Early Osseous Proliferation in Spiraled Healing Chambers Resulted After the Insertion of Titanium Implants in Cortical Bone of a Rabbit
Abstract
1. Introduction
2. Materials and Methods
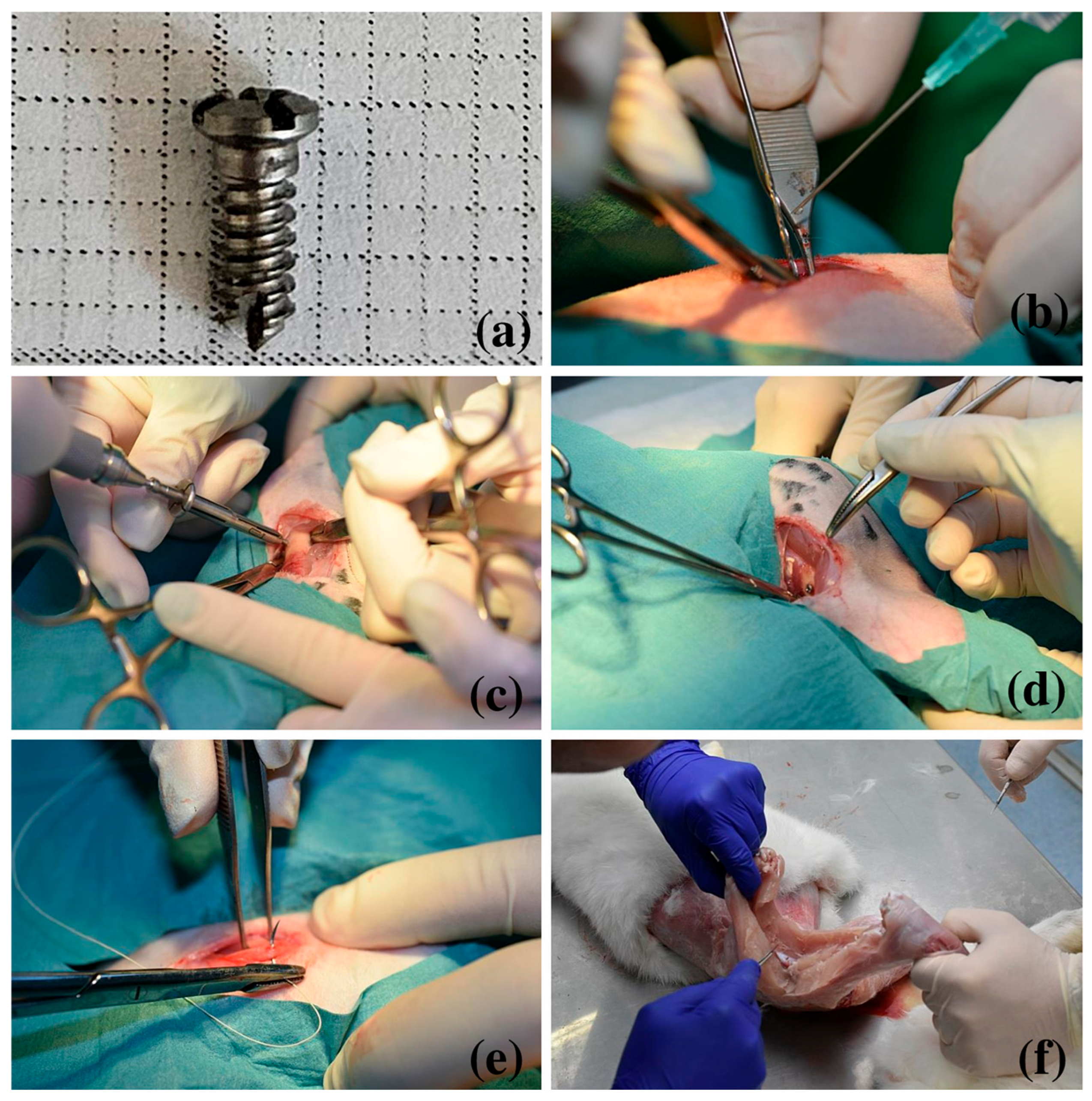
2.1. Surgical Procedure
2.2. Histological Analysis
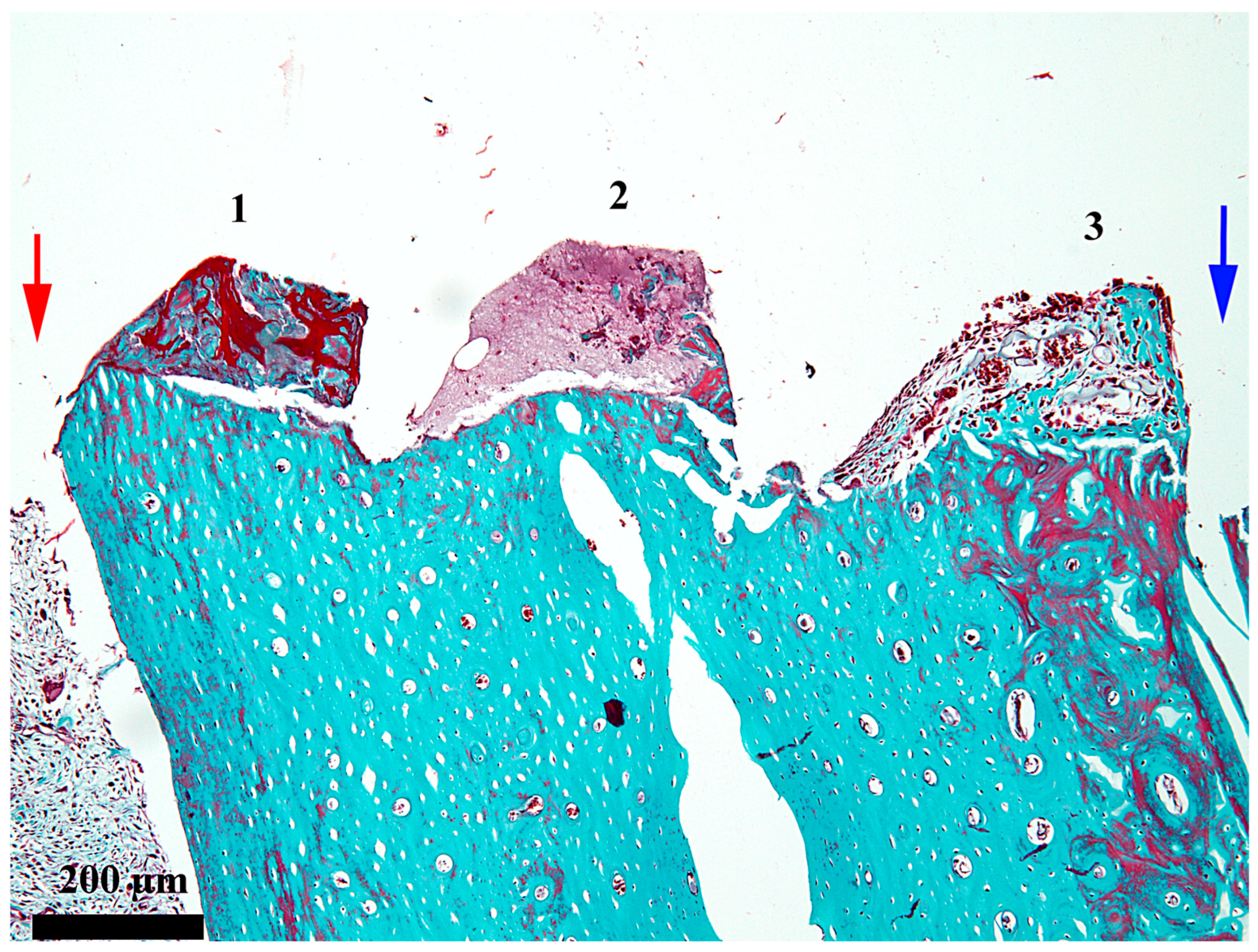
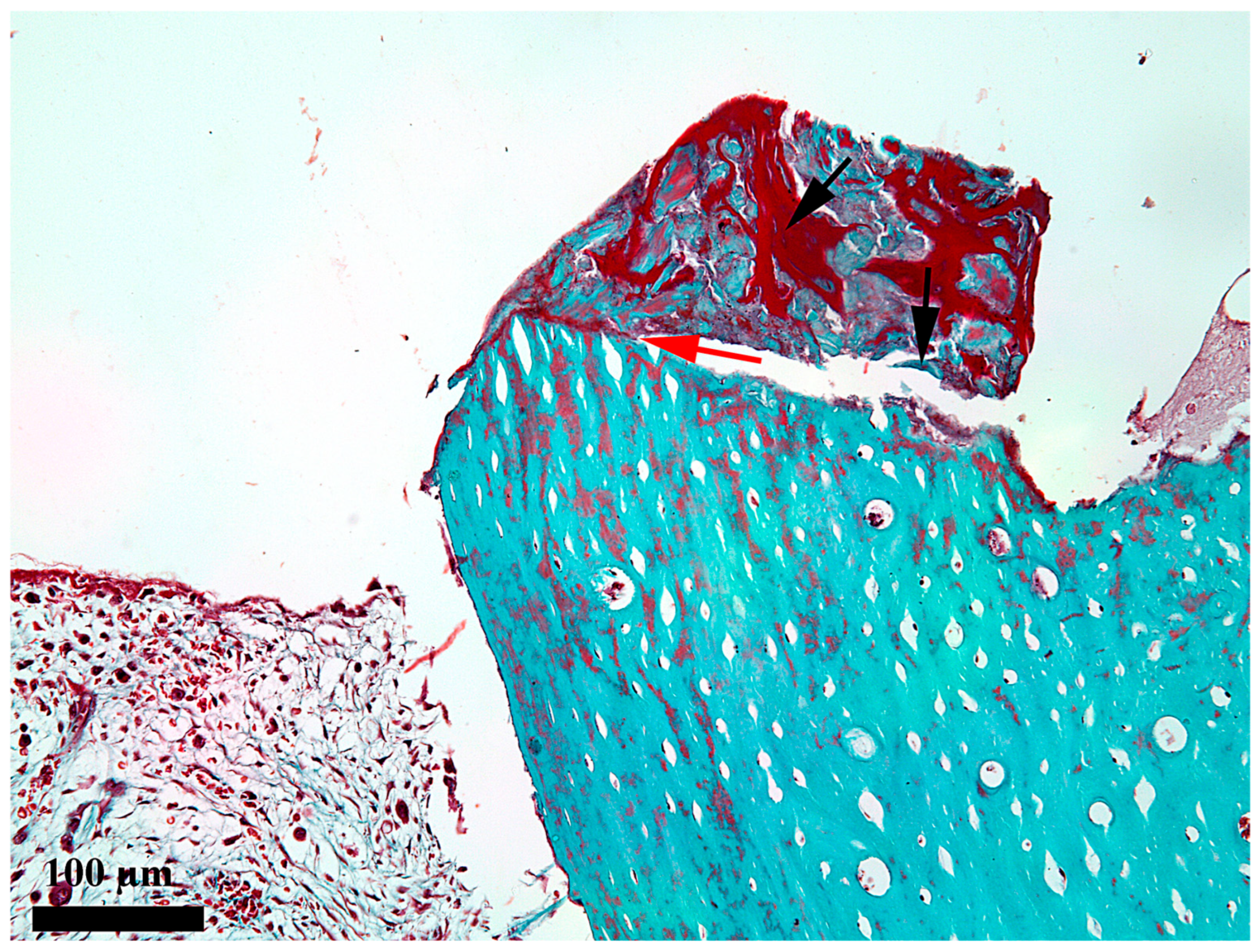
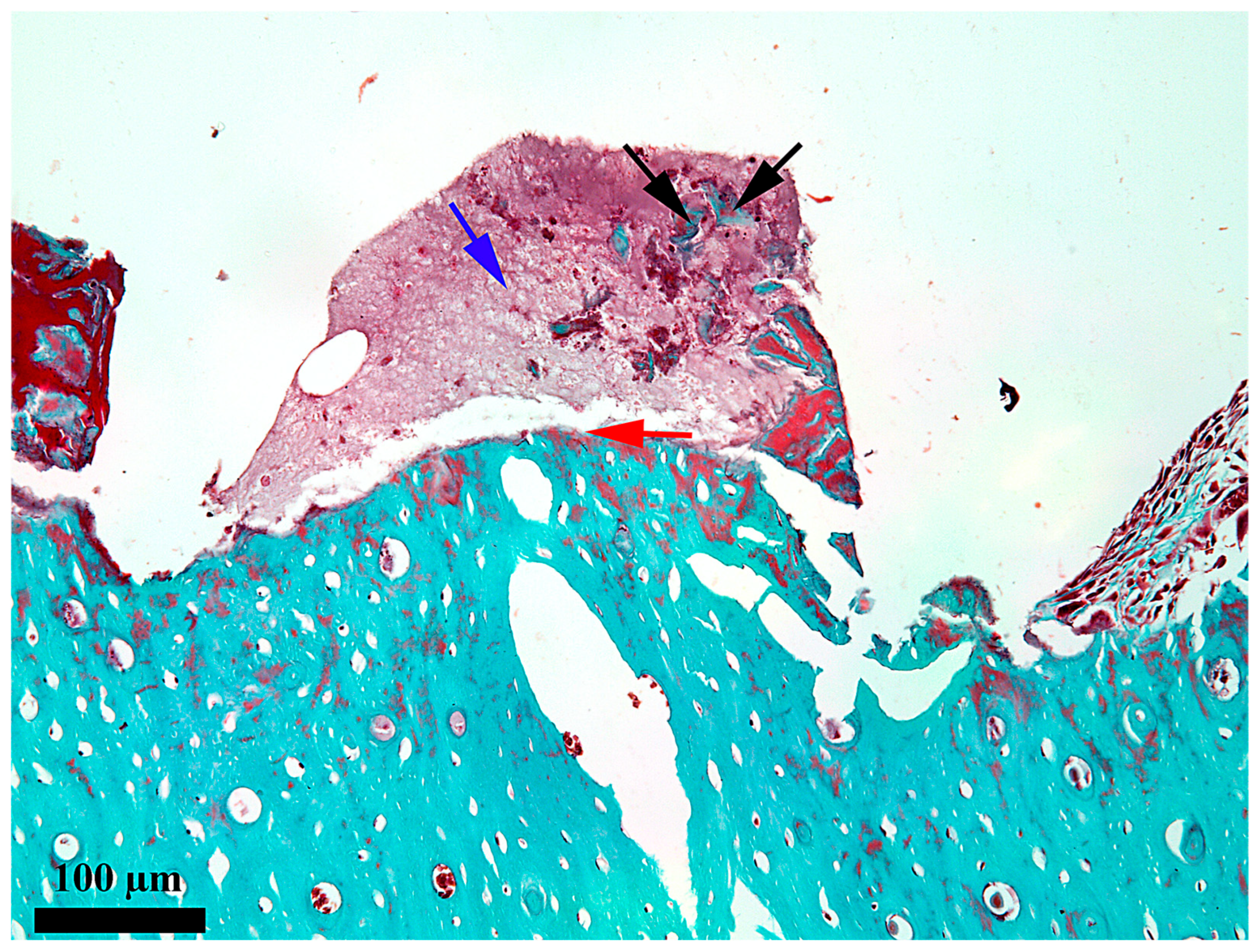
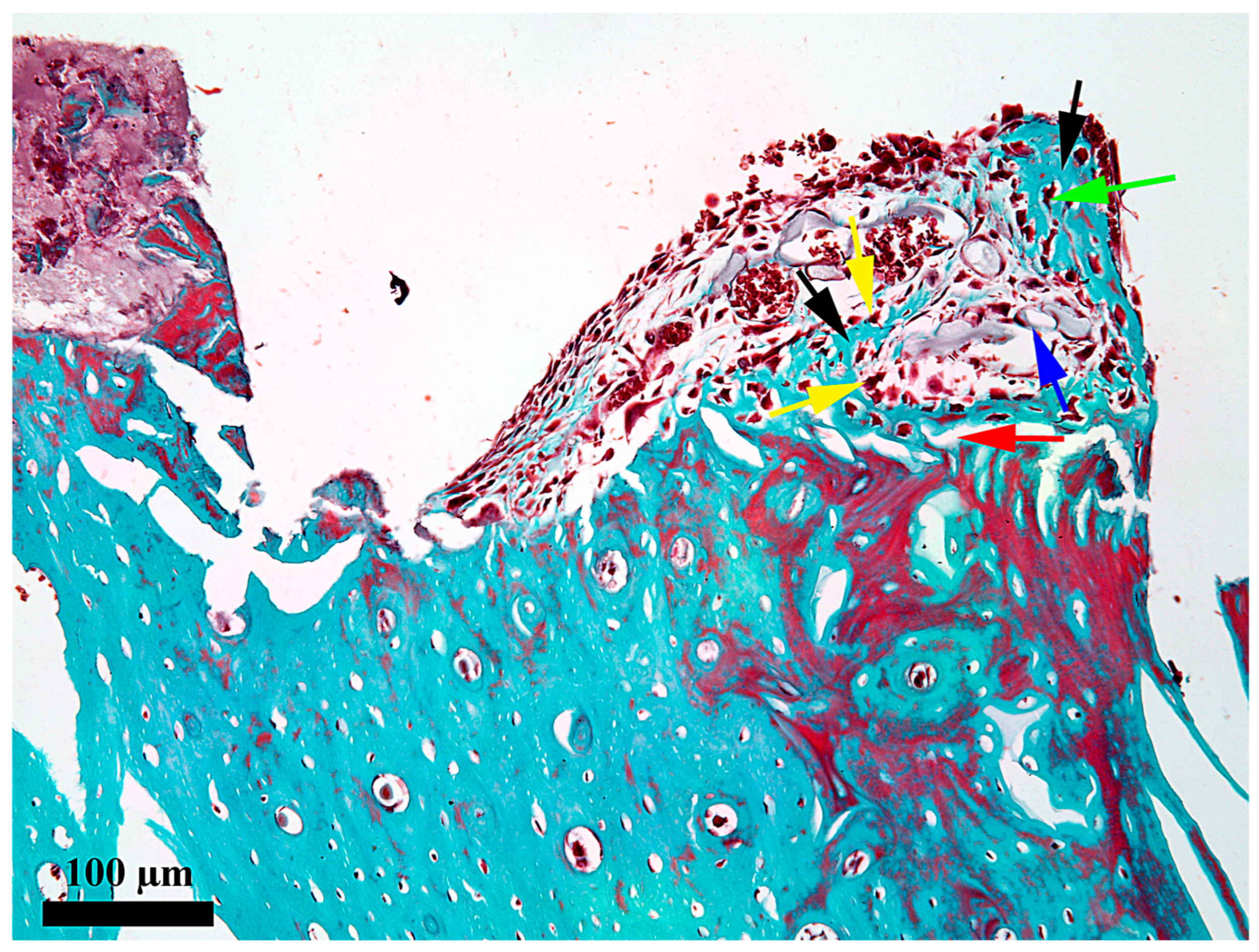
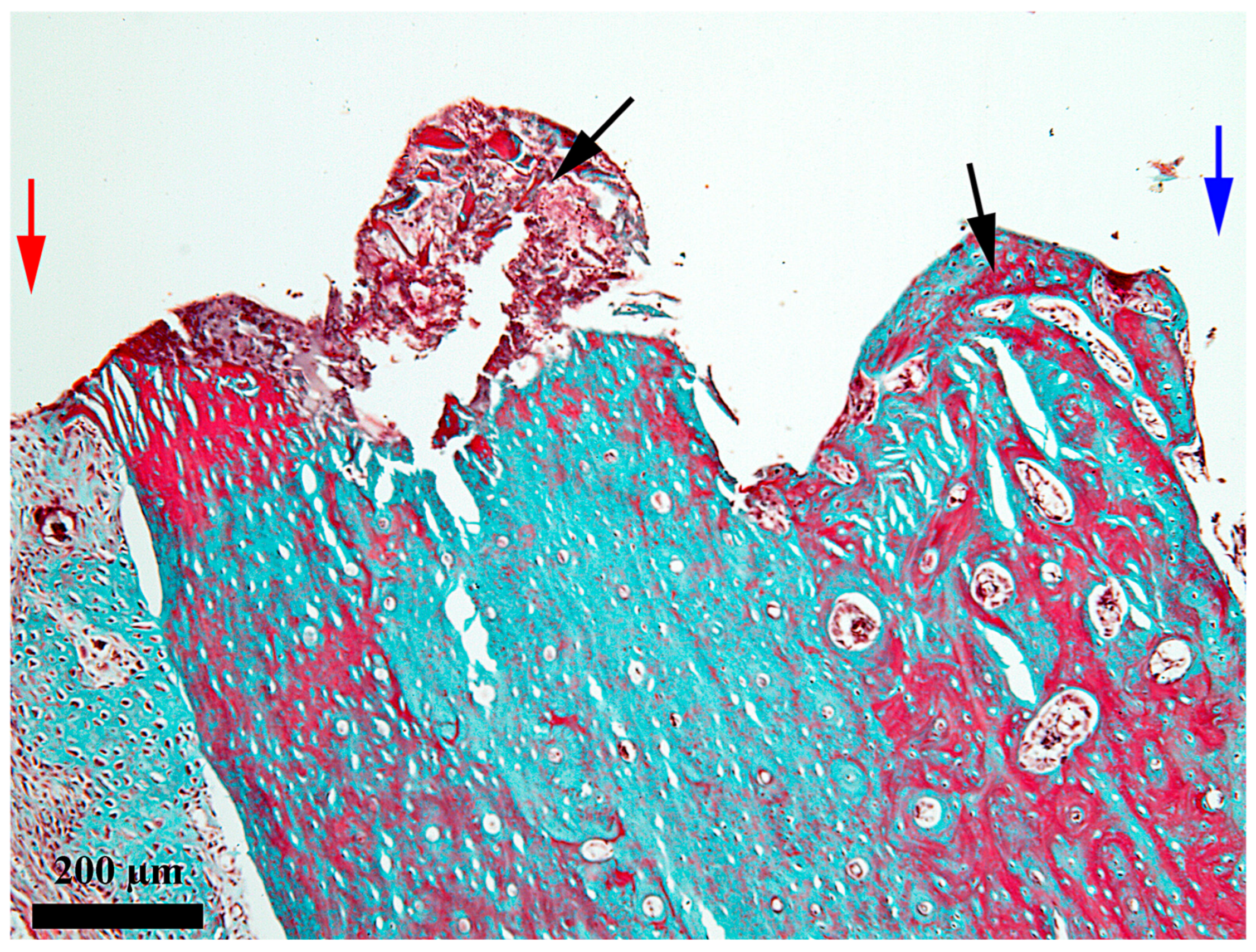
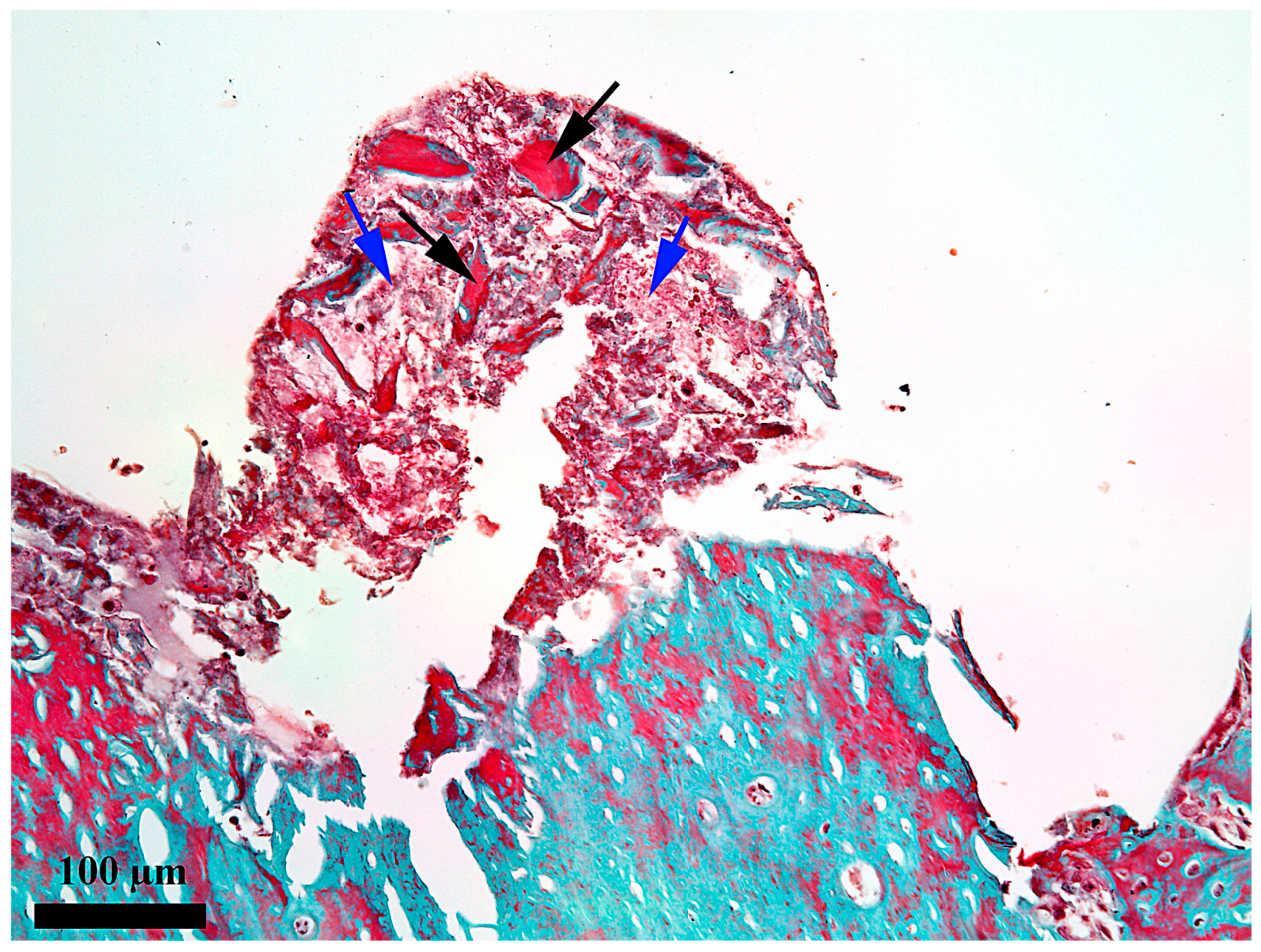
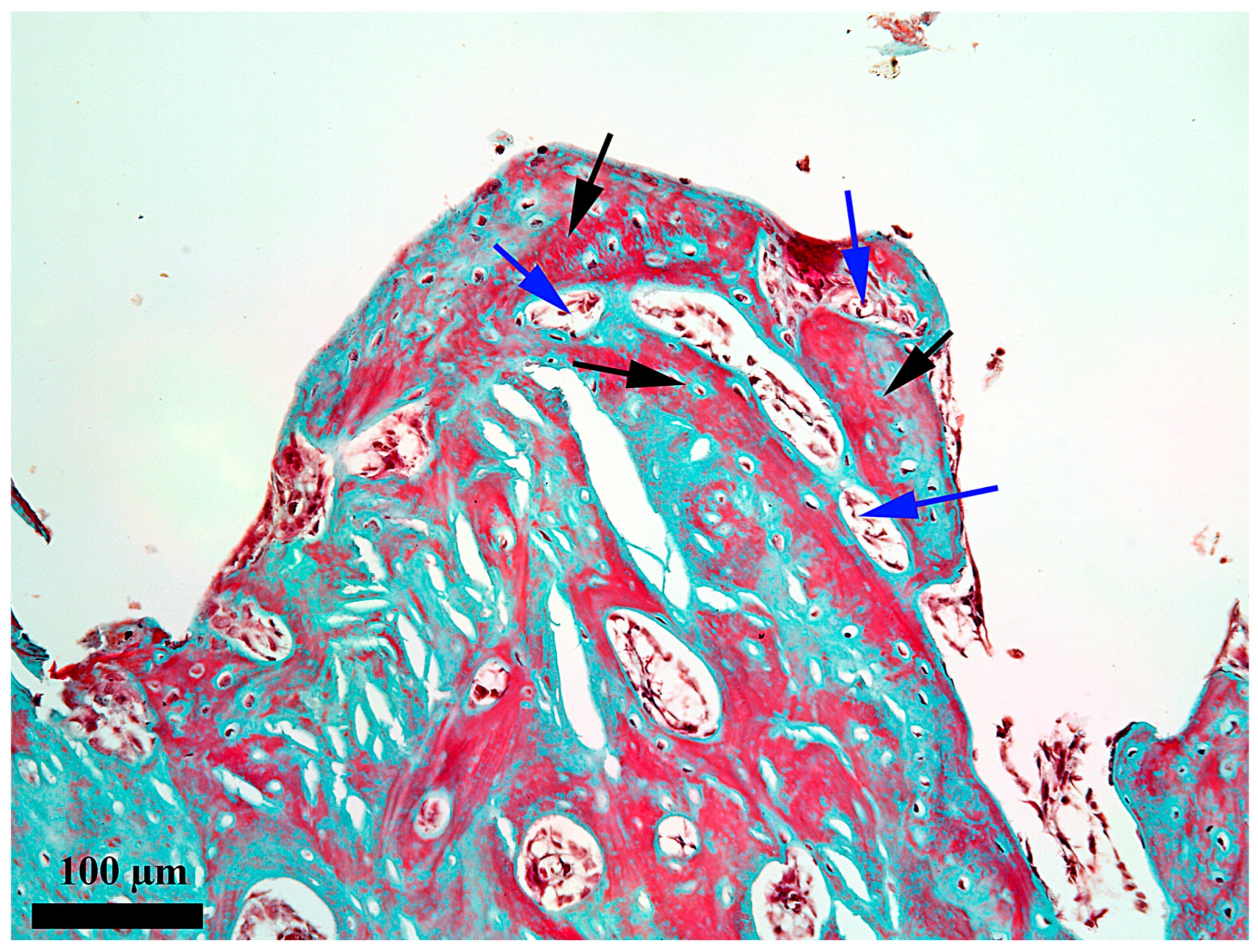
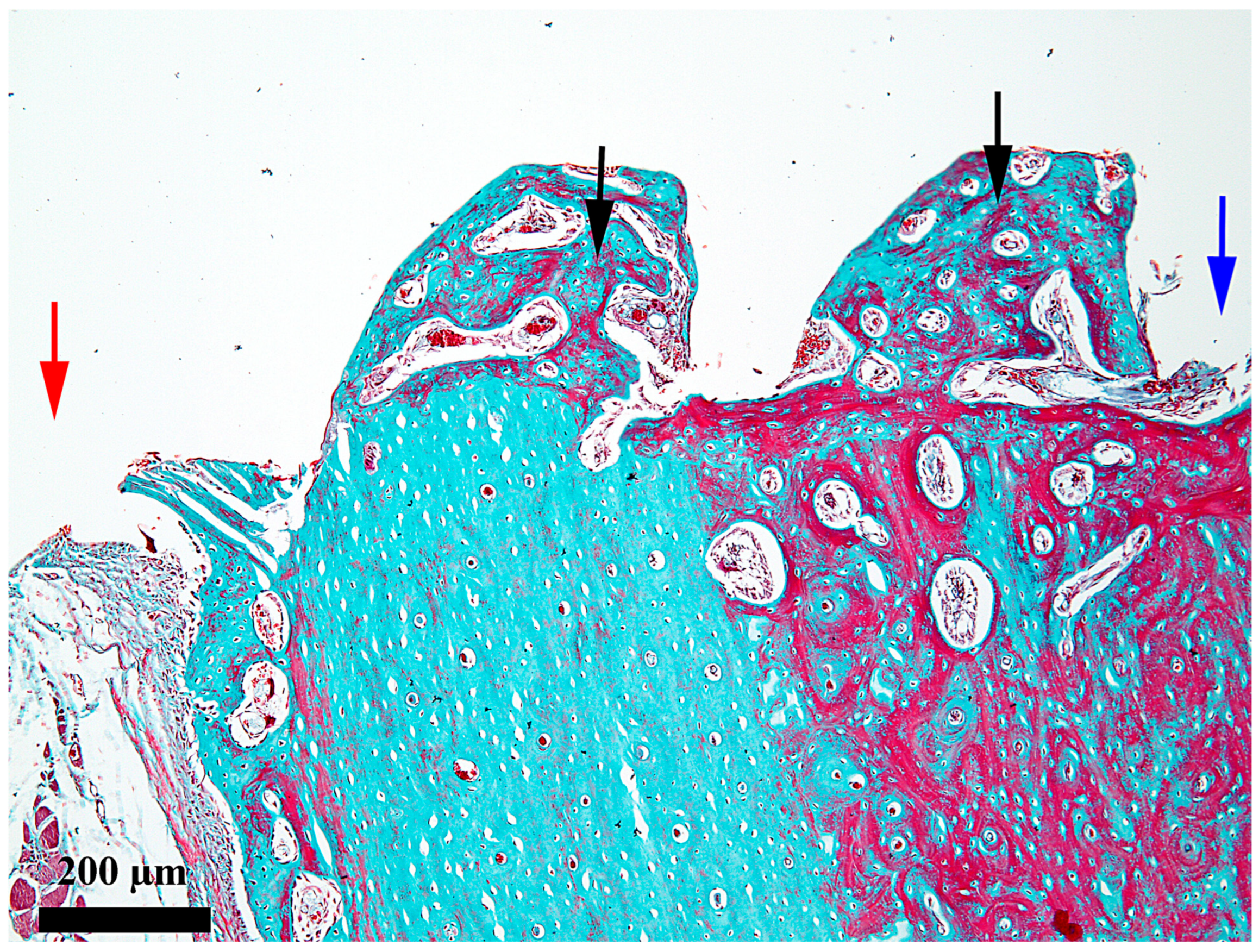
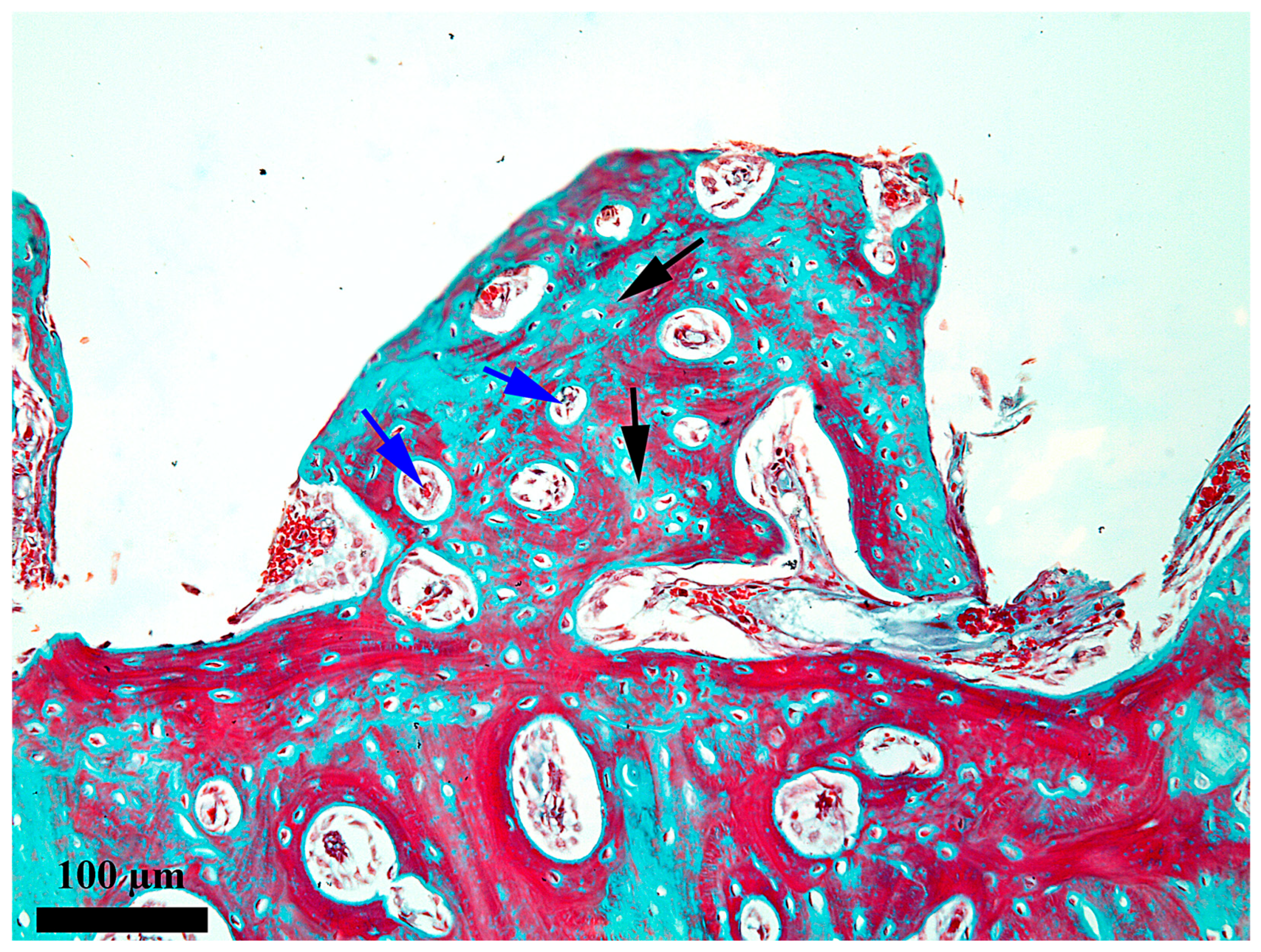
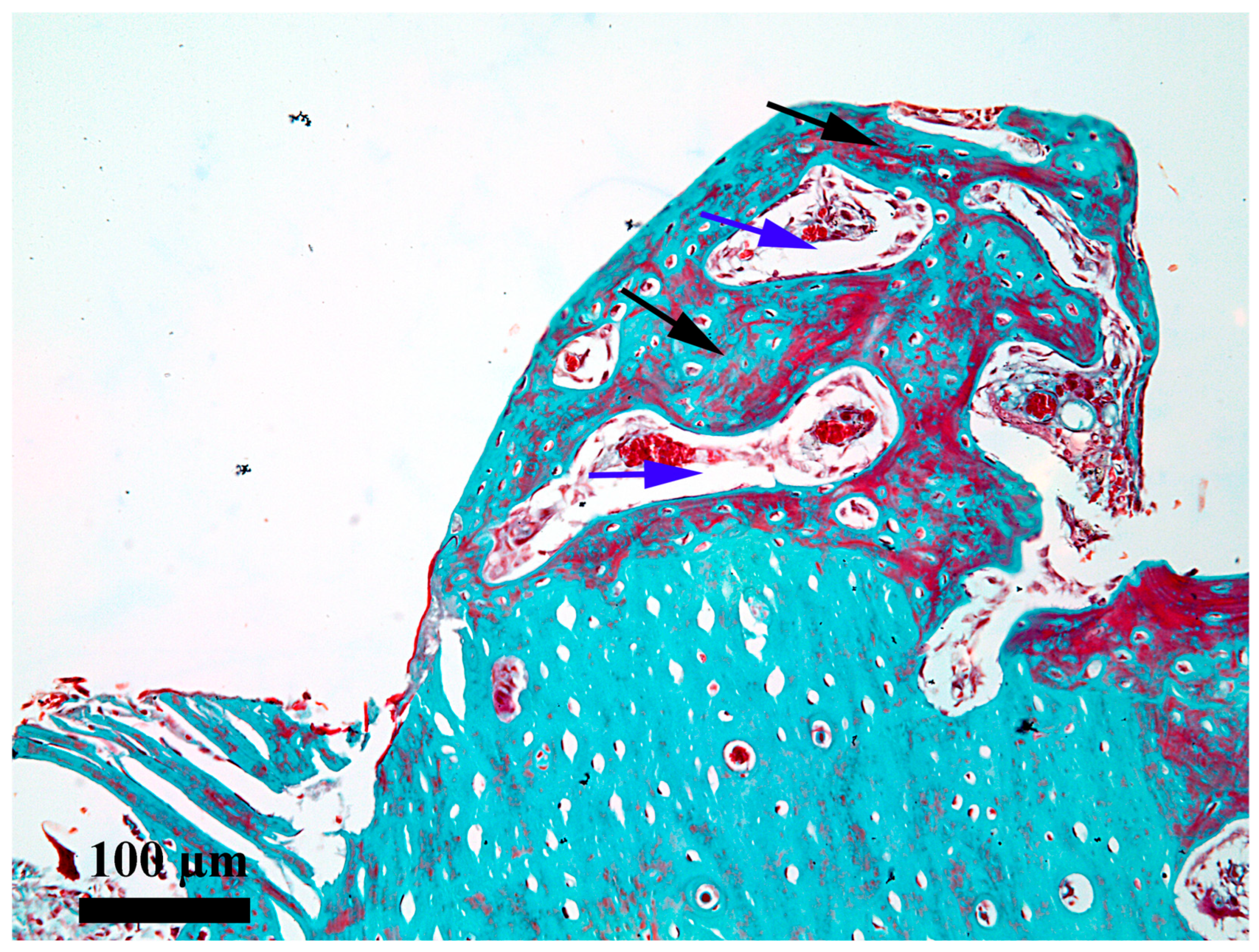
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liddell, R.S.; Davies, J.E. Biological Fixation: The Role of Screw Surface Design. In Manufacturing, and Industry Perspectives, Orthopedic Biomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 381–400. ISBN 978-3-319-89541-3. [Google Scholar] [CrossRef]
- Futami, T.; Fujii, N.; Ohnishi, H.; Taguchi, N.; Kusakari, H.; Ohshima, H.; Maeda, T. Tissue response to titanium implants in the rat maxilla: Ultrastructural and histochemical observations of the bone-titanium interface. J. Periodontol. 2000, 71, 287–298. [Google Scholar] [CrossRef] [PubMed]
- D’Albis, G.; Forte, M.; Alrashadah, A.O.; Marini, L.; Corsalini, M.; Pilloni, A.; Capodiferro, S. Immediate Loading of Implants-Supported Fixed Partial Prostheses in Posterior Regions: A Systematic Review. Dent. J. 2025, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, P.; Khorshidparast, S.; Rouhi, G. Investigation on primary stability of dental implants through considering peri-implant bone damage, caused by small and large deformations: A validated non-linear micro finite element study. J. Mech. Behav. Biomed. Mater. 2023, 146, 106062. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.W.; Inayat, N.; Zafar, M.S.; Zaigham, A.M. A resonance frequency analysis to investigate the impact of implant size on primary and secondary stability. Pak. J. Med. Sci. 2024, 40, 1261–1266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franchi, M.; Bacchelli, B.; Martini, D.; Pasquale, V.D.; Orsini, E.; Ottani, V.; Fini, M.; Giavaresi, G.; Giardino, R.; Ruggeri, A. Early detachment of titanium particles from various different surfaces of endosseous dental implants. Biomaterials 2004, 25, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Bijelic, A.; Ziebart, T.; Koch, F.; Kämmerer, P.W.; Wieland, M.; Konerding, M.A.; Al-Nawas, B. Submicron scale-structured hydrophilic titanium surfaces promote early osteogenic gene response for cell adhesion and cell differentiation. Clin. Implant Dent. Relat. Res. 2013, 15, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A. Characteristics of Implant Systems That Can Accelerate and Improve the Osseointegration Proces. In Current Concepts in Dental Implantology—From Science to Clinical Research; IntechOpen: London, UK, 2022; pp. 1–21. ISBN 978-1-83969-863-7. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Arunachalam, S.; Babu, Y.R.; Srivalli, L.; Srikanth, L.; Soni, S. A brief history of osseointegration: A review. IP Ann. Prosthodont. Restor. Dent. 2021, 7, 29–36. [Google Scholar] [CrossRef]
- Wilson, T.G., Jr.; Miller, R.J.; Trushkowsky, R.; Dard, M. Tapered Implants in Dentistry: Revitalizing Concepts with Technology: A Review. Adv. Dent. Res. 2016, 28, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E. Implant design considerations for the posterior regions of the mouth. Implant Dent. 1999, 8, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Climent, M.; Lázaro, P.; Vicente Rios, J.; Lluch, S.; Marqués, M.; Guillem-Martí, J.; Gil, F.J. Influence of acid-etching after grit-blasted on osseointegration of titanium dental implants: In vitro and in vivo studies. J. Mater. Sci. Mater. Med. 2013, 24, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Bonfante, E.; Granato, R.; Neiva, R.; Gil, L.F.; Marão, H.F.; Suzuki, M.; Coelho, P.G. The Effect of Osteotomy Dimension on Implant Insertion Torque, Healing Mode, and Osseointegration Indicators: A Study in Dogs. Implant Dent. 2016, 25, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Júnior, J.A.; Pérez-Díaz, L.; Treichel, T.L.E.; Dedavid, B.A.; de Aza, P.N.; Prados-Frutos, J.C. New Implant Macrogeometry to Improve and Accelerate the Osseointegration: An In Vivo Experimental Study. Appl. Sci. 2019, 9, 3181. [Google Scholar] [CrossRef]
- Mello-Machado, R.C.; de Almeida Barros Mourão, C.F.; Javid, K.; Ferreira, H.T.; Montemezzi, P.; Calasans-Maia, M.D.; Senna, P.M. Clinical Assessment of Dental Implants Placed in Low-Quality Bone Sites Prepared for the Healing Chamber with Osseodensification Concept: A Double-Blind, Randomized Clinical Trial. Appl. Sci. 2021, 11, 640. [Google Scholar] [CrossRef]
- Marin, C.; Granato, R.; Suzuki, M.; Gil, J.N.; Janal, M.N.; Coelho, P.G. Histomorphologic and histomorphometric evaluation of various endosseous implant healing chamber configurations at early implantation times: A study in dogs. Clin. Oral Implant. Res. 2010, 21, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Beutel, B.G.; Danna, N.R.; Granato, R.; Bonfante, E.A.; Marin, C.; Tovar, N.; Suzuki, M.; Coelho, P.G. Implant design and its effects on osseointegration over time within cortical and trabecular bone. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Mello-Machado, R.C.; Sartoretto, S.C.; Granjeiro, J.M.; Calasans-Maia, J.A.; de Uzeda, M.J.P.G.; Mourão, C.F.A.B.; Ghiraldini, B.; Bezerra, F.J.B.; Senna, P.M.; Calasans-Maia, M.D. Osseodensification enables bone healing chambers with improved low-density bone site primary stability: An in vivo study. Sci. Rep. 2021, 11, 15436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pilliar, R.M.; Lee, J.M.; Maniatopoulos, C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin. Orthop. Relat. Res. 1986, 208, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Søballe, K.; Hansen, E.S.; Brockstedt-Rasmussen, H.; Bünger, C. Hydroxyapatite coating converts fibrous tissue to bone around loaded implants. J. Bone Joint Surg. Br. 1993, 75, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Marcu, T.; Gal, A.F.; Ratiu, C.A.; Damian, A.; Ratiu, I.A. Adaptive structures proliferated in the rabbit shoulder after 8 weeks from the insertion of a titanium implant. J. Osseointegr. 2022, 14, 180–184. Available online: https://api.semanticscholar.org/CorpusID:268236368 (accessed on 28 November 2025).
- Kalsi, H.S.; Kalsi, S.K.; Patankar, V. Factors Influencing the Primary Success of Implant Stability in Dental Practice—A Review of Literature. IOSR J. Dent. Med. Sci. (IOSR-JDMS) 2020, 19, 8–14. [Google Scholar]
- Soto-Peñaloza, D.; Martín-de-Llano, J.J.; Carda-Batalla, C.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. Basic Bone Biology Healing During Osseointegration of Titanium Dental Implants. In Atlas of Immediate Dental Implant Loading; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Shah, F.A.; Snis, A.; Matic, A.; Thomsen, P.; Palmquist, A. 3D printed Ti6Al4V implant surface promotes bone maturation and retains a higher density of less aged osteocytes at the bone-implant interface. Acta Biomater. 2016, 30, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Stenlund, P.; Kurosu, S.; Koizumi, Y.; Suska, F.; Matsumoto, H.; Chiba, A.; Palmquist, A. Osseointegration Enhancement by Zr doping of Co-Cr-Mo Implants Fabricated by Electron Beam Melting. Addit. Manuf. 2015, 6, 6–15. [Google Scholar] [CrossRef]
- Stangl, R.; Pries, A.; Loos, B.; Müller, M.; Erben, R.G. Influence of pores created by laser superfinishing on osseointegration of titanium alloy implants. J. Biomed. Mater. Res. A 2004, 69, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, P.R.; Schemitsch, E.H. The basic science of peri-implant bone healing. Indian J. Orthop. 2011, 45, 108–115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, J.E.; Hosseini, M.M. Histodynamics of endosseous wound healing. In Proceedings of the International Bone Engineering Workshop; Davies, J.E., Ed.; Em Squared Incorporated: Toronto, ON, Canada, 2000; pp. 1–14. [Google Scholar]
- Batool, F.; Özçelik, H.; Stutz, C.; Gegout, P.Y.; Benkirane-Jessel, N.; Petit, C.; Huck, O. Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J. Tissue Eng. 2021, 12, 20417314211041428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J. Systematic approach to characterize the dynamics of protein adsorption on the surface of biomaterials using proteomics. Colloids Surf. B Biointerfaces 2020, 188, 110756. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.B.; Kyriakides, T.R. Molecular Characterization of Macrophage-Biomaterial Interactions. Adv. Exp. Med. Biol. 2015, 865, 109–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wahab, M.A.B.; Yusof, E.M.; Ahmad, R.; Salleh, M.Z.; Kek, T.L. Peri-implant Bone Healing: Its Basic Osteogenesis and Biomarkers. Malays. J. Med. Health Sci. 2022, 18, 324–331. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Davies, J.E. Red blood cell and platelet interactions with titanium implant surfaces. Clin. Oral Implant Res. 2000, 11, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, C.A.; Ratiu, I.A.; Miclaus, V.; Pantor, M.; Rus, V.; Martonos, C.O.; Lacatus, R.; Purdoiu, R.C.; Gal, A.F. The influence of haematogenous bone marrow on the early osseointegration of a titanium implant which penetrates the endosteum. Int. J. Morphol. 2022, 40, 188–193. [Google Scholar] [CrossRef]
- Scarano, A.; Degidi, M.; Perrotti, V.; Degidi, D.; Piattelli, A.; Iezzi, G. Experimental evaluation in rabbits of the effects of thread concavities in bone formation with different titanium implant surfaces. Clin. Implant Dent. Relat. Res. 2014, 16, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Moreo, P.; García-Aznar, J.M.; Doblaré, M. Bone ingrowth on the surface of endosseous implants. Part 1: Mathematical model. J. Theor. Biol. 2009, 260, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moreo, P.; García-Aznar, J.M.; Doblaré, M. Bone ingrowth on the surface of endosseous implants. Part 2: Theoretical and numerical analysis. J. Theor. Biol. 2009, 260, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Blood Inflammation, wound healing and foreign body response. In Biomaterials Science; Academic Press: New York, NY, USA, 2013; pp. 503–512. [Google Scholar] [CrossRef]
- Einhorn, T.A. The science of fracture healing. J. Orthop. Trauma 2005, 19, S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Cintra, C.V.; Sepúlveda, R.V.; Valente, F.L.; Reis, E.C.C.; Borges, A.P.B. Cellular interactions with implanted surfaces in the living organism and osseointegration of implants. Rev. Saúde Meio Ambiente 2021, 1, 102–120. Available online: https://periodicos.ufms.br/index.php/sameamb/article/view/12314 (accessed on 28 November 2025).
- Kopf, B.S.; Ruch, S.; Berner, S.; Spencer, N.D.; Maniura-Weber, K. The role of nanostructures and hydrophilicity in osseointegration: In-vitro protein-adsorption and blood-interaction studies. J. Biomed. Mater. Res. A 2015, 103, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Joos, U.; Mythili, J.; Stamm, T.; Hohoff, A.; Fillies, T.; Stratmann, U.; Wiesmann, H.P. Ultrastructural characterization of the implant/bone interface of immediately loaded dental implants. Biomaterials 2004, 25, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, D.; Lang, N.P. Dynamics of osseointegration in various human and animal models—A comparative analysis. Clin. Oral. Implant. Res. 2017, 28, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Ravidà, A.; Wang, H.L.; Helms, J.A.; Brunski, J.B. Relationship Between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral Maxillofac. Implants 2019, 34, s7–s23. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, C.A.; Sinescu, C.; Dejeu, D.; Tica, O.; Moisa, C.; Croitoru, C.A.; Ratiu, I.A.; Duma, V.F.; Todor, A.; Miclaus, V.; et al. Participation of the Periosteum, Endosteum, and Hematogenous Marrow in the Early Osseointegration of a Titanium Implant Inserted in Contact with the Hematogenous Marrow. Medicina 2025, 61, 1841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jimbo, R.; Tovar, N.; Anchieta, R.B.; Machado, L.S.; Marin, C.; Teixeira, H.S.; Coelho, P.G. The combined effects of undersized drilling and implant macrogeometry on bone healing around dental implants: An experimental study. Int. J. Oral Maxillofac. Surg. 2014, 43, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Tumedei, M.; Aramburú Júnior, J.; Treichel, T.L.E.; Kolerman, R.; Lepore, S.; Piattelli, A.; Iezzi, G. Histological and Histomorphometrical Evaluation of a New Implant Macrogeometry. A Sheep Study. Int. J. Environ. Res. Public Health 2020, 17, 3477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gehrke, S.A.; Aramburú JJúnior Pérez-Díaz, L.; do Prado, T.D.; Dedavid, B.A.; Mazon, P.; NDe Aza, P. Can changes in implant macrogeometry accelerate the osseointegration process?: An in vivo experimental biomechanical and histological evaluations. PLoS ONE 2020, 15, e0233304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonfante, E.A.; Granato, R.; Marin, C.; Suzuki, M.; Oliveira, S.R.; Giro, G.; Coelho, P.G. Early bone healing and biomechanical fixation of dual acid-etched and as-machined implants with healing chambers: An experimental study in dogs. Int. J. Oral Maxillofac. Implant. 2011, 26, 75–82. [Google Scholar] [PubMed]
- Ribeiro, A.K.C.; Costa, R.T.F.; Vasconcelos, B.C.D.E.; de Moraes, S.L.D.; Carreiro, A.D.F.P.; Pellizzer, E.P. Patient-reported outcome measures and prosthetic events in implant-supported mandibular overdenture patients after immediate versus delayed loading: A systematic review and meta-analysis. J. Prosthet. Dent. 2024, 131, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, F.; Zhao, Y.; Sun, Q.; Xia, H.; Xia, D.; Bai, Y. Immediate Versus Non-immediate Loading Protocols for Reduced-Diameter Implants Supporting Overdentures: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2024, 39, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Sadilina, S.; Park, S.H.; Chantler, J.; Park, J.Y.; Thoma, D.; Cha, J.K.; Strauss, F.J. Immediate loading of definitive restorations in partially edentulous patients requiring an implant-supported prosthesis: A scoping review. J. Prosthet. Dent. 2025, 134, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Gonzaga, L.; Amorim, K.; Wittneben, J.G.; Martig, L.; Morton, D.; Martin, W.; Gallucci, G.O.; Wismeijer, D. Selection criteria for immediate implant placement and immediate loading for single tooth replacement in the maxillary esthetic zone: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2023, 26, 304–348. [Google Scholar] [CrossRef] [PubMed]
- Huwais, S.; Meyer, E.G. A Novel Osseous Densification Approach in Implant Osteotomy Preparation to Increase Biomechanical Primary Stability, Bone Mineral Density, and Bone-to-Implant Contact. Int. J. Oral Maxillofac. Implants 2017, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zarei, I.; Khajehpour, S.; Sabouri, A.; Haghnegahdar Az Jafari, K. Assessing the Effect of Dental Implants Thread Design on Distribution of Stress in Impact Loadings Using Three Dimensional Finite Element Method. J. Dent. Biomater. 2016, 3, 233–240. [Google Scholar] [PubMed] [PubMed Central]
- Zhang, G.; Yuan, H.; Chen, X.; Wang, W.; Chen, J.; Liang, J.; Zhang, P. A Three-Dimensional Finite Element Study on the Biomechanical Simulation of Various Structured Dental Implants and Their Surrounding Bone Tissues. Int. J. Dent. 2016, 2016, 4867402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.C.; Tsai, P.I.; Huang, C.C.; Chen, S.Y.; Chao, C.G.; Tsou, N.T. Numerical Method for the Design of Healing Chamber in Additive-Manufactured Dental Implants. Biomed. Res. Int. 2017, 2017, 1970680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duma, V.; Gal, A.F.; Rus, V.; Matei-Latiu, M.C.; Rațiu, C.A.; Alexandru, B.C.; Latiu, C.; Martonos, C.; Oană, L.I. Comparative assessment of contact osteogenesis at the titanium implant-bone junction in male rabbits with dissimilar femoral defects. Int. J. Morphol. 2023, 41, 1317–1322. Available online: https://www.scielo.cl/pdf/ijmorphol/v41n5/0717-9502-ijmorphol-41-05-1317.pdf (accessed on 28 November 2025). [CrossRef]
- Hartung, T. Thoughts on limitations of animal models. Park. Relat Disord. 2008, 2, S81–S83. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ratiu, C.A.; Dejeu, D.; Croitoru, C.A.; Todor, A.; Ratiu, I.A.; Luca, R.E.; Moisa, C.; Miclaus, V.; Rus, V. Early Osseous Proliferation in Spiraled Healing Chambers Resulted After the Insertion of Titanium Implants in Cortical Bone of a Rabbit. Medicina 2026, 62, 72. https://doi.org/10.3390/medicina62010072
Ratiu CA, Dejeu D, Croitoru CA, Todor A, Ratiu IA, Luca RE, Moisa C, Miclaus V, Rus V. Early Osseous Proliferation in Spiraled Healing Chambers Resulted After the Insertion of Titanium Implants in Cortical Bone of a Rabbit. Medicina. 2026; 62(1):72. https://doi.org/10.3390/medicina62010072
Chicago/Turabian StyleRatiu, Cristian Adrian, Danut Dejeu, Camelia Anca Croitoru, Adrian Todor, Ioana Adela Ratiu, Ruxandra Elena Luca, Corina Moisa, Viorel Miclaus, and Vasile Rus. 2026. "Early Osseous Proliferation in Spiraled Healing Chambers Resulted After the Insertion of Titanium Implants in Cortical Bone of a Rabbit" Medicina 62, no. 1: 72. https://doi.org/10.3390/medicina62010072
APA StyleRatiu, C. A., Dejeu, D., Croitoru, C. A., Todor, A., Ratiu, I. A., Luca, R. E., Moisa, C., Miclaus, V., & Rus, V. (2026). Early Osseous Proliferation in Spiraled Healing Chambers Resulted After the Insertion of Titanium Implants in Cortical Bone of a Rabbit. Medicina, 62(1), 72. https://doi.org/10.3390/medicina62010072






