Heat Treatment of Geopolymer Samples Obtained by Varying Concentration of Sodium Hydroxide as Constituent of Alkali Activator
Abstract
:1. Introduction
2. Results and Discussion
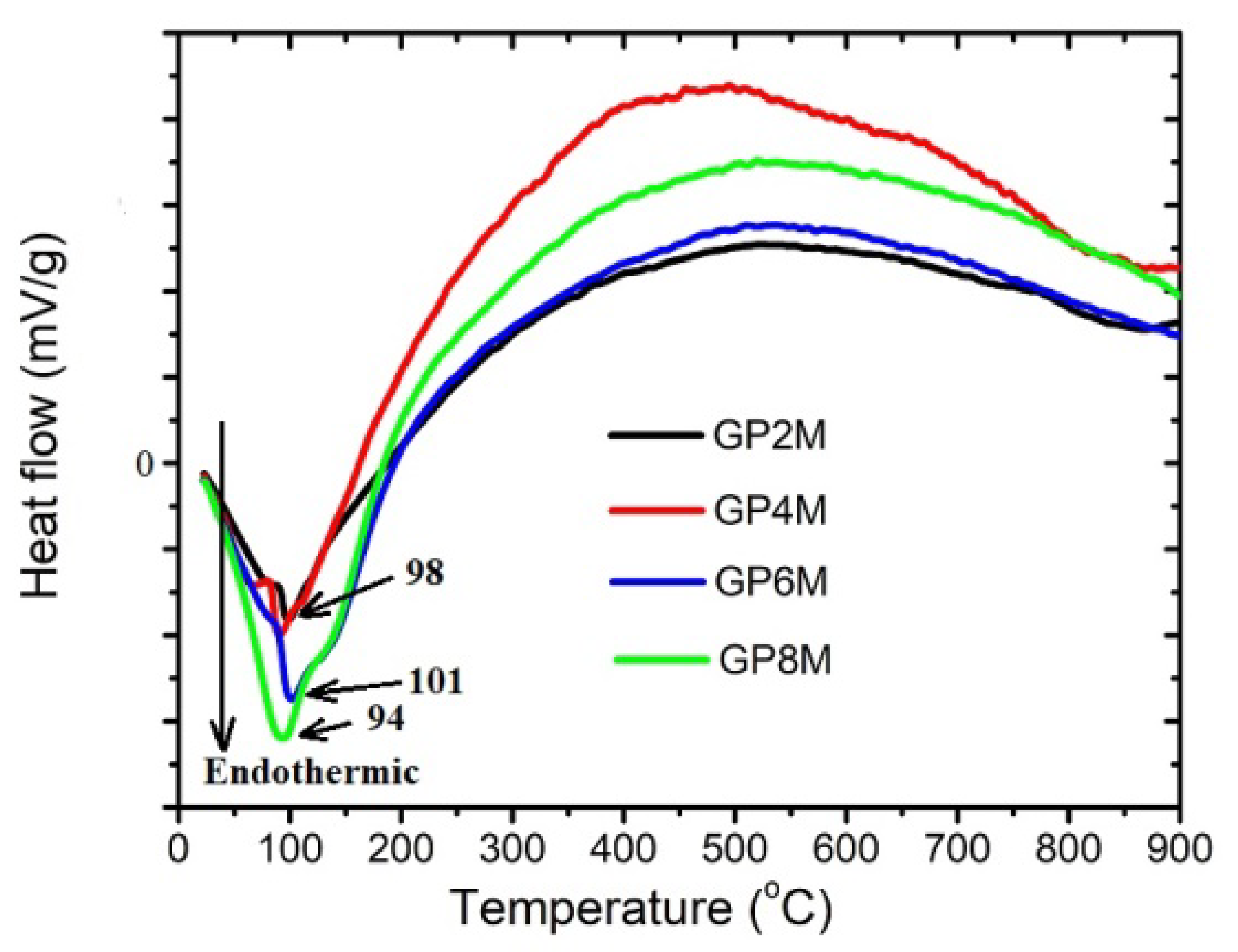
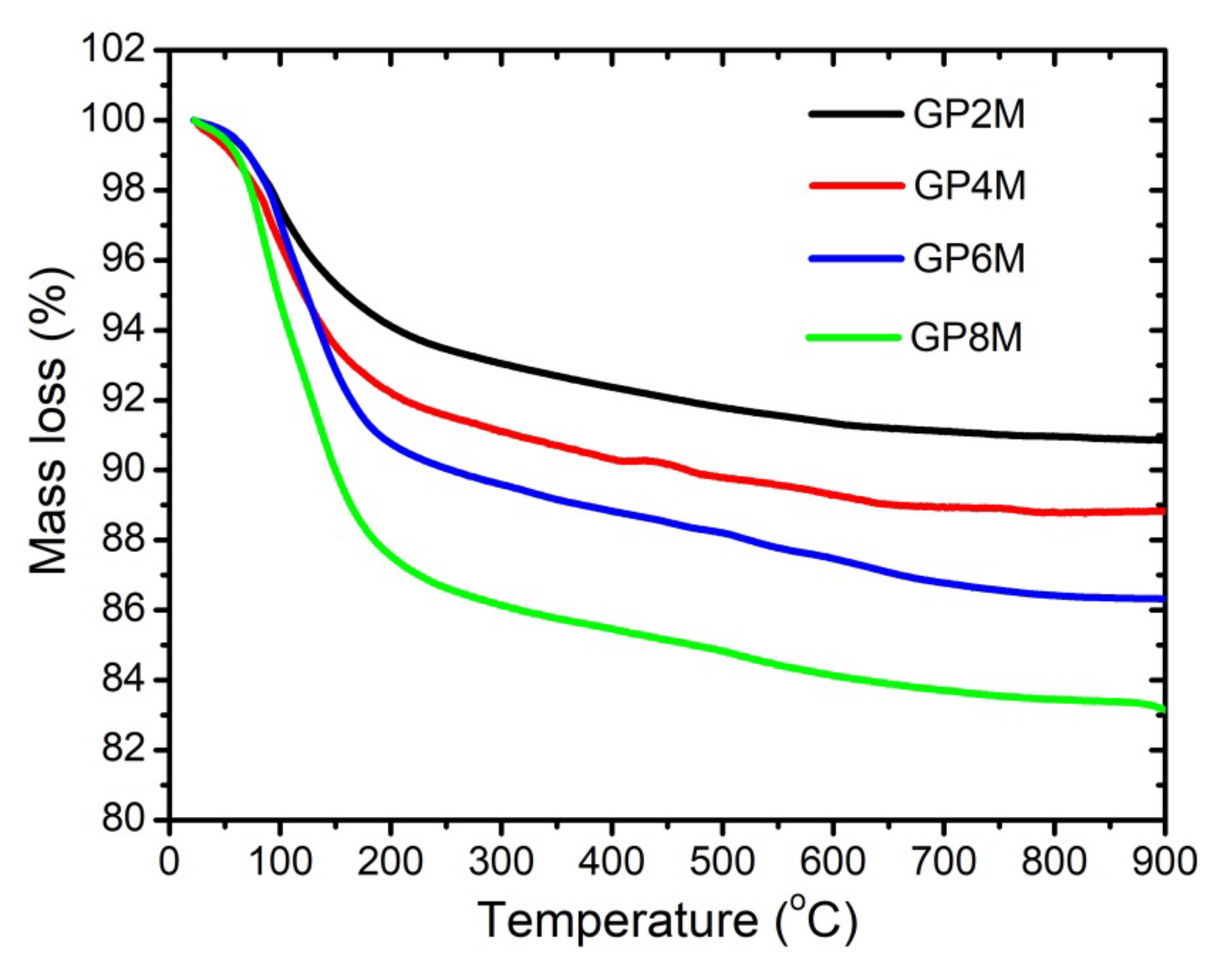
2.1. TGA/DTA Analysis
2.2. Structural and Chemical Characterization of Referent and Thermally Treated Geopolymer Samples
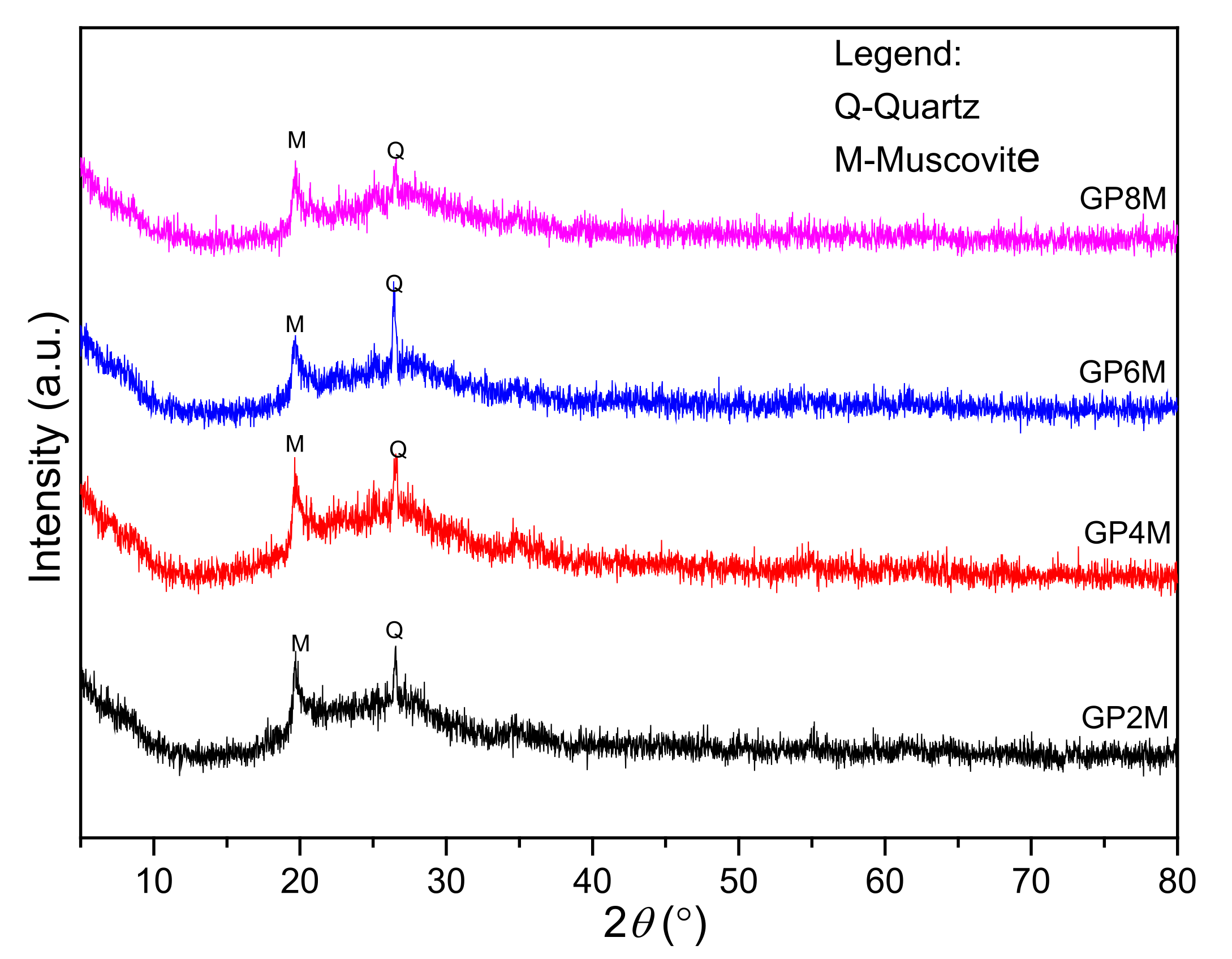
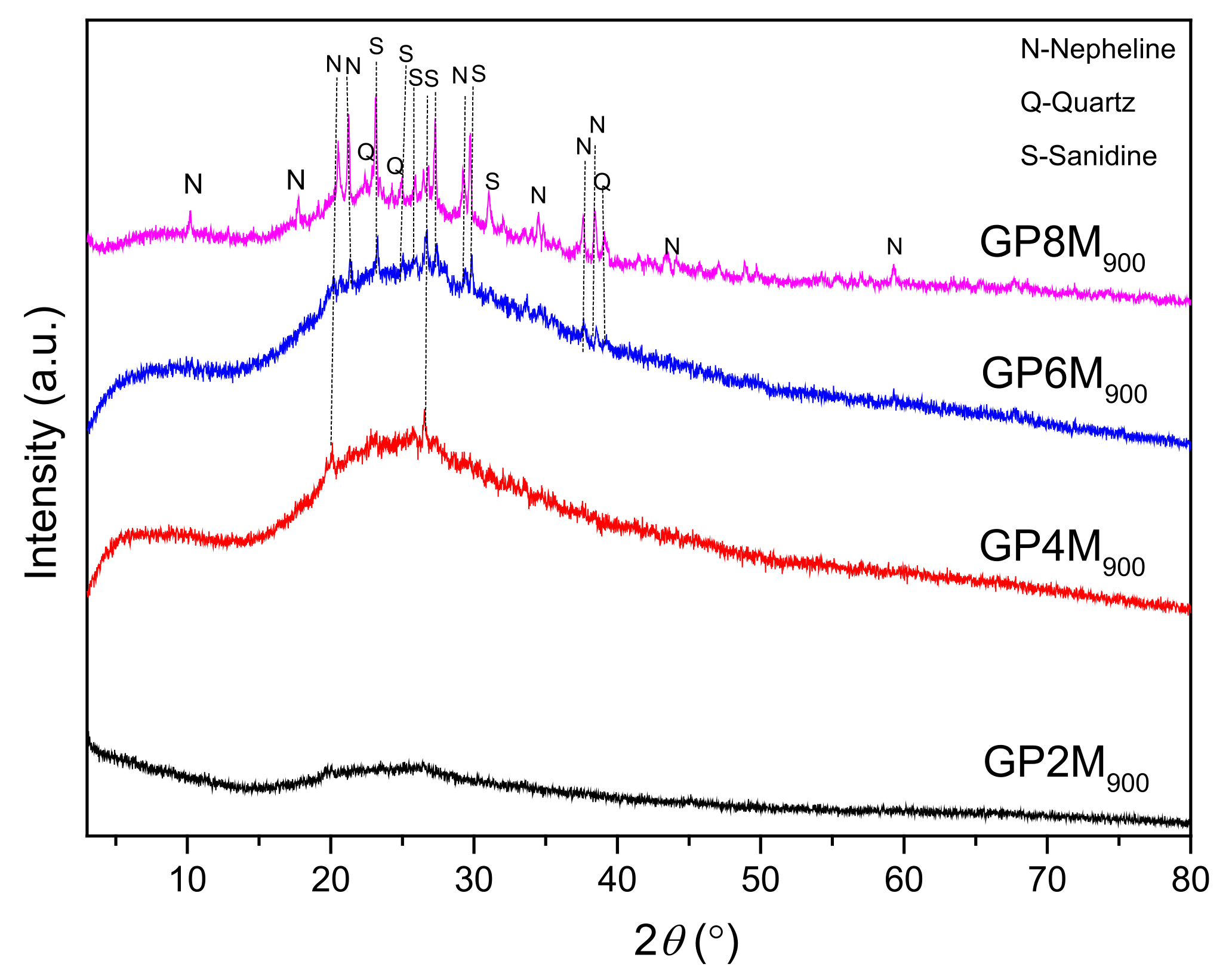
2.2.1. XRD Analysis
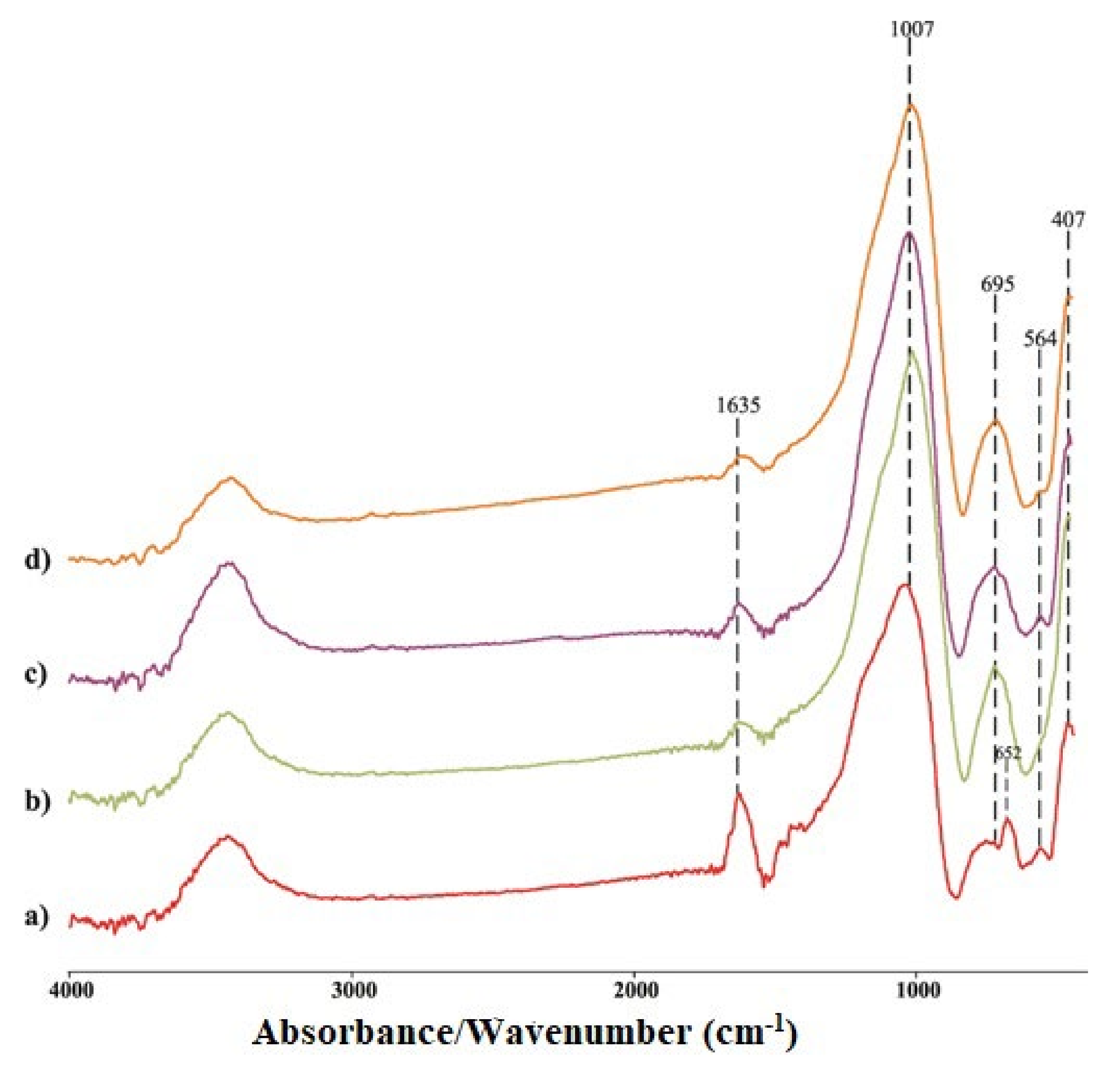
2.2.2. FTIR Analysis
2.2.3. Morphological Analysis
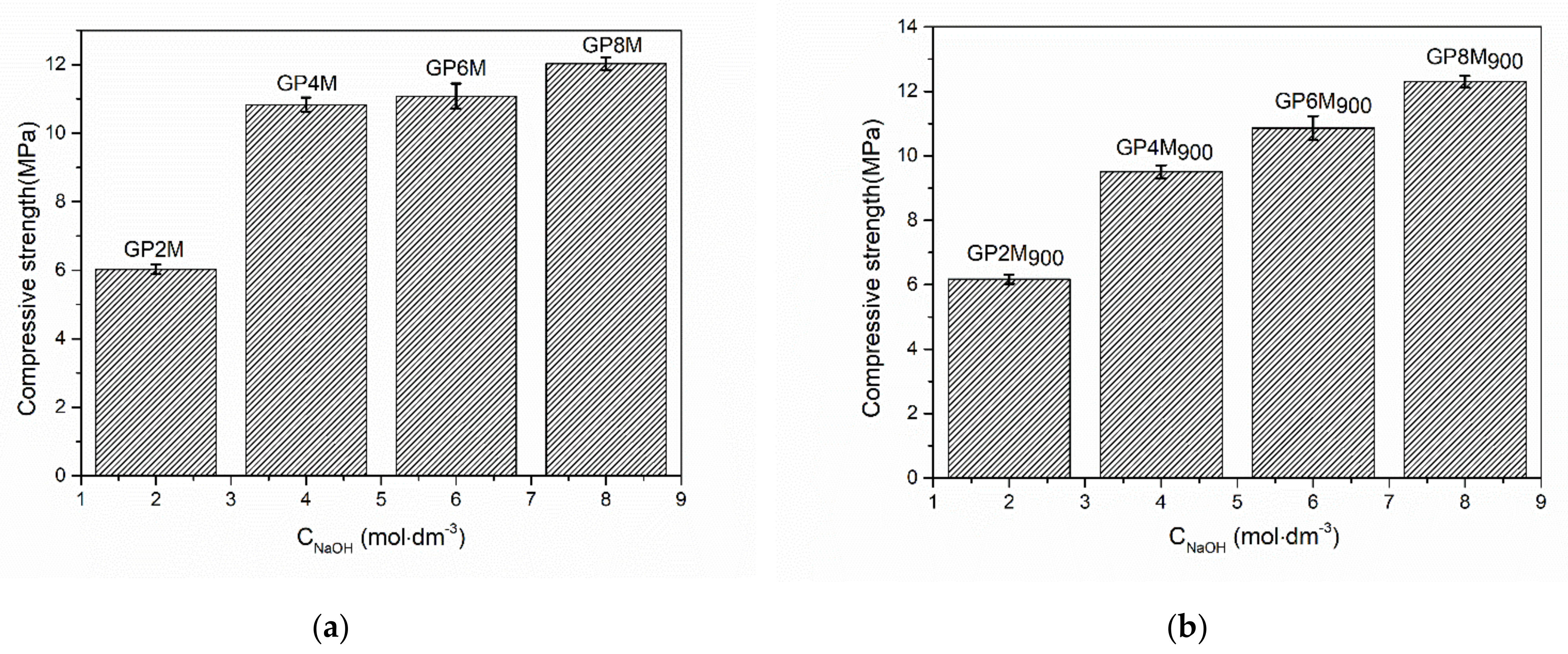
2.3. Compressive Strength of Geopolymer Samples
3. Conclusions
- The XRD results of all investigated GPRef samples show almost the same mineralogical contents. GPRef samples consist of an amorphous structure with quartz and muscovite as an impurity. After heating GPRef until 900 °C, new semi-crystalline phases appeared in GP4M900–GP8M900.
- FTIR analysis of both groups of samples confirmed the formation of a new amorphous gel with a certain proportion of crystalline phases after heating. The shift of wavenumbers towards higher or lower values is an indicator of these changes. Additionally, some bands do not appear in GPnM900, as a consequence of the process of dehydroxylation and further condensation.
- The microstructure of geopolymer samples treated thermally up to 900 °C was significantly changed in relation to the microstructure of GPRef samples. Samples GP6M900 and GP8M900 are more compact than others and crystallites are observed, as is the predominant dense glass matrix. The effect of alkaline activator concentration is visible in both GPRef samples and GPnM900 samples.
- Based on the DTA results, it can be concluded that among the examined geopolymers, there are small differences which occur during thermal treatment, which are most likely a function of the structural composition as well as thermal stability of geopolymers.
- The compressive strength of the thermally treated geopolymer samples increases as a function of the concentration of NaOH as a component of an alkali activator solution. There are no significant changes in the corresponding values for the compression strength of GPRef samples and GPnM900.
- Given the obtained results, we continue research in the direction of further increasing the temperature of thermal treatment, as well as other conditions that may affect the change in compressive strength.
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar]
- Zhang, X.; Bai, C.; Qiaoa, Y.; Wang, X.; Jia, D.; Li, H.; Colombo, P. Porous geopolymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106629. [Google Scholar] [CrossRef]
- Cheng, T.W.; Chiu, J.P. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Amran, M.; Fediuk, R.; Murali, G.; Avudaiappan, S.; Ozbakkaloglu, T.; Vatin, N.; Karelina, M.; Klyuev, S.; Gholampour, A. Fly Ash-Based Eco-Efficient Concretes: A Comprehensive Review of the Short-Term Properties. Materials 2021, 14, 4264. [Google Scholar] [CrossRef]
- He, R.; Dai, N.; Wang, Z. Thermal and Mechanical Properties of Geopolymers Exposed to High Temperature. A Literature Review. Adv. Civil Eng. 2020, 2020, 7532703. [Google Scholar] [CrossRef] [Green Version]
- Azimi, E.A.; Al Bakri Abdullah, M.M.; Ming, L.Y.; Yong, H.C.; Hussin, K.; Aziz, I.H. Processing and properties of geopolymers as thermal insulating materials. Rev. Adv. Mater. Sci. 2016, 44, 273–285. [Google Scholar]
- Fernández-Jiménez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; Mackenzie, K.J.D. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Kljajević, L.M.; Nenadović, S.S.; Nenadović, M.T.; Bundaleski, N.; Todorović, B.Ž.; Pavlović, V.B.; Rakočević, Z.L. Structural and chemical properties of thermally treated geopolymer samples. Ceram. Int. 2017, 43, 6700–6708. [Google Scholar] [CrossRef]
- Serra, J.; Gonzalez, P.; Liste, S.; Chiussi, S.; Leon, B.; Perez-Amor, M.; Ylanen, H.O.; Hupa, M. Influence of the non brindging oxygen groups on the bioactivity of silicateglasses. J. Mater. Sci. Mater. Med. 2002, 13, 1221–1225. [Google Scholar] [CrossRef]
- Provis, J.; van Deventer, J.S.J. Alkali Activated Materials. State of the Art Report, RILEM TC 224-AAM; Springer: New York, NY, USA, 2014; ISBN 978-94-007-7671-5. [Google Scholar]
- Bakharev, T. Thermal behaviour of geopolymers prepared using class F fly ash and elevated temperature curing. Cem. Concr. Res. 2006, 36, 1134–1147. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Pastor, J.Y.; Martín, A. New cementations materials based on alkali activated fly ash: Performance at high temperature. J. Am. Ceram. Soc. 2008, 91, 3308–3314. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, H.; Wang, Y.; Yan, F. Preparation of macroporous ceramic from metakaolinite-based geopolymer by calcination. Ceram. Int. 2015, 41, 11177–11183. [Google Scholar] [CrossRef]
- Rahier, H.; Wastiels, J.; Biesemans, M.; Willem, R.; van Assche, G.; van Mele, B. Reaction, mechanism, kinetics and high temperature transformations of geopolymers. J. Mater. Sci. 2007, 42, 2982–2996. [Google Scholar] [CrossRef]
- Kuenzel, C.; Grover, L.M.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Production of nepheline/quartz ceramics from geopolymer mortars. J. Eur. Ceram. Soc. 2013, 33, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Lahoti, M.; Wong, K.K.; Yang, E.-H.; Tan, K.H. Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Jaya, N.A.; Al Bakri Abdullah, M.M.; Ruzaidi Ghazali, C.H.; Binhussain, M.; Hussin, K.; Ahmad, R. Effect of NaOH concentration on flexural strength, phase formation and microstructural development of kaolin geopolymer ceramic. Mat. Sci. Forum 2016, 857, 405–411. [Google Scholar] [CrossRef]
- Elimbi, A.; Kouamo, H.T.; Kondoh, M.; Dika Manga, J. Thermal behavior and characteristics of fired geopolymers produced from local Cameroonian metakaolin. Ceram. Int. 2014, 40, 4515–4520. [Google Scholar] [CrossRef]
- Hussain, M.; Varley, R.J.; Cheng, Y.B.; Mathys, Z.; Simon, G.P. Synthesis and thermal behavior of inorganic–organic hybrid geopolymer composites. J. Appl. Polym. Sci. 2005, 96, 112–121. [Google Scholar] [CrossRef]
- Redaoui, D.; Sahnoune, F.; Heraiz, M.; Raghdi, A. Mechanism and Kinetic Parameters of the Thermal Decomposition of Gibbsite Al(OH)3 by Thermogravimetric Analysis. Acta Phys. Pol. Ser. 2017, 131, 562–565. [Google Scholar] [CrossRef]
- Hao, H.C.; Lin, K.-L.; Wang, D.; Chao, S.-J.; Shiu, H.-S.; Cheng, T.-W.; Hwang, C.-L. Elucidating Characteristics of Geopolymer with Solar Panel Waste Glass. Environ. Eng. Manag. 2015, 14, 79–87. [Google Scholar]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Adediran, A.; Yliniem, J.; Ismailov, A.; Levanen, E.; Tanskanen, P.; Kinnunen, P.; Roning, J.; Illikainen, M. Thermal stability of one-part metakaolin geopolymer composites containing high volume of spodumene tailings and glass wool. Cem. Concr. Compos. 2020, 114, 103792. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Roviello, G.; Capasso, I.; Caputo, D.; Aprea, P.; Liguori, B.; Ferone, C. Thermal cycling stability of fly ash based geopolymer mortars. Compos. Part B Eng. 2017, 129, 11–17. [Google Scholar] [CrossRef]
- Ivanović, M.; Kljajević, L.; Gulicovski, J.; Petkovic, M.; Jankovic-Castvan, I.; Bučevac, D.; Nenadović, S. The effect of the concentration of alkaline activator and aging time on the structure of metakaolin based geopolymer. Sci. Sinter. 2020, 52, 219–229. [Google Scholar] [CrossRef]
- Babič, D. Mineralogija, 1st ed.; Cicero: Belgrade, Serbia, 2003. [Google Scholar]
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The characterization of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Mazzucato, E.; Artioli, G.; Gualtieri, A. High-temperature dehydroxylation of muscovite-2M1: A kinetic study by in situ XRPD. Phys. Chem. Miner. 1999, 26, 375–381. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterization of materials based on inorganic polymers of alumina and silica: Sodiumpolysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Zaharaki, D.; Komnitsas, K.; Perdikatsis, V. Use of analytical techniques for identification of inorganic polymer gel composition. J. Mater. Sci. 2010, 45, 2715–2724. [Google Scholar] [CrossRef]
- Provis, J.L.; Rees, C.A. Geopolymer Synthesis Kinetics. In Geopolymers: Structures, Processing, Properties and Industrial Applications; Provis, J.L., van Deventer, J.S.J., Eds.; Woodhead Publishing: Abingdon UK, 2009; pp. 118–136. [Google Scholar]
- Karlsson, C.; Zanghellini, E.; Swenson, J.; Roling, B.; Bowron, D.T.; Borjesson, L. Structure of mixed alkali/alkaline-earth silicate glasses from neutron diffraction and vibrational spectroscopy. Phys. Rev. B 2005, 72, 064206. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared Spectroscopy of Sol-Gel Derived Silica-Based Films: A Spectra-Microstructure Overview. J. Non Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkaline activated fly ash structure. Micropor. Mesopor. Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Kosor, T.; Nakic-Alfirević, B.; Svilović, S. Geopolymer depolymerization index. Vib. Spectrosc. 2016, 86, 143–148. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals; Mineralogical Society: London, UK, 1974. [Google Scholar]
- Kioupis, D.; Kavakakis, C.; Tsivilis, S.; Kakali, G. Synthesis and Characterization of Porous Fly Ash-Based Geopolymers Using Si as Foaming Agent. Adv. Mater. Sci. Eng. 2018, 2018, 1942898. [Google Scholar] [CrossRef] [Green Version]
- Criado, M.; Palomo, A.; Fernandez-Jimenez, A. Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Xu, H.; van Deventer, J.S.J. The geopolymerization of alumino- silicate minerals. Int. J. Min. Process. 2000, 59, 247–258. [Google Scholar] [CrossRef] [Green Version]
- García-Mejía, T.A.; Chávez-García, M.-L. Compressive Strength of Metakaolin-Based Geopolymers: Influence of KOH Concentration, Temperature, Time and Relative Humidity. Mat. Sci. Appl. 2016, 7, 772–791. [Google Scholar] [CrossRef] [Green Version]
- Rovnanik, P.; Šafránková, K. Thermal Behaviour of Metakaolin/Fly Ash Geopolymers with Chamotte Aggregate. Materials 2016, 9, 535. [Google Scholar] [CrossRef]
- Rashad, A.M.; Ouda, A.S. Research on alkali-activated fly ash pastes modified with quartz powder subjected to elevated temperatures. Constr. Build. Mat. 2016, 122, 417–425. [Google Scholar] [CrossRef]
- Kovarik, T.; Rieger, D.; Kadlec, J.; Krenek, T.; Kullova, L.; Pola, M.; Belsky, P.; Franče, P.; Riha, J. Thermomechanical properties of particle-reinforced geopolymer composite with different grades of fine ceramic aggregate aggregates. Constr. Build. Mat. 2017, 143, 599–606. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodriguez, E.D.; de Gutierrez, R.M.; Gordillo, M.; Provis, J.L. Mechanical and thermal characterisation of geopolymers based on silicate-activated metakaolin/slag blends. J. Mater. Sci. 2011, 46, 5477–5486. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Thermal evolution of metakaolin geopolymers: Part 1-Physical evolution. J. Non. Cryst. Solids 2006, 352, 5541–5555. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Wu, B.; Cao, L. Comparative thermal and mechanical performance of geopolymers derived from metakaolin and fly ash. J. Mater. Civil. Eng. 2016, 28, 04015092. [Google Scholar] [CrossRef]
- Zhang, H.-I.; Kodur, V.; Cao, L.; Ki, S.-L. Fiber-reinforced geopolymers for fire resistance applications. Proc. Eng. 2014, 71, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Nenadović, S.S.; Kljajević, L.M.; Nenadović, M.T.; Mirković, M.M.; Marković, S.B.; Rakočević, Z.L. Mechanochemical treatment and structural properties of lead adsorption on kaolinite (Rudovci, Serbia). Environ. Earth Sci. 2015, 73, 7669–7677. [Google Scholar] [CrossRef]
- Rigaku. PDXL Integrated X-Ray Powder Diffraction Software; Rigaku: Tokyo, Japan, 2011. [Google Scholar]
- International Crystallographic Database (ICDD); PSDS: Newtown Township, PA, USA, 2012.




| Sample | GP2M | GP4M | GP6M | GP8M | GP2M900 | GP4M900 | GP6M900 | GP8M900 |
|---|---|---|---|---|---|---|---|---|
| Si/Al | 1.910 | 1.930 | 1.960 | 2.090 | 1.710 | 1.770 | 1.630 | 1.580 |
| Si/Na | 5.283 | 3.146 | 2.670 | 2.521 | 4.651 | 2.398 | 2.435 | 2.005 |
| Oxide | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | *LoI |
|---|---|---|---|---|---|---|---|---|
| % wt | 53.03 | 35.44 | 4.39 | 1.25 | 1.38 | 0.01 | 2.06 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kljajević, L.; Nenadović, M.; Ivanović, M.; Bučevac, D.; Mirković, M.; Mladenović Nikolić, N.; Nenadović, S. Heat Treatment of Geopolymer Samples Obtained by Varying Concentration of Sodium Hydroxide as Constituent of Alkali Activator. Gels 2022, 8, 333. https://doi.org/10.3390/gels8060333
Kljajević L, Nenadović M, Ivanović M, Bučevac D, Mirković M, Mladenović Nikolić N, Nenadović S. Heat Treatment of Geopolymer Samples Obtained by Varying Concentration of Sodium Hydroxide as Constituent of Alkali Activator. Gels. 2022; 8(6):333. https://doi.org/10.3390/gels8060333
Chicago/Turabian StyleKljajević, Ljiljana, Miloš Nenadović, Marija Ivanović, Dušan Bučevac, Miljana Mirković, Nataša Mladenović Nikolić, and Snežana Nenadović. 2022. "Heat Treatment of Geopolymer Samples Obtained by Varying Concentration of Sodium Hydroxide as Constituent of Alkali Activator" Gels 8, no. 6: 333. https://doi.org/10.3390/gels8060333








