1. Introduction
Minimally invasive surgery is regarded as the gold standard for the treatment of gynecological conditions. Although robot-assisted surgery (RAS) offers enhanced precision and visualization, conventional systems often lack haptic feedback, which may impair delicate tissue handling and increase the risk of inadvertent injury [
1]. The Saroa surgical system (Riverfield Inc., Tokyo, Japan), introduced in 2023, represents a next-generation robotic surgical platform specially engineered to overcome these limitations. Unlike previous systems, the Saroa surgical system utilizes a pneumatically actuated mechanism that provides real-time haptic feedback, restoring the surgeon’s ability to perceive the grasping force during manipulation [
2]. The compact and lightweight configuration of the system, coupled with its streamlined setup, offers significant logistical and cost-related benefits.
The development of the Saroa surgical system originated from a collaborative research initiative between the former Tokyo Medical and Dental University and the Tokyo Institute of Technology (now Institute of Science Tokyo). However, during the development phase, validation specifically focused on gynecologic procedures had not been conducted. This study represents the first report worldwide on the clinical application of this system in gynecologic surgery.
Our study presents the initial clinical experience with the Saroa surgical system in robot-assisted hysterectomy (RAH) and evaluates its feasibility, safety, and integration within contemporary laparoscopic workflows.
2. Materials and Methods
2.1. Patient Selection
This study enrolled five consecutive patients who underwent robot-assisted laparoscopic hysterectomy using the Saroa surgical system between October 2023 and December 2024 at the Institute of Science Tokyo Hospital. The inclusion criteria encompassed benign gynecological conditions warranting hysterectomy, including uterine myoma, endometrial hyperplasia, and endometrial polyps. The exclusion criteria included malignancy, pregnancy, and the inability to tolerate pneumoperitoneum. The study was approved by the Institutional Ethics Committee, and written informed consent was obtained from all patients. Clinical data, including patient demographics, operative duration, blood loss, complications, and device performance, were retrospectively collected.
2.2. Preoperative Preparation
All patients underwent standard preoperative evaluations, including imaging, laboratory testing, and anesthesia clearance. Additionally, prophylactic antibiotics were administered preoperatively, according to the institutional protocol. Informed discussions with the patients included specific consent regarding the use of the novel surgical system.
2.3. Surgical Procedure
A schematic of the surgical setup and port placement is provided in
Figure 1.
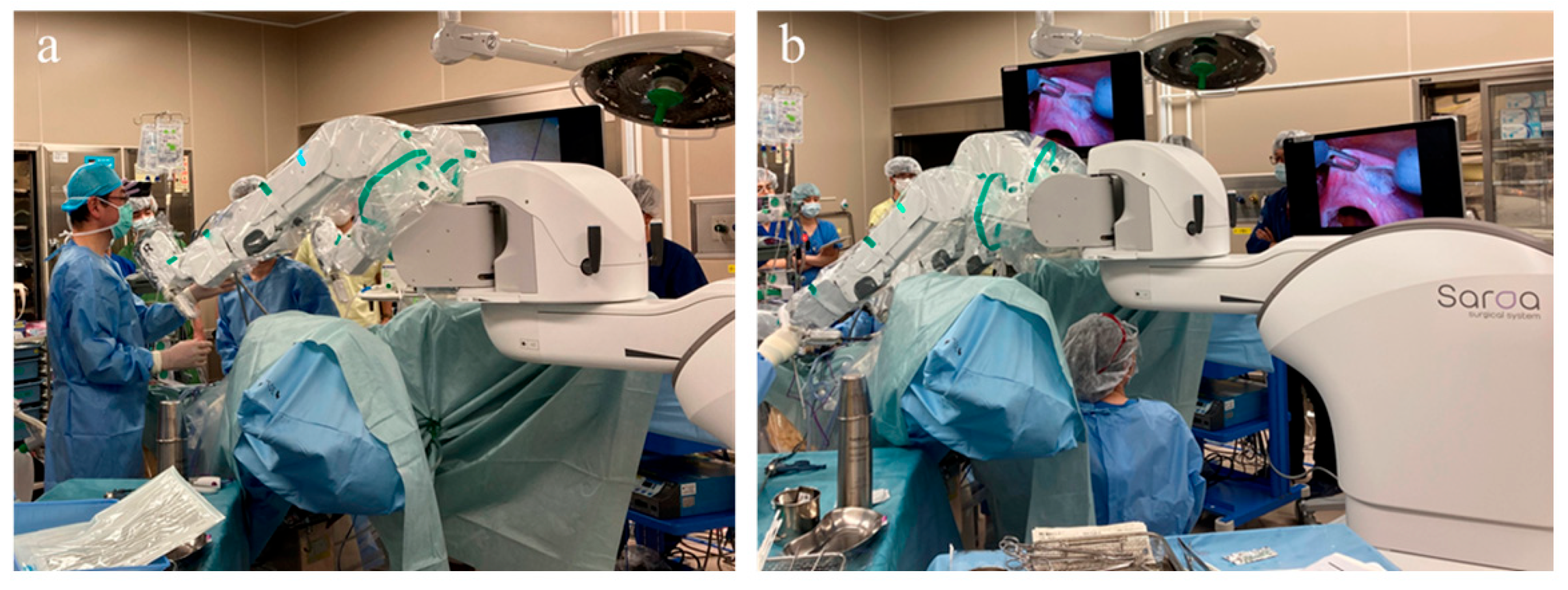
After induction of general anesthesia, the patients were placed in the lithotomy position with a 15-degree Trendelenburg tilt. The overall layout of the operating room is shown in
Figure 1a. To optimize access to the three robotic arms, the patient cart was positioned on the patient’s left side. The first assistant stood on the patient’s left side to perform suction, retraction, and instrument exchange, while the second assistant was positioned between the patient’s legs to operate the uterine manipulator. The scrub nurse was positioned on the opposite side of the first assistant. The anesthesiologist was located at the head of the patient, with a main monitor positioned for shared viewing by the assistants and scrub nurse, and a sub-monitor providing supplementary visual information. The laparoscopic tower was placed near the anesthesiologist to facilitate easy access to equipment. A 12 mm trocar was inserted through the umbilicus, followed by the placement of two 11 mm trocars, each positioned 8 cm lateral and 3 cm caudal to the umbilical port on each side (
Figure 1b). A 12 mm trocar was inserted through the umbilicus, followed by the placement of two 11 mm trocars, each positioned 8 cm lateral and 3 cm caudal to the umbilical port on each side. An additional 5 mm port for the auxiliary forceps was inserted at a point midway between the umbilical and left lateral abdominal ports. A 10 mm, 30-degree oblique-view laparoscope (Olympus, Tokyo, Japan) was introduced through the umbilical port. All trocars used were ENDOPATH XCEL™ bladeless trocars (ETHICON, Johnson & Johnson, Cincinnati, OH, USA).
The cart for the Saroa surgical system was subsequently positioned between the legs (
Figure 2a), allowing unobstructed access for the surgical assistants. Adequate space was also maintained beneath the robotic arms (
Figure 2b) to accommodate a second assistant, enabling the use of a vaginal pipe or uterine manipulator when required.
Due to its compact size, the system can be rolled in with ease, providing unobstructed access to the surgical field. The instruments were mounted on the three robotic arms of the Saroa surgical system and inserted through the ports without docking. The surgeon performed the procedure at an open console, wearing three-dimensional (3D) glasses and operating hand controllers equipped with real-time haptic feedback (
Figure 3).
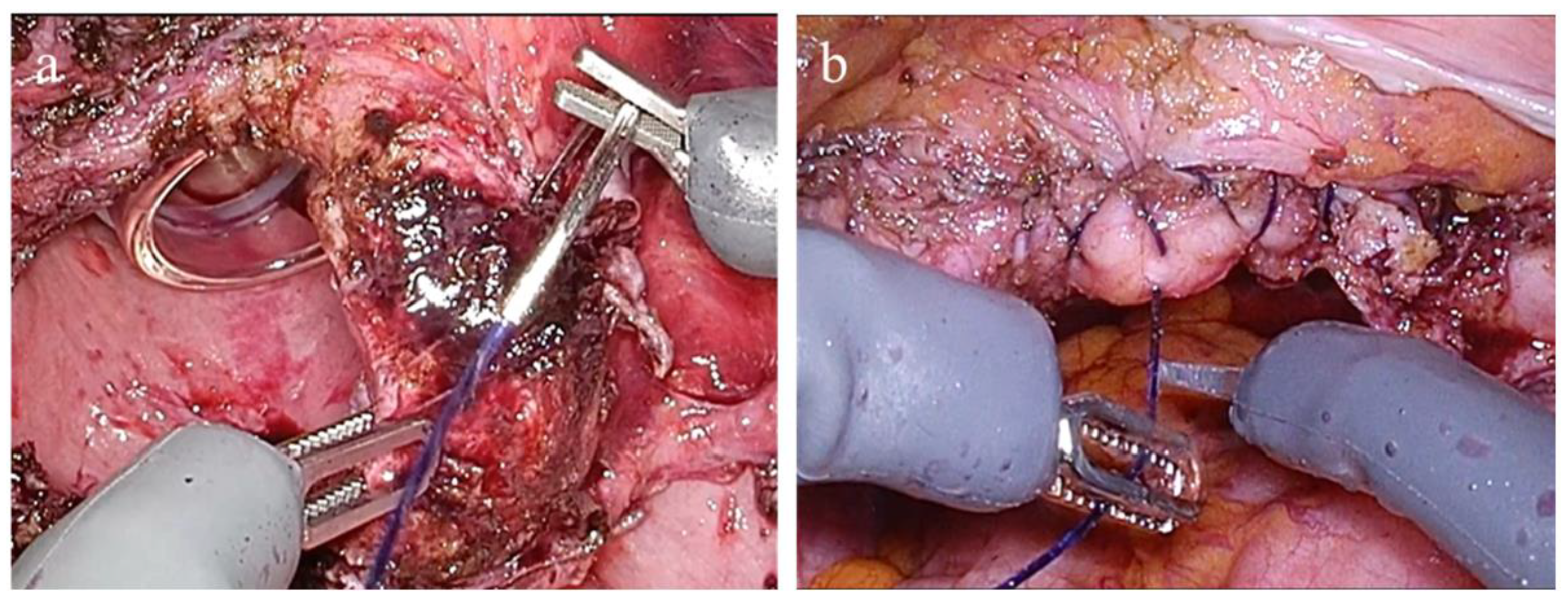
This configuration enabled precise instrument manipulation and intuitive control of the grasping force throughout the procedure. Representative intraoperative views of vaginal cuff suturing are displayed in
Figure 4. During this step, the surgeon perceives the grasping force applied to the vaginal cuff in real time through the system’s haptic feedback functionality. This sensory input allows for delicate tissue handling without excessive tension or the risk of suture breakage. The ability to modulate the grasping force throughout the suturing process enables secure and precise closure, contributing to atraumatic and controlled management of the vaginal cuff.
A vessel-sealing system was employed through the auxiliary port for vascular processing in all cases. In cases 4 and 5, the bipolar forceps of the Saroa surgical system became clinically available and were utilized to assist with hemostasis. In contrast, in cases 1–3, hemostasis was achieved solely with a vessel-sealing device operated by the assistant. The surgical approach employed in this study resembled a hybrid robot-assisted surgery model, integrating both robotic and conventional laparoscopic techniques. In this configuration, while the primary procedural steps were executed using the Saroa surgical system, certain tasks, such as the transection of major vessels, were performed by the assistant through conventional laparoscopic access ports. A dedicated vessel-sealing system for the Saroa surgical platform is currently under development.
2.4. Data Collection and Statistical Analysis
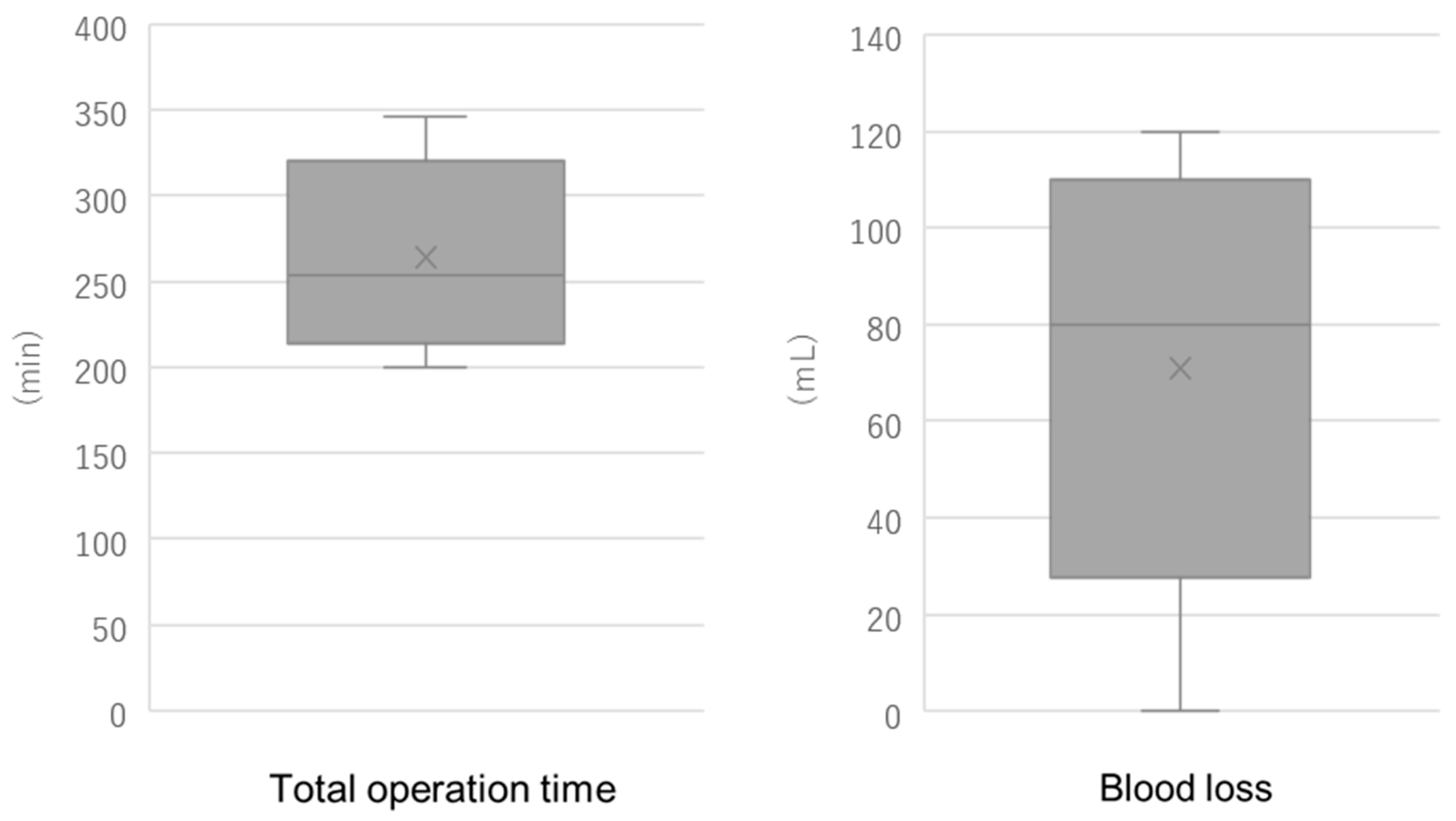
Demographic variables, operative duration (setup, console, and total), estimated blood loss, complications, and length of hospital stay were recorded. Inferential statistical analysis was not performed due to the limited sample size.
4. Discussion
This study demonstrated that the Saroa surgical system is safe and feasible for RAH. Although we previously performed robotic surgery using the da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA), the initial clinical outcomes achieved with the Saroa surgical system were comparable to those observed during the early adoption of da Vinci-assisted procedures.
The Saroa surgical system is characterized by two principal advantages.
First, the system incorporated real-time haptic feedback. In this system, a pneumatic cylinder mounted on a robotic arm actuated the wires embedded within the instrument shaft and transmitted force to the grasping jaw at the instrument tip [
3]. The grasping force was generated by the pressure inside the pneumatic cylinder, which was continuously monitored and converted into an electrical signal. These signals were then transmitted to the surgeon’s hand controller, where they were used to reproduce the forces applied at the instrument tip, thereby enabling real-time haptic feedback during surgical manipulation. The Saroa surgical system delivers real-time pneumatic grip force feedback, in contrast to the da Vinci 5 System, which primarily relies on software-mediated sensing to estimate contact and traction forces. This allows surgeons to perceive and modulate the pressure applied during tissue handling physically, thereby offering a direct and intuitive tactile experience [
4]. A preclinical study by Sakai et al. demonstrated that the Saroa surgical system’s real-time contact-force feedback significantly reduced excessive tissue compression and enhanced precision in grasping maneuvers compared with conventional robotic systems lacking haptic feedback [
5]. Histological assessments confirmed that such feedback substantially decreased tissue trauma, providing experimental evidence for the system’s potential to mitigate injury. These results complement the qualitative impressions reported in the present clinical series. In addition to minimizing tissue injury, the ability of the system to assess the grasping force in Newtons provides a quantifiable parameter for evaluating intraoperative tissue handling. This creates opportunities to investigate the relationship between the applied force and surgical proficiency. Notably, novice surgeons appear to benefit most from real-time perception of the grasping force, which may aid in preventing inadvertent tissue overcompression [
6]. Such feedback facilitates precise tissue handling and may accelerate skill acquisition. For surgeons transitioning from systems without haptic feedback, a brief adaptation period is anticipated; however, the intuitive nature and real-time presentation of the feedback suggest a relatively shallow learning curve. This feature has the potential to enhance training for inexperienced surgeons and refine precision in experienced operators. These findings suggest that the Saroa surgical system could serve as a particularly valuable platform for surgical education and skill acquisition, offering both safety advantages and objective feedback for performance evaluation. Although the system can record the grasping force in Newtons, data collection was not performed in this initial case series due to the regulatory and ethical constraints specific to our institution. Future studies with prospective approval are planned to validate the force-based metrics. In Japan, this system has been introduced not only for gynecologic surgery but also for gastrointestinal, thoracic, and urologic procedures, all of which have been performed safely [
7,
8,
9]. Unlike procedures in other specialties, gynecologic surgery frequently involves manipulation of the uterus, a relatively firm and heavy organ, and requires delicate maneuvers including grasping and suturing the vaginal cuff. Haptic feedback may be particularly advantageous during vaginal cuff suturing to avoid application of excessive force. These characteristics can benefit from real-time grasping force feedback, potentially improving surgical control and reducing the risk of tissue trauma [
10].
Second, the Saroa surgical system features a compact and lightweight design, offering advantages in terms of cost efficiency and maneuverability, making the system particularly well suited for patients with small stature and low body weight, including slender adults. In contrast to traditional robotic systems that require docking, the Saroa surgical system is equipped with a lightweight patient cart that can be easily positioned between the patient’s legs. Weighing approximately 500 kg, the patient cart allows simple repositioning at the bedside without the need for complex docking procedures, thereby improving the operative efficiency [
11]. The compact design further improves maneuverability, particularly in operating rooms with limited space. Moreover, the system proves to be cost-effective and adaptable, rendering it a promising option for expanding access to RAS, particularly where conventional robotic systems are less readily available.
However, despite these benefits, a potential limitation of the Saroa surgical system includes the use of only three robotic arms, compared to the four arms available on conventional platforms such as the da Vinci Xi. In complex procedures, the absence of a fourth arm could theoretically limit the ability to perform retraction and dissection simultaneously, potentially requiring additional assistant maneuvers. However, in our experience with RAH, the compact three-arm design did not create significant challenges in retraction, as its smaller footprint reduced interference with assistant instruments and allowed precise and timely traction according to the surgeon’s instructions. Nevertheless, in more advanced procedures involving multiple dissection planes or extensive adhesiolysis, strategic operative planning may be necessary to mitigate this structural limitation. An additional single-arm auxiliary unit is currently under development to complement the existing three-arm system, with the aim of enhancing surgical versatility and overcoming current limitations in tissue retraction. The Saroa surgical system presents an accessible and pragmatic solution for broad clinical implementations. Future comparative studies are warranted to delineate the specific procedural advantages and cost–benefit profiles of each robotic system, including the potential utility of force-based metrics for surgical evaluation and training.
4.1. Clinical Implications and Future Directions
The integration of real-time force measurements into clinical robotics represents a major step toward quantitative data-driven surgery. In the future, intraoperatively recorded grasping force profiles may enable the creation of standardized metrics for evaluating surgical performance across institutions. Furthermore, these metrics may facilitate personalized training protocols and early detection of suboptimal techniques. Compared to existing robotic surgical systems without haptic feedback, platforms such as the Saroa system offer potential advantages in improving tissue handling precision and enhancing surgical safety. Continued investigation involving large cohorts and diverse procedures is needed to validate the clinical impact of these capabilities and to clarify their comparative benefits over conventional systems, as well as to define the optimal ranges of grasping force for varying tissue types.
In the context of other next-generation robotic platforms, our median operative time and blood loss fall within the ranges reported during early clinical adoption. For hysterectomy performed with the Hugo™ RAS system (Medtronic, Inc., Minneapolis, MN, USA), a median operative time of 127 min (range: 98–255 min) and a median estimated blood loss of 50 mL (range: 30–125 mL) have been reported [
12]. For the Versius system (CMR Surgical Ltd., Cambridge, UK), the median skin-to-skin operative time was 158.5 min (range: 50–385 min). When stratified by case sequence, the median operative time decreased from 191.5 min (range: 67–280 min) in the first 20 cases to 147 min (range: 90–273 min) in the last 20 cases, demonstrating a progressive reduction along the learning curve [
13]. For the Senhance system (Asensus Surgical, Inc., Research Triangle Park, NC, USA), the total operative time for hysterectomy cases ranged from 32 to 136 min, with a median of 57 min. The estimated blood loss ranged from 25 to 100 mL, with a median of 75 mL [
14]. Moreover, multi-platform comparisons suggest that newer systems (e.g., Hugo™, hinotori™, da Vinci SP™) yield broadly comparable perioperative outcomes in early use, although operative time may differ among systems and centers [
15]. Taken together, these data indicate that our early experience with Saroa, which showed a median blood loss under 100 mL and acceptable variability in operative time, is consistent with international reports from the introductory phases of other novel robotic systems.
4.2. Strengths and Limitations
The first clinical application of the Saroa surgical system in the RAH was evaluated using haptic feedback. This was a single-institution study with short-term follow-up. One important limitation of this study is the reliance on subjective feedback from the operating surgeons to assess the utility of the haptic feedback system. While qualitative impressions offer valuable insights, they are inherently influenced by surgeon experience, expectations, and potential confirmation bias, especially in early-phase evaluations. Without objective data—such as force measurements or validated performance metrics—the reported improvements cannot be quantitatively confirmed. Future studies using blinded assessments and standardized tools will be necessary to reduce bias and more accurately evaluate the system’s impact.