Early Allograft Dysfunction After Liver Transplantation: Impact on Clinical Outcomes and Associated Risk Factors
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Clinico-Pathologic Characteristics of Patients
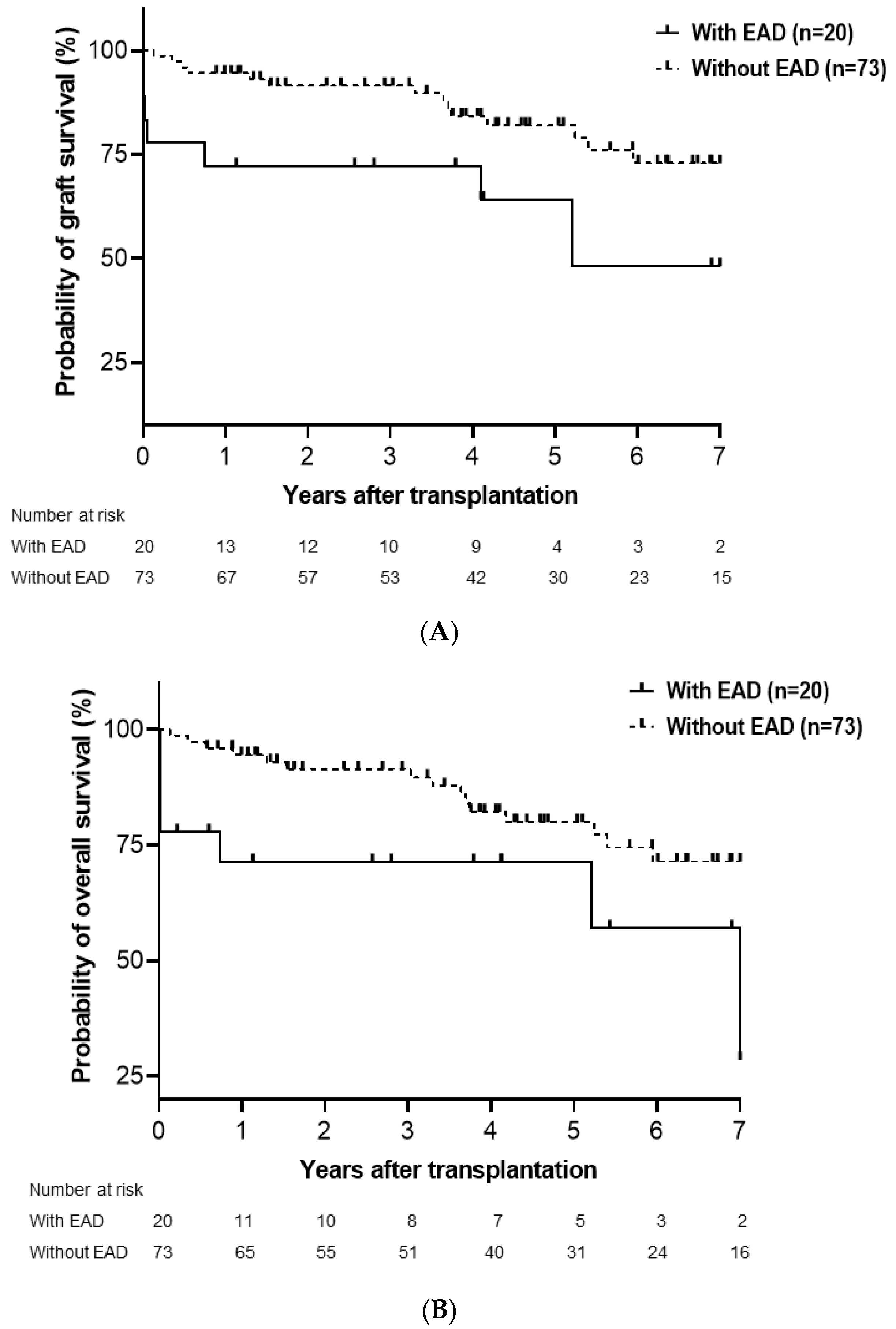
3.2. Cumulative Probability of Graft and Overall Survival
3.3. Cumulative Probability of Graft and Overall Survival in LDLT and DDLT
3.4. Risk Factor Analysis for Graft and Overall Survival
3.5. Risk Factor Analysis for EAD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fayek, S.A.; Quintini, C.; Chavin, K.D.; Marsh, C.L. The Current State of Liver Transplantation in the United States: Perspective From American Society of Transplant Surgeons (ASTS) Scientific Studies Committee and Endorsed by ASTS Council. Am. J. Transpl. 2016, 16, 3093–3104. [Google Scholar] [CrossRef]
- Brea-Gomez, E.; Villar-Quintana, R.; Plata-Illescas, C.; Zambudio-Carroll, N.; Lopez-Garrido, M.A.; Nogueras-Lopez, F. Analysis of the Predictive Ability for Graft Loss and Mortality of Two Criteria for Early Allograft Dysfunction After Liver Transplantation. Transpl. Proc. 2018, 50, 605–609. [Google Scholar] [CrossRef]
- Agopian, V.G.; Harlander-Locke, M.P.; Markovic, D.; Dumronggittigule, W.; Xia, V.; Kaldas, F.M. Evaluation of Early Allograft Function Using the Liver Graft Assessment Following Transplantation Risk Score Model. JAMA Surg. 2018, 153, 436–444. [Google Scholar] [CrossRef]
- Ali, J.M.; Davies, S.E.; Brais, R.J.; Randle, L.V.; Klinck, J.R.; Allison, M.E. Analysis of ischemia/reperfusion injury in time-zero biopsies predicts liver allograft outcomes. Liver Transpl. 2015, 21, 487–499. [Google Scholar] [CrossRef]
- Ito, T.; Naini, B.V.; Markovic, D.; Aziz, A.; Younan, S.; Lu, M. Ischemia-reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. Am. J. Transpl. 2021, 21, 614–625. [Google Scholar] [CrossRef]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010, 16, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Golse, N.; Guglielmo, N.; El Metni, A.; Frosio, F.; Cosse, C.; Naili, S. Arterial Lactate Concentration at the End of Liver Transplantation Is an Early Predictor of Primary Graft Dysfunction. Ann. Surg. 2019, 270, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, L.; Qi, B.; Zhang, Y.; Ni, C.; Zhang, Y. Dexmedetomidine use during orthotopic liver transplantation surgery on early allograft dysfunction: A randomized controlled trial. Int. J. Surg. 2024, 110, 5518–5526. [Google Scholar] [CrossRef] [PubMed]
- Al-Freah, M.A.B.; McPhail, M.J.W.; Dionigi, E.; Foxton, M.R.; Auzinger, G.; Rela, M. Improving the Diagnostic Criteria for Primary Liver Graft Nonfunction in Adults Utilizing Standard and Transportable Laboratory Parameters: An Outcome-Based Analysis. Am. J. Transpl. 2017, 17, 1255–1266. [Google Scholar] [CrossRef]
- Frick, K.; Beller, E.A.; Kalisvaart, M.; Dutkowski, P.; Schupbach, R.A.; Klinzing, S. Procalcitonin in early allograft dysfunction after orthotopic liver transplantation: A retrospective single center study. BMC Gastroenterol. 2022, 22, 404. [Google Scholar] [CrossRef]
- Agrawal, D.; Saigal, S. Early allograft dysfunction after living donor liver transplantation-current concepts and future directions. Liver Transpl. 2023, 29, 871–884. [Google Scholar] [CrossRef]
- Pomposelli, J.J.; Goodrich, N.P.; Emond, J.C.; Humar, A.; Baker, T.B.; Grant, D.R. Patterns of Early Allograft Dysfunction in Adult Live Donor Liver Transplantation: The A2ALL Experience. Transplantation 2016, 100, 1490–1499. [Google Scholar] [CrossRef]
- Masior, L.; Grat, M. Primary Nonfunction and Early Allograft Dysfunction after Liver Transplantation. Dig. Dis. 2022, 40, 766–776. [Google Scholar] [CrossRef]
- Choe, W.; Kwon, S.W.; Kim, S.S.; Hwang, S.; Song, G.W.; Lee, S.G. Effects of therapeutic plasma exchange on early allograft dysfunction after liver transplantation. J. Clin. Apher. 2017, 32, 147–153. [Google Scholar] [CrossRef]
- Kulik, U.; Lehner, F.; Klempnauer, J.; Borlak, J. Primary non-function is frequently associated with fatty liver allografts and high mortality after re-transplantation. Liver Int. 2017, 37, 1219–1228. [Google Scholar] [CrossRef]
- Kanneganti, M.; Olthoff, K.M.; Bittermann, T. Impact of Older Donor Age on Recipient and Graft Survival After LDLT: The US Experience. Transplantation 2023, 107, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Lee, D.D.; Croome, S.; Chadha, R.; Livingston, D.; Abader, P. The impact of postreperfusion syndrome during liver transplantation using livers with significant macrosteatosis. Am. J. Transpl. 2019, 19, 2550–2559. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Singh, A.; Burns, J.M.; Perry, D.K.; Nguyen, J.H.; Taner, C.B. Early allograft dysfunction in liver transplantation with donation after cardiac death donors results in inferior survival. Liver Transpl. 2014, 20, 1447–1453. [Google Scholar] [CrossRef]
- Agopian, V.G.; Markovic, D.; Klintmalm, G.B.; Saracino, G.; Chapman, W.C.; Vachharajani, N. Multicenter validation of the liver graft assessment following transplantation (L-GrAFT) score for assessment of early allograft dysfunction. J. Hepatol. 2021, 74, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Roll, G.R.; Spiro, M.; Raptis, D.A.; Jalal, A.; Yan, C.T.; Olthoff, K.M. Which recipient pretransplant factors, such as MELD, renal function, sarcopenia, and recent sepsis influence suitability for and outcome after living donor liver transplantation? A systematic review of the literature and expert panel recommendations. Clin. Transpl. 2022, 36, e14656. [Google Scholar] [CrossRef]
- Fu, Z.; Cheng, P.; Jian, Q.; Wang, H.; Ma, Y. High Systemic Immune-Inflammation Index, Predicting Early Allograft Dysfunction, Indicates High 90-Day Mortality for Acute-On-Chronic Liver Failure after Liver Transplantation. Dig. Dis. 2023, 41, 938–945. [Google Scholar] [CrossRef]
- Yuan, G.; Li, S.; Liang, P.; Chen, G.; Luo, Y.; Shen, Y. High visceral adipose tissue area is independently associated with early allograft dysfunction in liver transplantation recipients: A propensity score analysis. Insights Imaging 2022, 13, 165. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Wei, Q.; Saeb-Parsy, K.; Xu, X. The Role of Ischemia/Reperfusion Injury in Early Hepatic Allograft Dysfunction. Liver Transpl. 2020, 26, 1034–1048. [Google Scholar] [CrossRef]
- Al-Kurd, A.; Kitajima, T.; Delvecchio, K.; Tayseer Shamaa, M.; Ivanics, T.; Yeddula, S. Short recipient warm ischemia time improves outcomes in deceased donor liver transplantation. Transpl. Int. 2021, 34, 1422–1432. [Google Scholar] [CrossRef]



| With EAD (n = 20) | Without EAD (n = 73) | p | |
|---|---|---|---|
| Age, years | 53.6 ± 8.8 | 54.1 ± 11.9 | 0.852 |
| Sex (male) | 13 (65.0%) | 50 (68.5%) | 0.767 |
| BMI | 24.5 ± 4.2 | 23.6 ± 4.0 | 0.358 |
| Diabetes mellitus | 14 (70.0%) | 51 (69.9%) | 0.991 |
| Hypertension | 16 (80.0%) | 57 (78.1%) | 0.853 |
| Original liver disease | |||
| HBV | 5 (25.0%) | 10 (13.7%) | |
| HCV | 0 | 3 (4.1%) | |
| Alcoholic | 9 (45.0%) | 31 (42.5%) | |
| HCC | 1 (5.0%) | 20 (27.4%) | |
| Cholestatic | 0 | 6 (8.2%) | |
| Acute liver failure | 5 (25.0%) | 3 (4.1%) | 0.010 |
| Laboratory MELD | 29.5 (6–40) | 15 (6–40) | 0.027 |
| Donor age, years | 49.6 ± 16.5 | 39.2 ± 13.3 | 0.005 |
| ABOi | 2 (10.0%) | 8 (11.0%) | 0.902 |
| DDLT | 10 (50.0%) | 22 (30.1%) | 0.098 |
| GRWR < 0.8 | 2 (10.0%) | 7 (9.6%) | 0.956 |
| Hepatic steatosis > 10% | 2 (10.0%) | 6 (8.2%) | 0.801 |
| Operative time, min | 460 (225–815) | 475 (285–830) | 0.523 |
| CIT, h | 3.5 ± 2.7 | 2.3 ± 2.0 | 0.030 |
| WIT, min | 48.6 ± 31.8 | 37.3 ± 8.8 | 0.008 |
| Peak AST within 7 days, U/L | 3614 ± 5192 | 424 ± 336 | 0.000 |
| Peak ALT within 7 days, U/L | 1847 ± 2179 | 357 ± 274 | 0.000 |
| Postoperative hospital stay, days | 52.2 ± 73.2 | 38.8 ± 43.5 | 0.305 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age, years | 0.994 | 0.956−1.034 | 0.763 | |||
| Sex (male) | 1.580 | 0.584−4.271 | 0.367 | |||
| BMI | 0.982 | 0.885−1.091 | 0.740 | |||
| DM | 1.541 | 0.571−4.160 | 0.393 | |||
| HTN | 1.768 | 0.524−5.964 | 0.358 | |||
| Primary liver disease | 1.064 | 0.823−1.375 | 0.636 | |||
| Acute hepatic failure | 2.273 | 0.671−7.701 | 0.187 | |||
| Hepatocellular carcinoma | 0.653 | 0.221−1.928 | 0.441 | |||
| Laboratory MELD | 1.017 | 0.983−1.053 | 0.334 | |||
| Donor age, years | 1.017 | 0.989−1.047 | 0.242 | |||
| ABOi | 0.814 | 0.191−3.476 | 0.782 | |||
| DDLT | 1.130 | 0.479−2.667 | 0.781 | |||
| Hepatic steatosis > 10% | 0.426 | 0.057−3.158 | 0.403 | |||
| Operative time, min | 0.999 | 0.996−1.003 | 0.716 | |||
| CIT, h | 1.132 | 0.946−1.356 | 0.177 | |||
| WIT, min | 1.024 | 1.006−1.042 | 0.010 | |||
| EAD | 3.388 | 1.457−7.881 | 0.005 | 2.866 | 1.169−7.025 | 0.021 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age, years | 1.016 | 0.976−1.058 | 0.433 | |||
| Sex (male) | 2.182 | 0.745−6.392 | 0.155 | |||
| BMI | 1.033 | 0.938−1.138 | 0.512 | |||
| DM | 0.980 | 0.405−2.372 | 0.964 | |||
| HTN | 1.235 | 0.421−3.626 | 0.701 | |||
| Primary liver disease | 0.958 | 0.709−1.295 | 0.781 | |||
| Acute hepatic failure | 2.785 | 0.821−9.444 | 0.100 | |||
| Hepatocellular carcinoma | 1.018 | 0.403−2.573 | 0.969 | |||
| Laboratory MELD | 1.006 | 0.971−1.041 | 0.755 | |||
| Donor age, years | 1.017 | 0.989−1.045 | 0.232 | |||
| ABOi | 0.892 | 0.209−3.802 | 0.877 | |||
| DDLT | 0.853 | 0.353−2.061 | 0.724 | |||
| Hepatic steatosis > 10% | 0.856 | 0.200−3.655 | 0.834 | |||
| Operative time, min | 1.001 | 0.998−1.005 | 0.406 | |||
| CIT, h | 1.033 | 0.862−1.237 | 0.726 | |||
| WIT, min | 1.025 | 1.009−1.041 | 0.002 | 1.018 | 1.001−1.035 | 0.038 |
| EAD | 3.329 | 1.450−7.645 | 0.005 | 2.728 | 1.119−6.651 | 0.027 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age, years | 0.996 | 0.953−1.041 | 0.850 | |||
| Sex (male) | 0.854 | 0.301−2.425 | 0.767 | |||
| BMI | 1.059 | 0.938−1.196 | 0.355 | |||
| DM | 1.007 | 0.342−2.961 | 0.991 | |||
| HTN | 1.123 | 0.329−3.834 | 0.853 | |||
| Acute hepatic failure | 7.778 | 1.674−36.141 | 0.009 | 6.228 | 1.179−32.906 | 0.031 |
| Hepatocellular carcinoma | 0.139 | 0.018−1.112 | 0.063 | |||
| Laboratory MELD | 1.045 | 1.004−1.088 | 0.031 | |||
| Donor age, years | 1.053 | 1.014−1.093 | 0.008 | 1.051 | 1.008−1.096 | 0.020 |
| ABOi | 0.903 | 0.176−4.631 | 0.903 | |||
| DDLT | 2.318 | 0.845−6.359 | 0.102 | |||
| Hepatic steatosis > 10% | 1.241 | 0.231−6.676 | 0.802 | |||
| Operative time, min | 1.002 | 0.997−1.006 | 0.476 | |||
| CIT, h | 1.257 | 0.037−1.557 | 0.037 | |||
| WIT, min | 1.040 | 1.000−1.082 | 0.051 | 1.048 | 1.001−1.098 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Suh, S.-W. Early Allograft Dysfunction After Liver Transplantation: Impact on Clinical Outcomes and Associated Risk Factors. Medicina 2025, 61, 1710. https://doi.org/10.3390/medicina61091710
Shin J, Suh S-W. Early Allograft Dysfunction After Liver Transplantation: Impact on Clinical Outcomes and Associated Risk Factors. Medicina. 2025; 61(9):1710. https://doi.org/10.3390/medicina61091710
Chicago/Turabian StyleShin, Jungho, and Suk-Won Suh. 2025. "Early Allograft Dysfunction After Liver Transplantation: Impact on Clinical Outcomes and Associated Risk Factors" Medicina 61, no. 9: 1710. https://doi.org/10.3390/medicina61091710
APA StyleShin, J., & Suh, S.-W. (2025). Early Allograft Dysfunction After Liver Transplantation: Impact on Clinical Outcomes and Associated Risk Factors. Medicina, 61(9), 1710. https://doi.org/10.3390/medicina61091710







