Prognostic Significance of Peritumoral and Intratumoral Lymphatic Vessels Density in Clinically Node-Negative (cN0) Oral Squamous Cell Carcinoma: A Preliminary Report
Abstract
1. Introduction
2. Materials and Methods
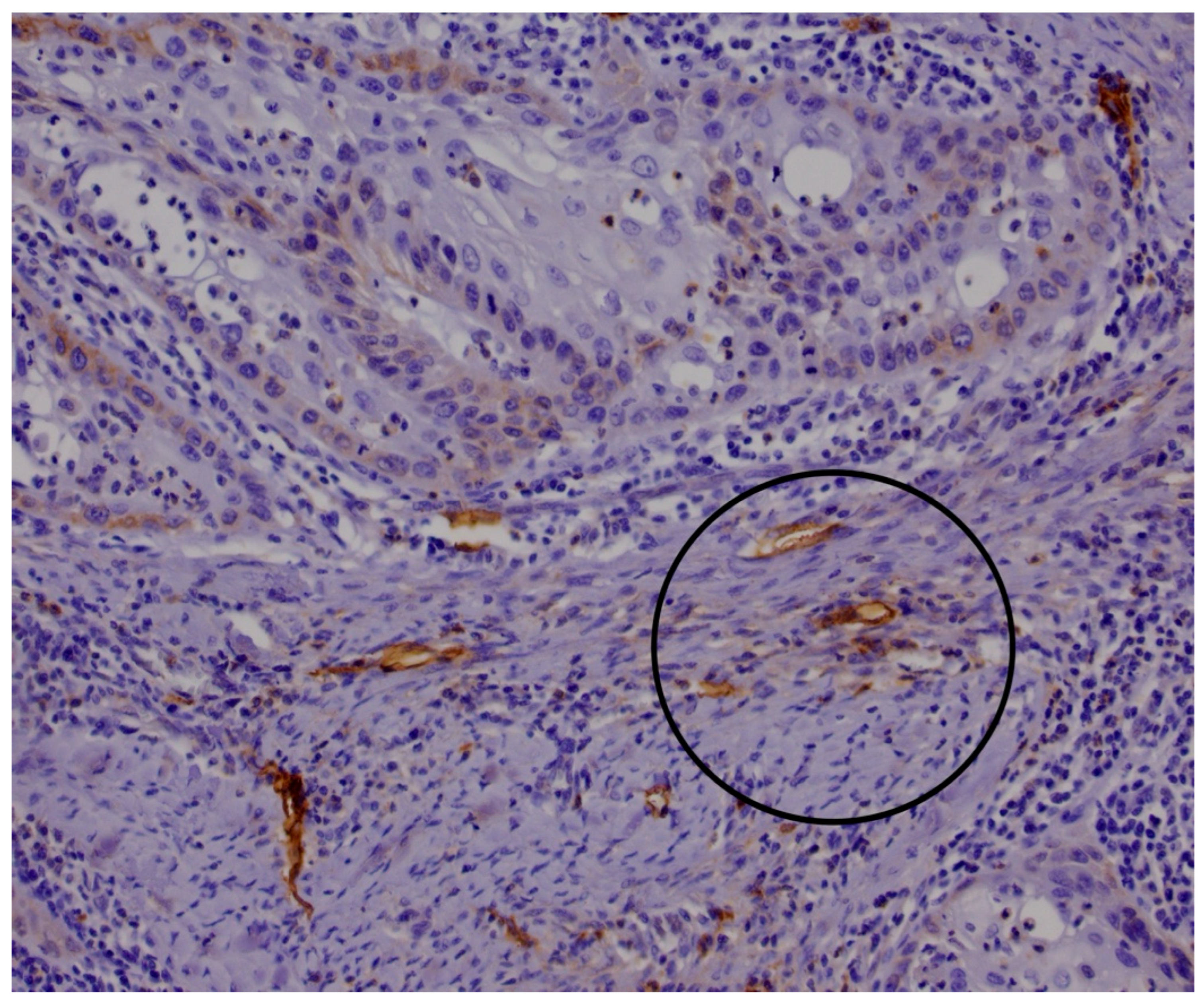
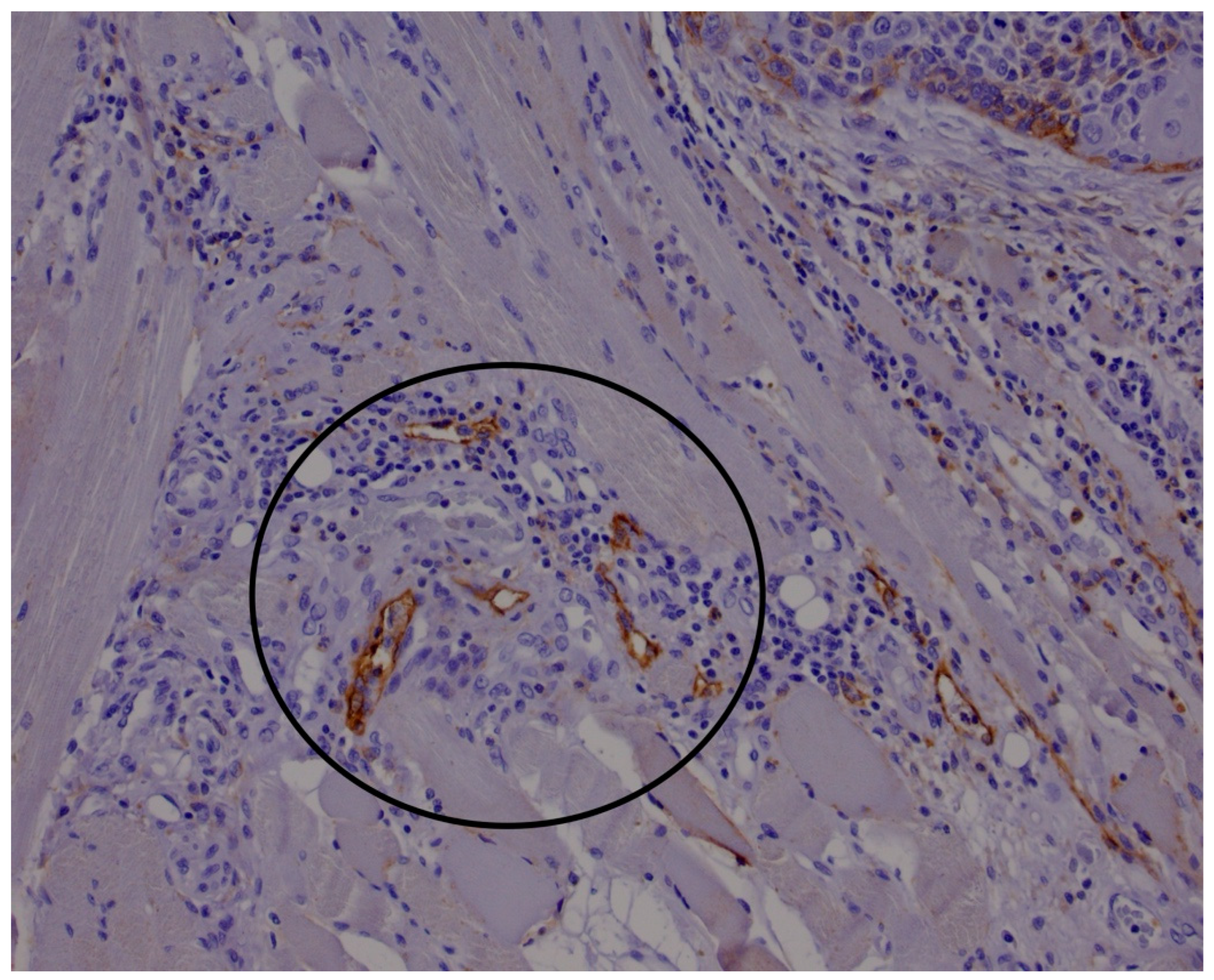
2.1. Histological and Immunohistological Analysis
2.2. Statistical Analysis
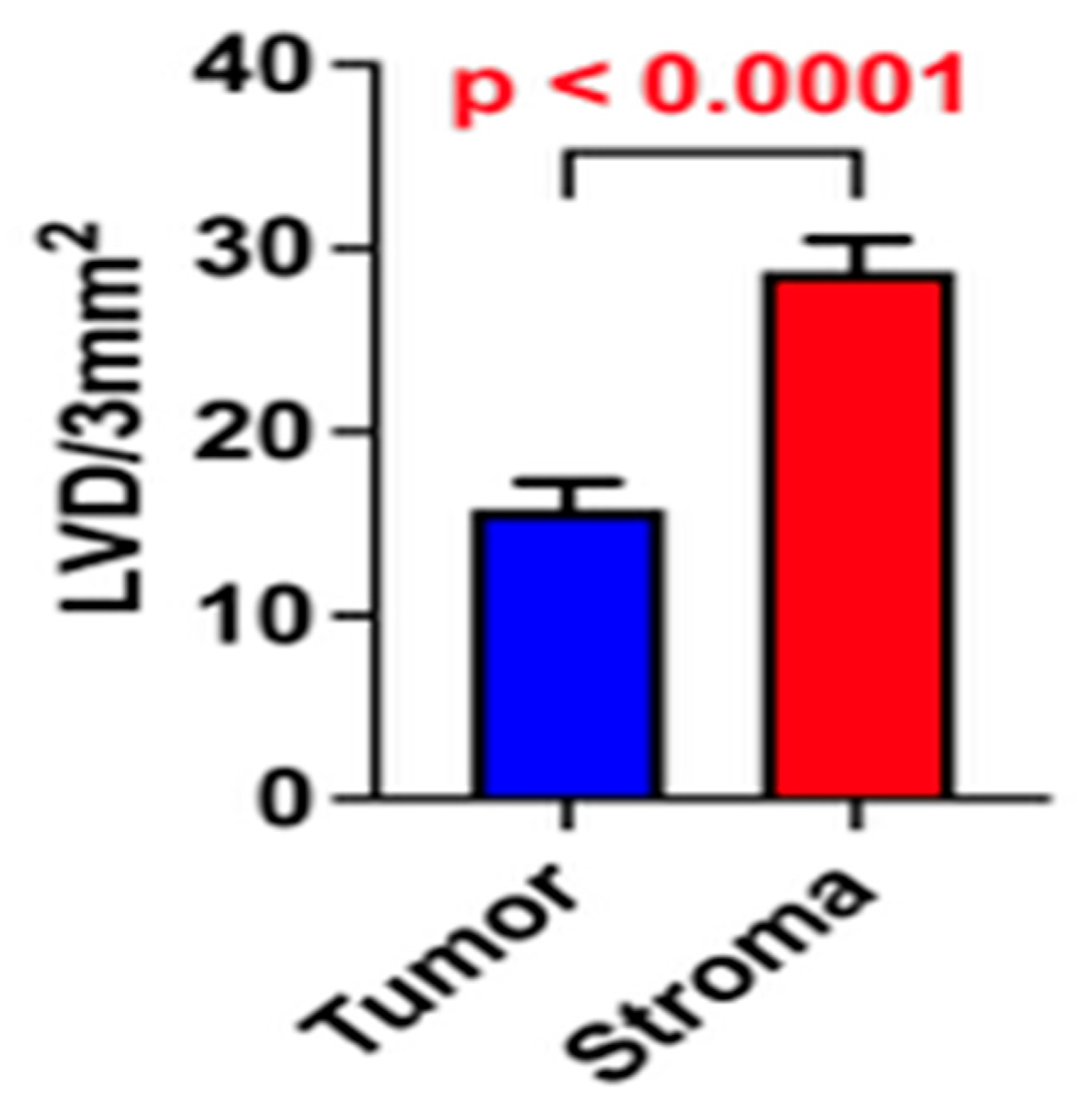
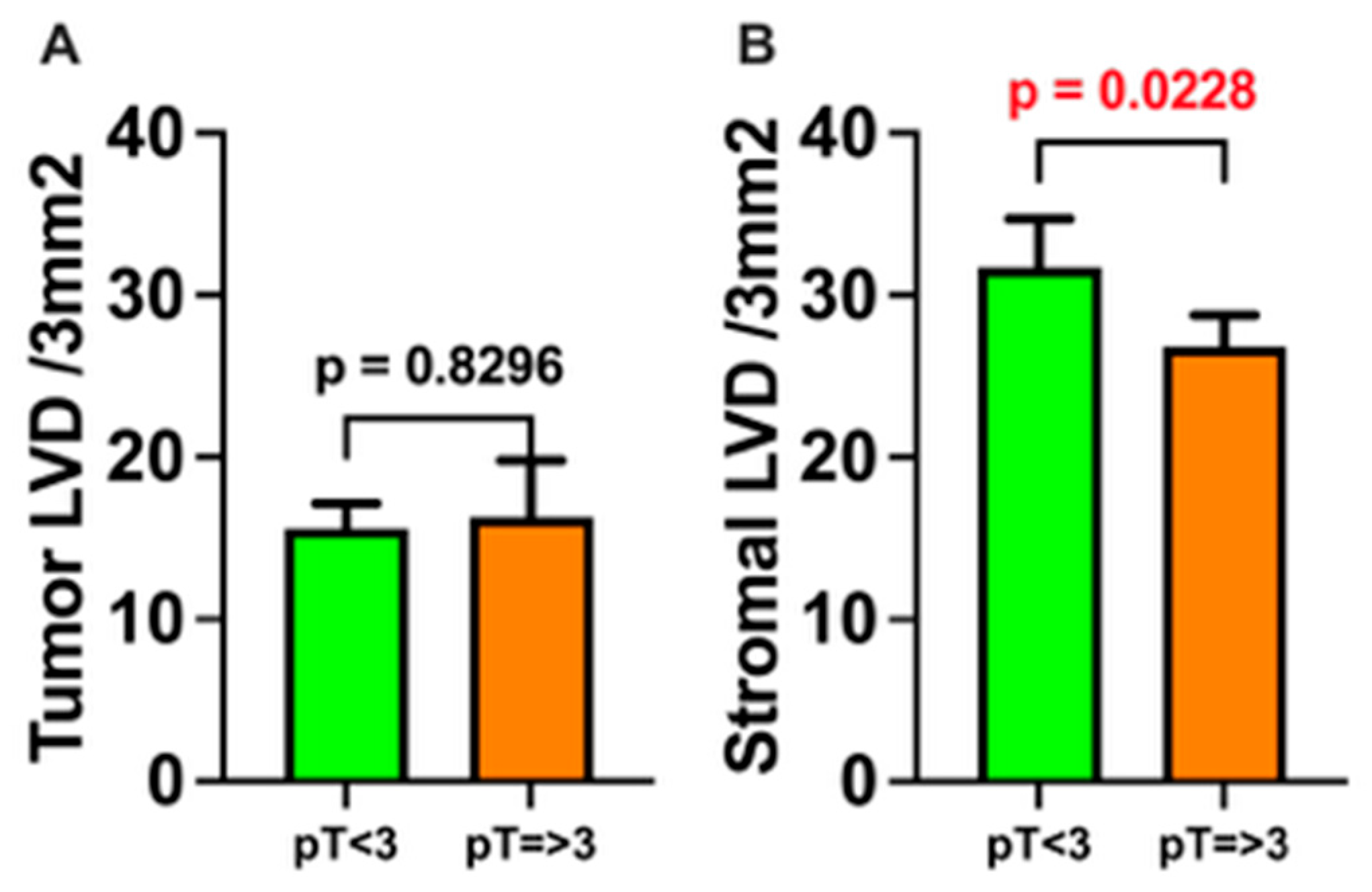
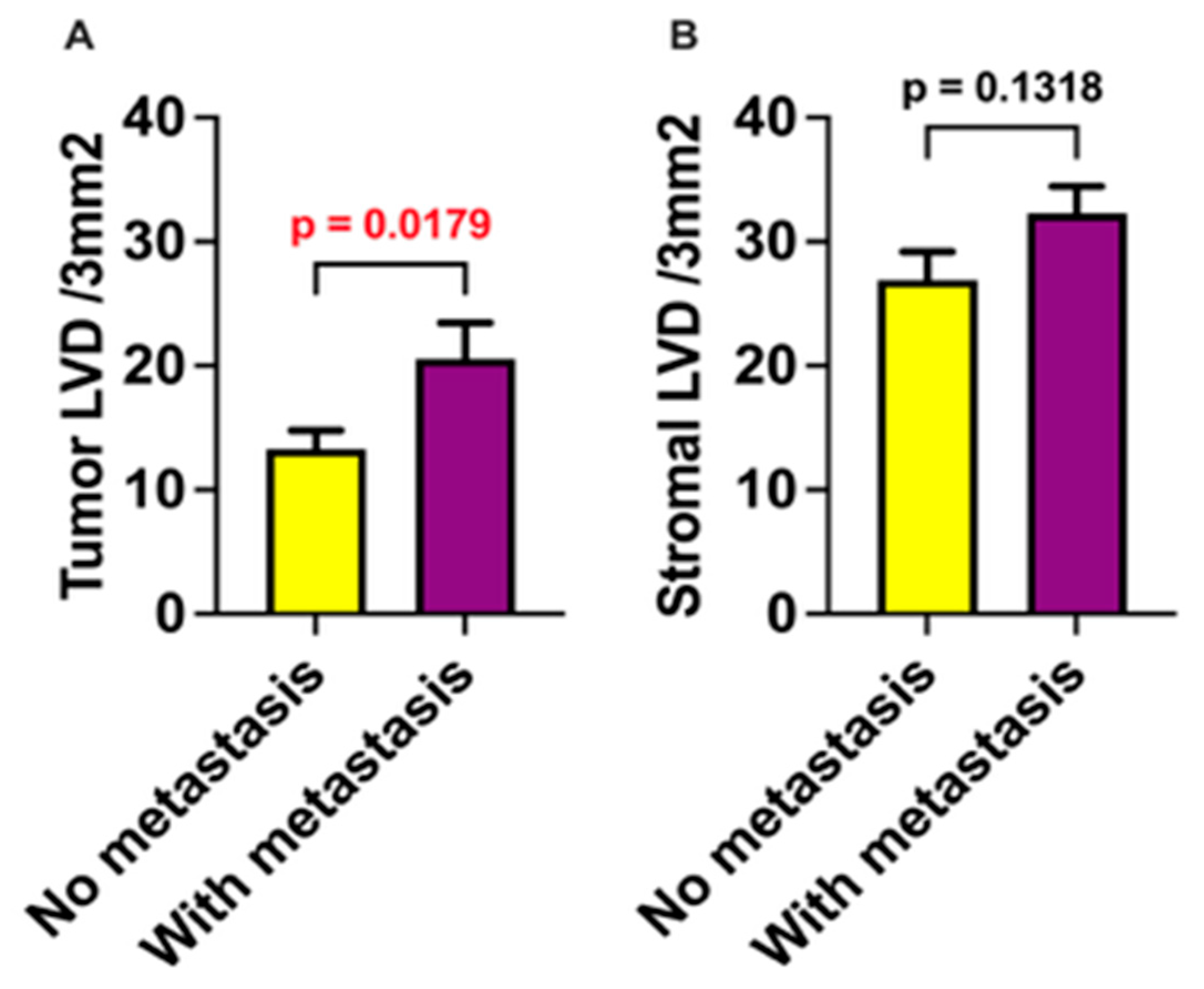
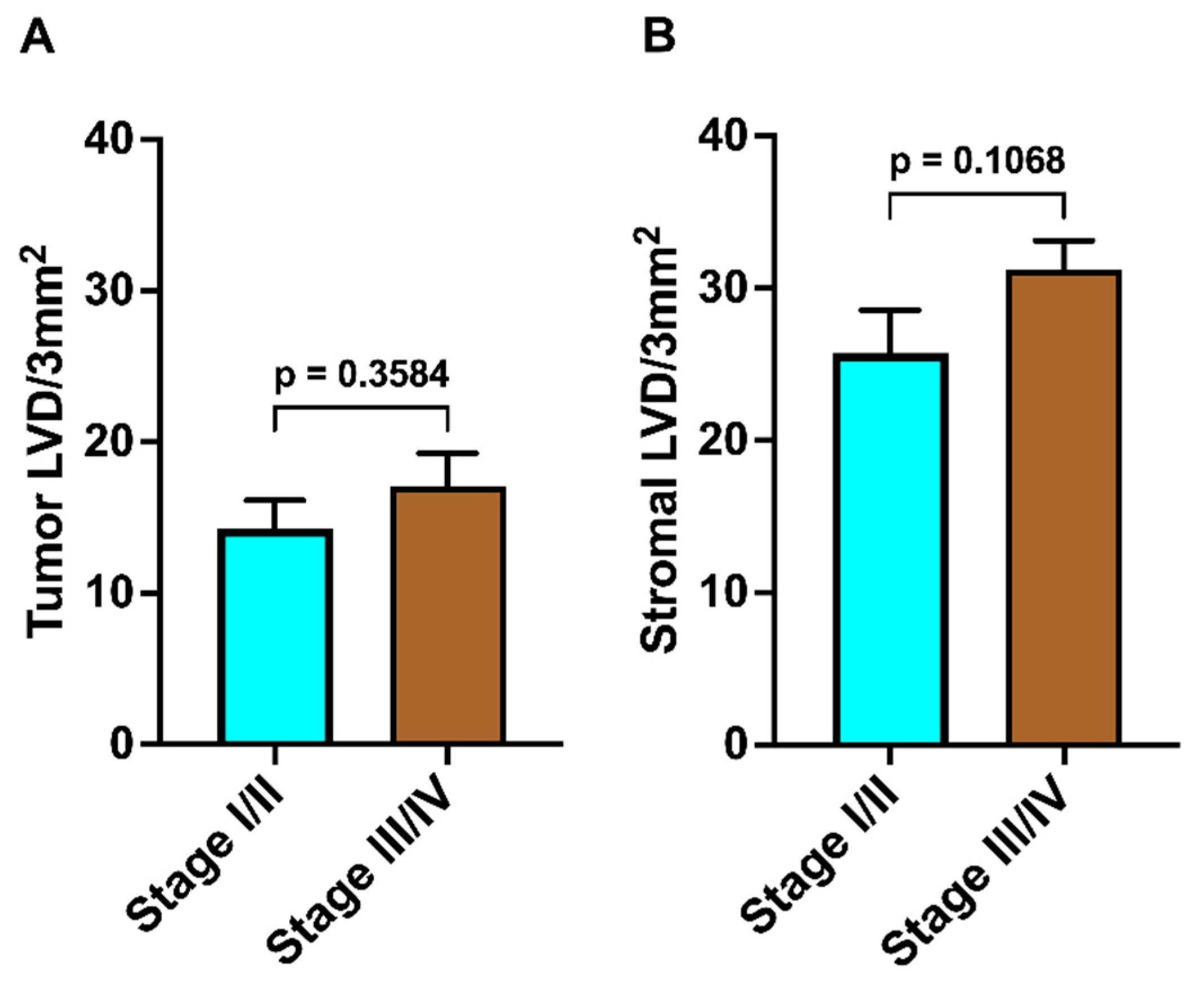
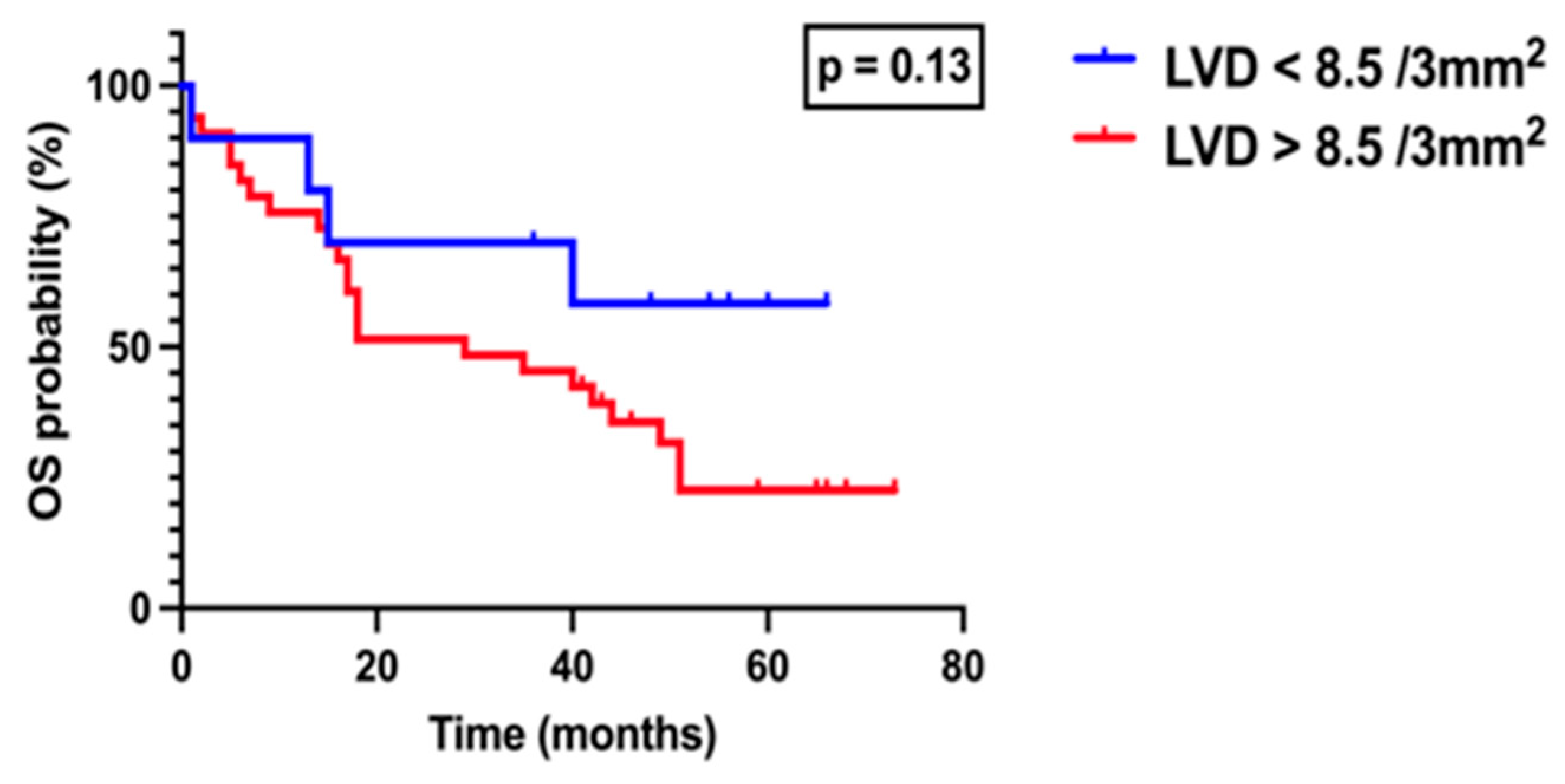
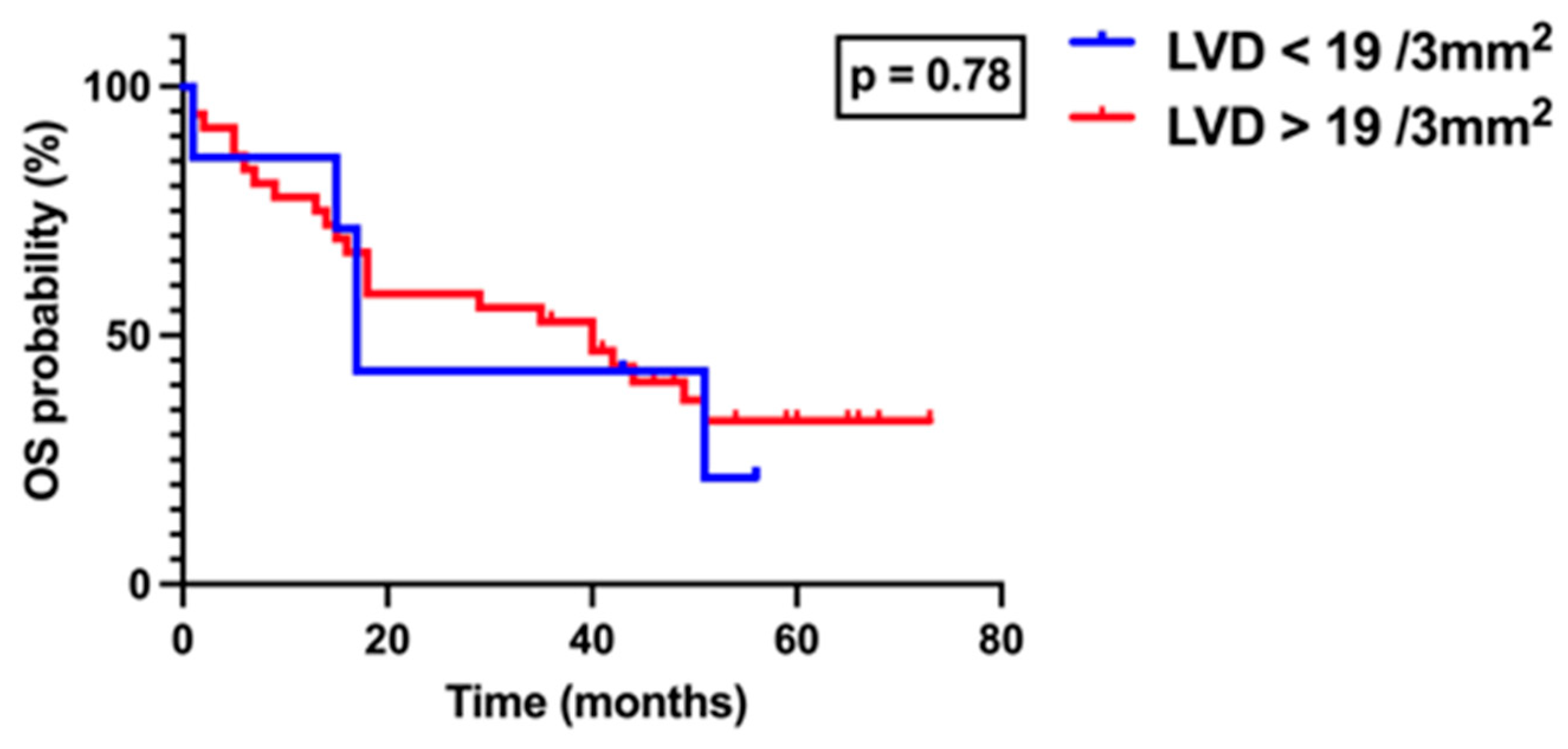
3. Results
4. Discussion
4.1. Future Research Directions
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OSCC | Oral Squamous Cell Carcinoma |
| cN0 | Clinically Node Negative Neck |
| OLNM | Occult Lymph Node Metastasis |
| END | Elective Neck Dissection |
| SLNB | Sentinel Lymph Node Biopsy |
| DOI | Depth of Invasion |
| LVD | Lymphovascular Density |
| ILVD | Intratumoral Lymphovascular Density |
| PLVD | Peritumoral Lymphovascular Density |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| (cN+) | Clinically Node Positive Neck |
| MSCT | Multi-Slice Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| HE | Hematoxylin and Eosin-stained |
| ENE | Extranodal Extension |
| DAB | Diaminobenzidine |
| UICC | Union for International Cancer Control |
| AJCC | American Joint Committee on Cancer |
| PNI | Perineural Invasion |
| LVI | Lymphovascular Invasion |
| DFS | Disease Free Survival |
| OS | Overall Survival |
| IQR | Interquartile Range |
| ROC | Receiver Operating Characteristic |
| HPV | Human Papillomavirus |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, F.; Hua, M.; Song, X.; Liu, S.; Dong, Z. Prognostic Value of Lymphatic Vessel Density in Oral Squamous Cell Carcinoma. Life Sci. 2021, 265, 118746. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.D.; Ferlay, J.; Jemal, A.; Sankaranarayanan, R.; Chaturvedi, A.K.; Bray, F.; Soerjomataram, I. The Global Incidence of Lip, Oral Cavity, and Pharyngeal Cancers by Subsite in 2012. CA Cancer J. Clin. 2017, 67, 51–64. [Google Scholar] [CrossRef]
- Duggan, M.A.; Anderson, W.F.; Altekruse, S.; Penberthy, L.; Sherman, M.E. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am. J. Surg. Pathol. 2016, 40, e94–e102. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- de Bree, R.; Takes, R.P.; Shah, J.P.; Hamoir, M.; Kowalski, L.P.; Robbins, K.T.; Rodrigo, J.P.; Sanabria, A.; Medina, J.E.; Rinaldo, A.; et al. Elective Neck Dissection in Oral Squamous Cell Carcinoma: Past, Present and Future. Oral Oncol. 2019, 90, 87–93. [Google Scholar] [CrossRef]
- Jose, J.; Coatesworth, A.P.; MacLennan, K. Cervical Metastases in Upper Aerodigestive Tract Squamous Cell Carcinoma: Histopathologic Analysis and Reporting. Head Neck 2003, 25, 194–197. [Google Scholar] [CrossRef]
- Andersen, P.E.; Shah, J.P.; Cambronero, E.; Spiro, R.H. The Role of Comprehensive Neck Dissection with Preservation of the Spinal Accessory Nerve in the Clinically Positive Neck. Am. J. Surg. 1994, 168, 499–502. [Google Scholar] [CrossRef]
- Mermod, M.; Bongiovanni, M.; Petrova, T.V.; Dubikovskaya, E.A.; Simon, C.; Tolstonog, G.; Monnier, Y. Prediction of Occult Lymph Node Metastasis in Squamous Cell Carcinoma of the Oral Cavity and the Oropharynx Using Peritumoral Prospero Homeobox Protein 1 Lymphatic Nuclear Quantification. Head Neck 2016, 38, 1407–1415. [Google Scholar] [CrossRef]
- Fasunla, A.J.; Greene, B.H.; Timmesfeld, N.; Wiegand, S.; Werner, J.A.; Sesterhenn, A.M. A Meta-Analysis of the Randomized Controlled Trials on Elective Neck Dissection versus Therapeutic Neck Dissection in Oral Cavity Cancers with Clinically Node-Negative Neck. Oral Oncol. 2011, 47, 320–324. [Google Scholar] [CrossRef]
- Goudakos, J.K.; Markou, K.; Nikolaou, A.; Themelis, C.; Vital, V. Management of the Clinically Negative Neck (N0) of Supraglottic Laryngeal Carcinoma: A Systematic Review. Eur. J. Surg. Oncol. 2009, 35, 223–229. [Google Scholar] [CrossRef]
- Psychogios, G.; Mantsopoulos, K.; Bohr, C.; Koch, M.; Zenk, J.; Iro, H. Incidence of Occult Cervical Metastasis in Head and Neck Carcinomas: Development over Time. J. Surg. Oncol. 2013, 107, 384–387. [Google Scholar] [CrossRef]
- El Ghani, F.; Van Den Brekel, M.W.M.; De Goede, C.J.T.; Kuik, J.; Leemans, C.R.; Smeele, L.E. Shoulder Function and Patient Well-Being after Various Types of Neck Dissections. Clin. Otolaryngol. Allied Sci. 2002, 27, 403–408. [Google Scholar] [CrossRef]
- Goldstein, D.P.; Ringash, J.; Bissada, E.; Jaquet, Y.; Irish, J.; Chepeha, D.; Davis, A.M. Scoping Review of the Literature on Shoulder Impairments and Disability after Neck Dissection. Head Neck 2014, 36, 299–308. [Google Scholar] [CrossRef]
- Leusink, F.K.J.; van Es, R.J.J.; de Bree, R.; Baatenburg de Jong, R.J.; van Hooff, S.R.; Holstege, F.C.P.; Slootweg, P.J.; Brakenhoff, R.H.; Takes, R.P. Novel Diagnostic Modalities for Assessment of the Clinically Node-Negative Neck in Oral Squamous-Cell Carcinoma. Lancet Oncol. 2012, 13, e554–e561. [Google Scholar] [CrossRef]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef]
- Alkureishi, L.W.T.; Ross, G.L.; Shoaib, T.; Soutar, D.S.; Robertson, A.G.; Thompson, R.; Hunter, K.D.; Sorensen, J.A.; Thomsen, J.; Krogdahl, A.; et al. Sentinel Node Biopsy in Head and Neck Squamous Cell Cancer: 5-Year Follow-up of a European Multicenter Trial. Ann. Surg. Oncol. 2010, 17, 2459–2464. [Google Scholar] [CrossRef]
- Civantos, F.J.; Zitsch, R.P.; Schuller, D.E.; Agrawal, A.; Smith, R.B.; Nason, R.; Petruzelli, G.; Gourin, C.G.; Wong, R.J.; Ferris, R.L.; et al. Sentinel Lymph Node Biopsy Accurately Stages the Regional Lymph Nodes for T1-T2 Oral Squamous Cell Carcinomas: Results of a Prospective Multi-Institutional Trial. J. Clin. Oncol. 2010, 28, 1395–1400. [Google Scholar] [CrossRef]
- Crissman, J.D.; Gluckman, J.; Whiteley, J.; Quenelle, D. Squamous-Cell Carcinoma of the Floor of the Mouth. Head Neck Surg. 1980, 3, 2–7. [Google Scholar] [CrossRef]
- Högmo, A.; Kuylenstierna, R.; Lindholm, J.; Munck-Wikland, E. Predictive Value of Malignancy Grading Systems, DNA Content, P53, and Angiogenesis for Stage I Tongue Carcinomas. J. Clin. Pathol. 1999, 52, 35–40. [Google Scholar] [CrossRef]
- Jakobsson, P.A.; Eneroth, C.M.; Killander, D.; Moberger, G.; Mårtensson, B. Histologic Classification and Grading of Malignancy in Carcinoma of the Larynx. Acta Radiol. Ther. Phys. Biol. 1973, 12, 1–8. [Google Scholar] [CrossRef]
- Willén, R.; Nathanson, A.; Moberger, G.; Anneroth, G. Squamous Cell Carcinoma of the Gingiva. Histological Classification and Grading of Malignancy. Acta Otolaryngol. 1975, 79, 146–154. [Google Scholar] [CrossRef]
- Huang, S.H.; Hwang, D.; Lockwood, G.; Goldstein, D.P.; O’Sullivan, B. Predictive Value of Tumor Thickness for Cervical Lymph-Node Involvement in Squamous Cell Carcinoma of the Oral Cavity: A Meta-Analysis of Reported Studies. Cancer 2009, 115, 1489–1497. [Google Scholar] [CrossRef]
- Stacker, S.A.; Achen, M.G.; Jussila, L.; Baldwin, M.E.; Alitalo, K. Lymphangiogenesis and Cancer Metastasis. Nat. Rev. Cancer 2002, 2, 573–583. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in Development and Human Disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef]
- de Sousa, S.F.; Gleber-Netto, F.O.; de Oliveira-Neto, H.H.; Batista, A.C.; Nogueira Guimarães Abreu, M.H.; de Aguiar, M.C.F. Lymphangiogenesis and Podoplanin Expression in Oral Squamous Cell Carcinoma and the Associated Lymph Nodes. Appl. Immunohistochem. Mol. Morphol. AIMM 2012, 20, 588–594. [Google Scholar] [CrossRef]
- Kyzas, P.A.; Geleff, S.; Batistatou, A.; Agnantis, N.J.; Stefanou, D. Evidence for Lymphangiogenesis and Its Prognostic Implications in Head and Neck Squamous Cell Carcinoma. J. Pathol. 2005, 206, 170–177. [Google Scholar] [CrossRef]
- Miyahara, M.; Tanuma, J.; Sugihara, K.; Semba, I. Tumor Lymphangiogenesis Correlates with Lymph Node Metastasis and Clinicopathologic Parameters in Oral Squamous Cell Carcinoma. Cancer 2007, 110, 1287–1294. [Google Scholar] [CrossRef]
- de Assis, E.M.; Rodrigues, M.; Vieira, J.C.; Pascoaloti, M.I.M.; Junior, H.M.; Souto, G.R.; Souza, P.E.A.; Horta, M.C.R. Lymphatic Vascular Density, the Expression of Podoplanin and Tumor Budding in Oral Squamous Cell Carcinoma. Head Neck Pathol. 2023, 17, 371–382. [Google Scholar] [CrossRef]
- Tandon, A.; Sandhya, K.; Singh, N.N.; Kumar, A. Prognostic Relevance of Lymphatic Vessel Density in Squamous Cell Carcinomas of the Oral Cavity: A Systematic Review and Meta-Analysis. Head Neck Pathol. 2022, 16, 1185–1194. [Google Scholar] [CrossRef]
- Cueni, L.N.; Hegyi, I.; Shin, J.W.; Albinger-Hegyi, A.; Gruber, S.; Kunstfeld, R.; Moch, H.; Detmar, M. Tumor Lymphangiogenesis and Metastasis to Lymph Nodes Induced by Cancer Cell Expression of Podoplanin. Am. J. Pathol. 2010, 177, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, M.; Scheer, M.; Drebber, U.; Ritter, L.; Zöller, J.E. Impact of Podoplanin Expression in Oral Squamous Cell Carcinoma: Clinical and Histopathologic Correlations. Virchows Arch. 2010, 456, 473–482. [Google Scholar] [CrossRef]
- Martín-Villar, E.; Scholl, F.G.; Gamallo, C.; Yurrita, M.M.; Muñoz-Guerra, M.; Cruces, J.; Quintanilla, M. Characterization of Human PA2.26 Antigen (T1α–2, Podoplanin), a Small Membrane Mucin Induced in Oral Squamous Cell Carcinomas. Int. J. Cancer 2005, 113, 899–910. [Google Scholar] [CrossRef]
- Wicki, A.; Lehembre, F.; Wick, N.; Hantusch, B.; Kerjaschki, D.; Christofori, G. Tumor Invasion in the Absence of Epithelial-Mesenchymal Transition: Podoplanin-Mediated Remodeling of the Actin Cytoskeleton. Cancer Cell 2006, 9, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Volk, L.; Hall, K.; Flister, M.J. Lymphangiogenesis and Lymphatic Metastasis in Breast Cancer. Pathophysiology 2010, 17, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Longatto Filho, A.; Oliveira, T.G.; Pinheiro, C.; de Carvalho, M.B.; Curioni, O.A.; Mercante, A.M.d.C.; Schmitt, F.C.; Gattás, G.J. How Useful Is the Assessment of Lymphatic Vascular Density in Oral Carcinoma Prognosis? World J. Surg. Oncol. 2007, 5, 140. [Google Scholar] [CrossRef]
- Zhao, D.; Pan, J.; Li, X.Q.; Wang, X.Y.; Tang, C.; Xuan, M. Intratumoral Lymphangiogenesis in Oral Squamous Cell Carcinoma and Its Clinicopathological Significance. J. Oral Pathol. Med. 2008, 37, 616–625. [Google Scholar] [CrossRef]
- Marinho, V.F.Z.; Sanches, F.S.F.; Rocha, G.F.S.; Metze, K.; Gobbi, H. D2-40, a Novel Lymphatic Endothelial Marker: Identification of Lymphovascular Invasion and Relationship with Axillary Metastases in Breast Cancer. J. Bras. Patol. E Med. Lab. 2008, 44, 45–50. [Google Scholar] [CrossRef]
- Sugiura, T.; Inoue, Y.; Matsuki, R.; Ishii, K.; Takahashi, M.; Abe, M.; Shirasuna, K. VEGF-C and VEGF-D Expression Is Correlated with Lymphatic Vessel Density and Lymph Node Metastasis in Oral Squamous Cell Carcinoma: Implications for Use as a Prognostic Marker. Int. J. Oncol. 2009, 34, 673–680. [Google Scholar] [CrossRef]
- Alaeddini, M.; Etemad-Moghadam, S. Lymphangiogenesis and Angiogenesis in Oral Cavity and Lower Lip Squamous Cell Carcinoma. Braz. J. Otorhinolaryngol. 2016, 82, 385–390. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, Y.; Ye, M.; Liu, W.; Feng, L. The Clinical Value of Lymphatic Vessel Density, Intercellular Adhesion Molecule 1 and Vascular Cell Adhesion Molecule 1 Expression in Patients with Oral Tongue Squamous Cell Carcinoma. J. Cancer Res. Ther. 2014, 10, C125. [Google Scholar] [CrossRef]
- Yuan, P.; Temam, S.; El-Naggar, A.; Zhou, X.; Liu, D.D.; Lee, J.J.; Mao, L. Overexpression of Podoplanin in Oral Cancer and Its Association with Poor Clinical Outcome. Cancer 2006, 107, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Maturana-Ramírez, A.; Espinoza, I.; Reyes, M.; Aitken, J.P.; Aguayo, F.; Hartel, S.; Rojas-Alcayaga, G. Higher Blood Vessel Density in Comparison to the Lymphatic Vessels in Oral Squamous Cell Carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 13677–13686. [Google Scholar] [PubMed]
- Agarwal, D.; Pardhe, N.; Bajpai, M.; Gupta, S.; Mathur, N.; Vanaki, S.S.; Puranik, R.S.; Mittal, M. Characterization, Localization and Patterning of Lymphatics and Blood Vessels in Oral Squamous Cell Carcinoma: A Comparative Study Using D2-40 and CD-34 IHC Marker. J. Clin. Diagn. Res. JCDR 2014, 8, ZC86–ZC89. [Google Scholar] [CrossRef]
- Zhang, Z.; Helman, J.I.; Li, L. Lymphangiogenesis, Lymphatic Endothelial Cells and Lymphatic Metastasis in Head and Neck Cancer—a Review of Mechanisms. Int. J. Oral Sci. 2010, 2, 5–14. [Google Scholar] [CrossRef]
- Leong, S.P.; Naxerova, K.; Keller, L.; Pantel, K.; Witte, M. Molecular Mechanisms of Cancer Metastasis via the Lymphatic versus the Blood Vessels. Clin. Exp. Metastasis 2022, 39, 159–179. [Google Scholar] [CrossRef]
- Mashhadiabbas, F.; Mahjour, F.; Mahjour, S.B.; Fereidooni, F.; Hosseini, F.S. The Immunohistochemical Characterization of MMP-2, MMP-10, TIMP-1, TIMP-2, and Podoplanin in Oral Squamous Cell Carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 240–250. [Google Scholar] [CrossRef]
- Muñoz-Guerra, M.F.; Marazuela, E.G.; Martín-Villar, E.; Quintanilla, M.; Gamallo, C. Prognostic Significance of Intratumoral Lymphangiogenesis in Squamous Cell Carcinoma of the Oral Cavity. Cancer 2004, 100, 553–560. [Google Scholar] [CrossRef]
- Mafra, R.P.; Serpa, M.S.; Lima, K.C.d.; Silveira, É.J.D.d.; Souza, L.B.d.; Pinto, L.P. Immunohistochemical Analysis of Lymphatic Vessel Density and Mast Cells in Oral Tongue Squamous Cell Carcinoma. J. Cranio-Maxillofac. Surg. 2018, 46, 2234–2239. [Google Scholar] [CrossRef]
- Franchi, A.; Gallo, O.; Massi, D.; Baroni, G.; Santucci, M. Tumor Lymphangiogenesis in Head and Neck Squamous Cell Carcinoma: A Morphometric Study with Clinical Correlations. Cancer 2004, 101, 973–978. [Google Scholar] [CrossRef]
- Jardim, J.F.; Galvis, M.M.; Fabelo, I.R.; Soares, F.A.; Pinto, C.A.L.; Kowalski, L.P. Intratumoral Lymphatic Vascular Density Is an Independent Factor for Disease-Free and Overall Survival in Advanced Stage Oral Squamous Cell Carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 580–588. [Google Scholar] [CrossRef]
- O’Donnell, R.K.; Feldman, M.; Mick, R.; Muschel, R.J. Immunohistochemical Method Identifies Lymphovascular Invasion in a Majority of Oral Squamous Cell Carcinomas and Discriminates between Blood and Lymphatic Vessel Invasion. J. Histochem. Cytochem. 2008, 56, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Beasley, N.J.P.; Prevo, R.; Banerji, S.; Leek, R.D.; Moore, J.; van Trappen, P.; Cox, G.; Harris, A.L.; Jackson, D.G. Intratumoral Lymphangiogenesis and Lymph Node Metastasis in Head and Neck Cancer. Cancer Res. 2002, 62, 1315–1320. [Google Scholar]
- Audet, N.; Beasley, N.J.; MacMillan, C.; Jackson, D.G.; Gullane, P.J.; Kamel-Reid, S. Lymphatic Vessel Density, Nodal Metastases, and Prognosis in Patients with Head and Neck Cancer. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 1065–1070. [Google Scholar] [CrossRef]
- Maula, S.-M.; Luukkaa, M.; Grénman, R.; Jackson, D.; Jalkanen, S.; Ristamäki, R. Intratumoral Lymphatics Are Essential for the Metastatic Spread and Prognosis in Squamous Cell Carcinomas of the Head and Neck Region. Cancer Res. 2003, 63, 1920–1926. [Google Scholar]
- Pepper, M.S. Lymphangiogenesis and Tumor Metastasis: Myth or Reality? Clin. Cancer Res. 2001, 7, 462–468. [Google Scholar]
- Clarijs, R.; Ruiter, D.J.; de Waal, R.M.W. Lymphangiogenesis in Malignant Tumours: Does It Occur? J. Pathol. 2001, 193, 143–146. [Google Scholar] [CrossRef]
- Padera, T.P.; Kadambi, A.; di Tomaso, E.; Carreira, C.M.; Brown, E.B.; Boucher, Y.; Choi, N.C.; Mathisen, D.; Wain, J.; Mark, E.J.; et al. Lymphatic Metastasis in the Absence of Functional Intratumor Lymphatics. Science 2002, 296, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Mandriota, S.J.; Jussila, L.; Jeltsch, M.; Compagni, A.; Baetens, D.; Prevo, R.; Banerji, S.; Huarte, J.; Montesano, R.; Jackson, D.G.; et al. Vascular Endothelial Growth Factor-C-Mediated Lymphangiogenesis Promotes Tumour Metastasis. EMBO J. 2001, 20, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Al-Shareef, H.; Hiraoka, S.-I.; Tanaka, N.; Shogen, Y.; Lee, A.-D.; Bakhshishayan, S.; Kogo, M. Use of NRP1, a Novel Biomarker, along with VEGF-C, VEGFR-3, CCR7 and SEMA3E, to Predict Lymph Node Metastasis in Squamous Cell Carcinoma of the Tongue. Oncol. Rep. 2016, 36, 2444–2454. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, B.; Suton, P.; Müller, D.; Mirošević, V.; Mamić, M.; Lukšić, I. Prognostic Significance of Peritumoral and Intratumoral Lymphatic Vessels Density in Clinically Node-Negative (cN0) Oral Squamous Cell Carcinoma: A Preliminary Report. Medicina 2025, 61, 1712. https://doi.org/10.3390/medicina61091712
Kos B, Suton P, Müller D, Mirošević V, Mamić M, Lukšić I. Prognostic Significance of Peritumoral and Intratumoral Lymphatic Vessels Density in Clinically Node-Negative (cN0) Oral Squamous Cell Carcinoma: A Preliminary Report. Medicina. 2025; 61(9):1712. https://doi.org/10.3390/medicina61091712
Chicago/Turabian StyleKos, Boris, Petar Suton, Danko Müller, Vid Mirošević, Matija Mamić, and Ivica Lukšić. 2025. "Prognostic Significance of Peritumoral and Intratumoral Lymphatic Vessels Density in Clinically Node-Negative (cN0) Oral Squamous Cell Carcinoma: A Preliminary Report" Medicina 61, no. 9: 1712. https://doi.org/10.3390/medicina61091712
APA StyleKos, B., Suton, P., Müller, D., Mirošević, V., Mamić, M., & Lukšić, I. (2025). Prognostic Significance of Peritumoral and Intratumoral Lymphatic Vessels Density in Clinically Node-Negative (cN0) Oral Squamous Cell Carcinoma: A Preliminary Report. Medicina, 61(9), 1712. https://doi.org/10.3390/medicina61091712






