. RSC Adv. 2014, 4, 35919–35927. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Mane, R.S.; Joo, O.-S. La2O3-encapsulated SnO2 nanocrystallite-based photoanodes for enhanced DSSCs performance. Dalton Trans. 2015, 44, 3075–3081. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, C.; Wu, Z.; Han, W.; Wang, Y.; Fu, W.; Li, X.; Xie, E. Toward efficient photoelectrochemical water-splitting by using screw-like SnO2 nanostructures as photoanode after being decorated with CdS quantum dots. Nano Energy 2016, 19, 318–327. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Tian, B.; Zhang, J. Core–shell structural CdS@SnO2 nanorods with excellent visible-light photocatalytic activity for the selective oxidation of benzyl alcohol to benzaldehyde. ACS Appl. Mater. Interfaces 2015, 7, 13849–13858. [Google Scholar] [CrossRef] [PubMed]
- Memarian, N.; Rozati, S.M.; Concina, I.; Vomiero, A. Deposition of nanostructured CdS thin films by thermal evaporation method: Effect of substrate temperature. Materials 2017, 10, 773. [Google Scholar] [CrossRef]
- Rubio, S.; Plaza, J.L.; Diéguez, E. Influence of CdS deposition technique for CdS/CdTe solar cells applications. J. Cryst. Growth 2014, 401, 550–553. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, W.; Yang, H.; Li, Y.; Chen, Y.; Sun, M.; Ma, J.; Yang, L.; Zhao, B.; Tian, L. CdS quantum dots sensitized SnO2 photoelectrode for photoelectrochemical application. Electrochim. Acta 2013, 89, 510–515. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Li, B.; Li, R.; Gao, L.; Zhang, S.; Zhang, M.; Mu, J.; Zhang, X. CdS/CdSe nanoparticles co-deposited SnO2(TiO2) spherical structure film for photoelectrochemical application. Mater. Lett. 2018, 239, 59–62. [Google Scholar] [CrossRef]
- Zaraska, L.; Bobruk, M.; Sulka, G.D. Formation of nanoporous tin oxide layers on different substrates during anodic oxidation in oxalic acid electrolyte. Adv. Condens. Matter Phys. 2015, 2015, 302560. [Google Scholar] [CrossRef] [Green Version]
- Barreca, D.; Garon, S.; Tondello, E.; Zanella, P. SnO2 nanocrystalline thin films by XPS. Surf. Sci. Spectra 2000, 7, 81–85. [Google Scholar] [CrossRef]
- Kwoka, M.; Ottaviano, L.; Passacantando, M.; Santucci, S.; Czempik, G.; Szuber, J. XPS study of the surface chemistry of L-CVD SnO2 thin films after oxidation. Thin Solid Films 2005, 490, 36–42. [Google Scholar] [CrossRef]
- Turishchev, S.; Chuvenkova, O.; Parinova, E.; Koyuda, D.; Chumakov, R.; Presselt, M.; Schleusener, A.; Sivakov, V. XPS investigations of MOCVD tin oxide thin layers on Si nanowires array. Results Phys. 2018, 11, 507–509. [Google Scholar] [CrossRef]
- Park, B.-E.; Park, J.; Lee, S.; Lee, S.; Kim, W.-H.; Kim, H. Phase-controlled synthesis of SnOx thin films by atomic layer deposition and post-treatment. Appl. Surf. Sci. 2019, 480, 472–477. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.W.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Barreca, D.; Gasparotto, A.; Maragno, C.; Tondello, E. Nanostructured cadmium sulfide thin films by XPS. Surf. Sci. Spectra 2002, 9, 46–53. [Google Scholar] [CrossRef]
- Stoev, M.; Katerski, A. XPS and XRD study of photoconductive CdS films obtained by a chemical bath deposition process. J. Mater. Chem. 1996, 6, 377–380. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Gurgul, M.; Gilek, D.; Kozieł, M.; Socha, R.P.; Sulka, G.D. Morphology of nanoporous anodic films formed on tin during anodic oxidation in less commonly used acidic and alkaline electrolytes. Surf. Coat. Technol. 2019, 362, 191–199. [Google Scholar] [CrossRef]
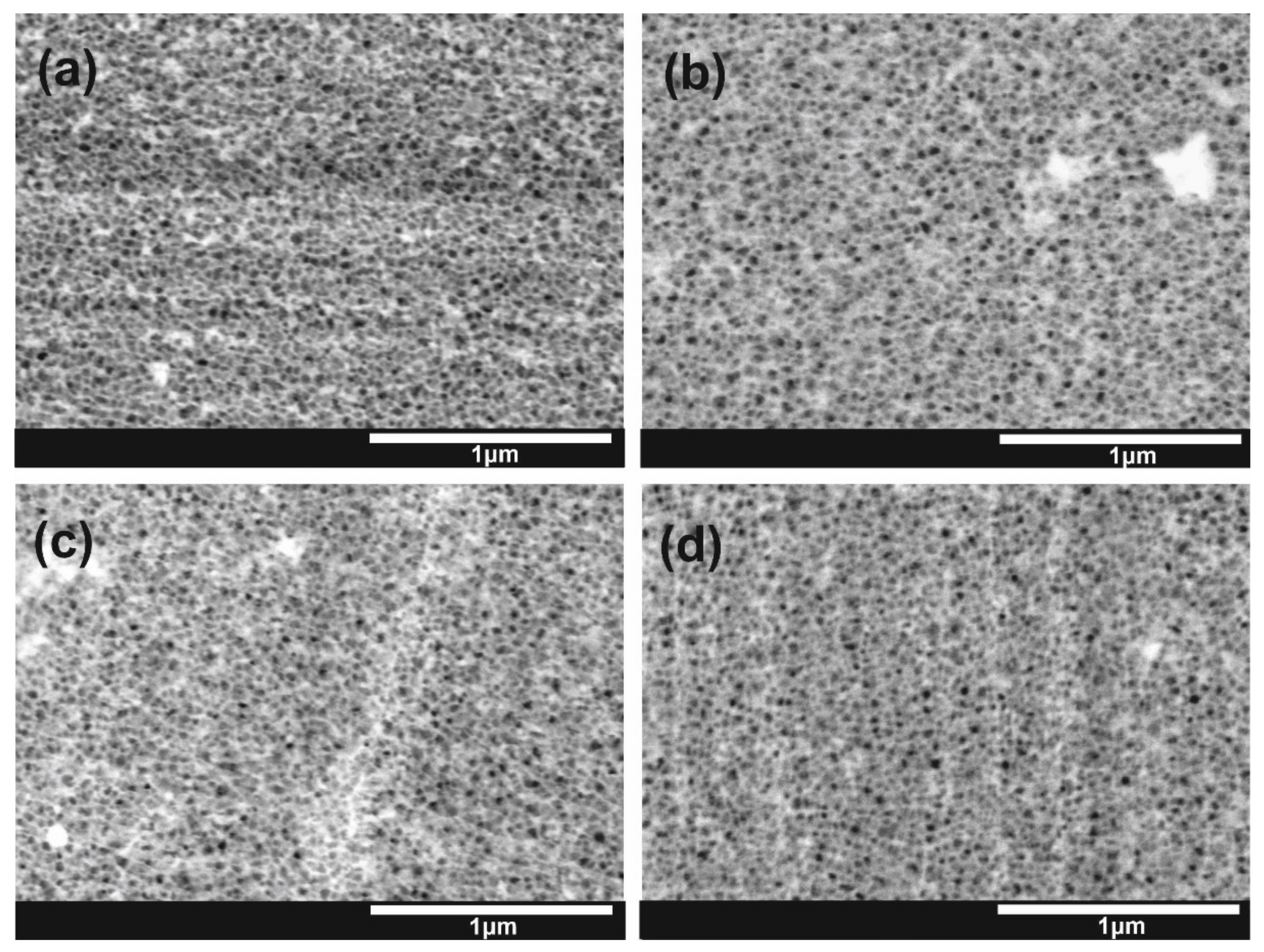
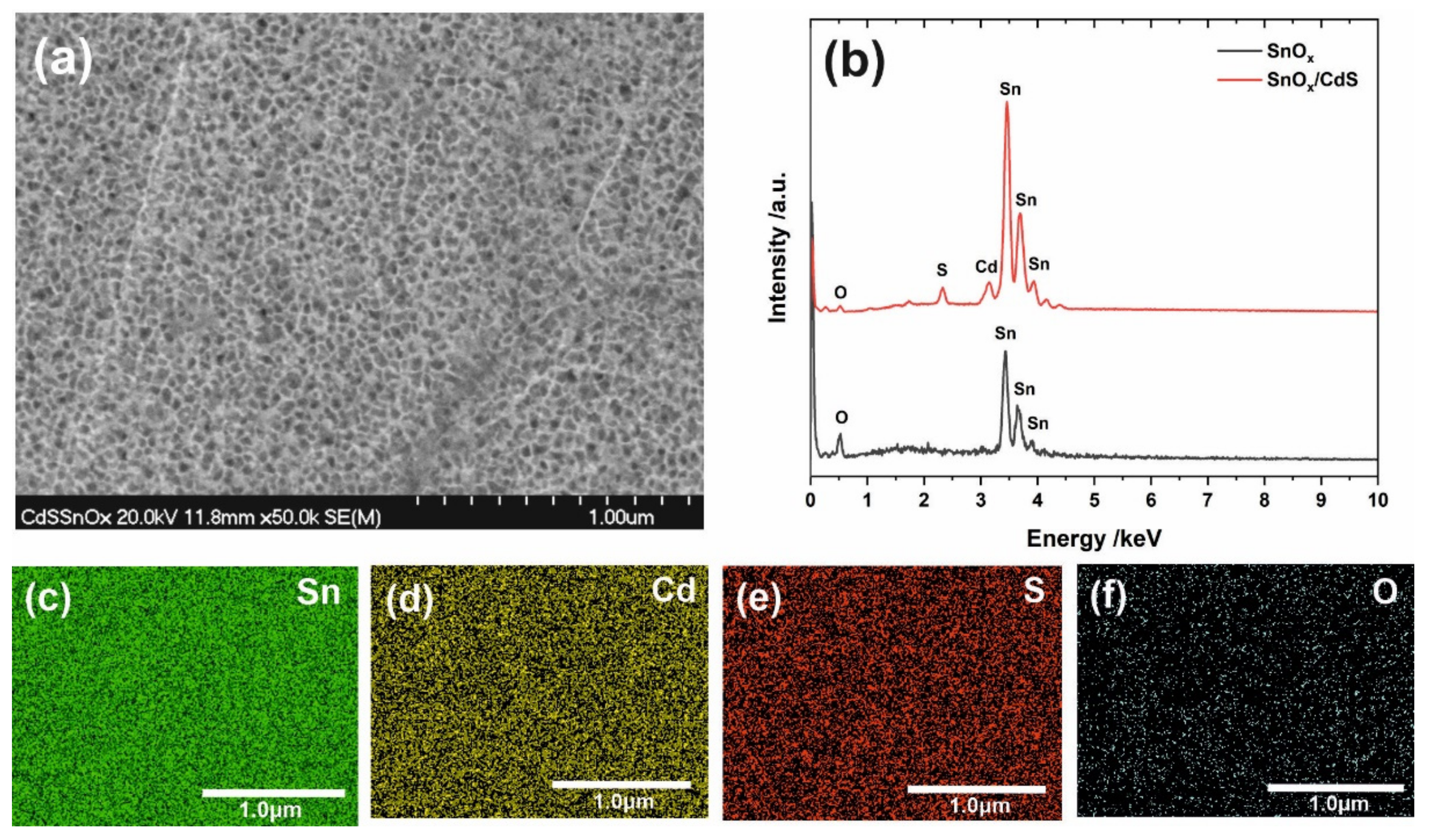
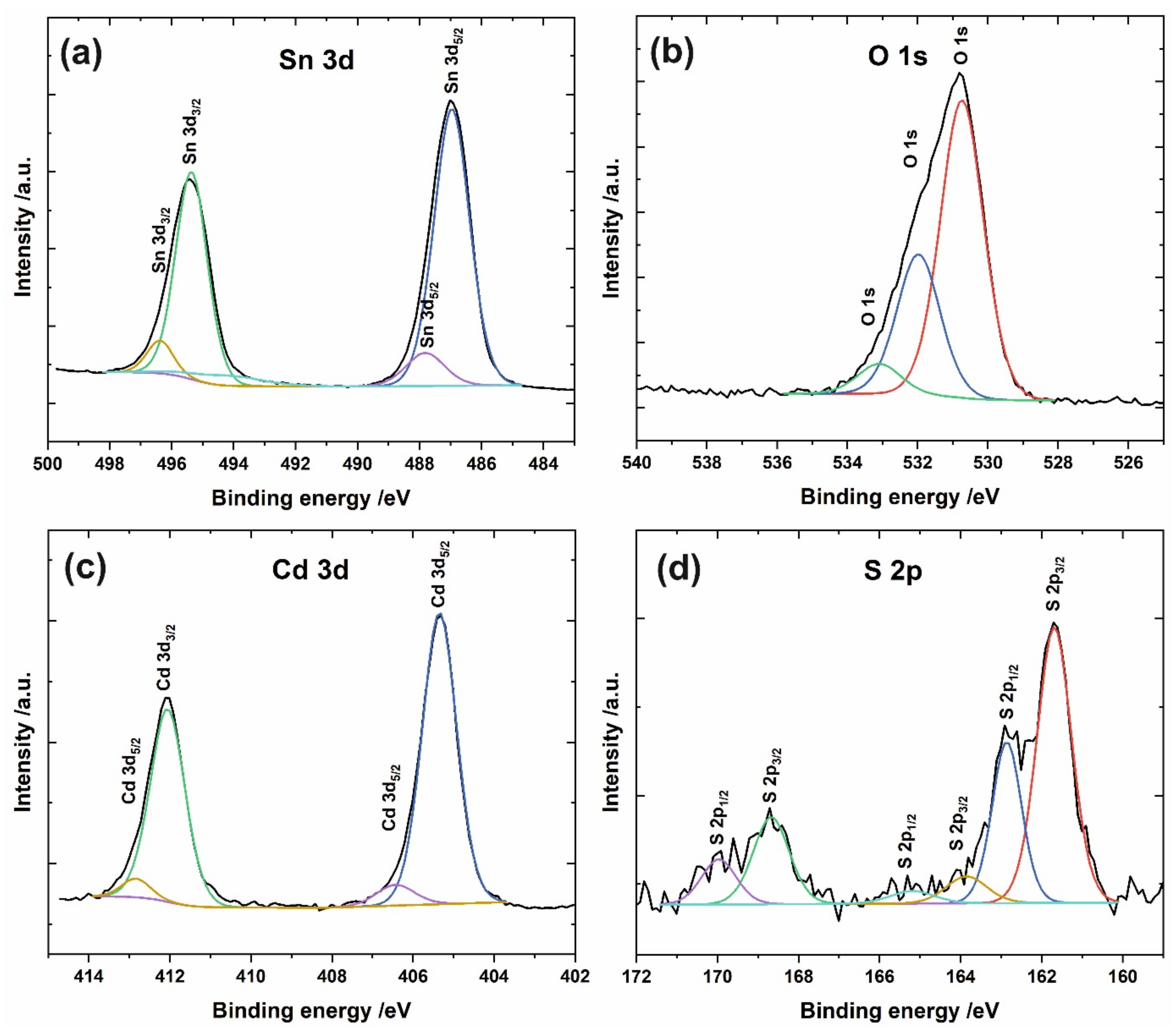


| Label of the Sample | |||
|---|---|---|---|
| -/- | a/- | -/a | a/a |
| Annealing after Anodization | |||
| No | Yes | No | Yes |
| Annealing after SILAR Procedure | |||
| No | No | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawlak, K.; Popiołek, D.; Pisarek, M.; Sulka, G.D.; Zaraska, L. CdS-Decorated Porous Anodic SnOx Photoanodes with Enhanced Performance under Visible Light. Materials 2022, 15, 3848. https://doi.org/10.3390/ma15113848
Gawlak K, Popiołek D, Pisarek M, Sulka GD, Zaraska L. CdS-Decorated Porous Anodic SnOx Photoanodes with Enhanced Performance under Visible Light. Materials. 2022; 15(11):3848. https://doi.org/10.3390/ma15113848
Chicago/Turabian StyleGawlak, Karolina, Dominika Popiołek, Marcin Pisarek, Grzegorz D. Sulka, and Leszek Zaraska. 2022. "CdS-Decorated Porous Anodic SnOx Photoanodes with Enhanced Performance under Visible Light" Materials 15, no. 11: 3848. https://doi.org/10.3390/ma15113848
APA StyleGawlak, K., Popiołek, D., Pisarek, M., Sulka, G. D., & Zaraska, L. (2022). CdS-Decorated Porous Anodic SnOx Photoanodes with Enhanced Performance under Visible Light. Materials, 15(11), 3848. https://doi.org/10.3390/ma15113848








