Rethinking Lymphadenectomy in Cutaneous Melanoma: From Routine Practice to Selective Indication: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
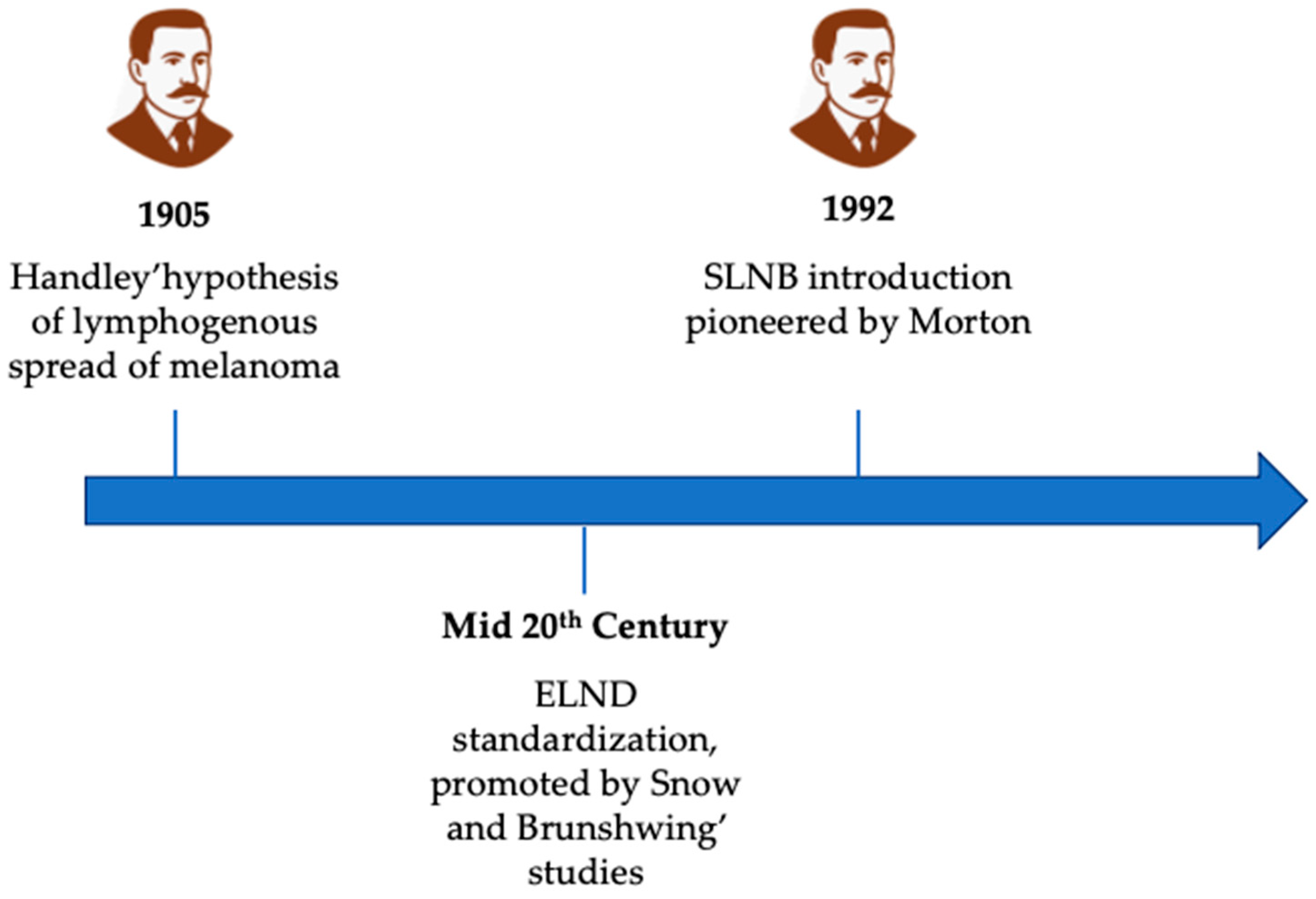
3. From Handley to Morton: The Development of Lymphatic Surgery in Melanoma
4. Sentinel Lymph Node Biopsy and Completion Lymph Node Dissection: Evidence from Clinical Trials
5. Complications Associated with Lymphadenectomy
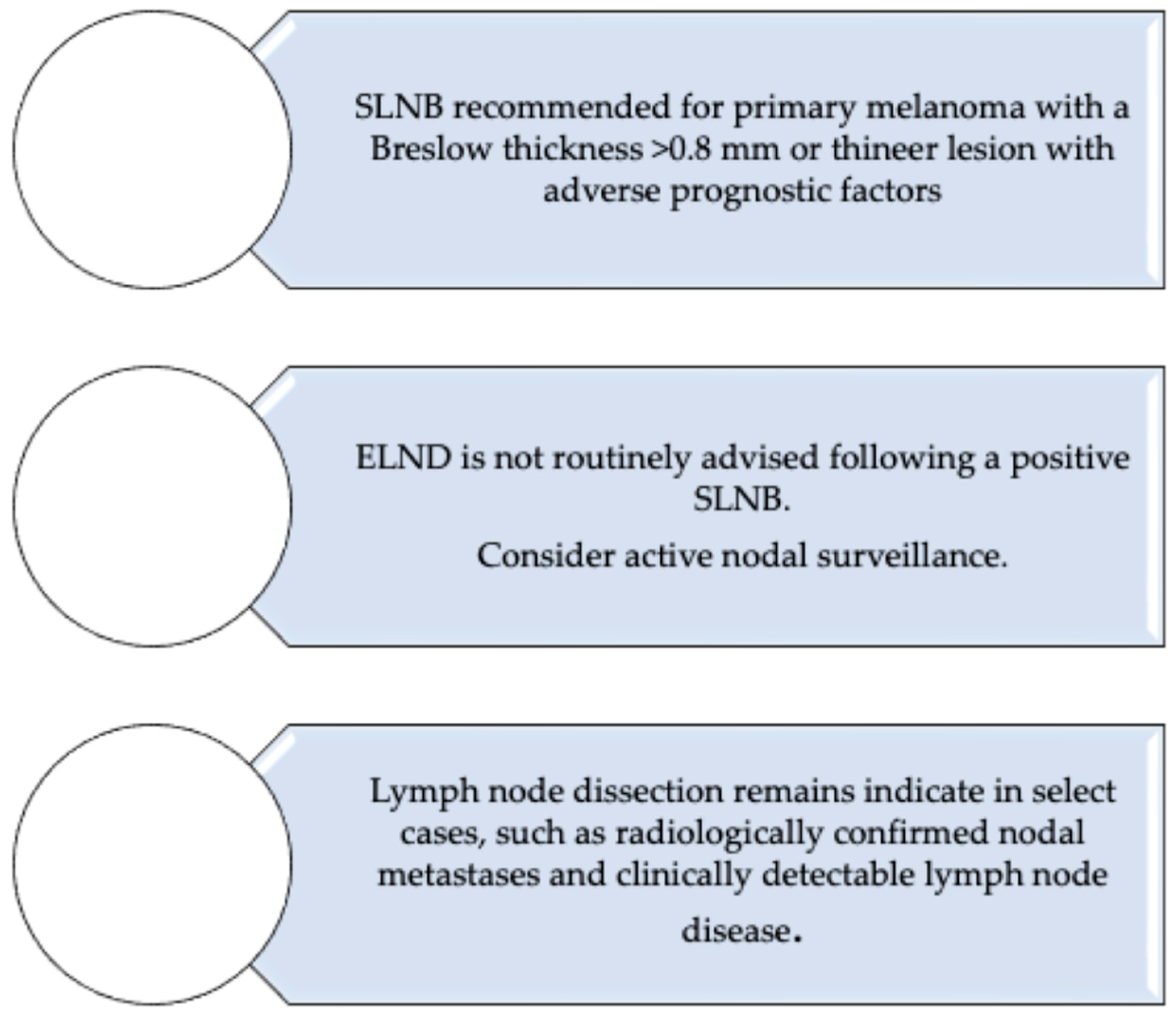
6. Current Guidelines on Sentinel Lymph Node Biopsy and Lymphadenectomy in Melanoma: AIOM, ESMO, and NCCN Perspectives
7. The Evolving Role of Lymph Node Dissection in the Era of Systemic Therapy for Melanoma
8. The Limits of Sentinel Node Prognostic Value and Imaging-Based Surveillance in Melanoma Care
9. From Completion Lymphadenectomy to Index Node Resection: Evolving Surgical Strategies and the MSLT-3 Trial
10. Melanin Pigmentation in Cutaneous Melanoma: Implications for Disease Progression, Lymph Node Involvement and Therapeutic Decision-Making
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Little, E.G.; Eide, M.J. Update on the current state of melanoma incidence. Dermatol. Clin. 2012, 30, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Saraiya, M.; Patel, P.; Cherala, S.S.; Barnholtz-Sloan, J.; Kim, J.; Wiggins, C.L.; Wingo, P.A. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J. Am. Acad. Dermatol. 2011, 65 (Suppl. 1), S17–S25.e1–3. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- AIOM. Linee Guida AIOM per il Trattamento del Melanoma; Associazione Italiana di Oncologia Medica: Milan, Italy, 2023; Available online: https://www.aiom.it/linee-guida-aiom/ (accessed on 1 June 2025).
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.-P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A landscape of driver mutations in melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clark, W.H., Jr.; From, L.; Bernardino, E.A.; Mihm, M.C. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969, 29, 705–727. [Google Scholar] [PubMed]
- Kühnl-Petzoldt, C.; Kalkoff, K.W. Neuklassifizierung des malignen Melanoms. Grundlagen und praktische Bedeutung [New classification of malignant melanoma. Basic concepts and practical significance]. Med. Klin. 1976, 71, 1707–1715. (In German) [Google Scholar] [PubMed]
- Ferrara, G.; Argenziano, G. The WHO 2018 Classification of Cutaneous Melanocytic Neoplasms: Suggestions From Routine Practice. Front. Oncol. 2021, 11, 675296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McGovern, V.J.; Cochran, A.; Van Der Esch, E.; Little, J.; MacLennan, R. The classification of malignant melanoma, its histological reporting and registration: A revision of the 1972 Sydney classification. Pathology 1986, 18, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Patuzzo, R.; Mattavelli, I.; Gallino, G.; Galeone, C.; Valeri, B.; Mocellin, S.; Del Fiore, P.; Ribero, S.; Mandalà, M.; Tauceri, F.; et al. The prognostic role of mitotic rate in cutaneous malignant melanoma: Evidence from a multicenter study on behalf of the Italian Melanoma Intergroup. Cancer 2023, 129, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Speijers, M.J.; Bastiaannet, E.; Sloot, S.; Suurmeijer, A.J.H.; Hoekstra, H.J. Tumor mitotic rate added to the equation: Melanoma prognostic factors changed?: A single-institution database study on the prognostic value of tumor mitotic rate for sentinel lymph node status and survival of cutaneous melanoma patients. Ann. Surg. Oncol. 2015, 22, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Wat, H.; Senthilselvan, A.; Salopek, T.G. A retrospective, multicenter analysis of the predictive value of mitotic rate for sentinel lymph node (SLN) positivity in thin melanomas. J. Am. Acad. Dermatol. 2016, 74, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Maurichi, A.; Barretta, F.; Patuzzo, R.; Miceli, R.; Gallino, G.; Mattavelli, I.; Barbieri, C.; Leva, A.; Angi, M.; Lanza, F.B.; et al. Survival in Patients With Sentinel Node-Positive Melanoma With Extranodal Extension. J. Natl. Compr. Cancer Netw. 2021, 19, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Madu, M.; Wouters, M.; van Akkooi, A.C. Sentinel node biopsy in melanoma: Current controversies addressed. Eur. J. Surg. Oncol. 2017, 43, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Palve, J.; Ylitalo, L.; Luukkaala, T.; Jernman, J.; Korhonen, N. Sentinel node tumor burden in prediction of prognosis in melanoma patients. Clin. Exp. Metastasis 2020, 37, 365–376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moncrieff, M.D.; Lo, S.N.; Scolyer, R.A.; Heaton, M.J.; Nobes, J.P.; Snelling, A.P.; Carr, M.J.; Nessim, C.; Wade, R.; Peach, A.H.; et al. Clinical Outcomes and Risk Stratification of Early-Stage Melanoma Micrometastases From an International Multicenter Study: Implications for the Management of American Joint Committee on Cancer IIIA Disease. J. Clin. Oncol. 2022, 40, 3940–3951. [Google Scholar] [CrossRef] [PubMed]
- Koshenkov, V.P.; Broucek, J.; Kaufman, H.L. Surgical Management of Melanoma. In Cancer Treatment and Research; Springer: Berlin/Heidelberg, Germany, 2016; Volume 167, pp. 149–179. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.L. Sentinel lymph node biopsy from the vantage point of an oncologic surgeon. Clin. Dermatol. 2009, 27, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, M.; Dummer, R.; Giovanoli, P. Die Rolle der Chirurgie in der Behandlung des kutanen Melanoms [The role of surgery in the treatment of cutaneous melanoma]. Praxis 2011, 100, 911–916. (In German) [Google Scholar] [CrossRef] [PubMed]
- Durham, A.B.; Wong, S.L. Sentinel lymph node biopsy in melanoma: Controversies and current guidelines. Future Oncol. 2014, 10, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Keijzer, R.; Bril, H.; van der Loo, E.M.; de Graaf, P.W. Belangrijke prognostische betekenis van schildwachtklierbiopsie bij patiënten met maligne melanoom [Important prognostic significance of a sentinel-node biopsy in patients with malignant melanoma]. Ned. Tijdschr. Geneeskd. 2004, 148, 884–888. (In Dutch) [Google Scholar] [PubMed]
- European Society for Medical Oncology (ESMO). Cutaneous Melanoma: ESMO Clinical Practice Guidelines. 2022. Available online: https://www.esmo.org/guidelines (accessed on 1 June 2025).
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Melanoma, Version 2.2024. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf (accessed on 1 June 2025).
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leiter, U.; Stadler, R.; Mauch, C.; Hohenberger, W.; Brockmeyer, N.H.; Berking, C.; Sunderkötter, C.; Kaatz, M.; Schatton, K.; Lehmann, P.; et al. Final Analysis of DeCOG-SLT Trial: No Survival Benefit for Complete Lymph Node Dissection in Patients With Melanoma With Positive Sentinel Node. J. Clin. Oncol. 2019, 37, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Handley, W.S. The Pathology of Melanotic Growths in Relation to Their Operative Treatment. Lancet 1907, 1, 927–933, 996–1003. [Google Scholar] [CrossRef]
- Balch, C.M.; Soong, S.-J.; Bartolucci, A.A.; Urist, M.M.; Karakousis, C.P.; Smith, T.J.; Temple, W.J.; Ross, M.I.; Jewell, W.R.; Mihm, M.C.; et al. Efficacy of an elective regional lymph node dissection of 1 to 4 mm thick melanomas for patients 60 years of age and younger. Ann. Surg. 1996, 224, 255–263, discussion 263–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reintgen, D.S.; Cox, E.B.; McCARTY, K.S., Jr.; Vollmer, R.T.; Seigler, H.F. Efficacy of elective lymph node dissection in patients with intermediate thickness primary melanoma. Ann. Surg. 1983, 198, 379–385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petres, J.; Rompel, R.; Büttner, P.; Teichelmann, K.; Garbe, C. Elektive Lymphknoten-dissektion bei primärem malignen Melanom [Elective lymph node dissection in primary malignant melanoma]. Hautarzt 1996, 47, 29–34. (In German) [Google Scholar] [CrossRef] [PubMed]
- Hochwald, S.N.; Coit, D.G. Role of elective lymph node dissection in melanoma. Semin. Surg. Oncol. 1998, 14, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Tanis, P.J.; Nieweg, O.E.; Brekel, M.W.M.v.D.; Balm, A.J.M. Dilemma of clinically node-negative head and neck melanoma: Outcome of “watch and wait” policy, elective lymph node dissection, and sentinel node biopsy--a systematic review. Head Neck 2008, 30, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.R. Elective, therapeutic, and delayed lymph node dissection for malignant melanoma of the head and neck: Analysis of 1444 patients from 1970 to 1998. Laryngoscope 2002, 112, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Sagowski, C.; Neuber, K.; Kehrl, W.; Metternich, F.U. Kutane maligne Melanome der Kopf-Hals-Region mit intermediärer Tumordicke: Die Bedeutung der elektiven Versorgung der Lymphabflusswege im klinischen Stadium I [Cutaneous malignant melanoma of the head and neck with intermediate tumor thickness: The role of elective lymph node dissection for clinical stage I]. Laryngorhinootologie 2003, 82, 19–24. (In German) [Google Scholar] [CrossRef] [PubMed]
- Snow, H.M. Melanotic cancerous disease. Lancet 1982, 140, 869–922. [Google Scholar]
- Brunschwig, A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1948, 1, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.J.; Baron, P.L. Surgical management of patients with intermediate thickness melanoma: Current role of elective lymph node dissection. Semin. Oncol. 1996, 23, 719–724. [Google Scholar] [PubMed]
- Lens, M.B.; Dawes, M.; Goodacre, T.; Newton-Bishop, J.A. Elective lymph node dissection in patients with melanoma: Systematic review and meta-analysis of randomized controlled trials. Arch. Surg. 2002, 137, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Kroon, B.B.; Jonk, A. Elective lymph node dissection in melanoma: Still a controversial issue. Neth. J. Surg. 1991, 43, 129–132. [Google Scholar] [PubMed]
- McCarthy, W.H.; Shaw, H.M.; Cascinelli, N.; Santinami, M.; Belli, F. Elective lymph node dissection for melanoma: Two perspectives. World J. Surg. 1992, 16, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Piepkorn, M.; Weinstock, M.A.; Barnhill, R.L. Theoretical and empirical arguments in relation to elective lymph node dissection for melanoma. Arch. Dermatol. 1997, 133, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Reintgen, D. The role of elective lymph node dissection in malignant melanoma: Who should undergo this nodal staging procedure? J. Am. Coll. Surg. 1999, 189, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Reintgen, D.S.; Brobeil, A. Lymphatic mapping and selective lymphadenectomy as an alternative to elective lymph node dissection in patients with malignant melanoma. Hematol. Oncol. Clin. N. Am. 1998, 12, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Soong, S.-J.; Smith, T.; Ross, M.I.; Urist, M.M.; Karakousis, C.P.; Temple, W.J.; Mihm, M.C.; Barnhill, R.L.; Jewell, W.R.; et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann. Surg. Oncol. 2001, 8, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Cascinelli, N.; Morabito, A.; Santinami, M.; MacKie, R.M.; Belli, F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: A randomised trial. Lancet 1998, 351, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Wen, D.-R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef]
- Crystal, J.; Faries, M.B. Sentinel Lymph Node Biopsy: Indications and Technique. Surg. Oncol. Clin. N. Am. 2020, 29, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Mariani, G.; Gipponi, M.; Moresco, L.; Villa, G.; Bartolomei, M.; Mazzarol, G.; Bagnara, M.C.; Romanini, A.; Cafiero, F.; Paganelli, G.; et al. Radioguided sentinel lymph node biopsy in malignant cutaneous melanoma. J. Nucl. Med. 2002, 43, 811–827. [Google Scholar] [PubMed]
- Mariani, G.; Erba, P.; Manca, G.; Villa, G.; Gipponi, M.; Boni, G.; Buffoni, F.; Suriano, S.; Castagnola, F.; Bartolomei, M.; et al. Radioguided sentinel lymph node biopsy in patients with malignant cutaneous melanoma: The nuclear medicine contribution. J. Surg. Oncol. 2004, 85, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Altino de Almeida, S.; Guimarães, M.; Resende, J.F.; Gutfilen, B.; Barbosa da Fonseca, L.M. Sentinel node identification by scintigraphic methods in cutaneous melanoma. J. Exp. Clin. Cancer Res. 2005, 24, 181–185. [Google Scholar] [PubMed]
- Rossi, C.R.; De Salvo, G.L.; Trifirò, G.; Mocellin, S.; Landi, G.; Macripò, G.; Carcoforo, P.; Ricotti, G.; Giudice, G.; Picciotto, F.; et al. The impact of lymphoscintigraphy technique on the outcome of sentinel node biopsy in 1,313 patients with cutaneous melanoma: An Italian Multicentric Study (SOLISM-IMI). J. Nucl. Med. 2006, 47, 234–241. [Google Scholar] [PubMed]
- Rasgon, B.M. Use of low-dose technetium Tc 99m sulfur colloid to locate sentinel lymph nodes in melanoma of the head and neck: Preliminary study. Laryngoscope 2001, 111, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.S.; Sample, W.F.; Fee, H.J.; Holmes, C.; Morton, D.L. Regional lymphatic drainage in primary malignant melanoma of the trunk determined by colloidal gold scanning. Surg. Forum. 1977, 28, 147–148. [Google Scholar] [PubMed]
- Fee, H.J.; Robinson, D.S.; Sample, W.F.; Graham, L.S.; Holmes, E.C.; Morton, D.L. The determination of lymph shed by colloidal gold scanning in patients with malignant melanoma: A preliminary study. Surgery 1978, 84, 626–632. [Google Scholar] [PubMed]
- Morton, D.L.; Wen, D.R.; Foshag, L.J.; Essner, R.; Cochran, A. Intraoperative lymphatic mapping and selective cervical lymphadenectomy for early-stage melanomas of the head and neck. J. Clin. Oncol. 1993, 11, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Berman, C.G.; Choi, J.; Hersh, M.R.; Clark, R.A. Melanoma lymphoscintigraphy and lymphatic mapping. Semin. Nucl. Med. 2000, 30, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kamath, D.; Brobeil, A.; Stall, A.; Lyman, G.; Cruse, C.W.; Glass, F.; Fenske, N.; Messina, J.; Berman, C.; Reintgen, D. Cutaneous lymphatic drainage in patients with grossly involved nodal basins. Ann. Surg. Oncol. 1999, 6, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, M.E.; Silbermins, D.; de Rosa, N.; Wong, S.L.; Lyman, G.H. Lymphatic mapping and sentinel lymph node biopsy in patients with melanoma: A meta-analysis. J. Clin. Oncol. 2011, 29, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Tseng, C.H.; Thompson, W.; Mansfield, P.F.; Lee, J.E.; Bouvet, M.; Lee, J.J.; Ross, M.I. Improved sentinel lymph node localization in patients with primary melanoma with the use of radiolabeled colloid. Surgery 1998, 124, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Mocellin, S.; Mozzillo, N.; Maurichi, A.; Quaglino, P.; Borgognoni, L.; Solari, N.; Piazzalunga, D.; Mascheroni, L.; Giudice, G.; et al. Nonsentinel lymph node status in patients with cutaneous melanoma: Results from a multi-institution prognostic study. J. Clin. Oncol. 2014, 32, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Schuitevoerder, D.; Bubic, I.; Fortino, J.; Massimino, K.P.; Vetto, J.T. Patients with sentinel lymph node positive melanoma: Who needs completion lymph node dissection? Am. J. Surg. 2018, 215, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; Puleo, C.A.; Coventry, B.J.; et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 2014, 370, 599–609. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vasiliadou, E.S.; Kakagia, D.; Lazaridis, L.; Skordoulis, G. Management of lower limb lymphedema following lymphadenectomy for melanoma. J. Vasc. Nurs. 2018, 36, 35–40. [Google Scholar]
- Fields, R.C.; Grotz, T.E.; Ruiz, R.M.; Pockaj, B.A.; Vetto, J.T.; Ross, M.I. Regional lymph node basin recurrence and lymphedema following complete lymph node dissection for melanoma. Ann. Surg. Oncol. 2016, 23, 2100–2106. [Google Scholar]
- Kirkley, K.S.; Shultz, D.B.; Chagpar, A.B.; Horowitz, D.P. Surgical complications and quality of life after inguinal versus axillary lymph node dissection for cutaneous melanoma. Ann. Surg. Oncol. 2014, 21, 473–479. [Google Scholar]
- Leung, A.M.; Morton, D.L.; Essner, R. Complications associated with groin dissection in melanoma patients. Ann. Surg. Oncol. 2015, 22, 2891–2897. [Google Scholar]
- Petrillo, L.A.; Glass, G.E.; Lees, V.C.; Grobmyer, S.R. Groin lymphadenectomy in melanoma: A prospective multicenter study (GOLM study). Ann. Surg. Oncol. 2019, 26, 1171–1178. [Google Scholar]
- Carson, W.E.; Benda, R.K.; Vasiliou, V.; Carson, C. Nerve injury associated with axillary lymph node dissection for melanoma: Incidence and clinical implications. J. Surg. Oncol. 2013, 107, 615–620. [Google Scholar]
- Ascierto, P.A.; Borgognoni, L.; Botti, G.; Guida, M.; Marchetti, P.; Mocellin, S.; Muto, P.; Palmieri, G.; Patuzzo, R.; Quaglino, P.; et al. New paradigm for stage III melanoma: From surgery to adjuvant treatment. J. Transl. Med. 2019, 17, 266, Erratum in J. Transl. Med. 2019, 17, 315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eggermont, A.M.; Dummer, R. The 2017 complete overhaul of adjuvant therapies for high-risk melanoma and its consequences for staging and management of melanoma patients. Eur. J. Cancer 2017, 86, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Amaria, R.N.; Prieto, P.A.; Tetzlaff, M.T.; Reuben, A.; Andrews, M.C.; Ross, M.I.; Glitza, I.C.; Cormier, J.; Hwu, W.J.; Tawbi, H.A.; et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: A single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Eggermont, A.M.M. Neoadjuvant therapy in melanoma: The next step? Lancet Oncol. 2018, 19, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Shajari, N.; Baradaran, B.; Tohidkia, M.R.; Nasiri, H.; Sepehri, M.; Setayesh, S.; Aghebati-Maleki, L. Advancements in Melanoma Therapies: From Surgery to Immunotherapy. Curr. Treat. Options Oncol. 2024, 25, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wu, H.T.; Rajurkar, S.; Tan, T.; Hsu, V. Immunotherapy in Melanoma. Cancer Treat. Res. 2025, 129, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477, Erratum in Lancet Oncol. 2021, 22, e428. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.; Adhikari, C.; Bierman, C.; van de Wiel, B.A.; Scolyer, R.A.; Krijgsman, O.; Sikorska, K.; Eriksson, H.; et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019, 20, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Reijers, I.L.M.; Menzies, A.M.; van Akkooi, A.C.J.; Versluis, J.M.; Heuvel, N.M.J.v.D.; Saw, R.P.M.; Pennington, T.E.; Kapiteijn, E.; van der Veldt, A.A.M.; Suijkerbuijk, K.P.M.; et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: The PRADO trial. Nat Med. 2022, 28, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- van Akkooi, A.; Ariyan, C.; Moncrieff, M. Melanoma Great Debate: Targeted Versus Complete Nodal Dissection Following Neoadjuvant Therapy for Melanoma: Is it Time to Push Forward or Hold Off on Continued De-Escalation of Surgery? Ann. Surg. Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Patuzzo, R.; Maurichi, A.; Camerini, T.; Gallino, G.; Ruggeri, R.; Baffa, G.; Mattavelli, I.; Tinti, M.C.; Crippa, F.; Moglia, D.; et al. Accuracy and prognostic value of sentinel lymph node biopsy in head and neck melanomas. J. Surg. Res. 2014, 187, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Wang, Z.; Zhu, G.; Zhang, Y.; Xu, Y.; Xu, X. Sentinel lymph node biopsy in head and neck cutaneous melanomas: A PRISMA-compliant systematic review and meta-analysis. Medicine 2021, 100, e24284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappello, Z.J.; Augenstein, A.C.; Potts, K.L.; McMasters, K.M.; Bumpous, J.M. Sentinel lymph node status is the most important prognostic factor in patients with melanoma of the scalp. Laryngoscope 2013, 123, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Cromwell, K.D.; Cormier, J.N. Review of diagnostic imaging modalities for the surveillance of melanoma patients. Dermatol. Res. Pract. 2012, 2012, 941921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Institute for Health and Care Excellence (NICE). Evidence Reviews for the Follow-Up of People with Melanoma: Melanoma: Assessment and Management: Evidence Review G.; National Institute for Health and Care Excellence (NICE): London, UK, 2022. [PubMed]
- Krüger, U.; Kretschmer, L.; Thoms, K.-M.; Padeken, M.; Bertsch, H.P.; Schön, M.P.; Zutt, M. Lymph node ultrasound during melanoma follow-up significantly improves metastasis detection compared with clinical examination alone: A study on 433 patients. Melanoma Res. 2011, 21, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Reijers, I.L.M.; Rawson, R.V.; Colebatch, A.J.; Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.J.; Shannon, K.F.; Wouters, M.W.; Saw, R.P.M.; van Houdt, W.J.; et al. Representativeness of the Index Lymph Node for Total Nodal Basin in Pathologic Response Assessment After Neoadjuvant Checkpoint Inhibitor Therapy in Patients with Stage III Melanoma. JAMA Surg. 2022, 157, 335–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.ctg.queensu.ca/public/melanoma/melanoma-disease-site (accessed on 1 June 2025).
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.-G.; et al. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; van de Wiel, B.A.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; ter Huurne, J.; Berkhout, M.; Gruis, N.; Bastiaens, M.; Bergman, W.; Willemze, R.; Bavinck, J.N. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J. Investig. Dermatol. 2001, 117, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Aebischer, V.; Abu-Ghazaleh, A.; Metzler, G.; Riedl, L.; Garbe, C.; Flatz, L.; Eigentler, T.; Forchhammer, S. Histopathologic abundance of pigmentation correlates with disease-specific survival in malignant melanoma but is not independent of current AJCC pT stage. Pigment Cell Melanoma Res. 2023, 36, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.A.; Jóźwicki, W.; Carlson, J.A.; Slominski, A.T. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum. Pathol. 2013, 44, 2071–2074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brożyna, A.A.; Jóźwicki, W.; Roszkowski, K.; Filipiak, J.; Slominski, A.T. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016, 7, 17844–17853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Outcomes | SLNB | CLND | Comments |
|---|---|---|---|
| Surgical Morbidity | Low | Moderate-High | None |
| Lymphedema | 3–7% | 20–30% | Risk higher after CLND, particularly in inguinal region (MSLT-II, DeCOG-SLT). |
| Seroma Formation | 1–5% | 10–20% | None |
| Wound Infection | <5% | 5–15% | None |
| Nerve Injuries | Rare | 5–10% | None |
| Hospital Stay | 1 day | 1–3 days | CLND often requires longer postoperative monitoring. |
| Trial/Guideline | Population/Scope | Intervention/Focus | Key Findings/Recommendations |
|---|---|---|---|
| MSLT-II (2017) [29] | 1900 patients with positive SLNB | CLND vs. nodal observation with ultrasound | CLND improved regional disease control but showed no melanoma-specific survival benefit compared to observation. |
| DeCOG-SLT (2016, 2019 final) [30] | Patients with positive SLNB | CLND vs. observation | No survival advantage for CLND; reinforced shift towards active surveillance after SLNB positivity. |
| COMBI-AD (2017) [81] | BRAF V600-mutant resected Stage III melanoma | Adjuvant dabrafenib + Trametinib Vs. Placebo | 3-year RFS 58% Vs. 39% with placebo; established targeted therapy as standard adjuvant option in BRAF + patients. |
| OpACIN-neo (2019, 2023 update) [82] | Stage III melanoma | Neoadjuvant ipilimumab nivolumab (different dosing arms) | High pathologic response rates; responders had markedly improved RFS, supporting neoadjuvant immunotherapy as feasible and effective. |
| PRADO (2022) [83] | Stage III melanoma (extension of OpACIN-neo) | Neoadjuvant ipilimumab + nivolumab; response-directed surgery | 61% major pathologic response; in responders, TLND was safely omitted without increased recurrence, supporting response-adapted surgery. |
| AIOM, ESMO, NCCN Guidelines [4,27,28] | Clinical practice recommendations | SLNB, CLND, surveillance, adjuvant and neoadjuvant therapy | All endorse SLNB for staging. CLND no longer routine after positive SLN (observation with ultrasound preferred). Adjuvant: anti–PD-1 or BRAF/MEK targeted therapy. Neoadjuvant: ICI in resectable Stage III, with potential for surgery de-escalation in responders. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteucci, M.; Pesce, A.; Guarino, S.; Cassini, D.; Cirillo, B.; Boselli, C.; D’Andrea, V.; Artico, M.; Forte, F.; Covarelli, P.; et al. Rethinking Lymphadenectomy in Cutaneous Melanoma: From Routine Practice to Selective Indication: A Narrative Review. Medicina 2025, 61, 1722. https://doi.org/10.3390/medicina61091722
Matteucci M, Pesce A, Guarino S, Cassini D, Cirillo B, Boselli C, D’Andrea V, Artico M, Forte F, Covarelli P, et al. Rethinking Lymphadenectomy in Cutaneous Melanoma: From Routine Practice to Selective Indication: A Narrative Review. Medicina. 2025; 61(9):1722. https://doi.org/10.3390/medicina61091722
Chicago/Turabian StyleMatteucci, Matteo, Antonio Pesce, Salvatore Guarino, Diletta Cassini, Bruno Cirillo, Carlo Boselli, Vito D’Andrea, Marco Artico, Flavio Forte, Piero Covarelli, and et al. 2025. "Rethinking Lymphadenectomy in Cutaneous Melanoma: From Routine Practice to Selective Indication: A Narrative Review" Medicina 61, no. 9: 1722. https://doi.org/10.3390/medicina61091722
APA StyleMatteucci, M., Pesce, A., Guarino, S., Cassini, D., Cirillo, B., Boselli, C., D’Andrea, V., Artico, M., Forte, F., Covarelli, P., & Cirocchi, R. (2025). Rethinking Lymphadenectomy in Cutaneous Melanoma: From Routine Practice to Selective Indication: A Narrative Review. Medicina, 61(9), 1722. https://doi.org/10.3390/medicina61091722








