Effects of Concurrent Training on Biomarkers, Morphological Variables, and Physical Performance in People with Sarcopenic Obesity: A Meta-Analysis with Meta-Regression
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Search Process and Databases
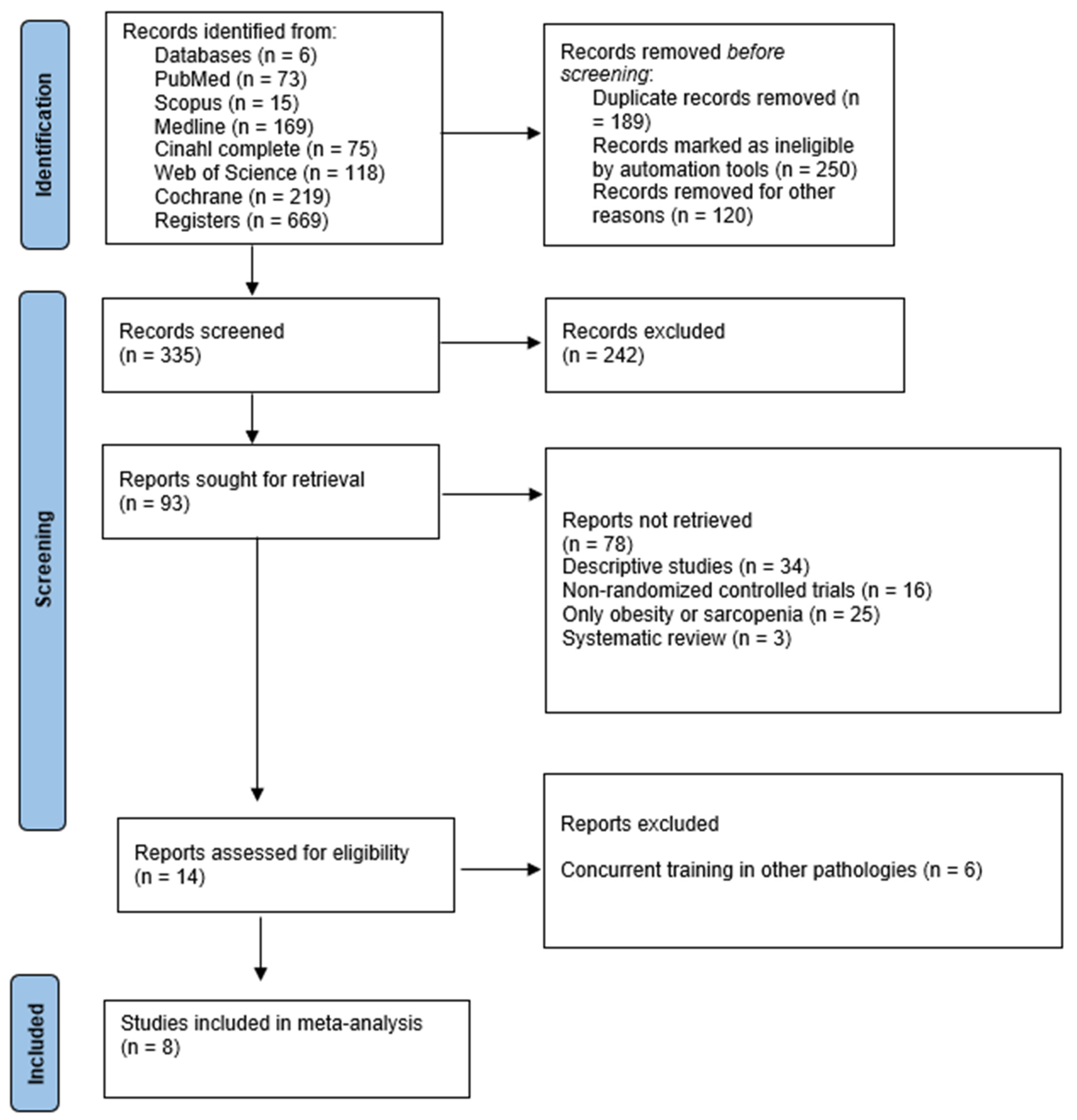
2.4. Study Selection and Data Collection Process
2.5. Assessment of Methodological Quality
2.6. Data Synthesis
2.7. Assessment of Bias Risk
2.8. Summary Measures for Meta-Analysis
2.9. Sensitivity Analyses
2.10. Moderator Analyses
2.11. Subgroup Analyses
2.12. Certainty of Evidence
3. Results
3.1. Methodological Quality
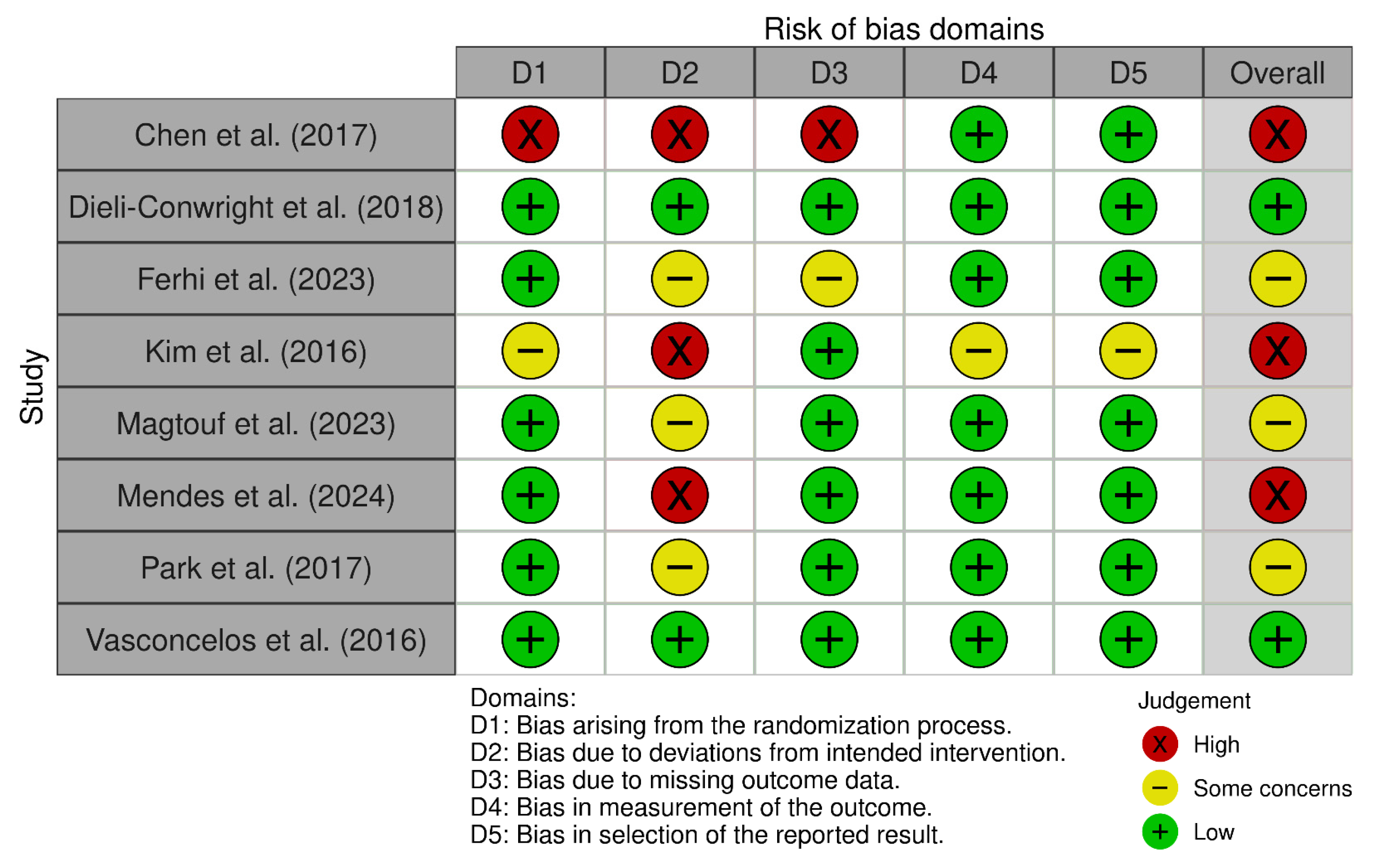
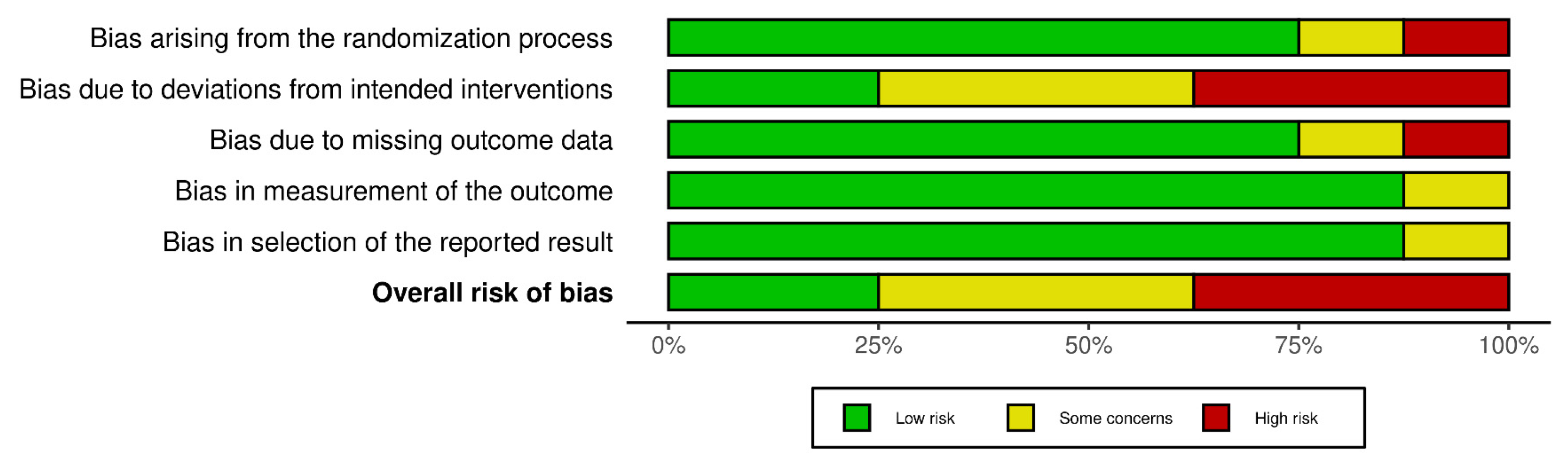
3.2. Risk of Bias
3.3. Studies and Sample Characteristics
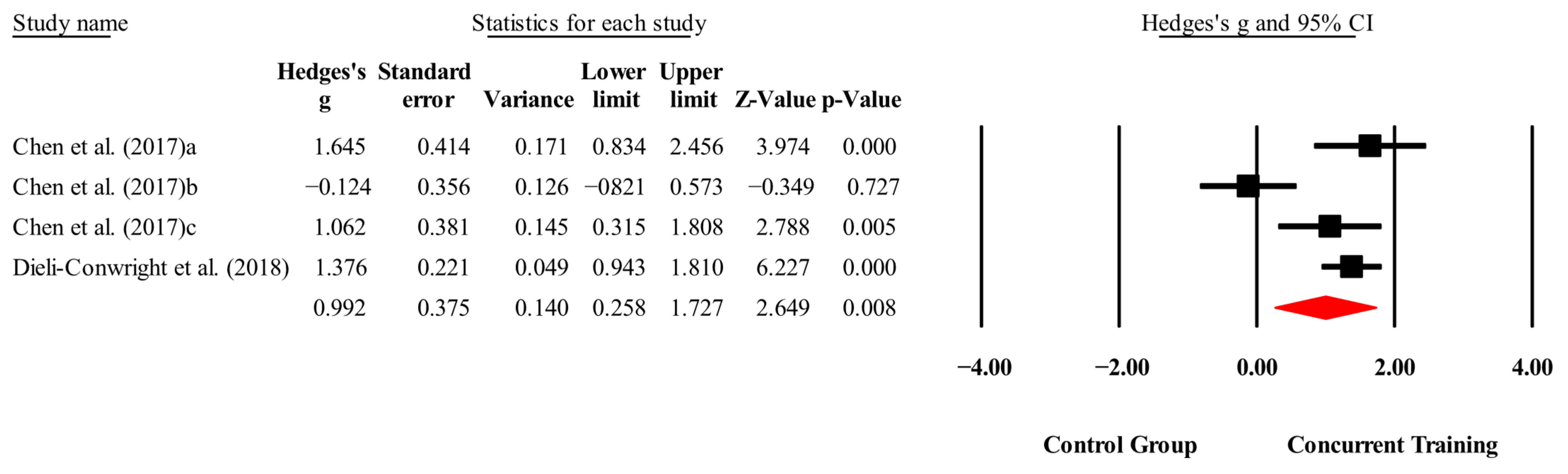
3.4. Meta-Analysis Results
3.5. Sensitivity Analysis for Overall Meta-Analysis
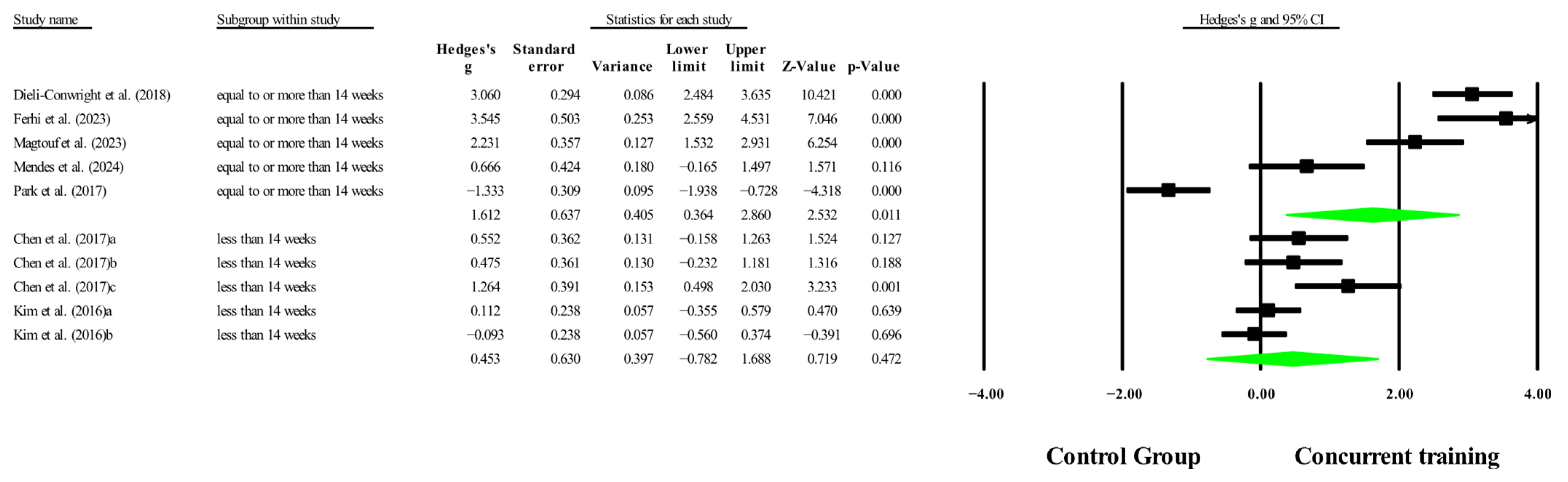
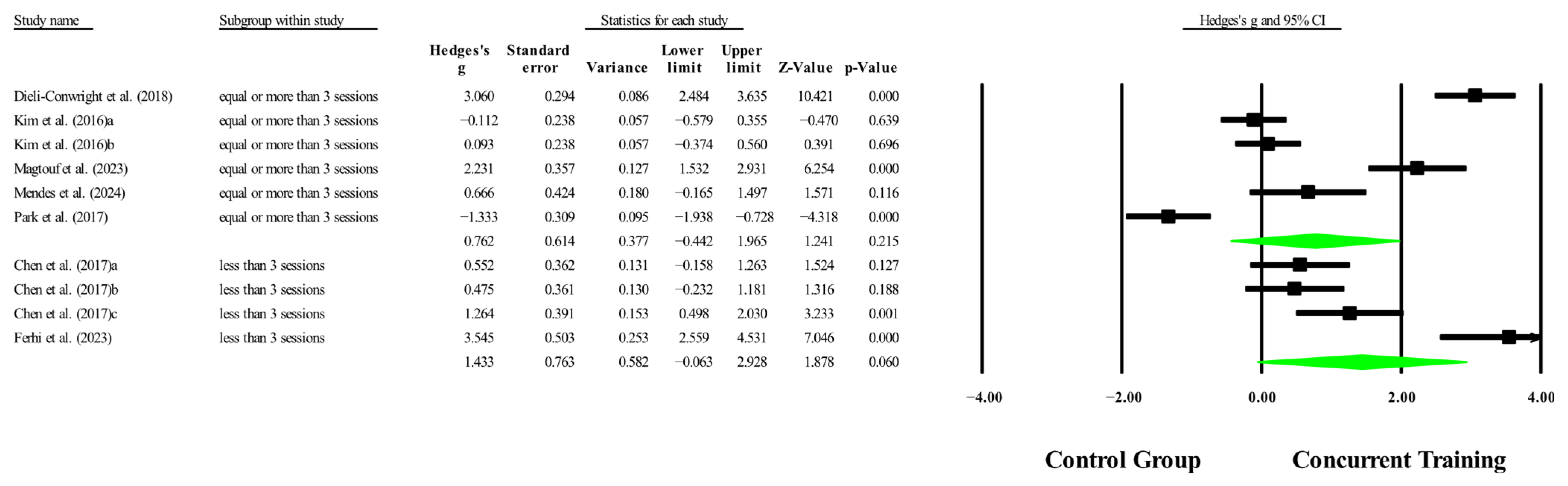
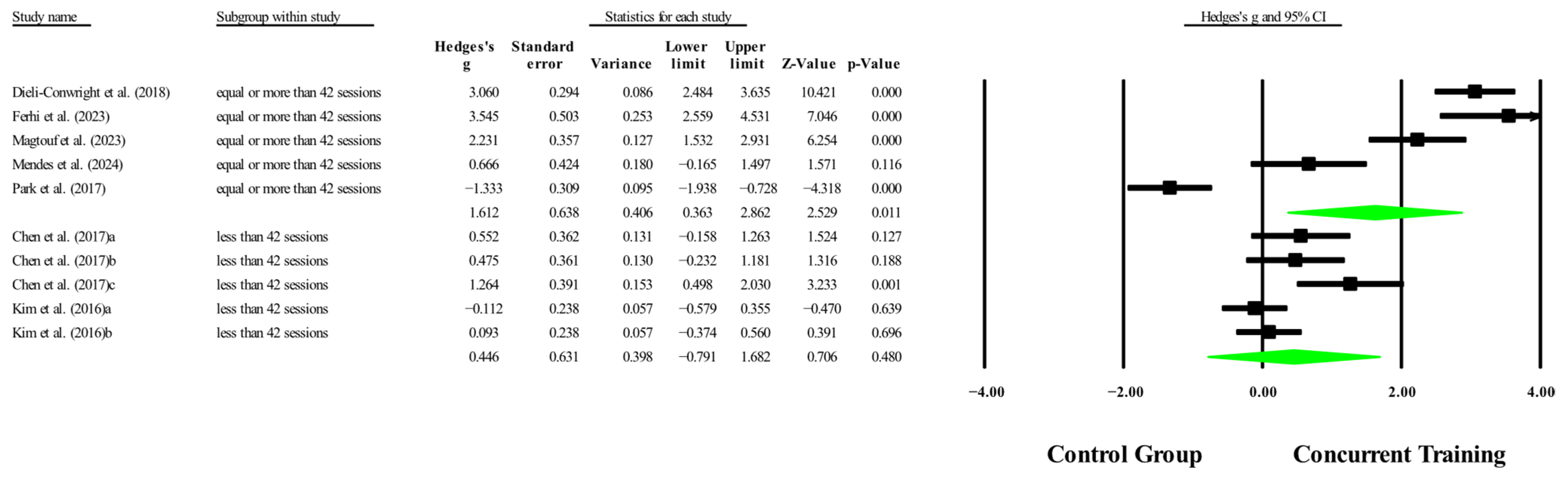
3.6. Meta-Analysis Subgroup
3.6.1. Subgroup Analysis by Dosage Training
3.6.2. Sensitivity Analysis for Subgroups
3.7. Meta-Regression
3.8. Certainty of Evidence
4. Discussion
4.1. Biomarkers
4.1.1. IGF-1
4.1.2. IL-6
4.1.3. CRP
4.1.4. Leptin
4.1.5. Total Cholesterol
4.1.6. Triglycerides
4.2. Morphological Variables
4.3. Physical Performance
4.4. Meta-Analysis Subgroup
4.4.1. Subgroup Analysis by Dosage Training
4.4.2. Meta-Regression
4.5. Limitations and Strengths
4.6. Practical Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briand, M.; Raffin, J.; Gonzalez-Bautista, E.; Ritz, P.; Abellan Van Kan, G.; Pillard, F.; Faruch-Bilfeld, M.; Guyonnet, S.; Dray, C.; Vellas, B.; et al. Body composition and aging: Cross-sectional results from the INSPIRE study in people 20 to 93 years old. GeroScience 2025, 47, 863–875. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Axelrod, C.L.; Dantas, W.S.; Kirwan, J.P. Sarcopenic obesity: Emerging mechanisms and therapeutic potential. Metabolism 2023, 146, 155639. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-W.; Lee, Y.-H.; Liao, C.-D.; Escorpizo, R.; Liou, T.-H.; Lin, H.-W. Association of physical functional activity impairment with severity of sarcopenic obesity: Findings from National Health and Nutrition Examination Survey. Sci. Rep. 2024, 14, 3787. [Google Scholar] [CrossRef]
- Salinas-Rodríguez, A.; Rivera-Almaraz, A.; Manrique-Espinoza, B. Sarcopenic obesity is associated with long-term trajectories of physical activity and sedentary behavior. Exp. Gerontol. 2025, 204, 112752. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.H.; Liu, J.Y.W.; Välimäki, M. Effectiveness of non-pharmacological interventions on the management of sarcopenic obesity: A systematic review and meta-analysis. Exp. Gerontol. 2020, 135, 110937. [Google Scholar] [CrossRef]
- Hsu, K.J.; Liao, C.D.; Tsai, M.W.; Chen, C.N. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019, 11, 2163. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Sakhaei, M.H.; Rosenkranz, S.K.; Symonds, M.E. Impact of concurrent training versus aerobic or resistance training on cardiorespiratory fitness and muscular strength in middle-aged to older adults: A systematic review and meta-analysis. Physiol. Behav. 2022, 254, 113888. [Google Scholar] [CrossRef]
- da Silva Gonçalves, L.; Santos Lopes da Silva, L.; Rodrigues Benjamim, C.J.; Tasinafo Junior, M.F.; Bohn, L.; Ferreira Abud, G.; Ortiz, G.U.; de Freitas, E.C. The Effects of Different Exercise Training Types on Body Composition and Physical Performance in Older Adults with Sarcopenic Obesity: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2023, 27, 1076–1090. [Google Scholar] [CrossRef]
- Tian, H.; Li, H.; Zhang, X.; Liu, H.; Huang, L.; Yu, H.; Wu, J.; Cao, Y.; Peng, L.; García-Ramos, A. Non-pharmacological treatment strategies for anthropometric, physical capacity and physiological indicators among sarcopenic obesity patients: A systematic review of rigorous randomized controlled trials. Age Ageing 2024, 53, afae278. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Smart, N.A.; Waldron, M.; Ismail, H.; Giallauria, F.; Vigorito, C.; Cornelissen, V.; Dieberg, G. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int. J. Evid.-Based Healthc. 2015, 13, 9–18. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.C.; Pigott, T.D.; Rothstein, H.R. How Many Studies Do You Need?: A Primer on Statistical Power for Meta-Analysis. J. Educ. Behav. Stat. 2010, 35, 215–247. [Google Scholar] [CrossRef]
- Verhagen, A.P.; de Vet, H.C.; de Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef]
- Ruppar, T. Meta-analysis: How to quantify and explain heterogeneity? Eur. J. Cardiovasc. Nurs. 2020, 19, 646–652. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Petré, H.; Löfving, P.; Psilander, N. The Effect of Two Different Concurrent Training Programs on Strength and Power Gains in Highly-Trained Individuals. J. Sports Sci. Med. 2018, 17, 167–173. [Google Scholar] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Chen, H.T.; Chung, Y.C.; Chen, Y.J.; Ho, S.Y.; Wu, H.J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef]
- Ferhi, H.; Gaied Chortane, S.; Durand, S.; Beaune, B.; Boyas, S.; Maktouf, W. Effects of Physical Activity Program on Body Composition, Physical Performance, and Neuromuscular Strategies during Walking in Older Adults with Sarcopenic Obesity: Randomized Controlled Trial. Healthcare 2023, 11, 2294. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Kojima, N.; Fujino, K.; Hosoi, E.; Kobayashi, H.; Somekawa, S.; Niki, Y.; Yamashiro, Y.; Yoshida, H. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women with Sarcopenic Obesity: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Magtouf, E.; Chortane, S.G.; Chortane, O.G.; Boyas, S.; Beaune, B.; Durand, S.; Maktouf, W. Influence of Concurrent Exercise Training on Ankle Muscle Activation during Static and Proactive Postural Control on Older Adults with Sarcopenic Obesity: A Multicenter, Randomized, and Controlled Trial. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 2779–2794. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.; Carvalho, M.; Bravo, J.; Martins, S.; Raimundo, A. Possible Interaction Between Physical Exercise and Leptin and Ghrelin Changes Following Roux-en-Y Gastric Bypass in Sarcopenic Obesity Patients—A Pilot Study. Nutrients 2024, 16, 3913. [Google Scholar] [CrossRef]
- Park, J.; Kwon, Y.; Park, H. Effects of 24-Week Aerobic and Resistance Training on Carotid Artery Intima-Media Thickness and Flow Velocity in Elderly Women with Sarcopenic Obesity. J. Atheroscler. Thromb. 2017, 24, 1117–1124. [Google Scholar] [CrossRef]
- Vasconcelos, K.S.; Dias, J.M.; Araújo, M.C.; Pinheiro, A.C.; Moreira, B.S.; Dias, R.C. Effects of a progressive resistance exercise program with high-speed component on the physical function of older women with sarcopenic obesity: A randomized controlled trial. Braz. J. Phys. Ther. 2016, 20, 432–440. [Google Scholar] [CrossRef]
- Annibalini, G.; Lucertini, F.; Agostini, D.; Vallorani, L.; Gioacchini, A.; Barbieri, E.; Guescini, M.; Casadei, L.; Passalia, A.; Del Sal, M.; et al. Concurrent Aerobic and Resistance Training Has Anti-Inflammatory Effects and Increases Both Plasma and Leukocyte Levels of IGF-1 in Late Middle-Aged Type 2 Diabetic Patients. Oxidative Med. Cell. Longev. 2017, 2017, 3937842. [Google Scholar] [CrossRef]
- Jiang, Q.; Lou, K.; Hou, L.; Lu, Y.; Sun, L.; Tan, S.C.; Low, T.Y.; Kord-Varkaneh, H.; Pang, S. The effect of resistance training on serum insulin-like growth factor 1(IGF-1): A systematic review and meta-analysis. Complement. Ther. Med. 2020, 50, 102360. [Google Scholar] [CrossRef]
- Rahmani, J.; Kord Varkaneh, H.; Clark, C.; Zand, H.; Bawadi, H.; Ryan, P.M.; Fatahi, S.; Zhang, Y. The influence of fasting and energy restricting diets on IGF-1 levels in humans: A systematic review and meta-analysis. Ageing Res. Rev. 2019, 53, 100910. [Google Scholar] [CrossRef]
- Gibney, J.; Healy, M.-L.; Sönksen, P.H. The Growth Hormone/Insulin-Like Growth Factor-I Axis in Exercise and Sport. Endocr. Rev. 2007, 28, 603–624. [Google Scholar] [CrossRef]
- Lang, T.; Streeper, T.; Cawthon, P.; Baldwin, K.; Taaffe, D.R.; Harris, T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010, 21, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Libardi, C.A.; De Souza, G.V.; CAVAGLIERI, C.R.; MADRUGA, V.A.; CHACON-MIKAHIL, M.P.T. Effect of Resistance, Endurance, and Concurrent Training on TNF-α, IL-6, and CRP. Med. Sci. Sports Exerc. 2012, 44, 50–56. [Google Scholar] [CrossRef]
- Trejos-Montoya, J.A. Aerobic Versus Concurrent Exercise on Interleukin-6 in Patients with Coronary Arterial Disease: A Systematic Review. MHSalud Rev. Cienc. Mov. Hum. Salud 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Prado, C.M.; Batsis, J.A.; Donini, L.M.; Gonzalez, M.C.; Siervo, M. Sarcopenic obesity in older adults: A clinical overview. Nat. Rev. Endocrinol. 2024, 20, 261–277. [Google Scholar] [CrossRef]
- Tang, J.E.; Hartman, J.W.; Phillips, S.M. Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men. Appl. Physiol. Nutr. Metab. 2006, 31, 495–501. [Google Scholar] [CrossRef]
- Zouhal, H.; Ben Abderrahman, A.; Khodamoradi, A.; Saeidi, A.; Jayavel, A.; Hackney, A.C.; Laher, I.; Algotar, A.M.; Jabbour, G. Effects of physical training on anthropometrics, physical and physiological capacities in individuals with obesity: A systematic review. Obes. Rev. 2020, 21, e13039. [Google Scholar] [CrossRef] [PubMed]
- Nakhostin-Roohi, B.; Havaskar, S. The Effect of Concurrent Training Program on Body Composition Indices in Overweight and Obese Female Students. J. Exerc. Physiol. Health 2018, 1, 6–12. [Google Scholar]
- Reljic, D.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Impact of Different Low-Volume Concurrent Training Regimens on Cardiometabolic Health, Inflammation, and Fitness in Obese Metabolic Syndrome Patients. Nutrients 2025, 17, 561. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Kargarfard, M.; Jalali, K.; Ashtary-Larky, D.; Cheraghloo, N.; Ghobadi, H.; Moghadam, B.H.; Wong, A.; Nordvall, M.; Dutheil, F. The Effects of 12 Weeks of Concurrent and Combined Training on Inflammatory Markers, Muscular Performance, and Body Composition in Middle-Aged Overweight and Obese Males. Nutrients 2023, 15, 1482. [Google Scholar] [CrossRef]
- Colato, A.; Abreu, F.; Medeiros, N.; Lemos, L.; Dorneles, G.; Ramis, T.; Vianna, P.; Chies, J.A.; Peres, A. Effects of concurrent training on inflammatory markers and expression of CD4, CD8, and HLA-DR in overweight and obese adults. J. Exerc. Sci. Fit. 2014, 12, 55–61. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Zouhal, H.; Lemoine-Morel, S.; Mathieu, M.-E.; Casazz, G.A.; Jabbour, G. Catecholamines and Obesity: Effects of Exercise and Training. Sports Med. 2013, 43, 591–600. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L.; Williams, T.D.; Dobbs, W.C. The Effect of Chronic Exercise Training on Leptin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2018, 48, 1437–1450. [Google Scholar] [CrossRef]
- Ferdosi, M.H.; Asad, M.R. The Effect of Endurance, Resistance and Concurrent Trainings on Plasma Leptin Levels of Non-Athlete Males. Procedia Soc. Behav. Sci. 2012, 46, 311–315. [Google Scholar] [CrossRef][Green Version]
- Toussirot, E.; Nguyen, N.U.; Dumoulin, G.; Aubin, F.; Cédoz, J.-P.; Wendling, D. Relationship between growth hormone–IGF-I–IGFBP-3 axis and serum leptin levels with bone mass and body composition in patients with rheumatoid arthritis. Rheumatology 2004, 44, 120–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flores, J.; Del, J.; Valdivia, R.; Rojas-Larios, F.; Gamboa-Gómez, C.; Martinez-Aguilar, G.; Moncada-Jiménez, J. The effect of concurrent training on glucose, lipid profile, liver enzymes, and lipid peroxidation in young men. Retos 2024, 58, 769–775. [Google Scholar] [CrossRef]
- Da Silva, M.A.R.; Baptista, L.C.; Neves, R.S.; De França, E.; Loureiro, H.; Lira, F.S.; Caperuto, E.C.; Veríssimo, M.T.; Martins, R.A. The Effects of Concurrent Training Combining Both Resistance Exercise and High-Intensity Interval Training or Moderate-Intensity Continuous Training on Metabolic Syndrome. Front. Physiol. 2020, 11, 572. [Google Scholar] [CrossRef]
- Gálvez, E.; Cifuentes-Silva, E.; González, F.; Bueno, D.; Foster, P.; Inostroza, M. [Effect of a 12-week concurrent planification exercise program in overweight and obese children and adolescents]. Andes Pediatr. 2022, 93, 658–667. [Google Scholar] [CrossRef]
- Neufer, P.D. The Effect of Detraining and Reduced Training on the Physiological Adaptations to Aerobic Exercise Training. Sports Med. 1989, 8, 302–320. [Google Scholar] [CrossRef]
- Moghadam, B.H.; Bagheri, R.; Ashtary-Larky, D.; Tinsley, G.M.; Eskandari, M.; Wong, A.; Moghadam, B.H.; Kreider, R.B.; Baker, J.S. The Effects of Concurrent Training Order on Satellite Cell-Related Markers, Body Composition, Muscular and Cardiorespiratory Fitness in Older Men with Sarcopenia. J. Nutr. Health Aging 2020, 24, 796–804. [Google Scholar] [CrossRef]
- Kaczorowska, A.; Kozieł, S.; Ignasiak, Z. Hand grip strength and quality of life among adults aged 50–90 years from South West Poland. Sci. Rep. 2025, 15, 882. [Google Scholar] [CrossRef]
- Yu, J.; Lee, E.; Choi, J.-H.; Sun, Y.; Woo, S.; Cho, S.; Hwang, D.; Kim, S.-W.; Kim, J.; Lim, K.; et al. Effects of a 6-Week Concurrent Training Program Combining Resistance and Various Modalities of Aerobic Exercise in Obese Women with Prehypertension: A Randomized Controlled Trial. Metabolites 2025, 15, 278. [Google Scholar] [CrossRef]
- Delgado-Floody, P.; Soriano-Maldonado, A.; Rodríguez-Pérez, M.A.; Latorre-Román, P.; Martínez-Salazar, C.; Vargas, C.A.; Caamaño-Navarrete, F.; Jerez-Mayorga, D.; Álvarez, C. The Effects of Two Different Concurrent Training Configurations on Markers of Metabolic Syndrome and Fitness in Women With Severe/Morbid Obesity: A Randomised Controlled Trial. Front. Physiol. 2021, 12, 694798. [Google Scholar] [CrossRef]
- Rogers, M.E.; Sherwood, H.S.; Rogers, N.L.; Bohlken, R.M. Effects of Dumbbell and Elastic Band Training on Physical Function in Older Inner-City African-American Women. Women Health 2002, 36, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Belfield, A.E.; Wilkinson, T.J.; Henson, J.; Sargeant, J.A.; Breen, L.; Hall, A.P.; Davies, M.J.; Yates, T. Sarcopenia prevalence using handgrip strength or chair stand performance in adults living with type 2 diabetes mellitus. Age Ageing 2024, 53, afae090. [Google Scholar] [CrossRef]
- Vaishya, R.; Misra, A.; Vaish, A.; Ursino, N.; D’Ambrosi, R. Hand grip strength as a proposed new vital sign of health: A narrative review of evidences. J. Health Popul. Nutr. 2024, 43, 7. [Google Scholar] [CrossRef] [PubMed]
- Borde, R.; Hortobágyi, T.; Granacher, U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, M.V.; Nickerson, B.S.; Esco, M.R. Associations of body adiposity index, waist circumference, and body mass index in young adults. Clin. Nutr. 2019, 38, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Merrigan, J.; Stute, N.; Eckerle, J.; Mackowski, N.; Walters, J.; O’Connor, M.; Barrett, K.; Robert, R.; Strang, A.; Hagen, J. Reliability and validity of contemporary bioelectrical impedance analysis devices for body composition assessment. J. Exerc. Nutr. 2022, 5, 103133. [Google Scholar] [CrossRef]
- Markov, A.; Hauser, L.; Chaabene, H. Effects of Concurrent Strength and Endurance Training on Measures of Physical Fitness in Healthy Middle-Aged and Older Adults: A Systematic Review with Meta-Analysis. Sports Med. 2023, 53, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol. 2002, 93, 1318–1326. [Google Scholar] [CrossRef]
- Bandy, W.D.; Lovelace-Chandler, V.; McKitrick-Bandy, B. Adaptation of Skeletal Muscle to Resistance Training. J. Orthop. Sports Phys. Ther. 1990, 12, 248–255. [Google Scholar] [CrossRef]
- Daussin, F.N.; Zoll, J.; Dufour, S.P.; Ponsot, E.; Lonsdorfer-Wolf, E.; Doutreleau, S.; Mettauer, B.; Piquard, F.; Geny, B.; Richard, R. Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: Relationship to aerobic performance improvements in sedentary subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R264–R272. [Google Scholar] [CrossRef]
- Grgic, J.; Garofolini, A.; Orazem, J.; Sabol, F.; Schoenfeld, B.J.; Pedisic, Z. Effects of Resistance Training on Muscle Size and Strength in Very Elderly Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2020, 50, 1983–1999. [Google Scholar] [CrossRef]
- Peterson, M.D.; Rhea, M.R.; Sen, A.; Gordon, P.M. Resistance exercise for muscular strength in older adults: A meta-analysis. Ageing Res. Rev. 2010, 9, 226–237. [Google Scholar] [CrossRef]
- Hernandez-Martinez, J.; Guzmán-Muñoz, E.; Cid-Calfucura, I.; Villalobos-Fuentes, F.; Diaz-Saldaña, D.; Alvarez-Martinez, I.; Castillo-Cerda, M.; Herrera-Valenzuela, T.; Branco, B.H.M.; Valdés-Badilla, P. Elastic Band Training Versus Multicomponent Training and Group-Based Dance on Morphological Variables and Physical Performance in Older Women: A Randomized Controlled Trial. Life 2024, 14, 1362. [Google Scholar] [CrossRef]
- Pijnappels, M.; Reeves, N.D.; Maganaris, C.N.; van Dieën, J.H. Tripping without falling; lower limb strength, a limitation for balance recovery and a target for training in the elderly. J. Electromyogr. Kinesiol. 2008, 18, 188–196. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, H.; Gong, Y.; Tang, Y.; Su, H.; Zhang, Z.; Tong, P.; Chen, G. Clinical outcome changes in sarcopenic obesity: A meta-analysis of exercise training methods. BMC Geriatr. 2025, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Baar, K.; Wende, A.R.; Jones, T.E.; Marison, M.; Nolte, L.A.; Chen, M.; Kelly, D.P.; Holloszy, J.O. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002, 16, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.-C. The Role of Skeletal Muscle Glycogen Breakdown for Regulation of Insulin Sensitivity by Exercise. Front. Physiol. 2011, 2, 112. [Google Scholar] [CrossRef] [PubMed]

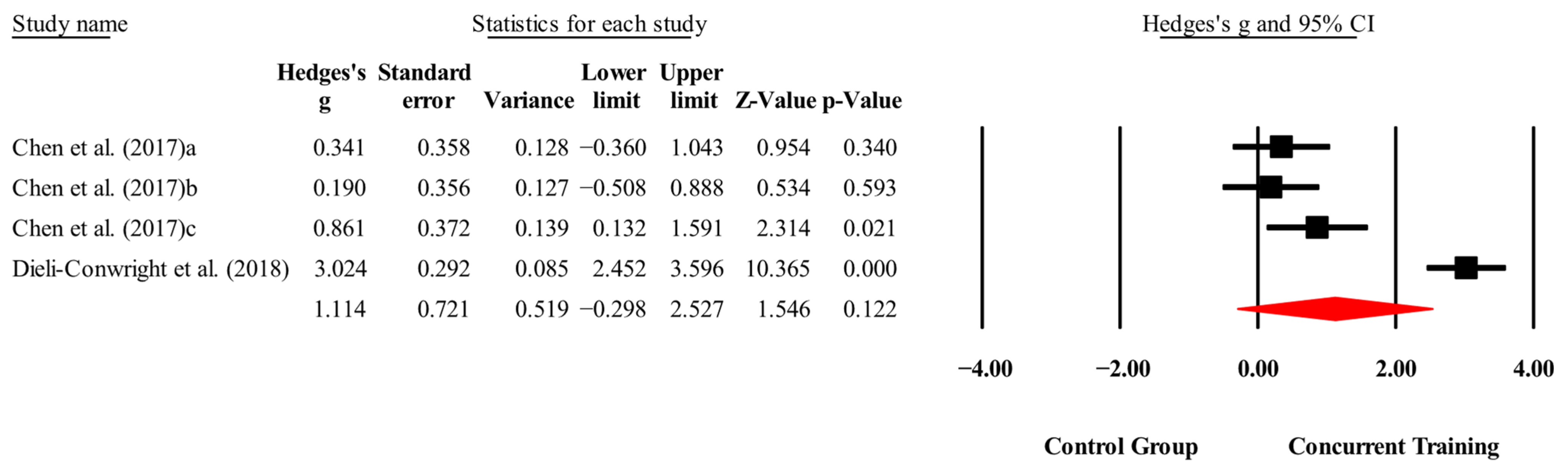

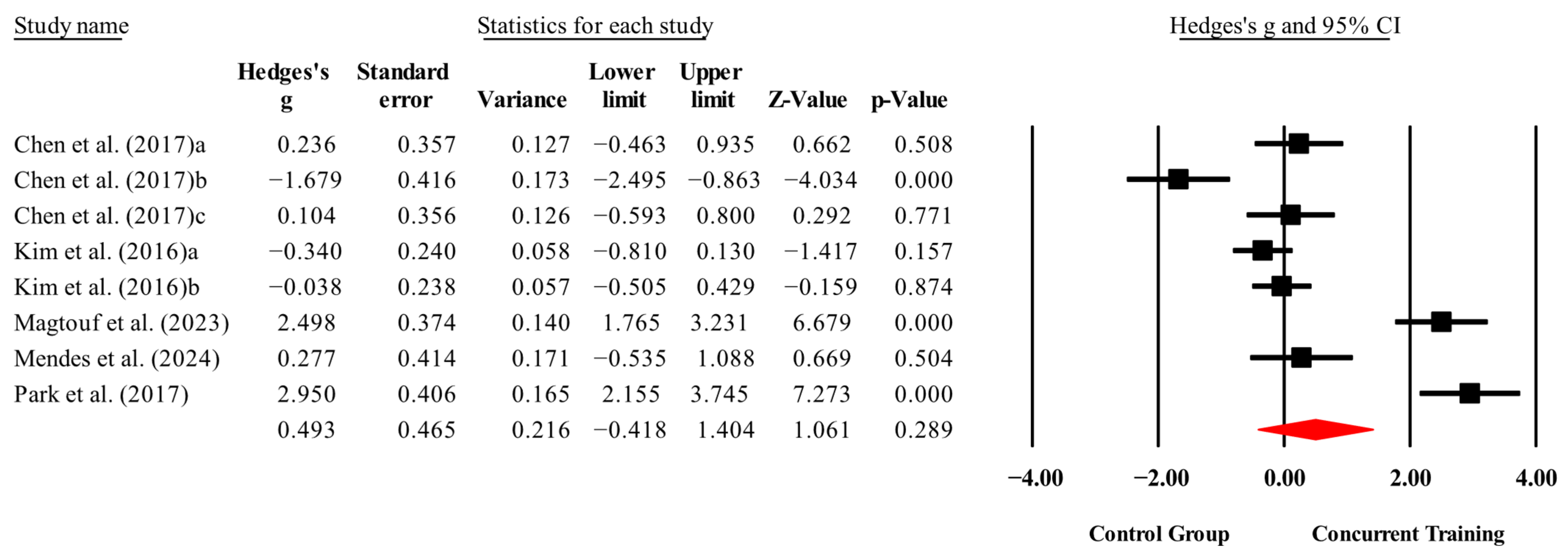
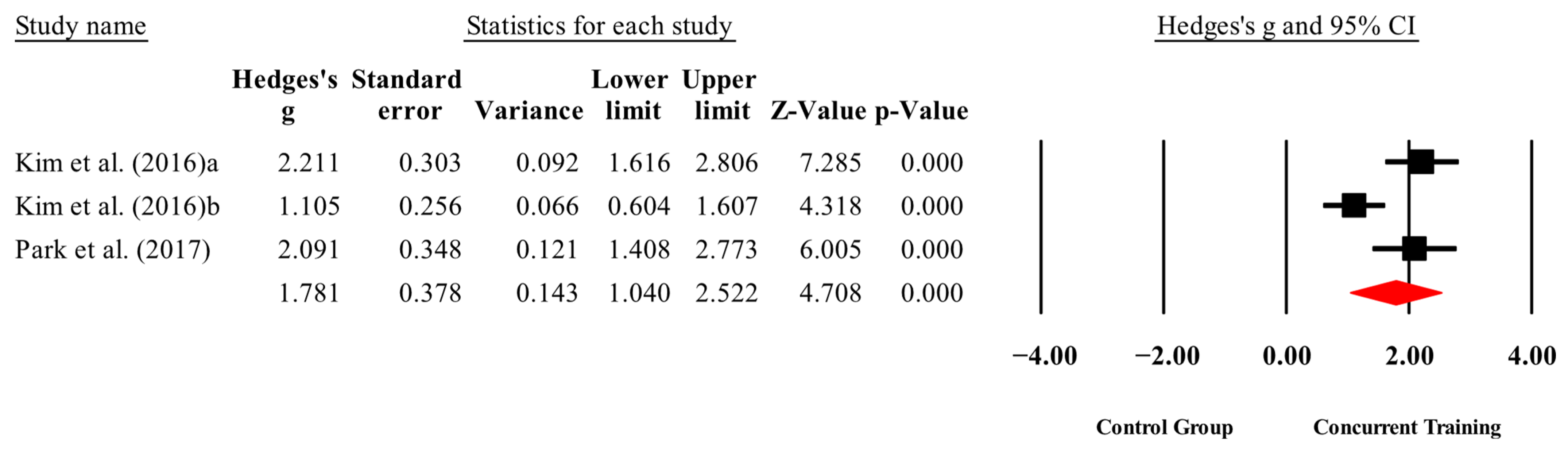
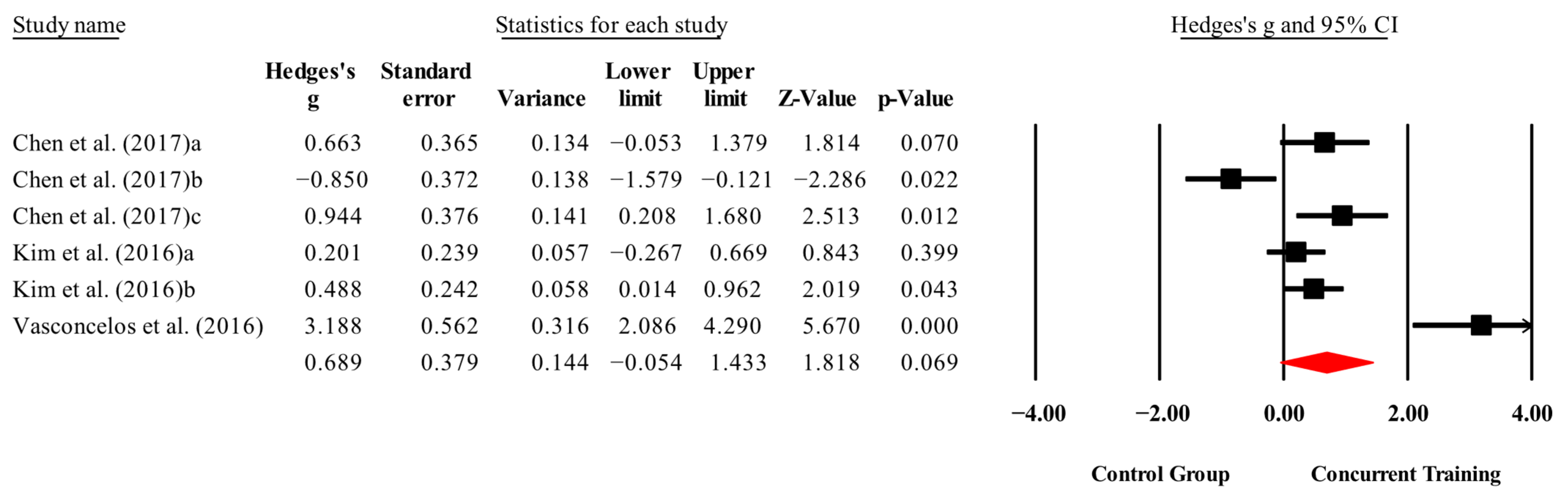
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Persons to have a combination of obesity, sarcopenia, and no other diseases, such as fractures and heart failure. Participants were required to be adults (age ≥ 18 years), but there were no restrictions by sex or setting (such as hospitals, communities, or nursing homes). | Population under 18 years of age only with obesity and/or sarcopenia. Persons ≥18 years of age apparently healthy. |
| Intervention | Interventions included strength training (resistance training, elastic band training, and progressive resistance training) and endurance training (aerobic training, treadmill training, walking training, gait training), with a minimum of 4 weeks of intervention at a frequency of 1 session per week with a minimum of 30 min per session. | Interventions that do not use strength training and endurance training. There are no details of the intervention procedure. |
| Comparator | Interventions with or without an active/inactive control groups or placebo. | Observational studies (i.e., cross-sectional, retrospective, and prospective studies) that do not include structured comparison pre/post analysis. |
| Outcome | Primary outcomes: biomarkers (e.g., IGF-1, IL-6, leptin, C-reactive protein, cholesterol, glycemia, and triglycerides), morphological variables (e.g., body mass index, waist circumference, body fat percentage, skeletal muscle mass index, and bone mineral density); physical performance (e.g., MIHS, gait speed, and knee extension). To determine the diagnosis of sarcopenic obesity, they followed the guidelines of the European Sarcopenia Group and/or Asian Sarcopenia Group and/or Brazilian Sarcopenia Group. | Lack of baseline data and/or follow-ups. |
| Study design | Experimental design studies (randomized controlled trials) with pre- and post-assessments. | Non-randomized controlled trials, cross-sectional, retrospective, and prospective studies. |
| Eligibility Criteria Specified | Randomly Allocated Participants | Allocation Concealed | Groups Similar at Baseline | Assessors Blinded | Outcome Measures Assessed >85% of Participants * | Intention to Treat Analysis | Reporting of Between Group Statistical Comparisons | Point Measures and Measures of Variability Reported ** | Activity Monitoring in Control Group | Relative Exercise Intensity Reviewed | Exercise Volume and Energy Expended | Overall TESTEX # |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen, Chung, Chen, Ho, and Wu [22] | Yes | Yes | Yes | Yes | Yes (1) | No | Yes | Yes (2) | Yes | Yes | Yes | 12/15 |
| Dieli-Conwright, Courneya, Demark-Wahnefried, Sami, Lee, Buchanan, Spicer, Tripathy, Bernstein, and Mortimer [23] | Yes | Yes | Yes | No | Yes (2) | No | Yes | Yes (2) | Yes | Yes | Yes | 12/15 |
| Ferhi, Gaied Chortane, Durand, Beaune, Boyas, and Maktouf [24] | Yes | Yes | Yes | Yes | Yes (1) | No | Yes | Yes (2) | Yes | Yes | Yes | 12/15 |
| Kim, Kim, Kojima, Fujino, Hosoi, Kobayashi, Somekawa, Niki, Yamashiro, and Yoshida [25] | Yes | Yes | Yes | No | Yes (2) | No | Yes | Yes (2) | Yes | No | Yes | 11/15 |
| Magtouf, Chortane, Chortane, Boyas, Beaune, Durand, and Maktouf [26] | Yes | Yes | Yes | Yes | Yes (1) | No | Yes | Yes (2) | Yes | Yes | Yes | 12/15 |
| Mendes, Carvalho, Bravo, Martins, and Raimundo [27] | Yes | Yes | Yes | Yes | Yes (1) | No | Yes | Yes (2) | Yes | Yes | Yes | 12/15 |
| Park, Kwon, and Park [28] | Yes | Yes | Yes | Yes | Yes (1) | No | Yes | Yes (2) | Yes | Yes | Yes | 12/15 |
| Vasconcelos, Dias, Araújo, Pinheiro, Moreira, and Dias [29] | Yes | Yes | Yes | Yes | Yes (1) | Yes | Yes | Yes (2) | Yes | Yes | Yes | 13/15 |
| Autor | Country | Study Design | Sex (Age) | Type of Training (Sample Size) | Diagnostic Criteria for Obesity Sarcopenic | Morphological Assessment Tool | Training | Intensity | Morphological Variables | Physical Performance | Biomarkers | Adverse Events and Adherence | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weeks | Frequency | Minutes | ||||||||||||
| Chen, Chung, Chen, Ho, and Wu [22] | Taiwan | RCT | Women 80% Men 20% CT: 68.5 ± 2.7 ST: 70.3 ± 11.2 AT: 62.8 ± 9.4 CG: 69.2 ± 9.6 | CT (15): strength training combination aerobic training ST (15): strength training with free weights AT (15): combination of dance steps and jumping exercises CG (15): continued with their daily life activities | Sarcopenia is defined as appendicular skeletal muscle mass (ASM) (kg)/weight (kg) 9100%. The determining threshold value is ≤32.5% for men and ≤25.7% for women. Obesity indicators are body mass index (BMI) ≥ 25 kg/m2,13 and visceral fat area (VFA) ≥ 100 cm2. | Electrical bioimpedance (Inbody 720, Biospace Inc., Cerritos, CA, USA) | 8 | 2 | 60 | 60–70% 1RM Endurance training moderate intensity | Weight (kg) SMM (kg) ASM/weight (%) BFM (kg) BMI (kg/m2) BF (%) VFA (cm2) | Back extensor (kg) Knee extensor (kg) MIHS (kg) | IGF-1 (ng/mL) | NR >85 |
| Dieli-Conwright, Courneya, Demark-Wahnefried, Sami, Lee, Buchanan, Spicer, Tripathy, Bernstein, and Mortimer [23] | United States | RCT | Women 100% CT: 52.8 ± 10.6 CG: 53.6 ± 10.1 | CT (50): strength training with free weights and endurance training in cycling CG (50): continued with their daily life activities | Sarcopenic obesity was defined as appendicular skeletal mass index, <5.45 kg/m2 and BMI ≥ 30.0 kg/m2. | DEXA (Lunar GE iDXA; Fairfield, CT, USA) | 18 | 3 | 60 | 60–80% 1RM 65–80% heart rate maximum | Waist circumference (cm) BMI (kg/m2) ASM/weight (%) BFM (kg) Lean mass (kg) BF (%) Trunk fat (kg) | NR | HDL-C (mg/dL) Total cholesterol (mg/dL) Triglycerides (mg/dL) Glucose (mg/dL) ATP III-score IGF-1 (ng/mL) IGFBP-3, (ng/mL) CRP (mg/L) Leptin (ng/mL) Adiponectin (mg/mg) IL-6 (pg/mL) IL-8 (pg/mL) TNF-a (pg/mg) SHBG (nmol/L) Estradiol (pg/mL) Free testosterone (pg/mL) | No adverse events occurred >85 |
| Ferhi, Gaied Chortane, Durand, Beaune, Boyas, and Maktouf [24] | France | RCT | NR CT: 74.1 ± 3.7 CG: 76.6 ± 5.6 | CT (20): strength training with free weights and walking exercises CG (20): continued with their daily life activities | Sarcopenic obesity was defined having a BMI > 30 kg/m2, a Handgrip force (HF) < 17 N, gait speed < 1.0 m/s, being | Electrical bioimpedance (Tanita; SC 240-Class III; Tanita Europe B.V., Amsterdam, The Netherlands). | 24 | 2 | 60 | 6 (RPE of the 10 points) | Weight (kg) BMI (kg/m2) BF (%) Waist circumference (cm) | Knee extensor (kg) Absolute PT 60 s 1 (Nm) Relative PT 60 s 1 (Nm/kg 100) | NR | NR >85 |
| Kim, Kim, Kojima, Fujino, Hosoi, Kobayashi, Somekawa, Niki, Yamashiro, and Yoshida [25] | Japan | RCT | Women 100% CT: 81.4 ± 4.3 N: 81.2 ± 4.9 CG: 81.1 ± 5.1 | CT (35): strength training with elastic band training and endurance training in cycling N (34): Supplementation with amino acids and tea Catechin CG (34): A general health education class | Sarcopenic obesity was operationally defined as body fat percent of 32% or greater, combined with skeletal muscle mass index less than 5.67 kg/m2; body fat percent of 32% or greater and grip strength less than 17.0 kg; and body fat percent of 32% or greater and walking speed under 1.0 m/s. | Electrical bioimpedance (Inbody 720, Biospace, Seoul, Republic of Korea) | 12 | 3 | 60 | NR | ASM (kg) BF (%) Trunk fat (kg) Total body fat (kg) Weight (kg) | Walking speed (m/s) MIHS (kg) Knee extension (kg) | Total cholesterol (mg/dL) Triglycerides (mg/dL) Hemoglobin A1c (%) Leptin (ng/mL) Cystatin C (mg/L) Vitamin D (ng/mL) IL-6 (pg/mL) CRP (mg/L) | No adverse events occurred >85 |
| Magtouf, Chortane, Chortane, Boyas, Beaune, Durand, and Maktouf [26] | France | RCT | NR CT: 76.3 ± 3.5 CG: 75.9 ± 5.4 | CT (25): strength training with free weights with elastic band training and walking exercises CG (25): continued with their daily life activities | Sarcopenic obesity was defined having a BMI > 30 kg/m2, a Handgrip force (HF) < 17 N, gait speed < 1.0 m/s, being | Electrical bioimpedance (Tanita; SC 24, Amsterdam, The Netherlands) | 16 | 3 | 60 | 6 (RPE of the 10 points) | Body mass (kg) Waist circumference (cm) Body mass (kg) BF (%) FBM (kg) LBM (kg) | TUG (s) Gait speed (m/s) MIHS (kg) Romberg test (s) | NR | NR >85 |
| Mendes, Carvalho, Bravo, Martins, and Raimundo [27] | Portugal | RCT | Women 85% Men 15% CT: 44.08 ± 13.2 CG: 50.4 ± 11.1 | CT (12): strength training with free weights with machine and endurance training in cycling CG (10): continued with their daily life activities | Sarcopenic obesity was defined as a high BMI or waist circumference, combined with low muscle mass and low muscle strength | DEXA (DXA, Hologic QDR, Hologic, Inc., Bedford, MA, USA) | 16 | 3 | 55 | 4–6 (RPE of the 10 points) 50–60% heart rate maximum | BMI (kg/m2) Weight (kg) BF (%) Lean mass (kg) BMC (kg) BMD (g/cm2) | MIHS (kg) | Leptin (ng/mL) Ghrelin (pg/mL) | NR >85 |
| Park, Kwon, and Park [28] | South Korea | RCT | Women 100% CT: 73.5 ± 7.1 CG: 74.7 ± 5.1 | CT (25): strength training with elastic band training and walking exercises CG (25): continued with their daily life activities | Sarcopenic obesity was defined as a body mass index (BMI) ≥ 25.0 kg/m2 and ASM/weight < 25.1% | Electrical bioimpedance (Inbody 720, Biospace, Seoul, Republic of Korea) | 24 | 5 | 50 to 80 | 13–15 (RPE of the 20 points) | Waist circumference (cm) BF (%) ASM (kg) | MIHS (kg) Sit and Reach (cm) Walking speed (m/s) 2-Minute step | HDL-C (mg/dL) LDL-C (mg/dL) Total cholesterol (mg/dL) Triglycerides (mg/dL) CRP (mg/L) | NR >85 |
| Vasconcelos, Dias, Araújo, Pinheiro, Moreira, and Dias [29] | Brazil | RCT | Women 100% CT: 72 ± 4.6 CG: 72 ± 3.6 | CT (14): strength training with free weights and walking exercises CG (14): continued with their daily life activities | Sarcopenic obesity was defined by a body mass index (BMI) ≥30 kg/m2 and handgrip strength ≤21 kg | Calibrated balance (FilizolaTM, São Paulo, SP, Brazil) | 10 | 2 | 60 | 40–75% 1RM | NR | Knee extension (kg) Gait speed (m/s) | NR | NR >85 |
| Biomarkers | |||||||
|---|---|---|---|---|---|---|---|
| n a | Model Effect | ES (95%CI) | p | I2 (%) | Egger’s Test (p) | RW (%) | |
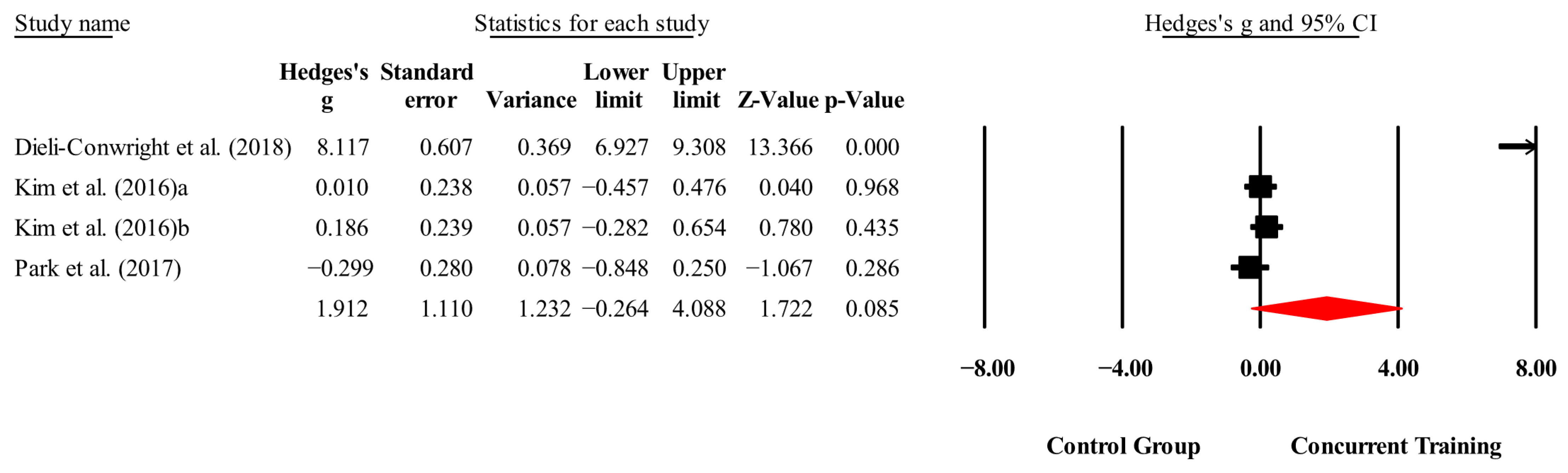
| IGF-1(ng/mL) | 2,2,4, 160. | Random | 1.01 (0.26 to 1.75) | 0.008 | 79.8 | 0.002 | 43.4 to 62.4 |
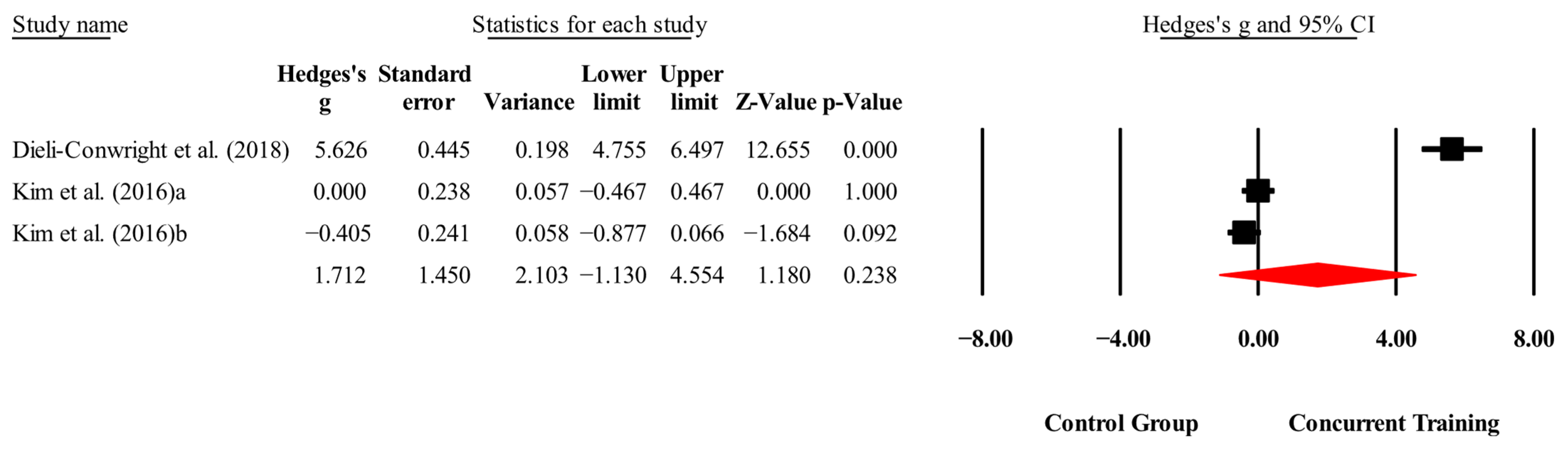
| IL-6 (pg/mL) | 2,2,3, 203. | Random | 1.72 (−1.14 to 4.59) | 0.23 | 98.6 | 0.000 | 0.81 to 4.97 |
| CRP (mg/L) | 3,3,4, 288. | Random | 1.32 (−0.63 to 3.31) | 0.18 | 97.9 | 0.000 | 1.31 to 7.70 |
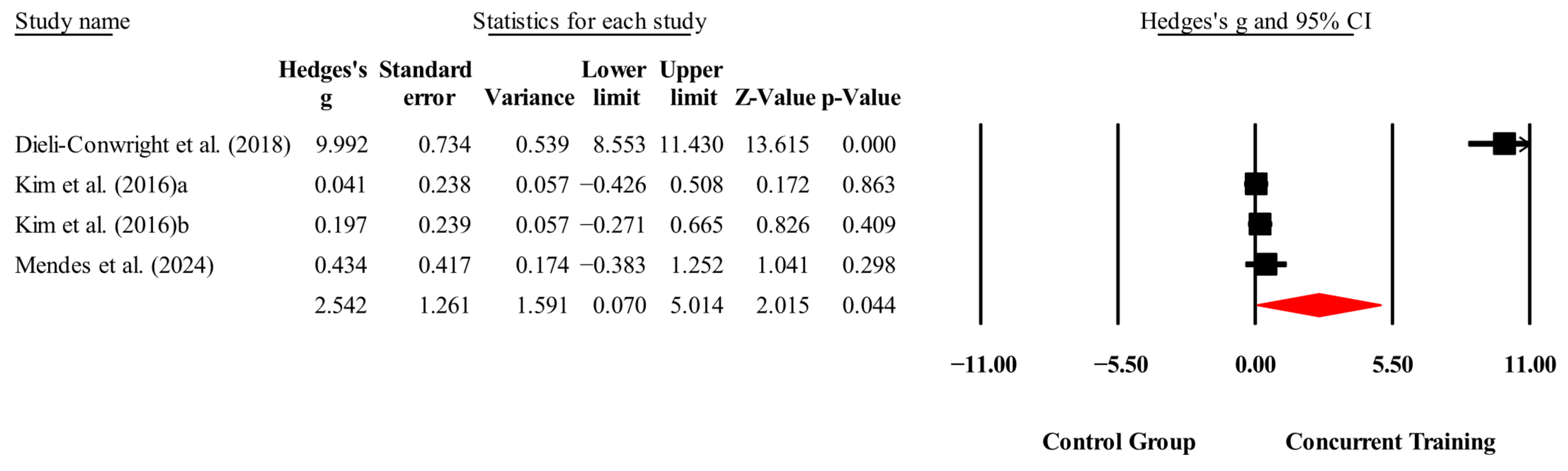
| Leptin (ng/mL) | 3,3,4, 260. | Random | 2.54 (0.07 to 5.01) | 0.04 | 98.2 | 0.000 | 1.59 to 14.9 |
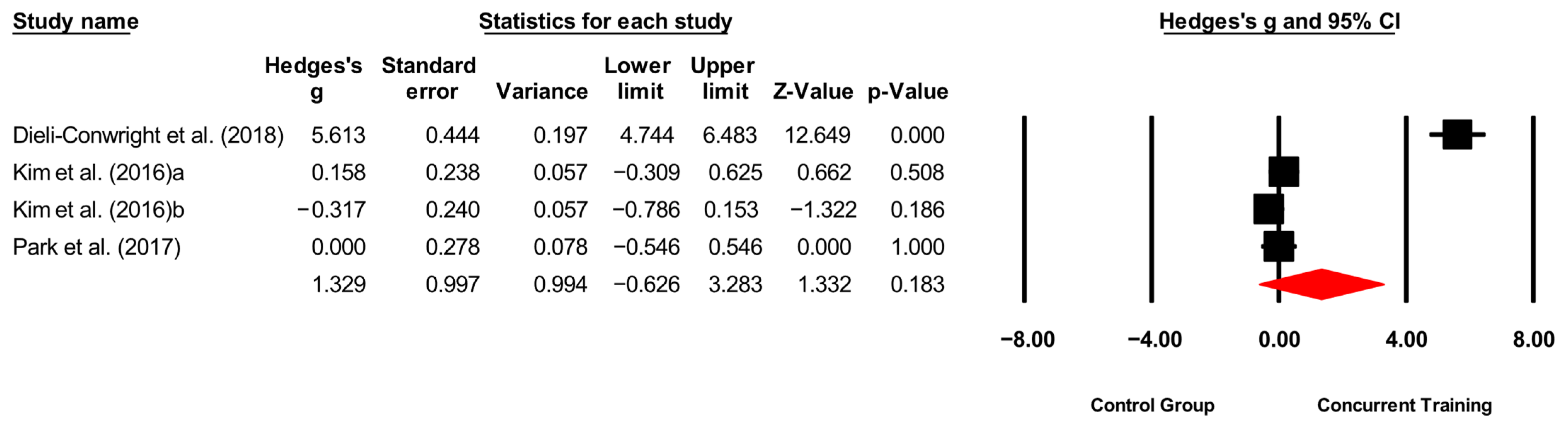
| Total cholesterol (mg/dL) | 3,3,4, 288. | Random | 0.57 (−0.60 to 1.75) | 0.34 | 95.5 | 0.000 | 1.58 to 3.81 |
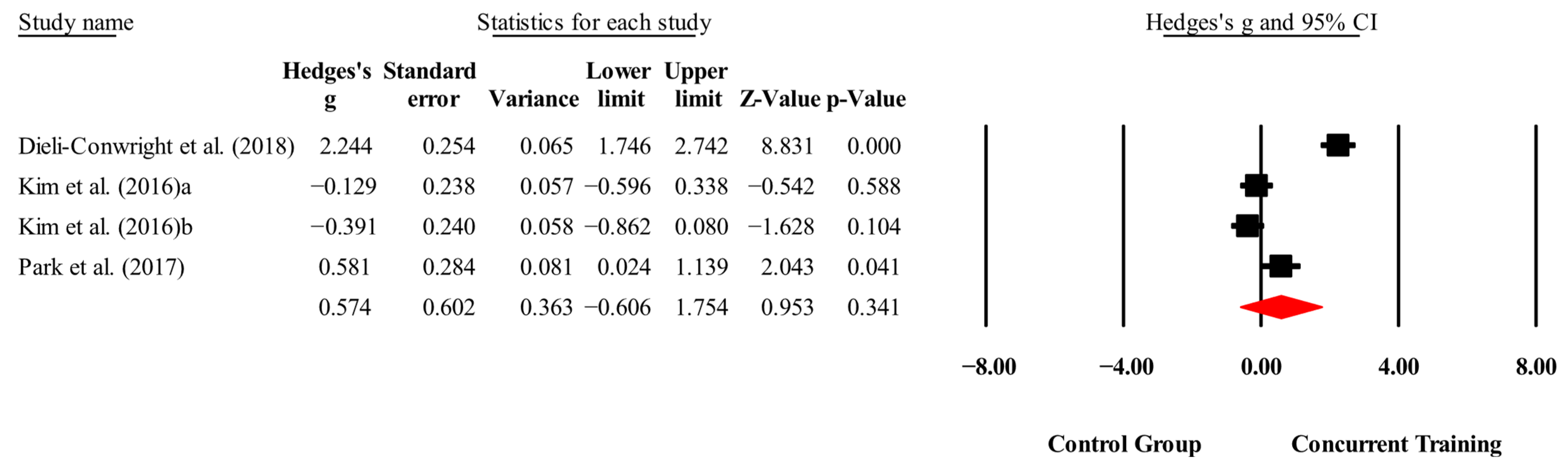
| Triglycerides (mg/dL) | 3,3,4, 288. | Random | 1.92 (−0.27 to 4.12) | 0.08 | 98.2 | 0.000 | 21.4 to 80.3 |
| Morphological variables | |||||||
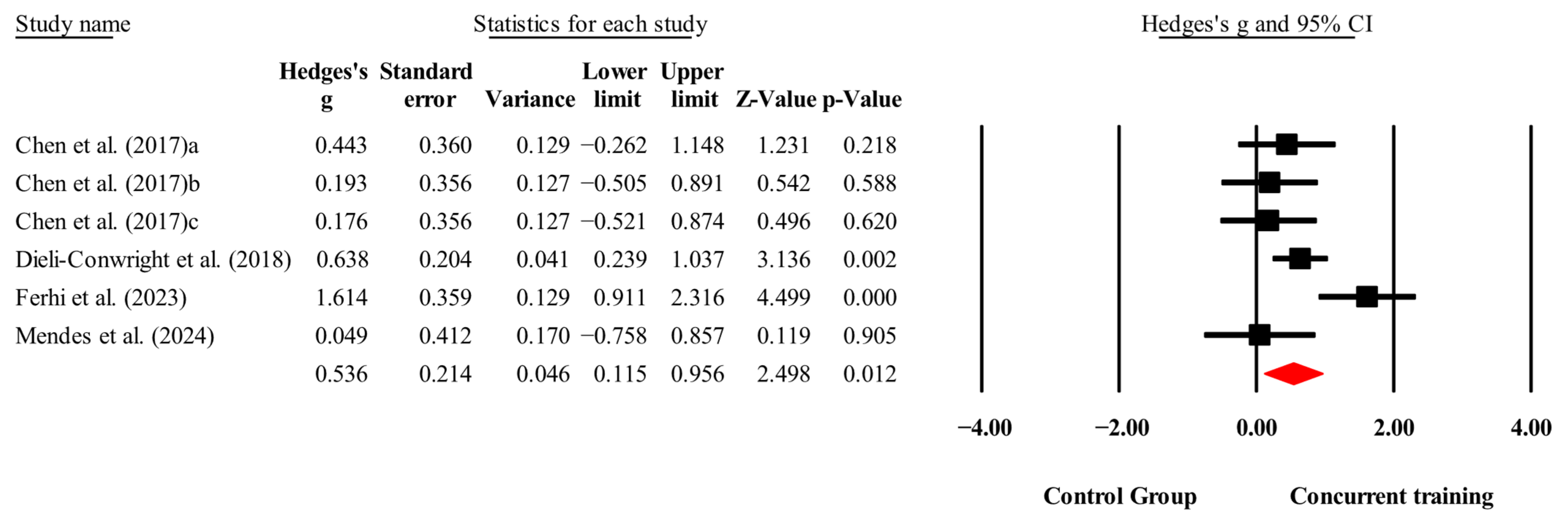
| BMI (kg/m2) | 4,4,6, 252. | Random | 0.54 (0.12 to 0.97) | 0.01 | 59.9 | 0.02 | 32.1 to 34 |
| Waist circumference (cm) | 4,4,4, 240. | Random | 1.22 (0.32 to 2.12) | 0.008 | 89.6 | 0.000 | 5.80 to 9.65 |
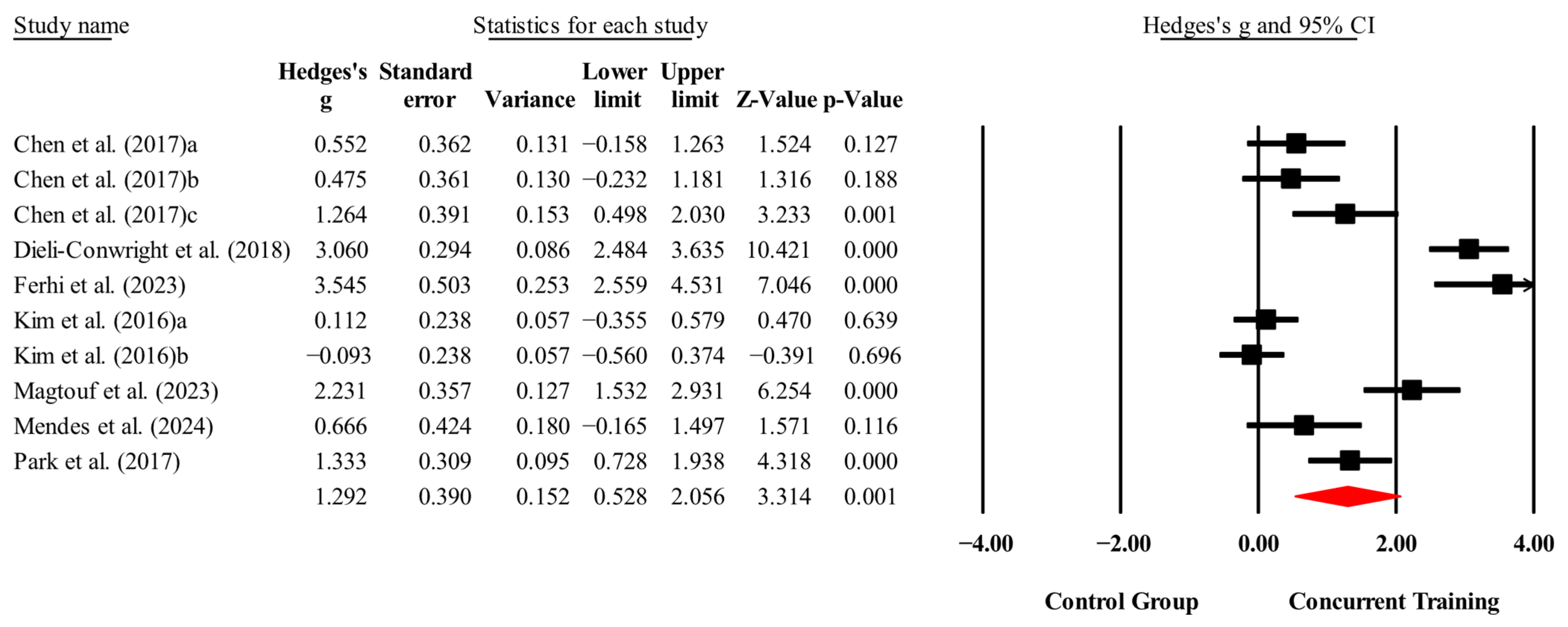
| Body fat (%) | 7,7,10, 460. | Random | 1.31 (0.53 to 2.09) | 0.001 | 92.9 | 0.000 | 8.36 to 20 |
| ASM/weight (%) | 2,2,4, 160. | Fixed | 0.42 (0.14 to 0.71) | 0.004 | 0.00 | 0.80 | 9.49 to 19.8 |
| Trunk fat (kg) | 2,2,3, 203. | Random | 0.76 (−0.25 to 1.78) | 0.14 | 92.8 | 0.000 | 2.38 to 4.13 |
| Body fat mass (kg) | 2,2,4, 160. | Random | 1.13 (−0.29 to 2.56) | 0.12 | 94.2 | 0.000 | 2.12 to 4.88 |
| Physical performance | |||||||
| Walking speed (m/s) | 2,2,3, 153. | Random | 1.80 (1.05 to 2.55) | 0.000 | 79 | 0.008 | 12.2 to 23.8 |
| MIHS (kg) | 5,5,8, 350. | Random | 0.50 (−0.42 to 1.43) | 0.29 | 93.5 | 0.000 | 2.22 to 10.2 |
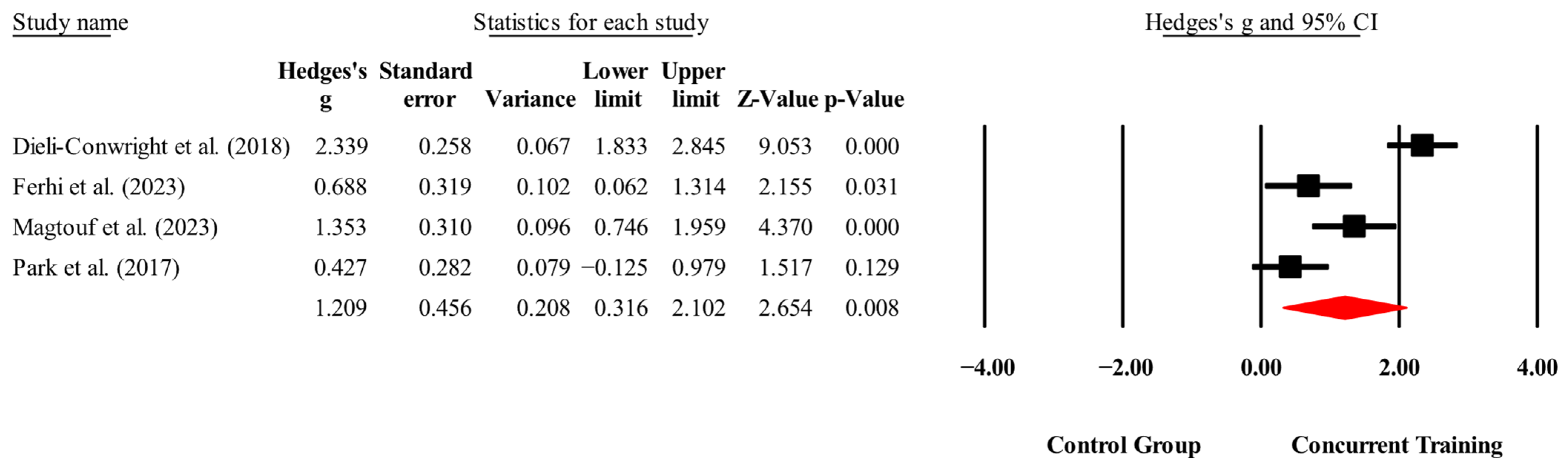
| Knee extension (kg) | 3,3,6, 231. | Random | 0.76 (0.09 to 1.42) | 0.02 | 85.8 | 0.000 | 6.57 to 15.3 |
| Biomarkers | |||||||
|---|---|---|---|---|---|---|---|
| Excluded Studies | DFBETA | Cook’s Distance | DFFITS | ES (95%CI) | p | I2 (%) | |
| IGF-1(ng/mL) | Chen, Chung, Chen, Ho, and Wu [22] | −2.34 | 0.95 | −2.07 | 1.35 (1.01 to 1.69) | <0.001 | 0 |
| IL-6 (pg/mL) | - | - | - | - | - | - | - |
| CRP (mg/L) | Dieli-Conwright, Courneya, Demark-Wahnefried, Sami, Lee, Buchanan, Spicer, Tripathy, Bernstein, and Mortimer [23] | 10.01 | 1.93 | 6.30 | −0.05 (−0.342 to 0.229) | 0.69 | 1.56 |
| Leptin (ng/mL) | Dieli-Conwright, Courneya, Demark-Wahnefried, Sami, Lee, Buchanan, Spicer, Tripathy, Bernstein, and Mortimer [23] | 15.56 | 3.56 | 6.65 | 0.16 (−0.143 to 0.469) | 0.29 | 0 |
| Total cholesterol (mg/dL) | Dieli-Conwright, Courneya, Demark-Wahnefried, Sami, Lee, Buchanan, Spicer, Tripathy, Bernstein, and Mortimer [23] | 2.38 | 0.90 | 2.38 | 0.02 (−0.537 to 0.540) | 0.99 | 71.7 |
| Triglycerides (mg/dL) | Dieli-Conwright, Courneya, Demark-Wahnefried, Sami, Lee, Buchanan, Spicer, Tripathy, Bernstein, and Mortimer [23] | 13.66 | 2.99 | 6.47 | −0.08 (−0.291 to 0.275) | 0.95 | 0 |
| Morphological variables | |||||||
| BMI (kg/m2) | Ferhi, Gaied Chortane, Durand, Beaune, Boyas, and Maktouf [24] | 0.97 | 0.33 | 0.87 | 0.41 (0.142 to 0.679) | 0.00 | 0 |
| Waist circumference (cm) | Dieli-Conwright, Courneya, Demark-Wahnefried, Sami, Lee, Buchanan, Spicer, Tripathy, Bernstein, and Mortimer [23] | 1.66 | 0.75 | 1.71 | 0.81 (0.268 to 1.359) | 0.00 | 60.4 |
| Body fat (%) | - | - | - | - | - | - | - |
| ASM/weight (%) | - | - | - | - | - | - | - |
| Trunk fat (kg) | - | - | - | - | - | - | - |
| Body fat mass (kg) | Dieli-Conwright, Courneya, Demark-Wahnefried, Sami, Lee, Buchanan, Spicer, Tripathy, Bernstein, and Mortimer [23] | 3.89 | 0.84 | 4.49 | 0.45 (0.043 to 0.862) | 0.03 | 0 |
| Physical performance | |||||||
| Walking speed (m/s) | - | - | - | - | - | - | - |
| MIHS (kg) | Park, Kwon, and Park [28] | 0.91 | 0.54 | 0.91 | 0.15 (−0.634 to 0.935) | 0.707 | 90.5 |
| Knee extension (kg) | Chen, Chung, Chen, Ho, and Wu [22] Vasconcelos, Dias, Araújo, Pinheiro, Moreira, and Dias [29] | 0.83 1.60 | 0.54 1.08 | 0.83 1.43 | 0.48 (0.194 to 0.769) | 0.001 | 4.39 |
| Covariate | Coefficient | 95% Cl | Z | p | R2 |
|---|---|---|---|---|---|
| Body Fat (%) (n = 10) | |||||
| Intercept | −3.14 | −26.8 to 20.5 | −0.26 | 0.79 | 0.61 |
| Weeks | 0.13 | 0.02 to 0.24 | 2.30 | 0.02 | 0.32 |
| Frequency of training | −0.06 | −1.00 to 0.88 | −0.13 | 0.89 | −0.13 |
| Minutes per session | 0.06 | −0.30 to 0.43 | 0.35 | 0.72 | −0.11 |
| Total, sessions | 0.01 | −0.01 to 0.03 | 0.83 | 0.40 | −0.02 |
| Certainty Assessment | Number of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Studies | Study Design | Risk of Bias | Inconsistency | Indirect evidence | Vagueness | Other Considerations | [Intervention] | [Comparison] | Relative (95% CI) | Absolute (95% CI) | ||
| Biomarkers | ||||||||||||
| 3 | Randomized trials | Serious to | It is not serious | It is not serious | It is not serious | None | 148/288 (51.4%) | 140/288 (48.6%) | Not estimable | Moderate | IMPORTANT | |
| Morphological variables | ||||||||||||
| 7 | Randomized trials | Serious to | It is not serious | It is not serious | It is not serious | None | 237/460 (51.5%) | 223/460 (48.5%) | Not estimable | Moderate | IMPORTANT | |
| Physical performance | ||||||||||||
| 5 | Randomized trials | Serious to | It is not serious | It is not serious | It is not serious | None | 178/350 (50.9%) | 172/350 (49.1%) | Not estimable | Moderate | IMPORTANT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Martinez, J.; Vásquez-Carrasco, E.; Cid-Calfucura, I.; Sandoval, C.; Herrera-Valenzuela, T.; Núñez-Espinosa, C.; Branco, B.H.M.; Valdés-Badilla, P. Effects of Concurrent Training on Biomarkers, Morphological Variables, and Physical Performance in People with Sarcopenic Obesity: A Meta-Analysis with Meta-Regression. Medicina 2025, 61, 1697. https://doi.org/10.3390/medicina61091697
Hernandez-Martinez J, Vásquez-Carrasco E, Cid-Calfucura I, Sandoval C, Herrera-Valenzuela T, Núñez-Espinosa C, Branco BHM, Valdés-Badilla P. Effects of Concurrent Training on Biomarkers, Morphological Variables, and Physical Performance in People with Sarcopenic Obesity: A Meta-Analysis with Meta-Regression. Medicina. 2025; 61(9):1697. https://doi.org/10.3390/medicina61091697
Chicago/Turabian StyleHernandez-Martinez, Jordan, Edgar Vásquez-Carrasco, Izham Cid-Calfucura, Cristian Sandoval, Tomás Herrera-Valenzuela, Cristian Núñez-Espinosa, Braulio Henrique Magnani Branco, and Pablo Valdés-Badilla. 2025. "Effects of Concurrent Training on Biomarkers, Morphological Variables, and Physical Performance in People with Sarcopenic Obesity: A Meta-Analysis with Meta-Regression" Medicina 61, no. 9: 1697. https://doi.org/10.3390/medicina61091697
APA StyleHernandez-Martinez, J., Vásquez-Carrasco, E., Cid-Calfucura, I., Sandoval, C., Herrera-Valenzuela, T., Núñez-Espinosa, C., Branco, B. H. M., & Valdés-Badilla, P. (2025). Effects of Concurrent Training on Biomarkers, Morphological Variables, and Physical Performance in People with Sarcopenic Obesity: A Meta-Analysis with Meta-Regression. Medicina, 61(9), 1697. https://doi.org/10.3390/medicina61091697












