Impact of Leukapheresis and Biological Risk Markers on Early Mortality in Patients with Hyperleukocytic Acute Myeloid Leukemia
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient-Related and Leukemia-Related Characteristics
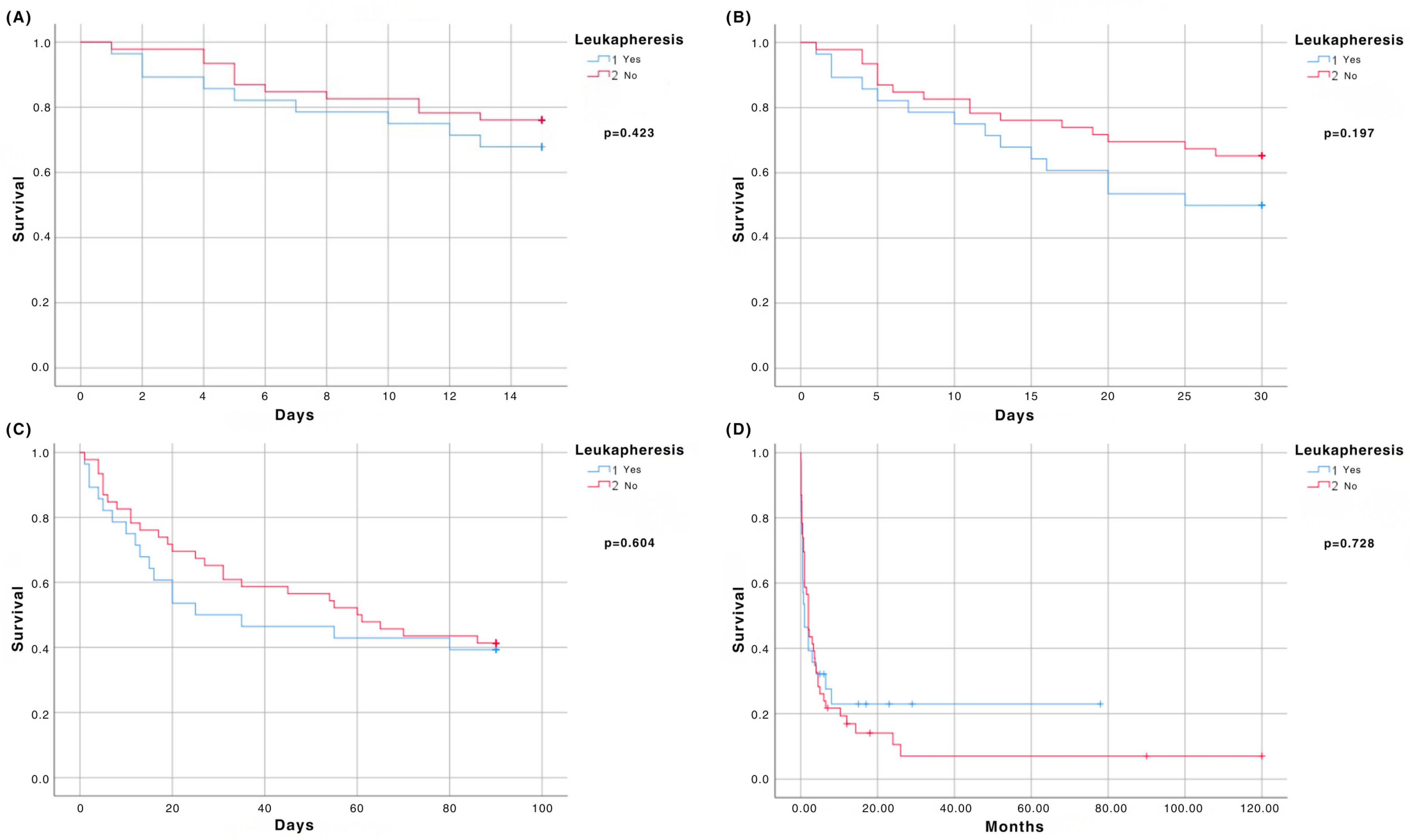
3.2. Early Mortality Analysis (15-, 30-, 90-Day)
3.3. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| AML | Acute myeloid leukemia |
| BM | Bone marrow |
| DIC | Disseminated intravascular coagulation |
| ECOG PS | Eastern Cooperative Oncology Group performance status |
| ELN | European LeukemiaNet |
| EMD | Extramedullary disease |
| FLT3 | FMS-like tyrosine kinase 3 |
| Hb | Hemoglobin |
| HCT-CI | Hematopoietic cell transplantation specific comorbidity index |
| HL | Hyperleukocytosis |
| LA | Leukapheresis |
| LDH | Lactate dehydrogenase |
| OS | Overall survival |
| PB | Peripheral blood |
| Plt | Platelet |
| TLS | Tumor lysis syndrome |
| WBC | White blood cell |
| WHO | World Health Organization |
References
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; Stahl, M.; Stone, R.M. Acute Myeloid Leukemia: 2025 Update on Diagnosis, Risk-stratification, and Management. Am. J. Hematol. 2025, 100, 860–891. [Google Scholar] [CrossRef] [PubMed]
- Pastore, F.; Pastore, A.; Wittmann, G.; Hiddemann, W.; Spiekermann, K. The Role of Therapeutic Leukapheresis in Hyperleukocytotic AML. PLoS ONE 2014, 9, e95062. [Google Scholar] [CrossRef] [PubMed]
- Ganzel, C.; Becker, J.; Mintz, P.D.; Lazarus, H.M.; Rowe, J.M. Hyperleukocytosis, Leukostasis and Leukapheresis: Practice Management. Blood Rev. 2012, 26, 117–122. [Google Scholar] [CrossRef]
- Rinaldi, I.; Sutandyo, N.; Winston, K. Comparison of Early Mortality between Leukapheresis and Non-Leukapheresis in Adult Acute Myeloid Leukemia Patients with Hyperleukocytosis: A Systematic Review and Meta-Analysis. Hematology 2022, 27, 141–149. [Google Scholar] [CrossRef]
- Röllig, C.; Ehninger, G. How I Treat Hyperleukocytosis in Acute Myeloid Leukemia. Blood 2015, 125, 3246–3252. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Zeidan, A.M. Hyperleukocytosis and Leukostasis in Acute Myeloid Leukemia: Can a Better Understanding of the Underlying Molecular Pathophysiology Lead to Novel Treatments? Cells 2020, 9, 2310. [Google Scholar] [CrossRef]
- Shallis, R.M.; Stahl, M.; Bewersdorf, J.P.; Hendrickson, J.E.; Zeidan, A.M. Leukocytapheresis for Patients with Acute Myeloid Leukemia Presenting with Hyperleukocytosis and Leukostasis: A Contemporary Appraisal of Outcomes and Benefits. Expert Rev. Hematol. 2020, 13, 489–499. [Google Scholar] [CrossRef]
- Lichtman, M.A.; Rowe, J.M. Hyperleukocytic Leukemias: Rheological, Clinical, and Therapeutic Considerations. Blood 1982, 60, 279–283. [Google Scholar] [CrossRef]
- Korkmaz, S. The Management of Hyperleukocytosis in 2017: Do We Still Need Leukapheresis? Transfus. Apher. Sci. 2018, 57, 4–7. [Google Scholar] [CrossRef]
- Porcu, P.; Cripe, L.D.; Ng, E.W.; Bhatia, S.; Danielson, C.M.; Orazi, A.; McCarthy, L.J. Hyperleukocytic Leukemias and Leukostasis: A Review of Pathophysiology, Clinical Presentation and Management. Leuk. Lymphoma 2000, 39, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stucki, A.; Rivier, A.-S.; Gikic, M.; Monai, N.; Schapira, M.; Spertini, O. Endothelial Cell Activation by Myeloblasts: Molecular Mechanisms of Leukostasis and Leukemic Cell Dissemination. Blood 2001, 97, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Hölig, K.; Moog, R. Leukocyte Depletion by Therapeutic Leukocytapheresis in Patients with Leukemia. Transfus. Med. Hemother. 2012, 39, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Powell, B.L.; Gregory, B.W.; Evans, J.K.; White, J.C.; Lyerly, E.S.; Chorley, H.M.; Russell, G.B.; Capizzi, R.L. Leukapheresis Induced Changes in Cell Cycle Distribution and Nucleoside Transporters in Patients with Untreated Acute Myeloid Leukemia. Leukemia 1991, 5, 1037–1042. [Google Scholar]
- Padmanabhan, A.; Connelly-Smith, L.; Aqui, N.; Balogun, R.A.; Klingel, R.; Meyer, E.; Pham, H.P.; Schneiderman, J.; Witt, V.; Wu, Y.; et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J. Clin. Apher. 2019, 34, 171–354. [Google Scholar] [CrossRef]
- Bug, G.; Anargyrou, K.; Tonn, T.; Bialleck, H.; Seifried, E.; Hoelzer, D.; Ottmann, O.G. Impact of Leukapheresis on Early Death Rate in Adult Acute Myeloid Leukemia Presenting with Hyperleukocytosis. Transfusion 2007, 47, 1843–1850. [Google Scholar] [CrossRef]
- Nan, X.; Qin, Q.; Gentille, C.; Ensor, J.; Leveque, C.; Pingali, S.R.; Phan, A.T.; Rice, L.; Iyer, S. Leukapheresis Reduces 4-Week Mortality in Acute Myeloid Leukemia Patients with Hyperleukocytosis—A Retrospective Study from a Tertiary Center. Leuk. Lymphoma 2017, 58, 2110–2117. [Google Scholar] [CrossRef]
- Lee, H.; Han, J.H.; Kim, J.K.; Yoo, J.; Cho, H.S.; Yoon, J.-H.; Cho, B.S.; Kim, H.-J.; Lim, J.; Jekarl, D.W.; et al. Effectiveness of Leukapheresis on Early Survival in Acute Myeloid Leukemia: An Observational Propensity Score Matching Cohort Study. J. Clin. Apher. 2023, 38, 727–737. [Google Scholar] [CrossRef]
- Stahl, M.; Shallis, R.M.; Wei, W.; Montesinos, P.; Lengline, E.; Neukirchen, J.; Bhatt, V.R.; Sekeres, M.A.; Fathi, A.T.; Konig, H.; et al. Management of Hyperleukocytosis and Impact of Leukapheresis among Patients with Acute Myeloid Leukemia (AML) on Short- and Long-Term Clinical Outcomes: A Large, Retrospective, Multicenter, International Study. Leukemia 2020, 34, 3149–3160. [Google Scholar] [CrossRef]
- Rinaldi, I.; Sari, R.M.; Tedhy, V.U.; Winston, K. Leukapheresis Does Not Improve Early Survival Outcome of Acute Myeloid Leukemia with Leukostasis Patients—A Dual-Center Retrospective Cohort Study. J. Blood Med. 2021, 12, 623–633. [Google Scholar] [CrossRef]
- Choi, M.H.; Choe, Y.H.; Park, Y.; Nah, H.; Kim, S.; Jeong, S.H.; Kim, H.O. The Effect of Therapeutic Leukapheresis on Early Complications and Outcomes in Patients with Acute Leukemia and Hyperleukocytosis: A Propensity Score-matched Study. Transfusion 2018, 58, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.H.M.; Callum, J.L.; Panzarella, T.; Jacks, L.M.; Brandwein, J.; Crump, M.; Curtis, J.E.; Gupta, V.; Lipton, J.H.; Minden, M.D.; et al. A Retrospective Observational Study of Leucoreductive Strategies to Manage Patients with Acute Myeloid Leukaemia Presenting with Hyperleucocytosis. Br. J. Haematol. 2015, 168, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cuadrón, D.; Montesinos, P.; Moscardo, F.; Lopez, L.; Martin, G.; Solves, P.; Boluda, B.; Pérez-Sirvent, M.; Vera, B.; Navarro, I.; et al. Treatment with Leukapheresis in Patients Diagnosed with Hyperleukocytic Acute Myeloid Leukemia. Blood 2013, 122, 5046. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations from an International Expert Panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and Response Criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Sorror, M.L.; Storer, B.E.; Fathi, A.T.; Gerds, A.T.; Medeiros, B.C.; Shami, P.; Brunner, A.M.; Sekeres, M.A.; Mukherjee, S.; Peña, E.; et al. Development and Validation of a Novel Acute Myeloid Leukemia–Composite Model to Estimate Risks of Mortality. JAMA Oncol. 2017, 3, 1675–1682. [Google Scholar] [CrossRef]
- Suzuki, K.; Wada, H.; Imai, H.; Iba, T.; Thachil, J.; Toh, C.-H. Subcommittee on Disseminated Intravascular Coagulation. A Re-Evaluation of the D-Dimer Cut-off Value for Making a Diagnosis According to the ISTH Overt-DIC Diagnostic Criteria: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1442–1444. [Google Scholar] [CrossRef]
- Cairo, M.S.; Coiffier, B.; Reiter, A.; Younes, A. TLS Expert Panel. Recommendations for the Evaluation of Risk and Prophylaxis of Tumour Lysis Syndrome (TLS) in Adults and Children with Malignant Diseases: An Expert TLS Panel Consensus. Br. J. Haematol. 2010, 149, 578–586. [Google Scholar] [CrossRef]
- Pereira, M.; Rodrigues, N.; Godinho, I.; Gameiro, J.; Neves, M.; Gouveia, J.; Costa E Silva, Z.; Lopes, J.A. Acute Kidney Injury in Patients with Severe Sepsis or Septic Shock: A Comparison between the “Risk, Injury, Failure, Loss of Kidney Function, End-Stage Kidney Disease” (RIFLE), Acute Kidney Injury Network (AKIN) and Kidney Disease: Improving Global Outcomes (KDIGO) Classifications. Clin. Kidney J. 2017, 10, 332–340. [Google Scholar] [CrossRef]
- Pastore, F.; Pastore, A.; Rothenberg-Thurley, M.; Metzeler, K.H.; Ksienzyk, B.; Schneider, S.; Bohlander, S.K.; Braess, J.; Sauerland, M.C.; Görlich, D.; et al. Molecular Profiling of Patients with Cytogenetically Normal Acute Myeloid Leukemia and Hyperleukocytosis. Cancer 2022, 128, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Pagano, L. The Important Role of Intensive Induction Chemotherapy in the Treatment of Acute Myeloid Leukemia. Expert Rev. Hematol. 2021, 14, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Shallis, R.M.; Stahl, M.; Wei, W.; Montesinos, P.; Lengline, E.; Neukirchen, J.; Bhatt, V.R.; Sekeres, M.A.; Fathi, A.T.; Konig, H.; et al. Patterns of Care and Clinical Outcomes of Patients with Newly Diagnosed Acute Myeloid Leukemia Presenting with Hyperleukocytosis Who Do Not Receive Intensive Chemotherapy. Leuk. Lymphoma 2020, 61, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Porcu, P.; Farag, S.; Marcucci, G.; Cataland, S.R.; Kennedy, M.S.; Bissell, M. Leukocytoreduction for Acute Leukemia. Ther. Apher. 2002, 6, 15–23. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, Y.; Jin, Y.; Kaweme, N.M.; Dong, Y. Leukapheresis and Hyperleukocytosis, Past and Future. Int. J. Gen. Med. 2021, 14, 3457–3467. [Google Scholar] [CrossRef]
- Gönen, M.; Sun, Z.; Figueroa, M.E.; Patel, J.P.; Abdel-Wahab, O.; Racevskis, J.; Ketterling, R.P.; Fernandez, H.; Rowe, J.M.; Tallman, M.S.; et al. CD25 Expression Status Improves Prognostic Risk Classification in AML Independent of Established Biomarkers: ECOG Phase 3 Trial, E1900. Blood 2012, 120, 2297–2306. [Google Scholar] [CrossRef]
- Li, J.; Ran, Q.; Xu, B.; Luo, X.; Song, S.; Xu, D.; Zhang, X. Role of CD25 Expression on Prognosis of Acute Myeloid Leukemia: A Literature Review and Meta-Analysis. PLoS ONE 2020, 15, e0236124. [Google Scholar] [CrossRef]
- Farid, K.M.N.; Sauer, T.; Schmitt, M.; Müller-Tidow, C.; Schmitt, A. Symptomatic Patients with Hyperleukocytic FLT3-ITD Mutated Acute Myeloid Leukemia Might Benefit from Leukapheresis. Cancers 2023, 16, 58. [Google Scholar] [CrossRef]
| All HL n = 74 | LA n = 28 | Non-LA n = 46 | p Value | |
|---|---|---|---|---|
| Age (median, range) | 55 (18–81) | 50 (19–80) | 58.5 (18–81) | 0.198 |
| Age > 60 years (n, %) | ||||
| Yes | 28 (37.8) | 8 (28.6) | 20 (43.5) | 0.200 |
| No | 46 (62.2) | 20 (71.4) | 26 (56.5) | |
| Sex (n, %) | ||||
| Male | 40 (54.1) | 14 (50) | 26 (56.5) | 0.585 |
| Female | 34 (45.9) | 14 (50) | 20 (43.5) | |
| M:F ratio | 1.17 | 1 | 1.3 | |
| ECOG PS ≥ 2 | ||||
| Yes | 26 (35.1) | 9 (32.1) | 17 (36.9) | 0.674 |
| No | 48 (64.9) | 19 (67.9) | 29 (63.1) | |
| HCT-CI (n,%) | ||||
| Low (0–2) | 49 (66.2) | 22 (78.6) | 27 (58.7) | 0.08 |
| High (>2) | 25 (33.8) | 6 (21.4) | 19 (41.3) | |
| Comorbidities (n, %) | ||||
| 0 | 36 (48.6) | 15 (53.6) | 21 (45.6) | 0.584 |
| 1 | 25 (33.8) | 9 (32.1) | 16 (34.8) | 0.816 |
| ≥2 | 13 (17.6) | 4 (14.3) | 9 (19.6) | 0.522 |
| CV comorbidity (n, %) | ||||
| Yes | 28 (37.8) | 10 (35.7) | 18 (39.1) | 0.769 |
| No | 46 (62.2%) | 18 (64.3) | 28 (60.9) | |
| WBC (×109/L) (median, range) | 161 (100.2–473.2) | 194 (103–470) | 141 (100.2–473.2) | 0.018 |
| Hemoglobin (g/L) (median, range) | 95.5 (30–140) | 95 (30–138) | 96 (41–140) | 0.369 |
| Platelet count (×109/L) (median, range) | 54 (11–284) | 45.5 (11–284) | 61 (14–244) | 0.107 |
| LDH (U/L) (median, range) | 1454.4 (219–8505) | 1802 (324–8505) | 1164 (219–6680) | 0.024 |
| Monocytes PB (median, range) | 4 (0–88) | 3 (0–88) | 4 (0–86) | 1.00 |
| PB blasts (median %, range) | 78 (0–100) | 85 (6–100) | 71 (0–98) | 0.477 |
| BM blasts (median %, range) | 75 (20–96) | 79.5 (28–96) | 73.5 (20–92) | 0.409 |
| Extramedullary disease (n, %) | ||||
| Yes | 50 (67.6) | 18 (64.3) | 32 (69.6) | 0.638 |
| No | 24 (32.4) | 10 (35.7) | 14 (30.4) | |
| Cytogenetics (n, %) | ||||
| Favorable and intermediate | 55 (74.3) | 20 (71.4) | 35 (76.1) | 1.00 |
| Unfavorable | 9 (12.2) | 3 (10.7) | 6 (13) | |
| Missing | 10 (13.5) | 5 (17.9) | 5 (10.9) | |
| NPM1 (n,%) | ||||
| Mutated | 15 (20.3) | 7 (25) | 8 (17.4) | 0.230 |
| Wild-type | 21 (28.4) | 14 (50) | 7 (15.2) | |
| Missing | 38 (51.3) | 7 (25) | 31 (67.4) | |
| FLT3 (n,%) | ||||
| Mutated | 25 (33.8) | 11 (39.3) | 14 (30.4) | 0.302 |
| Wild-type | 22 (29.7) | 13 (46.4) | 9 (19.6) | |
| Missing | 27 (36.5) | 4 (14.3) | 23 (50) | |
| CD56 (n,%) | ||||
| Positive | 27 (36.5) | 10 (35.7) | 17 (36.9) | 0.911 |
| Negative | 42 (56.7) | 15 (53.6) | 27 (58.7) | |
| Missing | 5 (6.8) | 3 (10.7) | 2 (4.4) | |
| CD117 (n,%) | ||||
| Positive | 59 (79.7) | 21 (75) | 38 (82.6) | 0.715 |
| Negative | 9 (12.2) | 4 (14.3) | 5 (10.9) | |
| Missing | 6 (8.1) | 3 (10.7) | 3 (6.5) | |
| CD7 (n,%) | ||||
| Positive | 22 (29.7) | 9 (32.2) | 13 (28.3) | 0.670 |
| Negative | 45 (60.8) | 16 (57.1) | 29 (63) | |
| Missing | 7 (9.5) | 3 (10.7) | 4 (8.7) | |
| CD25 (n,%) | ||||
| Positive | 28 (37.8) | 12 (42.9) | 16 (34.8) | 0.712 |
| Negative | 34 (45.9) | 13 (46.4) | 21 (45.6) | |
| Missing | 12 (16.2) | 3 (10.7) | 9 (19.6) | |
| CD11c (n, %) | ||||
| Positive | 54 (73) | 19 (67.9) | 35 (76.1) | |
| Negative | 9 (12.2) | 5 (17.8) | 4 (8.7) | 0.283 |
| Missing | 11 (14.8) | 4 (14.3) | 7 (15.2) | |
| TLS (n, %) | ||||
| Yes | 10 (13.5) | 4 (14.3) | 6 (13) | 1.00 |
| No | 64 (86.5) | 24 (85.7) | 40 (87) | |
| AKI (n, %) | ||||
| Yes | 19 (25.7) | 8 (28.6) | 11 (23.9) | 0.565 |
| No | 55 (74.3) | 20 (71.4) | 35 (76.1) | |
| DIC (n, %) | ||||
| Yes | 45 (60.8) | 18 (64.3) | 27 (58.7) | 0.633 |
| No | 29 (39.2) | 10 (35.7) | 19 (41.3) | |
| Treatment (n, %) | ||||
| Intensive | 44 (59.5) | 18 (64.3) | 26 (56.5) | 0.509 |
| Non-intensive and palliative | 30 (40.5) | 10 (35.7) | 20 (43.5) | |
| Allo-SCT (n, %) | ||||
| Yes | 7 (9.5) | 3 (10.7) | 4 (8.7) | 0.705 |
| No | 67 (90.5) | 25 (89.3) | 42 (91.3) |
| Parameters | 15-Day Mortality | 30-Day Mortality | 90-Day Mortality |
|---|---|---|---|
| Age | |||
| All | p = 0.375 | p = 0.616 | p = 0.345 |
| LA | p = 0.264 | p = 0.342 | p = 0.120 |
| non-LA | p = 0.739 | p = 0.916 | p = 0.939 |
| ECOG PS ≥ 2 | |||
| All | p < 0.01 | p < 0.01 | p < 0.01 |
| LA | p = 0.01 | p = 0.003 | p < 0.01 |
| non-LA | p = 0.002 | p < 0.01 | p = 0.003 |
| HCT-CI > 2 | |||
| All | p = 0.871 | p = 0.420 | p = 0.809 |
| LA | p = 0.407 | p = 0.419 | p = 0.492 |
| non-LA | p = 0.301 | p = 0.888 | p = 0.357 |
| ≥2 comorbidities | |||
| All | p = 0.462 | p = 0.897 | p = 0.297 |
| LA | p = 0.455 | p = 0.806 | p = 0.904 |
| non-LA | p = 0.657 | p = 0.810 | p = 0.156 |
| CV comorbidity | |||
| All | p = 0.416 | p = 0.657 | p = 0.182 |
| LA | p = 0.456 | p = 0.833 | p = 0.913 |
| non-LA | p = 0.652 | p = 0.638 | p = 0.093 |
| EMD | |||
| All | p = 0.205 | p = 0.110 | p = 0.259 |
| LA | p = 0.205 | p = 0.404 | p = 0.398 |
| non-LA | p = 0.654 | p = 0.182 | p = 0.479 |
| WBC count | |||
| All | p = 0.295 | p = 0.032 | p = 0.071 |
| LA | p = 0.669 | p = 0.334 | p = 0.109 |
| non-LA | p = 0.072 | p = 0.097 | p = 0.540 |
| Hb | |||
| All | p = 0.444 | p = 0.354 | p = 0.263 |
| LA | p = 0.641 | p = 0.638 | p = 0.813 |
| non-LA | p = 0.421 | p = 0.256 | p = 0.118 |
| PLT count | |||
| All | p = 0.198 | p = 0.225 | p = 0.118 |
| LA | p = 0.596 | p = 0.935 | p = 0.472 |
| non-LA | p = 0.157 | p = 0.055 | p = 0.096 |
| Monocytes PB | |||
| All | p = 0.897 | p = 0.506 | p = 0.125 |
| LA | p = 0.031 | p = 0.107 | p = 0.285 |
| Non-LA | p = 0.096 | p = 0.088 | p = 0.017 |
| LDH | |||
| All | p < 0.01 | p < 0.01 | p < 0.01 |
| LA | p = 0.021 | p = 0.097 | p = 0.068 |
| non-LA | p < 0.01 | p < 0.01 | p < 0.01 |
| DIC | |||
| All | p = 0.01 | p = 0.018 | p = 0.207 |
| LA | p = 0.131 | p = 0.116 | p = 0.247 |
| non-LA | p = 0.045 | p = 0.089 | p = 0.520 |
| TLS | |||
| All | p = 0.01 | p = 0.002 | p = 0.017 |
| LA | p = 0.033 | p = 0.004 | p = 0.004 |
| non-LA | p = 0.120 | p = 0.075 | p = 0.382 |
| AKI | |||
| All | p = 0.001 | p = 0.001 | p = 0.015 |
| LA | p = 0.037 | p = 0.037 | p = 0.025 |
| Non-LA | p = 0.007 | p = 0.010 | p = 0.225 |
| PB blast % | |||
| All | p = 0.375 | p = 0.072 | p = 0.036 |
| LA | p = 0.150 | p = 0.545 | p = 0.943 |
| non-LA | p = 0.056 | p = 0.021 | p = 0.014 |
| BM blast % | |||
| All | p = 0.679 | p = 0.279 | p = 0.238 |
| LA | p = 0.261 | p = 0.786 | p = 0.950 |
| non-LA | p = 0.138 | p = 0.137 | p = 0.175 |
| Unfavorable cytogenetics | |||
| All | p = 0.525 | p = 0.848 | p = 0.892 |
| LA | p = 0.052 | p = 0.284 | p = 0.118 |
| non-LA | p = 0.506 | p = 0.812 | p = 0.872 |
| FLT3 | |||
| All | p = 0.069 | p = 0.107 | p = 0.144 |
| LA | p = 0.065 | p = 0.177 | p = 0.083 |
| non-LA | p = 0.521 | p = 0.351 | p = 0.573 |
| NPM1 | |||
| All | p = 0.125 | p = 0.329 | p = 0.169 |
| LA | p = 0.057 | p = 0.099 | p = 0.094 |
| non-LA | p = 0.953 | p = 0.201 | p = 0.460 |
| CD56 | |||
| All | p = 0.513 | p = 0.769 | p = 0.435 |
| LA | p = 0.930 | p = 0.936 | p = 0.936 |
| non-LA | p = 0.762 | p = 0.783 | p = 0.817 |
| CD117 | |||
| All | p = 0.985 | p = 0.668 | p = 0.619 |
| LA | p = 0.936 | p = 0.936 | p = 0.935 |
| non-LA | p = 0.972 | p = 0.779 | p = 0.576 |
| CD7 | |||
| All | p = 0.815 | p = 0.568 | p = 0.365 |
| LA | p = 0.936 | p = 0.936 | p = 0.936 |
| non-LA | p = 0.254 | p = 0.459 | p = 0.216 |
| CD25 | |||
| All | p = 0.067 | p = 0.145 | p = 0.064 |
| LA | p = 0.932 | p = 0.934 | p = 0.933 |
| non-LA | p = 0.034 | p = 0.153 | p = 0.048 |
| CD11c | |||
| All | p = 0.470 | p = 0.360 | p = 0.876 |
| LA | p = 0.057 | p = 0.086 | p = 0.170 |
| non-LA | p = 0.547 | p = 0.410 | p = 0.884 |
| Intensive induction therapy | |||
| All | p < 0.01 | p < 0.01 | p < 0.01 |
| LA | p = 0.004 | p = 0.022 | p = 0.05 |
| non-LA | p = 0.007 | p = 0.002 | p = 0.008 |
| HR | 95% CI | p Value | |
|---|---|---|---|
| WBC | 0.998 | 0.994–1.002 | p = 0.406 |
| PB blast % | 1.017 | 1.005–1.029 | p = 0.004 |
| ECOG PS ≥ 2 | 4.588 | 1.675–12.569 | p = 0.003 |
| LDH | 1.000 | 1.000–1.000 | p = 0.231 |
| CD25 | 2.075 | 1.152–3.738 | p = 0.015 |
| Intensive induction therapy | 1.462 | 0.639–3.346 | p = 0.369 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Čučaković, M.; Trajković, L.; Dinić, M.; Pantić, N.; Sabljić, N.; Pravdić, Z.; Rajić, J.; Milošević, V.; Mitrović, M.; Vidović, A.; et al. Impact of Leukapheresis and Biological Risk Markers on Early Mortality in Patients with Hyperleukocytic Acute Myeloid Leukemia. Medicina 2026, 62, 35. https://doi.org/10.3390/medicina62010035
Čučaković M, Trajković L, Dinić M, Pantić N, Sabljić N, Pravdić Z, Rajić J, Milošević V, Mitrović M, Vidović A, et al. Impact of Leukapheresis and Biological Risk Markers on Early Mortality in Patients with Hyperleukocytic Acute Myeloid Leukemia. Medicina. 2026; 62(1):35. https://doi.org/10.3390/medicina62010035
Chicago/Turabian StyleČučaković, Mirjana, Lazar Trajković, Marija Dinić, Nikola Pantić, Nikica Sabljić, Zlatko Pravdić, Jovan Rajić, Violeta Milošević, Mirjana Mitrović, Ana Vidović, and et al. 2026. "Impact of Leukapheresis and Biological Risk Markers on Early Mortality in Patients with Hyperleukocytic Acute Myeloid Leukemia" Medicina 62, no. 1: 35. https://doi.org/10.3390/medicina62010035
APA StyleČučaković, M., Trajković, L., Dinić, M., Pantić, N., Sabljić, N., Pravdić, Z., Rajić, J., Milošević, V., Mitrović, M., Vidović, A., Suvajdžić-Vuković, N., Bogdanović, A., Jaković, L., & Virijević, M. (2026). Impact of Leukapheresis and Biological Risk Markers on Early Mortality in Patients with Hyperleukocytic Acute Myeloid Leukemia. Medicina, 62(1), 35. https://doi.org/10.3390/medicina62010035






