Bacterial Colonization of Orthodontic Devices (Molar Bands, Nance Buttons, and Acrylic Plates) and Its Impact on the Marginal Periodontium and Palatal Fibromucosa in Teenagers: A Cross-Sectional Clinical–Microbiological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, Participants, and Calibration
2.2. Clinical Examination and Indices
2.3. Microbiological Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Literature Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luchian, I.; Surlari, Z.; Goriuc, A.; Ioanid, N.; Zetu, I.; Butnaru, O.; Scutariu, M.M.; Tatarciuc, M.; Budala, D.G. The Influence of Orthodontic Treatment on Periodontal Health between Challenge and Synergy: A Narrative Review. Dent. J. 2024, 12, 112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Widhianingsih, D.; Koontongkaew, S. Enhancement of cariogenic virulence properties of dental plaque in asthmatics. J. Asthma 2021, 58, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Chen, S.; Bai, R.; Lu, Y.; Peng, L.; Han, B.; Yu, T. Dynamics of the oral microbiome during orthodontic treatment and antimicrobial advances for orthodontic appliances. iScience 2024, 27, 111458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maret, D.; Marchal-Sixou, C.; Vergnes, J.N.; Hamel, O.; Georgelin-Gurgel, M.; Van Der Sluis, L.; Sixou, M. Effect of fixed orthodontic appliances on salivary microbial parameters at 6 months: A controlled observational study. J. Appl. Oral Sci. 2014, 22, 38–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thanetchaloempong, W.; Koontongkaew, S.; Utispan, K. Fixed Orthodontic Treatment Increases Cariogenicity and Virulence Gene Expression in Dental Biofilm. J. Clin. Med. 2022, 11, 5860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shokeen, B.; Viloria, E.; Duong, E.; Rizvi, M.; Murillo, G.; Mullen, J.; Shi, B.; Dinis, M.; Li, H.; Tran, N.C.; et al. The impact of fixed orthodontic appliances and clear aligners on the oral microbiome and the association with clinical parameters: A longitudinal comparative study. Am. J. Orthod. Dentofac. Orthop. 2022, 161, e475–e485. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zhou, K.; Li, S.; Yue, Z.; Zhang, Q.; Li, Y.; Mi, X. Different Effects of Fixed Appliances and Clear Aligners on the Microbiome and Metabolome of Dental Plaque. Orthod. Craniofacial Res. 2025, 28, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Vico, R.M.; Iglesias-Linares, A.; Ballesta-Mudarra, S.; Ortiz-Ariza, E.; Solano-Reina, E.; Perea, E.J. Short-term effect of removal of fixed orthodontic appliances on gingival health and subgingival microbiota: A prospective cohort study. Acta Odontol. Scand. 2015, 73, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Catunda, R.Q.; Altabtbaei, K.; Flores-Mir, C.; Febbraio, M. Pre-treatment oral microbiome analysis and salivary Stephan curve kinetics in white spot lesion development in orthodontic patients wearing fixed appliances. A pilot study. BMC Oral Health 2023, 23, 239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kado, I.; Hisatsune, J.; Tsuruda, K.; Tanimoto, K.; Sugai, M. The impact of fixed orthodontic appliances on oral microbiome dynamics in Japanese patients. Sci. Rep. 2020, 10, 21989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alrehaili, R.; Alhujaili, A.; Almanjhi, W.; Alnami, H.; Alsaiyari, S.; Alqahtani, H.; Alabdan, R.; Baamer, D.; Khalil, A. How Effective Are the Nance Appliance and Transpalatal Arch at Reinforcing Anchorage in Extraction Cases? Cureus 2024, 16, e61171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patini, R.; Bonetti, A.A.; Camodeca, A.; Staderini, E.; Gallenzi, P. Haematemesis related to orthodontic treatment with Nance palatal arch: A case report. J. Orthod. 2018, 45, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Pellissari, B.A.; Sabino, G.S.P.; de Souza Lima, R.N.; Motta, R.H.L.; Suzuki, S.S.; Garcez, A.S.; Basting, R.T.; Barbosa, J.A.; Martins Montalli, V.A. Antimicrobial resistance of bacterial strains in patients undergoing orthodontic treatment with and without fixed appliances. Angle Orthod. 2021, 91, 672–679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jing, D.; Hao, J.; Shen, Y.; Tang, G.; Lei, L.; Zhao, Z. Effect of fixed orthodontic treatment on oral microbiota and salivary proteins. Exp. Ther. Med. 2019, 17, 4237–4243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arab, S.; Nouhzadeh Malekshah, S.; Abouei Mehrizi, E.; Ebrahimi Khanghah, A.; Naseh, R.; Imani, M.M. Effect of Fixed Orthodontic Treatment on Salivary Flow, pH and Microbial Count. J. Dent. 2016, 13, 18–22. [Google Scholar] [PubMed] [PubMed Central]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Yano, A.; Kaneko, N.; Ida, H.; Yamaguchi, T.; Hanada, N. Real-time PCR for quantification of Streptococcus mutans. FEMS Microbiol. Lett. 2002, 217, 23–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577, Erratum in Ann. Intern. Med. 2008, 148, 168. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Sharma, D.S. Biofilm associated microorganisms on removable oral orthodontic appliances in children in the mixed dentition. J. Clin. Pediatr. Dent. 2013, 37, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Charavet, C.; Graveline, L.; Gourdain, Z.; Lupi, L. What Are the Cleaning and Disinfection Methods for Acrylic Orthodontic Removable Appliance? A Systematic Review. Children 2021, 8, 967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khawwam, S.I.; Al-Groosh, D.H. Effect of Different Cleaning Regimes on Biofilm Formation of Acrylic-Based Removable Orthodontic Appliance: A Randomized Clinical Trial. Sci. World J. 2023, 2023, 9920850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crego-Ruiz, M.; Jorba-García, A. Assessment of the periodontal health status and gingival recession during orthodontic treatment with clear aligners and fixed appliances: A systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 2023, 28, e330–e340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Q.; Li, J.; Mei, L.; Du, J.; Levrini, L.; Abbate, G.M.; Li, H. Periodontal health during orthodontic treatment with clear aligners and fixed appliances: A meta-analysis. J. Am. Dent. Assoc. 2018, 149, 712–720.e12. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, J.; Lin, X.; Diao, S.; Cao, Y.; Dong, R.; Wang, L.; Wang, S.; Fan, Z. Demethylation of SFRP2 by histone demethylase KDM2A regulated osteo-/dentinogenic differentiation of stem cells of the apical papilla. Cell Prolif. 2016, 49, 330–340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mummolo, S.; Nota, A.; Albani, F.; Marchetti, E.; Gatto, R.; Marzo, G.; Quinzi, V.; Tecco, S. Salivary levels of Streptococcus mutans and Lactobacilli and other salivary indices in patients wearing clear aligners versus fixed orthodontic appliances: An observational study. PLoS ONE 2020, 15, e0228798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sifakakis, I.; Papaioannou, W.; Papadimitriou, A.; Kloukos, D.; Papageorgiou, S.N.; Eliades, T. Salivary levels of cariogenic bacterial species during orthodontic treatment with thermoplastic aligners or fixed appliances: A prospective cohort study. Prog. Orthod. 2018, 19, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paterlini, M. Italy toughens sanctions against perpetrators of violent attacks on hospital staff. BMJ 2024, 387, q2216. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, F.; Turk, T.; Karadeniz, E.I.; Elekdag-Turk, S.; Darendeliler, M.A. Physical properties of root cementum: Part 24. Root resorption of the first premolars after 4 weeks of occlusal trauma. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Marda, A.; Elhamzaoui, S.; El Mansari, A.; Souly, K.; Farissi, F.; Zouhdi, M.; Zaoui, F.; Bahije, L. Evaluation of Changes in Cariogenic Bacteria in a Young Moroccan Population with Fixed Orthodontic Appliances. Int. J. Dent. 2018, 2018, 5939015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, S.; Rogers, M.J.; He, J. Microbial reductive dehalogenation of trihalomethanes by a Dehalobacter-containing co-culture. Appl. Microbiol. Biotechnol. 2017, 101, 5481–5492. [Google Scholar] [CrossRef] [PubMed]
- Seethamraju, H.; Paul, S. Wrap it up! Should we take it? J. Thorac. Cardiovasc. Surg. 2018, 155, 2760–2761. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, H.K.; Arshad, T.; Merajoddin, M.; Mohammad, Z.S.; Usman, R.; Hasan, M.M. Docking analysis of aryl derivatives of diepoxide alkylating agents. Pak. J. Pharm. Sci. 2020, 33, 2017–2021. [Google Scholar] [PubMed]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Plante-Hébert, J.; Boucher, V.J.; Jemel, B. The processing of intimately familiar and unfamiliar voices: Specific neural responses of speaker recognition and identification. PLoS ONE 2021, 16, e0250214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Group | Age (y) | Female (%) | Wear Duration (Months) | Toothbrushing (Times/Day) | Sugary Drinks (Times/Week) | Treatment Stage, n (%) | Parental Education >Secondary, % | Baseline DMFT, Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Band (n = 44) | 15.3 ± 1.9 | 45.5 | 7.4 ± 2.9 | 2.1 ± 0.4 | 4.1 ± 1.8 | Leveling 21 (47.7); Space-closure 15 (34.1); Finishing 8 (18.2) | 56.8 | 2.1 ± 1.2 |
| Nance (n = 41) | 14.6 ± 1.6 | 63.4 | 9.6 ± 3.8 | 2.1 ± 0.4 | 4.4 ± 1.9 | Leveling 15 (36.6); Space-closure 18 (43.9); Finishing 8 (19.5) | 51.2 | 2.3 ± 1.3 |
| Acrylic (n = 43) | 14.9 ± 2.1 | 46.5 | 5.6 ± 2.6 | 2.2 ± 0.4 | 3.9 ± 2.1 | Leveling 18 (41.9); Space-closure 15 (34.9); Finishing 10 (23.2) | 58.1 | 2.0 ± 1.1 |
| Overall p-value | 0.261 | 0.182 | <0.001 | 0.371 | 0.410 | 0.642 | 0.712 | 0.568 |
| Group | Device Biofilm (log10 CFU/cm2) | Adjacent Enamel (log10 CFU/cm2) | Palatal Mucosa (log10 CFU/cm2) | S. mutans Load (log10 copies/mL) |
|---|---|---|---|---|
| Band | 4.6 ± 0.6 | 3.7 ± 0.6 | 3.2 ± 0.4 | 5.2 ± 0.6 |
| Nance | 5.3 ± 0.6 | 4.3 ± 0.4 | 4.8 ± 0.6 | 5.9 ± 0.7 |
| Acrylic | 5.6 ± 0.6 | 4.6 ± 0.6 | 4.9 ± 0.7 | 6.2 ± 0.7 |
| Group | Plaque Index (0–3) | Gingival Index (0–3) | Bleeding on Probing (%) | Probing Depth (mm) | Palatal Erythema (0–3) |
|---|---|---|---|---|---|
| Band | 1.6 ± 0.3 | 1.2 ± 0.3 | 22.3 ± 11.3 | 2.6 ± 0.4 | 0.6 ± 0.4 |
| Nance | 2.1 ± 0.2 | 1.7 ± 0.3 | 32.9 ± 13.9 | 2.9 ± 0.4 | 1.6 ± 0.8 |
| Acrylic | 1.7 ± 0.3 | 1.6 ± 0.3 | 28.9 ± 12.2 | 2.8 ± 0.6 | 1.9 ± 0.7 |
| Group Type | Palatal Mucosa (log10 CFU/cm2) | Palatal Erythema (0–3) | Gingival Index (0–3) |
|---|---|---|---|
| Fixed (bands + Nance) | 4.1 ± 0.9 | 1.1 ± 0.8 | 1.6 ± 0.4 |
| Removable (acrylic) | 4.9 ± 0.7 | 1.9 ± 0.7 | 1.6 ± 0.3 |
| Correlation | Spearman ρ | p-Value |
|---|---|---|
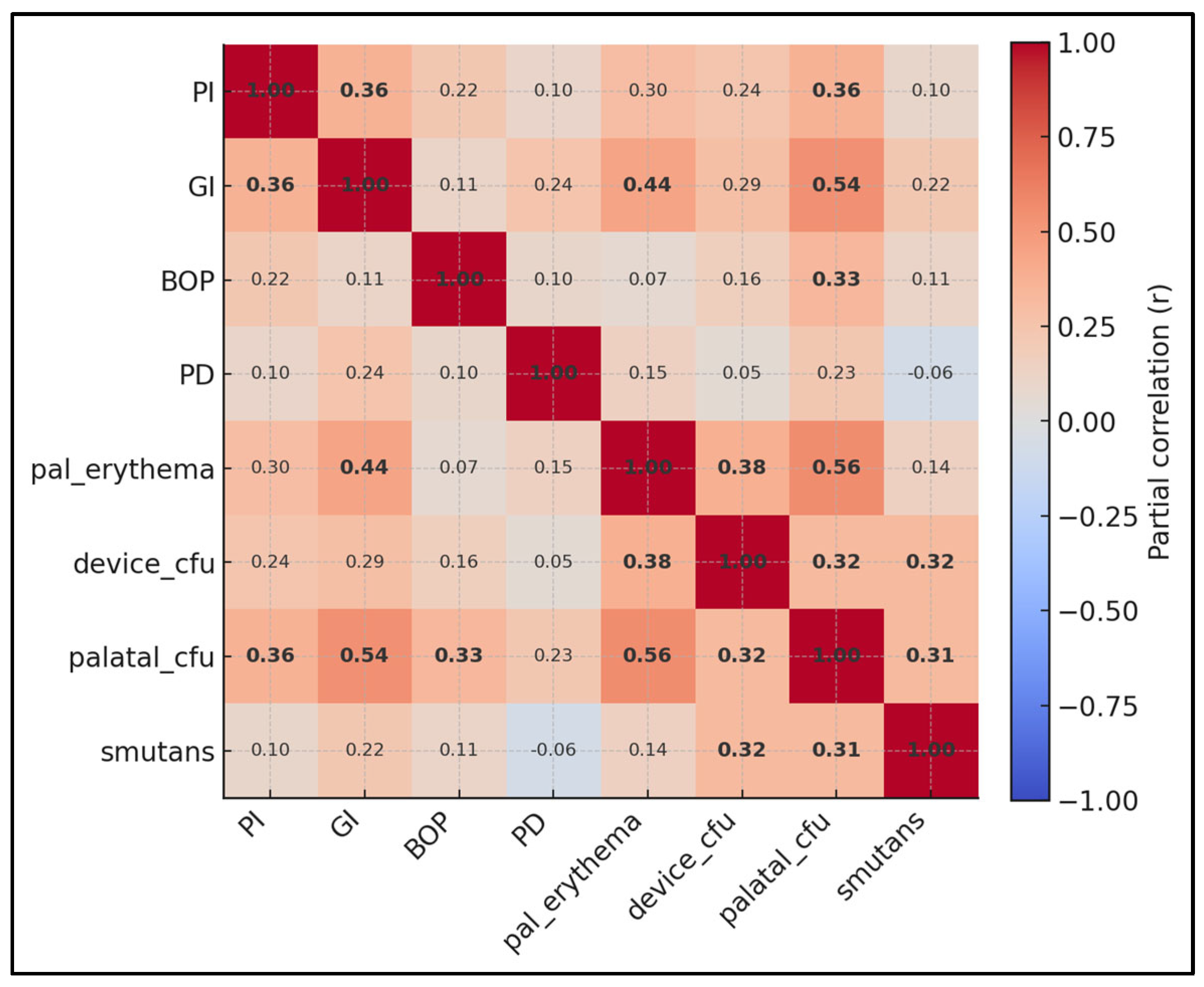
| CFU_Device vs. GI | 0.3 | <0.001 |
| CFU_Device vs. BOP% | 0.1 | 0.359 |
| CFU_Device vs. PD_mm | 0.2 | 0.029 |
| CFU_Device vs. Palatal Erythema | 0.4 | <0.001 |
| CFU_Device vs. Wear months | 0.1 | 0.993 |
| CFU_Device vs. SSB/week | 0.1 | 0.106 |
| CFU_Device vs. Brush/day | 0.1 | 0.838 |
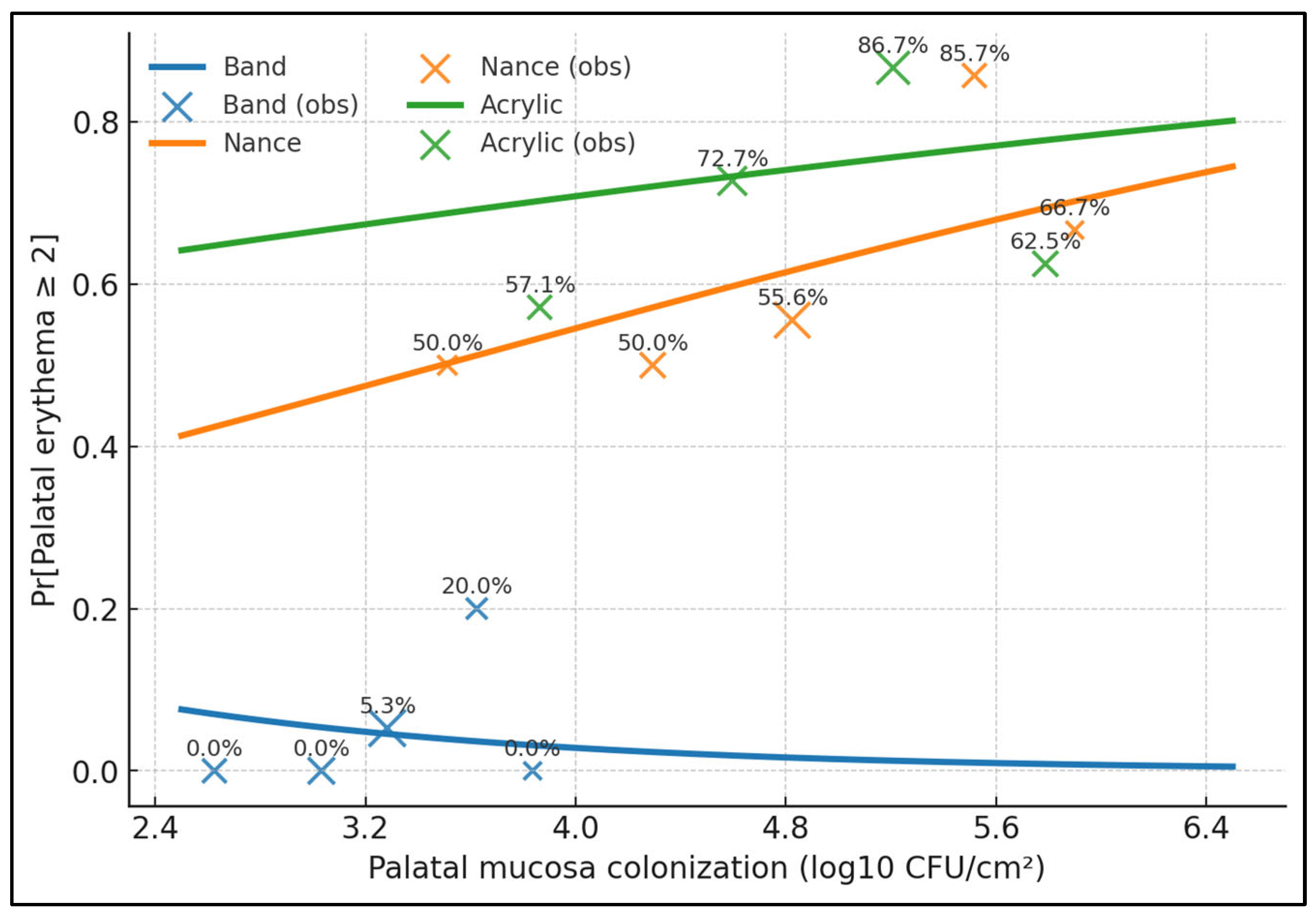
| Palatal CFU vs. Erythema | 0.6 | <0.001 |
| S. mutans vs. GI | 0.3 | 0.003 |
| Term | Beta | 95% CI | p-Value |
|---|---|---|---|
| Intercept | 0.98 | 0.22 to 1.74 | 0.013 |
| Device: Acrylic vs. Band | +0.38 | +0.20 to +0.56 | <0.001 |
| Device: Nance vs. Band | +0.52 | +0.33 to +0.71 | <0.001 |
| Device biofilm (per log10 CFU) | +0.06 | −0.06 to +0.18 | 0.327 |
| Toothbrushing (per time/day) | −0.03 | −0.16 to +0.10 | 0.637 |
| Sugary drinks (per time/week) | +0.02 | −0.02 to +0.06 | 0.335 |
| Age (per year) | +0.01 | −0.03 to +0.05 | 0.585 |
| Female (vs. male) | +0.04 | −0.07 to +0.15 | 0.493 |
| Wear duration (per month) | +0.02 | −0.01 to +0.04 | 0.164 |
| Treatment stage: Space-closure vs. Leveling | +0.08 | −0.06 to +0.22 | 0.258 |
| Treatment stage: Finishing vs. Leveling | +0.05 | −0.12 to +0.22 | 0.566 |
| Parental education (>secondary) | −0.06 | −0.17 to +0.05 | 0.278 |
| Baseline DMFT (per unit) | +0.04 | −0.02 to +0.10 | 0.179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragos, B.; Bratu, D.-C.; Popa, G.; Luca, M.-M.; Bratu, R.-C.; Sinescu, C. Bacterial Colonization of Orthodontic Devices (Molar Bands, Nance Buttons, and Acrylic Plates) and Its Impact on the Marginal Periodontium and Palatal Fibromucosa in Teenagers: A Cross-Sectional Clinical–Microbiological Study. Medicina 2025, 61, 1717. https://doi.org/10.3390/medicina61091717
Dragos B, Bratu D-C, Popa G, Luca M-M, Bratu R-C, Sinescu C. Bacterial Colonization of Orthodontic Devices (Molar Bands, Nance Buttons, and Acrylic Plates) and Its Impact on the Marginal Periodontium and Palatal Fibromucosa in Teenagers: A Cross-Sectional Clinical–Microbiological Study. Medicina. 2025; 61(9):1717. https://doi.org/10.3390/medicina61091717
Chicago/Turabian StyleDragos, Bianca, Dana-Cristina Bratu, George Popa, Magda-Mihaela Luca, Remus-Christian Bratu, and Cosmin Sinescu. 2025. "Bacterial Colonization of Orthodontic Devices (Molar Bands, Nance Buttons, and Acrylic Plates) and Its Impact on the Marginal Periodontium and Palatal Fibromucosa in Teenagers: A Cross-Sectional Clinical–Microbiological Study" Medicina 61, no. 9: 1717. https://doi.org/10.3390/medicina61091717
APA StyleDragos, B., Bratu, D.-C., Popa, G., Luca, M.-M., Bratu, R.-C., & Sinescu, C. (2025). Bacterial Colonization of Orthodontic Devices (Molar Bands, Nance Buttons, and Acrylic Plates) and Its Impact on the Marginal Periodontium and Palatal Fibromucosa in Teenagers: A Cross-Sectional Clinical–Microbiological Study. Medicina, 61(9), 1717. https://doi.org/10.3390/medicina61091717







