The Link Between Oxidative Stress and Male Infertility in Lithuania: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
- 1.
- Male infertility diagnosed according to the World Health Organization (WHO) criteria.
- 2.
- Age between 19 and 42 years.
- 3.
- First-time patients seeking medical assistance for infertility.
- 4.
- Comprehensive clinical examinations, including semen analysis, hormonal testing (FSH, LH, testosterone).
- 5.
- Infections, varicocele, and other anatomical abnormalities potentially affecting male fertility were evaluated during a urologist consultation, including ultrasound examination.
- 1.
- Couples with incomplete or insufficient medical records, preventing full assessment according to the inclusion criteria.
- 2.
- Patients who had previously undergone infertility treatment.
- 1.
- Male infertility diagnosed according to WHO criteria.
- 2.
- Age between 19 and 42 years.
- 3.
- First-time consultation about infertility.
- 4.
- Semen analysis, hormonal testing (FSH, LH, testosterone).
- 5.
- Infections, varicocele, and other anatomical abnormalities potentially affecting male fertility were evaluated during a urologist consultation, including ultrasound examination.
- 6.
- Oxidative stress assessment using the MiOXSYS system.
- 1.
- Patients who had previously received infertility treatment or undergone assisted reproductive procedures.
- 2.
- Incomplete or insufficient medical records.
- 3.
- Patients taking antioxidant supplements during the study period (due to the potential risk of oxidative stress that may affect test results).
Laboratory and Diagnostic Tests
3. Results
4. Discussion
4.1. Differences in Diagnostic Methods in Lithuania and International Practices
4.2. Relationship Between Oxidative Stress and Male Infertility
4.3. Limitations and Clinical Relevance of ORP Measurement
4.4. Study Significance and Future Directions
- Optimization of the ORP testing methodology to reduce diagnostic inaccuracies related to extreme sperm concentrations.
- Integration of ORP testing with sperm DNA fragmentation analysis to assess whether oxidative stress contributes to genetic sperm damage.
- Expansion of genetic, epigenetic, and proteomic studies to identify novel molecular biomarkers of male infertility.
- Comprehensive evaluation of environmental and lifestyle factors contributing to oxidative stress.
- Consideration of population-specific genetic and ethnic characteristics to ensure the relevance and applicability of findings.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Infertility. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 22 May 2024).
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef]
- Actan, G.; Abbosoglu, D.S.; Küçükgergin, C.; Kadıoğlu, A.; Erata, G.O.; Toker, N.K. Mystery of idiopathic male infertility: Is oxidative stress an actual risk? Fertil. Steril. 2013, 99, 1211–1215. [Google Scholar] [CrossRef]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of reactive oxygen species in male infertility: An updated review of literature. Arab. J. Urol. 2018, 16, 35–43. [Google Scholar] [CrossRef]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yuan, Y.; Yang, X.; Wang, Y.; Liu, L. Diagnostic value of oxidation-reduction potential for male infertility: A systematic review and meta-analysis. Transl. Androl. Urol. 2024, 13, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.H.; Tobler, K.J. Female Infertility. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556033/ (accessed on 19 December 2022).
- Jungwirth, A.; Diemer, T.; Kopa, Z.; Krausz, C.; Minhas, S.; Tournay, H. Male Infertility. Guidelines of the European Association of Urology. World J. Men’s Health 2019, 37, 296–312. [Google Scholar]
- Kothandaraman, N.; Agarwal, A.; Abu-Elmagd, M.; Al-Qahtani, M.H. Pathogenic landscape of idiopathic male infertility: New insight towards its regulatory networks. npj Genom. Med. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Kaiyal, R.S.; Mukherjee, S.D.; Panner Selvam, M.K.; Lundy, S.D. Mitochondrial dysfunction signatures in idiopathic primary male infertility: Proteomic insights. Front. Reprod. Health 2024, 6, 1479568. [Google Scholar] [CrossRef]
- Corda, P.O.; Moreira, J.; Howl, J.; Oliveira, P.F.; Fardilha, M.; Silva, J.V. Differential Proteomic Analysis of Human Sperm: A Systematic Review to Identify Candidate Targets to Monitor Sperm Quality. World J. Men’s Health 2024, 42, 71–91. [Google Scholar] [CrossRef]
- Ventimiglia, E.; Pozzi, E.; Capogrosso, P.; Boeri, L.; Alfano, M.; Cazzaniga, W.; Matloob, R.; Abbate, C.; Viganò, P.; Montorsi, F.; et al. Extensive Assessment of Underlying Etiological Factors in Primary Infertile Men Reduces the Proportion of Men With Idiopathic Infertility. Front. Endocrinol. 2021, 12, 801125. [Google Scholar] [CrossRef] [PubMed]
- Corsini, C.; Boeri, L.; Candela, L.; Pozzi, E.; Belladelli, F.; Capogrosso, P.; Fallara, G.; Schifano, N.; Cignoli, D.; Ventimiglia, E.; et al. Is There a Relevant Clinical Impact in Differentiating Idiopathic versus Unexplained Male Infertility? World J. Men’s Health 2022, 41, 354–362. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Chen, L.; Mori, Y.; Nishii, S.; Sakamoto, M.; Ohara, M.; Yamagishi, S.-I.; Sekizawa, A. Impact of Oxidative Stress on Sperm Quality in Oligozoospermia and Normozoospermia Males Without Obvious Causes of Infertility. J. Clin. Med. 2024, 13, 7158. [Google Scholar] [CrossRef]
- Agarwal, A.; Selvam, M.P.; Arafa, M.; Okada, H.; Homa, S.; Killeen, A.; Balaban, B.; Saleh, R.; Armagan, A.; Roychoudhury, S.; et al. Multi-center evaluation of oxidation-reduction potential by the MiOXSYS in males with abnormal semen. Asian J. Androl. 2019, 21, 565–569. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Nada, E.A.; El-Tonsy, M.H.; Sharma, R.K.; Meyer, A.; Nelson, D.R.; Thomas, A.J. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil. Steril. 2003, 79, 1597–1605. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Panner Selvam, M.K.; Finelli, R.; Leisegang, K.; Barbarosie, C.; Alvarez, J.G.; Rambhatla, A.; Renzulli, J.F. Oxidative Stress and Assisted Reproduction: A Comprehensive Review of Its Pathophysiological Role and Strategies for Optimizing Embryo Culture Environment. Antioxidants 2022, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, K.; Xu, S.; Tu, W.; Lin, X.; Su, Y.; Huang, R.; Deng, Y.; Liu, Y. Double-Edged Sword: Effects of Human Sperm Reactive Oxygen Species on Embryo Development in IVF Cycles. Reprod. Biol. Endocrinol. 2023, 21, 1. [Google Scholar] [CrossRef]
- Agarwal, A.; Wang, S.M. Clinical relevance of oxidation-reduction potential in the evaluation of male infertility. Urology 2017, 104, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A.; Esteves, S.C.; Ko, E.; Ramasamy, R.; Zini, A. Clinical utility of sperm DNA fragmentation testing: Practice recommendations based on clinical scenarios. Transl. Androl. Urol. 2016, 5, 935–950. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Selvam, M.K.P.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Vassiliou, A.; Martin, C.H.; Homa, S.T.; Stone, J.; Dawkins, A.; Genkova, M.N.; Roca, H.S.D.; Parikh, S.; Patel, J.; Yap, T.; et al. Redox potential in human semen: Validation and qualification of the MiOXsys assay. Andrologia 2020, 53, e13938. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Sperm DNA fragmentation analysis using the TUNEL assay. Methods Mol. Biol. 2016, 927, 121–136. [Google Scholar]
- Balló, A.; Czétány, P.; Busznyákné, K.S.; Márk, L.; Mike, N.; Török, A.; Szántó, Á.; Máté, G. Oxido-reduction potential as a method to determine oxidative stress in semen samples. Int. J. Mol. Sci. 2023, 24, 11981. [Google Scholar] [CrossRef] [PubMed]
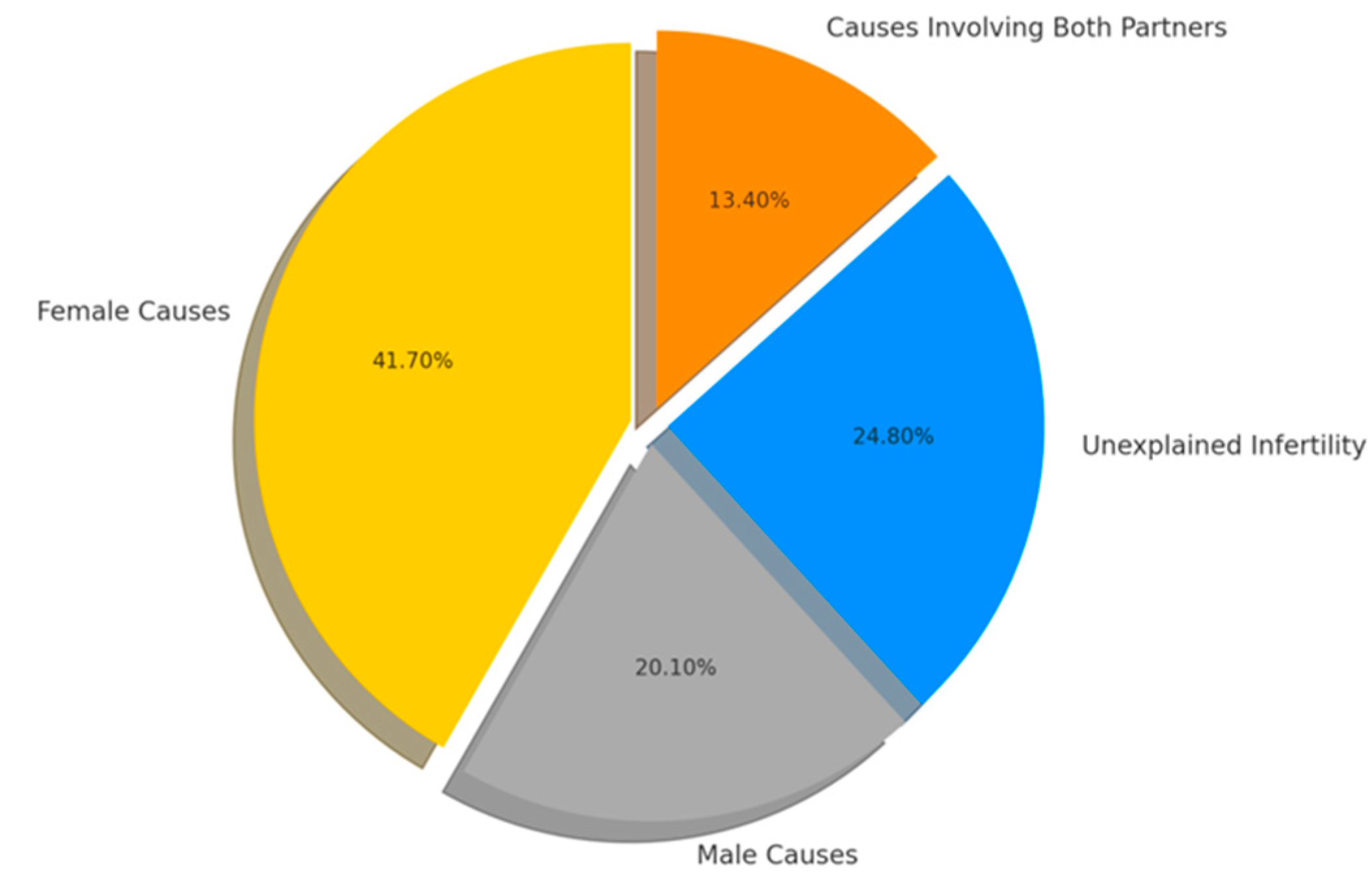
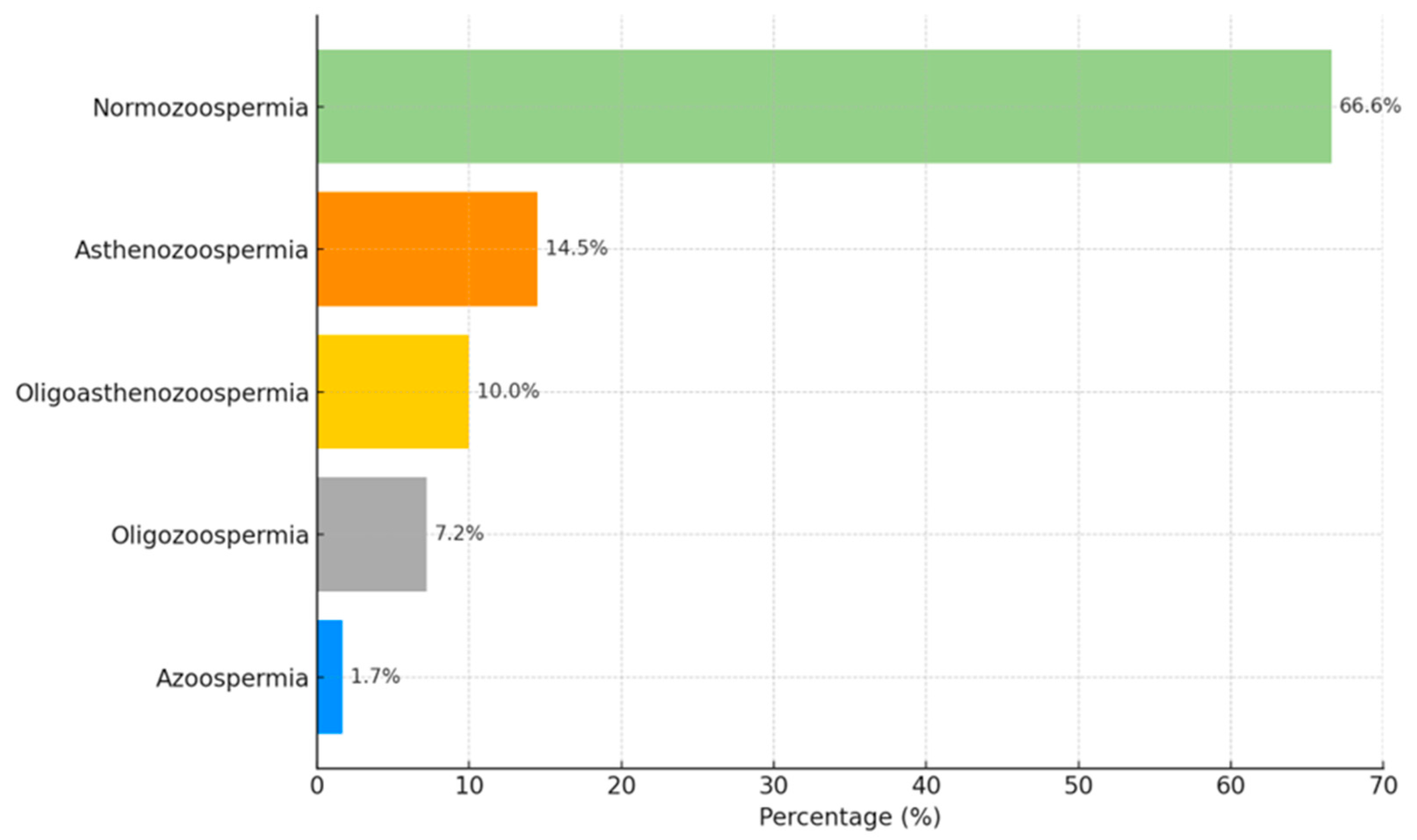

| Hormone | Lowest Detectable Concentration | Normal Interval |
|---|---|---|
| FSH | 0.1 mIU/mL | 0.7–11.1 mIU/mL |
| LH | 0.1 mIU/mL | 0.8–7.6 mIU/mL |
| Testosterone | 0.087 nmol/L (25 pg/mL) | 5.5–25.2 nmol/L (160–726 ng/dL) |
| Cause | Number of Cases (Percentage) (n-240) Current Study | Number of Cases (Percentage) (n-10.469) EAU Data |
|---|---|---|
| Idiopathic infertility | 86.7 | 31.0 |
| Varicocele | 4.2 | 15.6 |
| Cryptorchidism (undescended testes) | 1.7 | 7.8 |
| Infection | 0.8 | 5.9 |
| Endocrine causes | 2.5 | 8.9 |
| Immunological causes | - | 4.5 |
| Disturbance of erection/ejaculation | 0.8 | 5.9 |
| Obstructive causes | 0.8 | 1.7 |
| Systemic diseases | 1.7 | 3.1 |
| Others | 0.8 | 5.5 |
| Pathology | N | Normal ORP (≤1.34 mV/106/mL) | Elevated Oxidative Stress (>1.34 mV/106/mL) | χ2; p |
|---|---|---|---|---|
| Normozoospermia | 99 | 51 (51.5) | 48 (48.5) | 42.9; <0.001 |
| Asthenozoospermia | 76 | 32 (42.1) | 44 (57.9) | |
| Oligoasthenozoospermia | 52 | 5 (9.6) | 47 (90.4) | |
| Oligozoospermia | 26 | 0 | 26 (100) | |
| Oligoasthenoteratozoospermia | 4 | 1 (25.0) | 3 (75.0) | |
| Asthenoterazoospermia | 2 | 1 (50.0) | 1 (50.0) | |
| Teratozoospermia | 2 | 1 (50.0) | 1 (50.0) |
| Indicator | ORP | |
|---|---|---|
| r | p | |
| Sperm volume, mL | 0.011 | 0.854 |
| Sperm count, million/1 mL | −0.787 | <0.001 |
| Number of sperm in ejaculate, million | −0.676 | <0.001 |
| Percentage of progressively motile (A) sperm | −0.315 | <0.001 |
| Percentage of progressively motile (B) sperm | −0.219 | <0.001 |
| Morphology, %. | −0.346 | <0.001 |
| Agglutination, %. | −0.295 | <0.001 |
| Round cells, %. | 0.120 | 0.053 |
| Causes | Number of Cases (%) (n = 240) | Number of Cases (%) (n = 162) MiOXSYS | Z; p |
|---|---|---|---|
| Idiopathic infertility | 86.7% | 24.7% | 15.36; <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jašinskienė, E.; Čaplinskienė, M. The Link Between Oxidative Stress and Male Infertility in Lithuania: A Retrospective Study. Medicina 2025, 61, 1715. https://doi.org/10.3390/medicina61091715
Jašinskienė E, Čaplinskienė M. The Link Between Oxidative Stress and Male Infertility in Lithuania: A Retrospective Study. Medicina. 2025; 61(9):1715. https://doi.org/10.3390/medicina61091715
Chicago/Turabian StyleJašinskienė, Eglė, and Marija Čaplinskienė. 2025. "The Link Between Oxidative Stress and Male Infertility in Lithuania: A Retrospective Study" Medicina 61, no. 9: 1715. https://doi.org/10.3390/medicina61091715
APA StyleJašinskienė, E., & Čaplinskienė, M. (2025). The Link Between Oxidative Stress and Male Infertility in Lithuania: A Retrospective Study. Medicina, 61(9), 1715. https://doi.org/10.3390/medicina61091715







