Evaluating the Efficacy of Robot-Assisted Partial Nephrectomy in Complex Renal Tumours: A Single-Centre Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Surgical Techniques
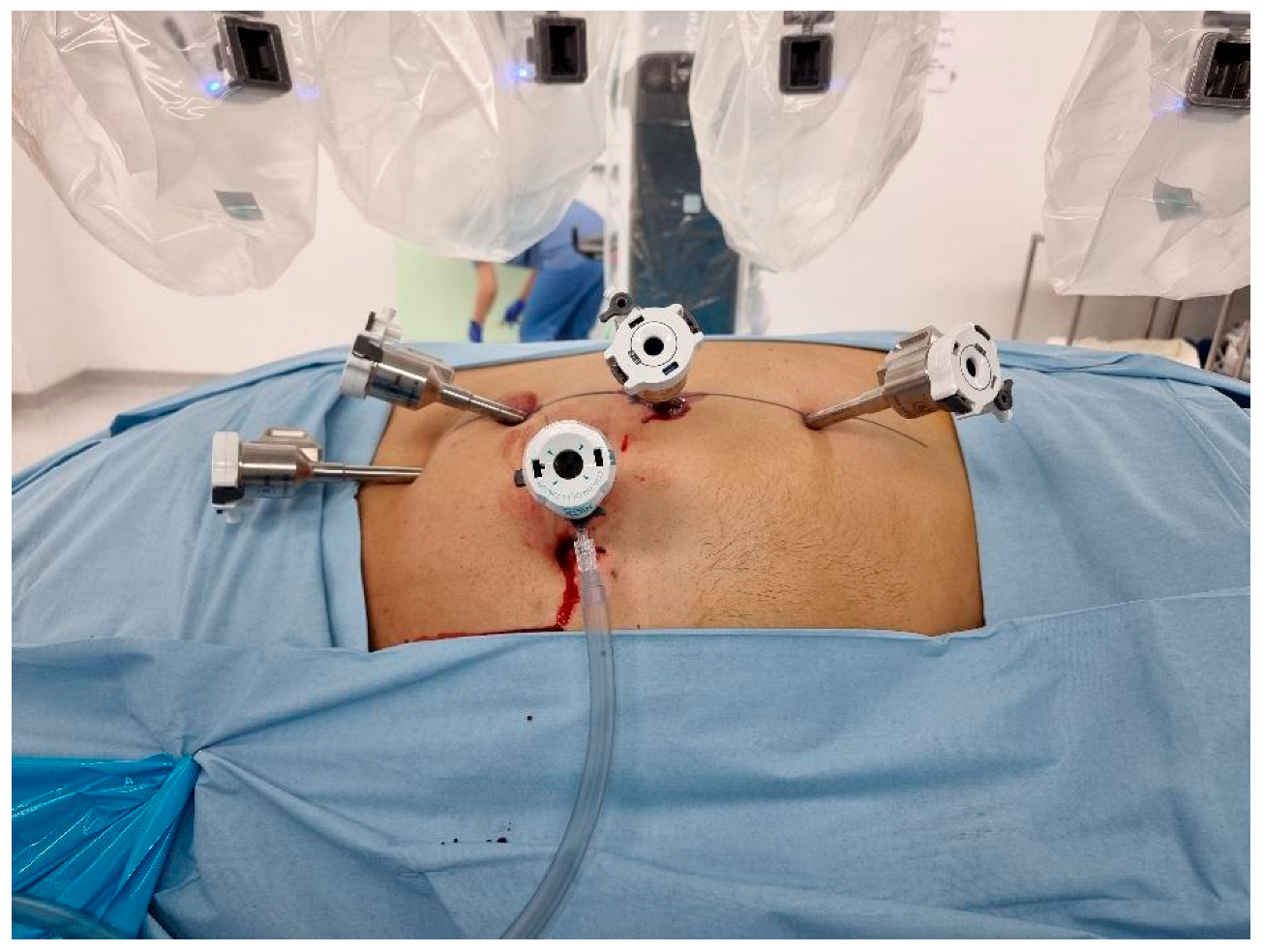
- Transperitoneal Approach: Patients were positioned in a 70-degree decubitus posture with their flank extended. Pneumoperitoneum was created using a Veress needle, and trocars were placed under direct vision at 8 cm intervals along the semilunar line. The Da Vinci robotic system was docked with targeting. The robotic instruments used consisted of a bipolar grasper, monopolar scissors, ProGrasp forceps, and a large needle driver. Once docking was performed, the colon was medially mobilized to reveal the kidney. The dissection of the renal hilum was performed to isolate the renal artery and vein. Clamping of the artery was then performed using a robotic bulldog clamp, followed by tumour excision using robotic scissors. Barbed sutures were used to repair the renal defect to achieve haemostasis and reconstruct the parenchyma (Figure 1).
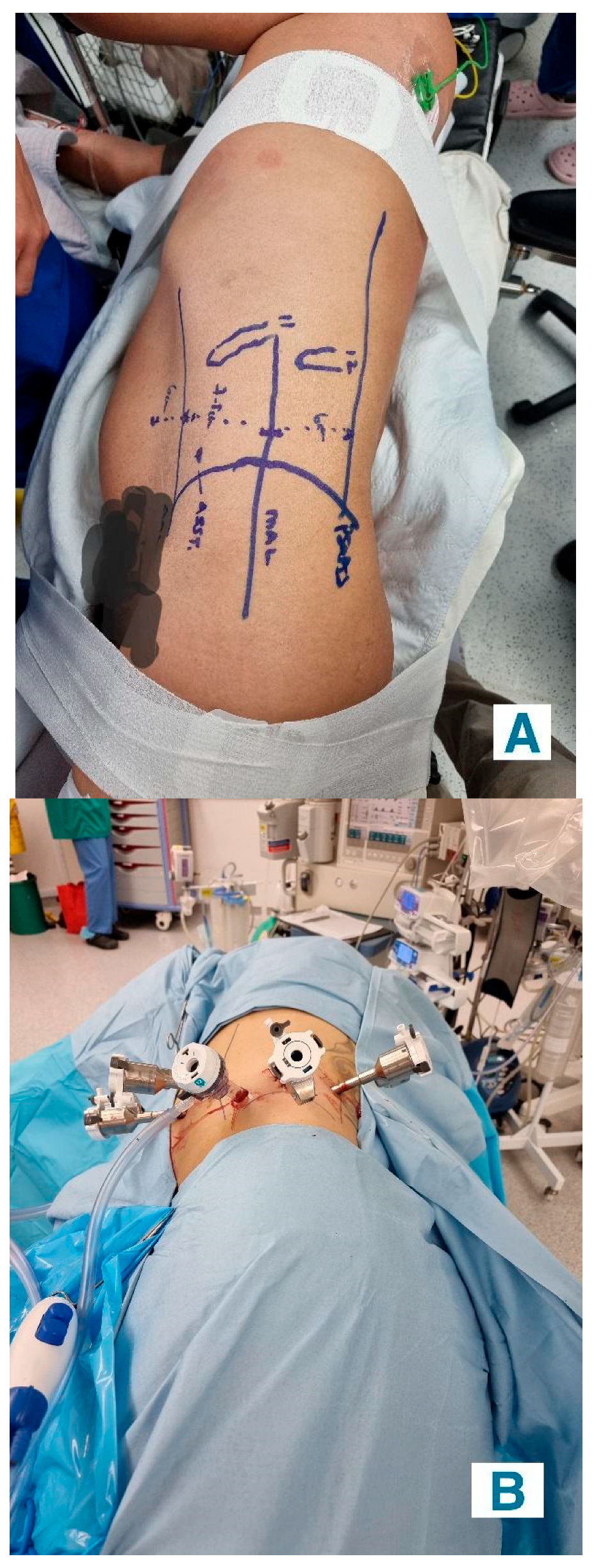
- Retroperitoneal Approach: Patients were positioned in a 90-degree lateral position, and port sites were marked according to the selected approach. A balloon dissector was used to create retroperitoneal space, and trocars were placed at 6 cm intervals using finger guidance or direct vision. Docking of the Da Vinci robotic system was then performed to provide an ideal working space to allow for the robotic arms and camera to obtain accurate surgical access. The bipolar grasper, monopolar scissors, ProGrasp forceps, and a large needle driver were the robotic instruments used for this procedure. The retroperitoneal fat was mobilized, and the lateral coronal fascia was incised. The perirenal fat was then carefully dissected to expose the kidney tumour. The renal hilum was accessed directly, allowing for precise isolation of the renal artery, which was clamped as needed to control blood flow (Figure 2A,B). The tumour was meticulously excised, and the renal defect was repaired in layers using barbed sutures to achieve haemostasis and reconstruct the parenchyma, following the same principles as the transperitoneal approach [19].
2.3. Statistical Analysis
2.4. Ethics Statement
2.5. Outcomes
3. Results
3.1. Correlation Analysis
3.2. Comparative Analysis of Surgical Approaches
3.3. Warm Ischemia Time and Renal Function
3.4. Operative Console Time and Tumour Complexity
3.5. “Trifecta” Achievement and Complications
3.6. Histopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RAPN | Robotic-assisted partial nephrectomy |
| LAPN | Laparoscopic partial nephrectomy |
| PN | Partial nephrectomy |
| NSS | Nephrometry scoring systems |
| PADUA | Preoperative Aspects and Dimensions Used for Anatomical |
| RENAL | Radius, Exophytic/Endophytic, Nearness, Anterior/Posterior, Location |
| DAP | Diameter-axial-polar |
| C-index | Centrality index |
| ABC | Arterial-Based Complexity |
| eGFR | Estimated glomerular filtration rate |
| WIT | Warm ischemia time |
References
- Koh, D.H.; Jang, W.S.; Park, J.W.; Ham, W.S.; Han, W.K.; Rha, K.H.; Choi, Y.D. Efficacy and Safety of Robotic Procedures Performed Using the da Vinci Robotic Surgical System at a Single Institute in Korea: Experience with 10,000 Cases. Yonsei Med. J. 2018, 59, 975–981. [Google Scholar] [CrossRef]
- Brenner, Z.R.; Salathiel, M.; Macey, B.A.; Krenzer, M. Postoperative care for the robotic surgery bowel resection patient. Gastroenterol. Nurs. 2011, 34, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, R.Z.; Averbach, M.; Ribeiro-Junior, U.; Machado, M.A.; Luca-Filho, C.R. Robotic abdominal surgery: A Brazilian initial experience. Arq. Bras. Cir. Dig. 2013, 26, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.; Bahadori, A.; Mao, D.; Ranasinghe, S.; Tracey, C. Benefits of Robotic Assisted vs. Traditional Laparoscopic Partial Nephrectomy: A Single Surgeon Comparative Study. J. Clin. Med. 2022, 11, 6974. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.G.; Khandwala, Y.S.; Kim, J.H.; Han, D.H.; Li, S.; Wang, Y.; Chang, S.L.; Chung, B.I. Association of Robotic-Assisted vs. Laparoscopic Radical Nephrectomy with Perioperative Outcomes and Health Care Costs, 2003 to 2015. JAMA 2017, 318, 1561–1568. [Google Scholar] [CrossRef]
- Huang, Q.; Gu, L.; Zhu, J.; Peng, C.; Du, S.; Liu, Q.; Chen, J.; Wang, B.; Fan, Y.; Gao, Y.; et al. A three-dimensional, anatomy-based nephrometry score to guide nephron-sparing surgery for renal sinus tumors. Cancer 2020, 126 (Suppl. S9), 2062–2072. [Google Scholar] [CrossRef]
- Diana, P.; Lughezzani, G.; Uleri, A.; Casale, P.; Saita, A.; Hurle, R.; Lazzeri, M.; Mottrie, A.; De Naeyer, G.; De Groote, R.; et al. Multi-institutional Retrospective Validation and Comparison of the Simplified PADUA REnal Nephrometry System for the Prediction of Surgical Success of Robot-assisted Partial Nephrectomy. Eur. Urol. Focus 2021, 7, 1100–1106. [Google Scholar] [CrossRef]
- Khoo, H.C.; Lim, L.Y.; Shukor, S.; Zainal Adwin, Z.A.; Zulkifli, M.Z.; Fam, X.I. Initial experience of laparoscopic retroperitoneal partial nephrectomy in an academic hospital in Malaysia. Med. J. Malaysia 2022, 77, 764–767. [Google Scholar]
- Carbonara, U.; Crocerossa, F.; Campi, R.; Veccia, A.; Cacciamani, G.E.; Amparore, D.; Checcucci, E.; Loizzo, D.; Pecoraro, A.; Marchioni, M.; et al. Retroperitoneal Robot-assisted Partial Nephrectomy: A Systematic Review and Pooled Analysis of Comparative Outcomes. Eur. Urol. Open Sci. 2022, 40, 27–37. [Google Scholar] [CrossRef]
- Shen, Z.; Sun, Z. Systematic review and meta-analysis of randomised trials of perioperative outcomes comparing robot-assisted versus open radical cystectomy. BMC Urol. 2016, 16, 59. [Google Scholar] [CrossRef]
- Rogers, C.; Sukumar, S.; Gill, I.S. Robotic partial nephrectomy: The real benefit. Curr. Opin. Urol. 2011, 21, 60–64. [Google Scholar] [CrossRef]
- Seo, I.Y.; Choi, H.; Boldbaatr, Y.; Lee, J.W.; Rim, J.S. Operative outcomes of robotic partial nephrectomy: A comparison with conventional laparoscopic partial nephrectomy. Korean J. Urol. 2011, 52, 279–283. [Google Scholar] [CrossRef]
- Benway, B.M.; Bhayani, S.B. Surgical outcomes of robot-assisted partial nephrectomy. BJU Int. 2011, 108, 955–961. [Google Scholar] [CrossRef]
- Lee, C.U.; Kang, M.; Sung, H.H.; Jeon, H.G.; Han, D.H.; Jeong, B.C.; Jeon, S.S.; Lee, H.M.; Choi, H.Y.; Seo, S.I. Comparison of 5-Year Outcomes of Robot-Assisted Laparoscopic and Laparoscopic Partial Nephrectomy in Patients with Localized Renal Cell Carcinoma. Korean J. Urol. Oncol. 2017, 15, 172–177. [Google Scholar] [CrossRef]
- Lv, Z.; Chen, G.; Chen, X.; Li, Y.; Bao, E.; Hu, K.; Yu, X. Open versus robot-assisted partial nephrectomy for highly complex renal masses: A meta-analysis of perioperitive and functional outcomes. J. Robot. Surg. 2023, 17, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.P.; Tyagi, S.; Bora, G.S.; Mavuduru, R.S.; Devana, S.K.; Singh, S.K. Robot-assisted partial nephrectomy for moderate to highly complex renal masses. A systematic review and meta-analysis. Indian J. Urol. 2022, 38, 174–183. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Wu, Z.; Campi, R.; Bertolo, R.; Amparore, D.; Mari, A.; Verze, P.; Manfredi, C.; Franco, A.; Ditonno, F.; et al. Outcomes and Techniques of Robotic-Assisted Partial Nephrectomy (RAPN) for Renal Hilar Masses: A Comprehensive Systematic Review. Cancers 2024, 16, 693. [Google Scholar] [CrossRef] [PubMed]
- Jabaji, R.B.; Fischer, H.; Kern, T.; Chien, G.W. Trend of Surgical Treatment of Localized Renal Cell Carcinoma. Perm. J. 2019, 23, 18–108. [Google Scholar] [CrossRef]
- Lyu, X.; Jia, Z.; Ao, L.; Ren, C.; Wu, Y.; Xu, Y.; Chen, K.; Gao, Y.; Wang, B.; Ma, X.; et al. Robot-assisted partial nephrectomy: Can retroperitoneal approach suit for renal tumors of all locations?—A large retrospective cohort study. BMC Urol. 2022, 22, 202. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, H.; Oh, J.J.; Lee, S.; Hong, S.K.; Lee, S.E.; Byun, S.S. Comparison of robotic and open partial nephrectomy for highly complex renal tumors (RENAL nephrometry score ≥ 10). PLoS ONE 2019, 14, e0210413. [Google Scholar] [CrossRef]
- Liu, X.; Jin, D.; Zhang, Y.; Zhang, S. Limited non-linear impact of warm ischemia time on renal functional decline after partial nephrectomy: A propensity score-matched study. Int. Urol. Nephrol. 2023, 55, 1699–1708. [Google Scholar] [CrossRef]
- Numakura, K.; Kobayashi, M.; Koizumi, A.; Kashima, S.; Yamamoto, R.; Nara, T.; Saito, M.; Narita, S.; Inoue, T.; Habuchi, T. Factors influencing warm ischemia time in robot-assisted partial nephrectomy change depending on the surgeon’s experience. World J. Surg. Oncol. 2022, 20, 202. [Google Scholar] [CrossRef]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Renal function after partial nephrectomy: Effect of warm ischemia relative to quantity and quality of preserved kidney. Urology 2012, 79, 356–360. [Google Scholar] [CrossRef]
- Benway, B.M.; Bhayani, S.B.; Rogers, C.G.; Dulabon, L.M.; Patel, M.N.; Lipkin, M.; Wang, A.J.; Stifelman, M.D. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: A multi-institutional analysis of perioperative outcomes. J. Urol. 2009, 182, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, D.B.; Wei, G.; Moon, D.; Kinnear, N.; Bolton, D.M.; Lawrentschuk, N.; Chan, Y.K. Strategies for success: A multi-institutional study on robot-assisted partial nephrectomy for complex renal lesions. BJU Int. 2018, 121 (Suppl. S3), 40–47. [Google Scholar] [CrossRef] [PubMed]
- Vartolomei, M.D.; Remzi, M.; Fajkovic, H.; Shariat, S.F. Robot-Assisted Partial Nephrectomy Mid-Term Oncologic Outcomes: A Systematic Review. J. Clin. Med. 2022, 11, 6165. [Google Scholar] [CrossRef] [PubMed]
- Tanabalan, C.; Raman, A.; Mumtaz, F. Robot-assisted partial nephrectomy: How to minimise renal ischaemia. Arab. J. Urol. 2018, 16, 350–356. [Google Scholar] [CrossRef]
- Furukawa, J.; Kanayama, H.; Azuma, H.; Inoue, K.; Kobayashi, Y.; Kashiwagi, A.; Segawa, T.; Takahashi, Y.; Horie, S.; Ogawa, O.; et al. ‘Trifecta’ outcomes of robot-assisted partial nephrectomy: A large Japanese multicenter study. Int. J. Clin. Oncol. 2020, 25, 347–353. [Google Scholar] [CrossRef]
- Jiang, X.L.; Ouyang, K.; Yang, R.; Yu, X.Y.; Yang, D.D.; Wu, J.T.; Zhao, H.W. The application of internal traction technique in retroperitoneal robot-assisted partial nephrectomy for renal ventral tumors. World J. Surg. Oncol. 2022, 20, 213. [Google Scholar] [CrossRef]
- Wang, L.; Deng, J.Y.; Liang, C.; Zhu, P.Y. Perioperative, functional, and oncological outcomes of robotic vs. laparoscopic partial nephrectomy for complex renal tumors (RENAL score ≥ 7): An evidence-based analysis. Front. Oncol. 2023, 13, 1195910. [Google Scholar] [CrossRef]
- Hinata, N.; Fujisawa, M. Robot assisted partial nephrectomy: Technique and outcomes. In Endourology Progress: Technique, Technology and Training; Springer: Berlin/Heidelberg, Germany, 2019; pp. 117–126. [Google Scholar]
| Variables | Number of Patients |
|---|---|
| Total number of patients (n) | 35 |
| Age (mean ± SD; range) | 53.37 ± 15.52 (22–78) years |
| Gender (number; percentage) | |
| Male | 21 (60.0%) |
| Female | 14 (40.0%) |
| Variables | Number of Patients (Percentage) |
|---|---|
| Tumour Size | |
| <4 cm | 13 (37.1) |
| 4–7 cm | 19 (54.3) |
| >7 cm | 3 (8.6) |
| Kidney side | |
| Right | 20 (57.1) |
| Left | 15 (42.9) |
| Anterior or Posterior | |
| Anterior | 16 (45.7) |
| Central | 12 (34.3) |
| Posterior | 7 (20.0) |
| Tumour Location | |
| Upper Pole | 6 (17.1) |
| Midpole | 18 (51.4) |
| Lower Pole | 11 (31.4) |
| Exophytic and Endophytic | |
| Exophytic | 19 (54.3) |
| Partially Endophytic | 11 (31.4) |
| Completely Endophytic | 5 (14.3) |
| Hilar Tumour | |
| Yes | 12 (34.3) |
| No | 23 (65.7) |
| RENAL Nephrometry Score | |
| 9 | 12 (34.3) |
| 10 | 16 (45.7) |
| 11 | 6 (17.1) |
| 12 | 1 (2.9) |
| Operative Approach | |
| Transperitoneal | 15 (42.9) |
| Retroperitoneal | 20 (57.1) |
| Mean | Standard Deviation (SD) | |
|---|---|---|
| Operative Console Time (min) | 145.91 | ±44.96 |
| Warm Ischaemic Time (min) | 15.03 | ±5.84 |
| Blood loss (mL) | 178.57 | ±135.82 |
| Duration of admission (Day) | 2.71 | ±0.79 |
| Creatinine change (µmol/L) | 5.69 | ±20.39 |
| Results (Percentage) | |
|---|---|
| Surgical Margin | |
| Positive | 0 (0) |
| Negative | 35 (100) |
| HPE Findings | |
| Clear Cell Renal Cell Carcinoma | 22 (62.9) |
| Papillary Renal Cell Carcinoma (RCC) | 2 (5.7) |
| Multiloculated cystic RCC | 2 (5.7) |
| Angiomyolipoma (AML) | 7 (20.0) |
| Ewing Sarcoma of Kidney | 1 (2.9) |
| Benign Vascular Lesion | 1 (2.9) |
| Clavien-Dindo Grade | Complication | Number of Patients | Management |
|---|---|---|---|
| Grade II | Postoperative fever | 1 | IV antibiotics for 7 days |
| Grade II | Ileus | 1 | Conservative: bowel rest, IV fluids |
| Grade II | Lung atelectasis | 1 | Non-invasive ventilation (NIV), supportive care |
| Grade II | Transient ischemic attack (TIA) | 1 | ICU monitoring, resolved without sequelae |
| Total | - | 4 (11.4%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Hashim, M.H.; Rizuana, I.H.; Md Zainuddin, Z.; Lim, L.Y.; Khoo, H.C.; Shukor, S.; Azizi, M.H.; Fam, X.I. Evaluating the Efficacy of Robot-Assisted Partial Nephrectomy in Complex Renal Tumours: A Single-Centre Retrospective Study. Medicina 2025, 61, 1702. https://doi.org/10.3390/medicina61091702
Mohd Hashim MH, Rizuana IH, Md Zainuddin Z, Lim LY, Khoo HC, Shukor S, Azizi MH, Fam XI. Evaluating the Efficacy of Robot-Assisted Partial Nephrectomy in Complex Renal Tumours: A Single-Centre Retrospective Study. Medicina. 2025; 61(9):1702. https://doi.org/10.3390/medicina61091702
Chicago/Turabian StyleMohd Hashim, Mohammad Hifzi, Iqbal Hussain Rizuana, Zulkifli Md Zainuddin, Li Yi Lim, Hau Chun Khoo, Suzliza Shukor, Muhammad Hasif Azizi, and Xeng Inn Fam. 2025. "Evaluating the Efficacy of Robot-Assisted Partial Nephrectomy in Complex Renal Tumours: A Single-Centre Retrospective Study" Medicina 61, no. 9: 1702. https://doi.org/10.3390/medicina61091702
APA StyleMohd Hashim, M. H., Rizuana, I. H., Md Zainuddin, Z., Lim, L. Y., Khoo, H. C., Shukor, S., Azizi, M. H., & Fam, X. I. (2025). Evaluating the Efficacy of Robot-Assisted Partial Nephrectomy in Complex Renal Tumours: A Single-Centre Retrospective Study. Medicina, 61(9), 1702. https://doi.org/10.3390/medicina61091702






