Beyond Motor Decline in ALS: Patient-Centered Insights into Non-Motor Manifestations
Abstract
1. Introduction
2. Materials and Methods
2.1. Neurological Assessment
2.2. Evaluation of Non-Motor Symptoms
2.3. Neuropsychological Assessment
2.4. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics of the Study Participants
3.2. Description of Non-Motor Symptoms
3.3. Association Between Non-Motor Symptoms and Functional Status in ALS Patients Assessed by ALSFRS-R Scores and Disease Progression Rate
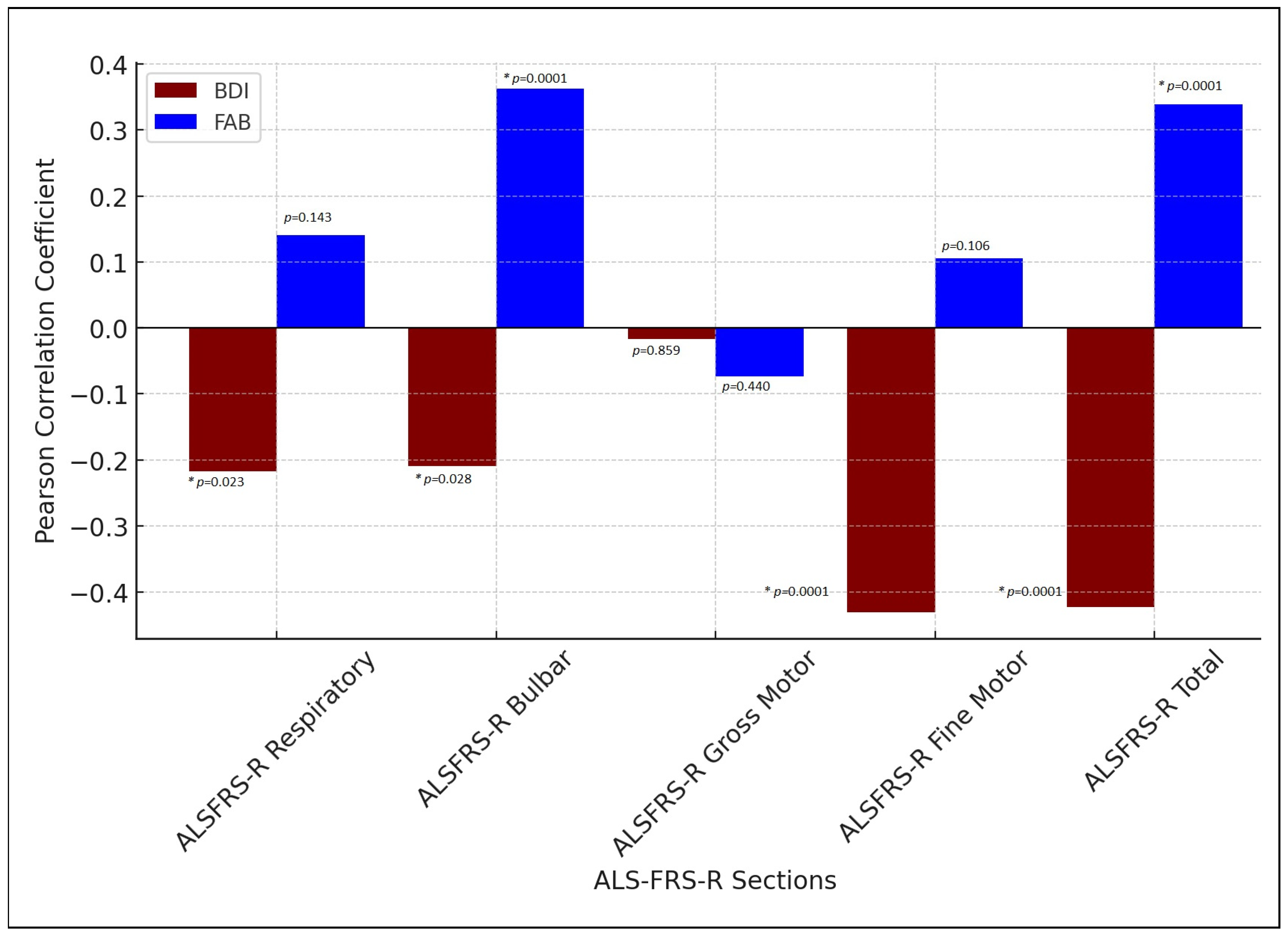
3.4. Correlation Between Affective Symptoms, Frontal Executive Function, and Functional Status in ALS Patients
4. Discussion
4.1. Autonomic Dysfunction
4.2. Sensory Dysfunction
4.3. Cognitive Impairment and Frontotemporal Dysfunction
4.4. Sleep Disturbances and Affective Symptoms
5. Conclusions
- There is a high prevalence of NMS in our ALS study patients, with the majority of them presenting with more than one non-motor symptom according to the survey results.
- The presence of NMS is not consistently reported by patients with greater functional impairment, as they do not uniformly correlate with lower ALS functional scores—somatosensory and urinary involvement may occur independently of the extent of motor disability.
- Affective, cognitive and behavioral changes co-occur with motor symptoms, and they are expected to be more prominent in patients with more severe motor impairment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 480–493. [Google Scholar] [CrossRef]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Fang, T.; Jozsa, F.; Al-Chalabi, A. Nonmotor Symptoms in Amyotrophic Lateral Sclerosis: A Systematic Review. Int. Rev. Neurobiol. 2017, 134, 1409–1441. [Google Scholar] [CrossRef]
- Piccione, E.A.; Sletten, D.M.; Staff, N.P.; Low, P.A. Autonomic system and amyotrophic lateral sclerosis. Muscle Nerve 2015, 51, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Caga, J.; Mahoney, C.J.; Kiernan, M.C.; Huynh, W. Behavioural changes predict poorer survival in amyotrophic lateral sclerosis. Brain Cogn. 2021, 150, 105710. [Google Scholar] [CrossRef]
- Rabkin, J.G.; Goetz, R.; Factor-Litvak, P.; Hupf, J.; McElhiney, M.; Singleton, J.; Mitsumoto, H.; Als Cosmos Study Group. Depression and wish to die in a multicenter cohort of ALS patients. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zheng, Z.; Guo, X.; Ou, R.; Chen, X.; Huang, R.; Yang, J.; Shang, H. Association between depression and survival in Chinese amyotrophic lateral sclerosis patients. Neurol. Sci. 2016, 37, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 69, 13–21. [Google Scholar] [CrossRef]
- Great Lakes ALS Study Group. A comparison of muscle strength testing techniques in amyotrophic lateral sclerosis. Neurology 2003, 61, 1503–1507. [Google Scholar] [CrossRef]
- Labra, J.; Menon, P.; Byth, K.; Morrison, S.; Vucic, S. Rate of disease progression: A prognostic biomarker in ALS. J. Neurol. Neurosurg. Psychiatry 2016, 87, 628–632. [Google Scholar] [CrossRef]
- Ravits, J.; Appel, S.; Baloh, R.H.; Barohn, R.; Brooks, B.R.; Elman, L.; Floeter, M.K.; Henderson, C.; Lomen-Hoerth, C.; Macklis, J.D.; et al. Deciphering amyotrophic lateral sclerosis: What phenotype, neuropathology and genetics are telling us about pathogenesis. Amyotroph. Lateral Scler. Front. Degener. 2013, 14 (Suppl. 1), 5–18. [Google Scholar] [CrossRef]
- Milella, G.; Zoccolella, S.; Urso, D.; Nigro, S.; Tamburrino, L.; Gnoni, V.; Filardi, M.; Logroscino, G. Different patterns of spreading direction and motor neurons involvement in a cohort of limb-onset amyotrophic lateral sclerosis patients from Southern Italy: Potential implication on disease course or progression? Brain Behav. 2023, 13, e2899. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Martinez-Martin, P.; Schapira, A.H.; Stocchi, F.; Sethi, K.; Odin, P.; Brown, R.G.; Koller, W.; Barone, P.; MacPhee, G.; et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov. Disord. 2006, 21, 916–923. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Martinez-Martin, P. Quantitation of non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15 (Suppl. 2), 2–7. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Naidu, Y. Early Parkinson’s disease and non-motor issues. J. Neurol. 2008, 255 (Suppl. 5), 33–38. [Google Scholar] [CrossRef]
- Ahn, S.W.; Kim, S.H.; Kim, J.E.; Kim, S.M.; Kim, S.H.; Sung, J.J.; Lee, K.W.; Hong, Y.H. Frontal assessment battery to evaluate frontal lobe dysfunction in ALS patients. Can. J. Neurol. Sci. 2011, 38, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Aiello, E.N.; Solca, F.; Torre, S.; Carelli, L.; Ferrucci, R.; Priori, A.; Verde, F.; Ticozzi, N.; Silani, V.; Poletti, B. Feasibility and diagnostics of the Frontal Assessment Battery (FAB) in amyotrophic lateral sclerosis. Neurol. Sci. 2023, 44, 587–592. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Heravi, F.S.; Naseri, K.; Hu, H. Gut Microbiota Composition in Patients with Neurodegenerative Disorders (Parkinson’s and Alzheimer’s) and Healthy Controls: A Systematic Review. Nutrients 2023, 15, 4365. [Google Scholar] [CrossRef]
- Boddy, S.L.; Giovannelli, I.; Sassani, M.; Cooper-Knock, J.; Snyder, M.P.; Segal, E.; Elinav, E.; Barker, L.A.; Shaw, P.J.; McDermott, C.J. The gut microbiome: A key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 2021, 19, 13. [Google Scholar] [CrossRef]
- Jin, M.; Günther, R.; Akgün, K.; Hermann, A.; Ziemssen, T. Peripheral proinflammatory Th1/Th17 immune cell shift is linked to disease severity in amyotrophic lateral sclerosis. Sci. Rep. 2020, 10, 5941. [Google Scholar] [CrossRef] [PubMed]
- Özaydin Aksun, Z.; Erdoğan, S.; Kalkanci, A.; Şahin, E.A.; Çuhadar, T.; Şener, H.Ö. Is gut microbiota of patients with ALS different from that of healthy individuals? Turk. J. Med. Sci. 2024, 54, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhuang, Z.; Zhang, G.; Huang, T.; Fan, D. Assessment of bidirectional relationships between 98 genera of the human gut microbiota and amyotrophic lateral sclerosis: A 2-sample Mendelian randomization study. BMC Neurol. 2022, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Xin, C.; Huo, J.; Liu, Q.; Dong, H.; Li, R.; Liu, Y. Neuroprotective Effect of a Multistrain Probiotic Mixture in SOD1G93A Mice by Reducing SOD1 Aggregation and Targeting the Microbiota-Gut-Brain Axis. Mol. Neurobiol. 2024, 61, 10051–10071. [Google Scholar] [CrossRef]
- Dubbioso, R.; Provitera, V.; Pacella, D.; Santoro, L.; Manganelli, F.; Nolano, M. Autonomic dysfunction is associated with disease progression and survival in amyotrophic lateral sclerosis: A prospective longitudinal cohort study. J. Neurol. 2023, 270, 4968–4977. [Google Scholar] [CrossRef]
- Ribichini, E.; Pallotta, N.; Badiali, D.; Carlucci, M.; Ceccanti, M.; Cambieri, C.; Libonati, L.; Corazziari, E.S.; Ruoppolo, G.; Inghilleri, M. Assessment of upper GI motor activity and GI symptoms in patients with amyotrophic lateral sclerosis: An observational study. Front. Neurol. 2025, 15, 1509917. [Google Scholar] [CrossRef]
- Niu, T.; Wang, P.; Zhou, X.; Liu, T.; Liu, Q.; Li, R.; Yang, H.; Dong, H.; Liu, Y. An overlap-weighted analysis on the association of constipation symptoms with disease progression and survival in amyotrophic lateral sclerosis: A nested case-control study. Ther. Adv. Neurol. Disord. 2025, 18, 17562864241309811. [Google Scholar] [CrossRef]
- Arlandis, S.; Vázquez-Costa, J.F.; Martínez-Cuenca, E.; Sevilla, T.; Boronat, F.; Broseta, E. Urodynamic findings in amyotrophic lateral sclerosis patients with lower urinary tract symptoms: Results from a pilot study. Neurourol. Urodyn. 2017, 36, 626–631. [Google Scholar] [CrossRef]
- Nakamura, M.; Nakayama, K.; Murakami, A.; Morise, S.; Kaneko, S.; Kusaka, H.; Yakushiji, Y. Early presentation of lower urinary tract and bowel dysfunction in sporadic amyotrophic lateral sclerosis: A case report. eNeurologicalSci 2022, 28, 100413. [Google Scholar] [CrossRef]
- Lopes de Carvalho, M.L.; Motta, R.; Battaglia, M.A.; Brichetto, G. Urinary disorders in amyotrophic lateral sclerosis subjects. Amyotroph. Lateral Scler. 2011, 12, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Costa, J.F.; Arlandis, S.; Hervas, D.; Martínez-Cuenca, E.; Cardona, F.; Pérez-Tur, J.; Broseta, E.; Sevilla, T. Clinical profile of motor neuron disease patients with lower urinary tract symptoms and neurogenic bladder. J. Neurol. Sci. 2017, 378, 130–136. [Google Scholar] [CrossRef]
- Hirayama, T.; Shibukawa, M.; Yanagihashi, M.; Warita, H.; Atsuta, N.; Yamanaka, K.; Kano, O. Investigation of non-motor symptoms in patients with amyotrophic lateral sclerosis. Acta Neurol. Belg. 2023, 123, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.; Holzberg, S.; Thirunavukkarasu, S.; Ciani, G. Perceptions of sexuality in individuals with Amyotrophic Lateral Sclerosis (ALS) and their treating clinicians. NeuroRehabilitation 2017, 41, 331–342. [Google Scholar] [CrossRef]
- Pavlovic, S.; Stevic, Z.; Milovanovic, B.; Milicic, B.; Rakocevic-Stojanovic, V.; Lavrnic, D.; Apostolski, S. Impairment of cardiac autonomic control in patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010, 11, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.J.; Potter, J.F. Cardiovascular causes of falls. Age Ageing 2001, 30 (Suppl. 4), 19–24. [Google Scholar] [CrossRef]
- Oey, P.L.; Vos, P.E.; Wieneke, G.H.; Wokke, J.H.; Blankestijn, P.J.; Karemaker, J.M. Subtle involvement of the sympathetic nervous system in amyotrophic lateral sclerosis. Muscle Nerve 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Merico, A.; Cavinato, M. Autonomic dysfunction in the early stage of ALS with bulbar involvement. Amyotroph. Lateral Scler. 2011, 12, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.C.; Moraes, Í.A.P.; Vidigal, G.P.; Simcsik, A.O.; Rosa, R.M.; Favero, F.M.; Fernandes, S.M.S.; Garner, D.M.; Araújo, L.V.; Massa, M.; et al. Cardiac Autonomic Modulation in Subjects with Amyotrophic Lateral Sclerosis (ALS) during an Upper Limb Virtual Reality Task: A Prospective Control Trial. Biomed. Res. Int. 2022, 2022, 4439681. [Google Scholar] [CrossRef]
- Kim, M.J.; Farrell, J. Orthostatic Hypotension: A Practical Approach. Am. Fam. Physician 2022, 106, 365. [Google Scholar]
- Pimentel, R.M.M.; Macedo, H., Jr.; Valenti, V.E.; Rocha, F.O.; Abreu, L.C.; de MMonteiro, C.B.; Ferreira, C. Decreased Heart Rate Variability in Individuals with Amyotrophic Lateral Sclerosis. Respir. Care 2019, 64, 1088–1095. [Google Scholar] [CrossRef]
- Kleinerova, J.; Chipika, R.H.; Tan, E.L.; Yunusova, Y.; Marchand-Pauvert, V.; Kassubek, J.; Pradat, P.F.; Bede, P. Sensory Dysfunction in ALS and Other Motor Neuron Diseases: Clinical Relevance, Histopathology, Neurophysiology, and Insights from Neuroimaging. Biomedicines 2025, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- Riancho, J.; Paz-Fajardo, L.; López de Munaín, A. Clinical and preclinical evidence of somatosensory involvement in amyotrophic lateral sclerosis. Br. J. Pharmacol. 2021, 178, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Silva, A.; Glass, J.; Sladky, J.T.; Benatar, M. Clinical, electrophysiologic, and pathologic evidence for sensory abnormalities in ALS. Neurology 2007, 69, 2236–2242. [Google Scholar] [CrossRef]
- Theys, P.A.; Peeters, E.; Robberecht, W. Evolution of motor and sensory deficits in amyotrophic lateral sclerosis estimated by neurophysiological techniques. J. Neurol. 1999, 246, 438–442. [Google Scholar] [CrossRef]
- Pugdahl, K.; Fuglsang-Frederiksen, A.; de Carvalho, M.; Johnsen, B.; Fawcett, P.R.; Labarre-Vila, A.; Liguori, R.; Nix, W.A.; Schofield, I.S. Generalised sensory system abnormalities in amyotrophic lateral sclerosis: A European multicentre study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 746–749. [Google Scholar] [CrossRef]
- Nolano, M.; Provitera, V.; Manganelli, F.; Iodice, R.; Caporaso, G.; Stancanelli, A.; Marinou, K.; Lanzillo, B.; Santoro, L.; Mora, G. Non-motor involvement in amyotrophic lateral sclerosis: New insight from nerve and vessel analysis in skin biopsy. Neuropathol. Appl. Neurobiol. 2017, 43, 119–132. [Google Scholar] [CrossRef]
- Isak, B.; Tankisi, H.; Johnsen, B.; Pugdahl, K.; Torvin MØLler, A.; Finnerup, N.B.; Christensen, P.B.; Fuglsang-Frederiksen, A. Involvement of distal sensory nerves in amyotrophic lateral sclerosis. Muscle Nerve 2016, 54, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Heads, T.; Pollock, M.; Robertson, A.; Sutherland, W.H.; Allpress, S. Sensory nerve pathology in amyotrophic lateral sclerosis. Acta Neuropathol. 1991, 82, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.K.; Kemp, Z.; Hatzipetros, T.; Vieira, F.; Valdez, G. Degeneration of proprioceptive sensory nerve endings in mice harboring amyotrophic lateral sclerosis-causing mutations. J. Comp. Neurol. 2015, 523, 2477–2494. [Google Scholar] [CrossRef]
- Ruiz-Soto, M.; Riancho, J.; Tapia, O.; Lafarga, M.; Berciano, M.T. Satellite Glial Cells of the Dorsal Root Ganglion: A New “Guest/Physiopathological Target” in ALS. Front. Aging Neurosci. 2020, 12, 595751. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, W.; Li, Y.; Sun, B.; Li, Y.; Yang, F.; Wang, H.; Li, M.; Cui, F.; Huang, X. Cutaneous somatic and autonomic nerve TDP-43 deposition in amyotrophic lateral sclerosis. J. Neurol. 2018, 265, 1753–1763. [Google Scholar] [CrossRef]
- Vaughan, S.K.; Sutherland, N.M.; Zhang, S.; Hatzipetros, T.; Vieira, F.; Valdez, G. The ALS-inducing factors, TDP43A315T and SOD1G93A, directly affect and sensitize sensory neurons to stress. Sci. Rep. 2018, 8, 16582. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Liu, M.Q.; Ma, L. Gray Matter Volume Changes over the Whole Brain in the Bulbar- and Spinal-onset Amyotrophic Lateral Sclerosis: A Voxel-based Morphometry Study. Chin. Med. Sci. J. 2018, 33, 20–28. [Google Scholar] [CrossRef]
- Rasoanandrianina, H.; Grapperon, A.M.; Taso, M.; Girard, O.M.; Duhamel, G.; Guye, M.; Ranjeva, J.P.; Attarian, S.; Verschueren, A.; Callot, V. Region-specific impairment of the cervical spinal cord (SC) in amyotrophic lateral sclerosis: A preliminary study using SC templates and quantitative MRI (diffusion tensor imaging/inhomogeneous magnetization transfer). NMR Biomed. 2017, 30, e3801. [Google Scholar] [CrossRef]
- Buhour, M.S.; Doidy, F.; Mondou, A.; Pélerin, A.; Carluer, L.; Eustache, F.; Viader, F.; Desgranges, B. Voxel-based mapping of grey matter volume and glucose metabolism profiles in amyotrophic lateral sclerosis. EJNMMI Res. 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Olney, N.T.; Bischof, A.; Rosen, H.; Caverzasi, E.; Stern, W.A.; Lomen-Hoerth, C.; Miller, B.L.; Henry, R.G.; Papinutto, N. Measurement of spinal cord atrophy using phase sensitive inversion recovery (PSIR) imaging in motor neuron disease. PLoS ONE 2018, 13, e0208255. [Google Scholar] [CrossRef] [PubMed]
- McAlary, L.; Yerbury, J.J.; Cashman, N.R. The prion-like nature of amyotrophic lateral sclerosis. Prog. Mol. Biol. Transl. Sci. 2020, 175, 261–296. [Google Scholar] [CrossRef]
- Cavallo, M.; Adenzato, M.; Macpherson, S.E.; Karwig, G.; Enrici, I.; Abrahams, S. Evidence of social understanding impairment in patients with amyotrophic lateral sclerosis. PLoS ONE 2011, 6, e25948. [Google Scholar] [CrossRef]
- Yang, W.; Sopper, M.M.; Leystra-Lantz, C.; Strong, M.J. Microtubule-associated tau protein positive neuronal and glial inclusions in ALS. Neurology 2003, 61, 1766–1773. [Google Scholar] [CrossRef]
- Root, J.; Merino, P.; Nuckols, A.; Johnson, M.; Kukar, T. Lysosome dysfunction as a cause of neurodegenerative diseases: Lessons from frontotemporal dementia and amyotrophic lateral sclerosis. Neurobiol. Dis. 2021, 154, 105360. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.C.; Polymenidou, M.; Cleveland, D.W. Converging mechanisms in ALS and FTD: Disrupted RNA and protein homeostasis. Neuron 2013, 79, 416–438. [Google Scholar] [CrossRef]
- Strong, M.J.; Abrahams, S.; Goldstein, L.H.; Woolley, S.; Mclaughlin, P.; Snowden, J.; Mioshi, E.; Roberts-South, A.; Benatar, M.; HortobáGyi, T.; et al. Amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 153–174. [Google Scholar] [CrossRef]
- Barulli, M.R.; Fontana, A.; Panza, F.; Copetti, M.; Bruno, S.; Tursi, M.; Iurillo, A.; Tortelli, R.; Capozzo, R.; Simone, I.L.; et al. Frontal assessment battery for detecting executive dysfunction in amyotrophic lateral sclerosis without dementia: A retrospective observational study. BMJ Open 2015, 5, e007069. [Google Scholar] [CrossRef]
- Goldstein, L.H.; Abrahams, S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: Nature of impairment and implications for assessment. Lancet Neurol. 2013, 12, 368–380. [Google Scholar] [CrossRef]
- Ringholz, G.M.; Appel, S.H.; Bradshaw, M.; Cooke, N.A.; Mosnik, D.M.; Schulz, P.E. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 2005, 65, 586–590. [Google Scholar] [CrossRef]
- Moțățăianu, A.; Ormenișan, I.; Bălașa, R. Apathy as Non-Motor Manifestation in Amyotrophic Lateral Sclerosis. Acta Marisiensis Ser. Medica 2021, 68, 3–5. [Google Scholar] [CrossRef]
- Chiò, A.; Moglia, C.; Canosa, A.; Manera, U.; Vasta, R.; Brunetti, M.; Barberis, M.; Corrado, L.; D’Alfonso, S.; Bersano, E.; et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology 2019, 93, e984–e994. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hou, Y.; Li, C.; Cao, B.; Cheng, Y.; Wei, Q.; Zhang, L.; Shang, H. Risk factors for cognitive impairment in amyotrophic lateral sclerosis: A systematic review and meta-analysis. Neurol. Neurosurg. Psychiatry 2021, 92, 688–693. [Google Scholar] [CrossRef]
- Benbrika, S.; Doidy, F.; Carluer, L.; Mondou, A.; Pélerin, A.; Eustache, F.; Viader, F.; Desgranges, B. Longitudinal Study of Cognitive and Emotional Alterations in Amyotrophic Lateral Sclerosis: Clinical and Imaging Data. Front. Neurol. 2021, 12, 620198. [Google Scholar] [CrossRef]
- Elamin, M.; Phukan, J.; Bede, P.; Jordan, N.; Byrne, S.; Pender, N.; Hardiman, O. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology 2011, 76, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.M.; Videnovic, A. Chronic sleep disturbance and neural injury: Links to neurodegenerative disease. Nat. Sci. Sleep. 2016, 8, 55–61. [Google Scholar] [CrossRef]
- Diaz-Abad, M.; Buczyner, J.R.; Venza, B.R.; Scharf, S.M.; Kwan, J.Y.; Lubinski, B.; Russell, J.W. Poor Sleep Quality in Patients with Amyotrophic Lateral Sclerosis at the Time of Diagnosis. J. Clin. Neuromuscul. Dis. 2018, 20, 60–68. [Google Scholar] [CrossRef]
- Lucia, D.; McCombe, P.A.; Henderson, R.D.; Ngo, S.T. Disorders of sleep and wakefulness in amyotrophic lateral sclerosis (ALS): A systematic review. Amyotroph Lateral Scler. Front. Degener. 2021, 22, 161–169. [Google Scholar] [CrossRef]
- Yang, T.; Pang, D.; Huang, J.; Xiao, Y.; Li, C.; Wei, Q.; Ou, R.; Cheng, Y.; Lin, J.; Che, N.; et al. Association between sleep and ALS-FTSD: A Prospective Cohort Study based on 396,918 UK biobank participants. Transl. Psychiatry 2025, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- Lo Coco, D.; Puligheddu, M.; Mattaliano, P.; Congiu, P.; Borghero, G.; Fantini, M.L.; La Bella, V.; Ferri, R. REM sleep behavior disorder and periodic leg movements during sleep in ALS. Acta Neurol. Scan 2017, 135, 219–224. [Google Scholar] [CrossRef]
- Gnoni, V.; Zoccolella, S.; Giugno, A.; Urso, D.; Tamburrino, L.; Filardi, M.; Logroscino, G. Hypothalamus and amyotrophic lateral sclerosis: Potential implications in sleep disorders. Front. Aging Neurosci. 2023, 15, 1193483. [Google Scholar] [CrossRef]
- Anghel, L.; Ciubară, A.; Nechita, A.; Nechita, L.; Manole, C.; Baroiu, L.; Ciubară, A.B.; Mușat, C.L. Sleep Disorders Associated with Neurodegenerative Diseases. Diagnostics 2023, 13, 2898. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Q.; Niu, T.; Jia, H.; Liu, T.; Xin, Z.; Li, Z.; Zhou, X.; Li, R.; Liu, Y.; et al. Sleep Disturbances as a Potential Risk Factor for Deterioration of Respiratory Function in Patients with Amyotrophic Lateral Sclerosis. Ann. Indian Acad. Neurol. 2023, 26, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Silva, J.; Salgueira, S.; Mendes, A.; Matos, E.; Conde, B. Sleep Disturbances in Amyotrophic Lateral Sclerosis and Prognostic Impact-A Retrospective Study. Life 2024, 14, 1284. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.E.; Nadali, J.; Parouhan, A.; Azarafraz, M.; Tabatabai, S.M.; Irvani, S.S.N.; Eskandari, F.; Gharebaghi, A. Prevalence of depression among amyotrophic lateral sclerosis (ALS) patients: A systematic review and meta-analysis. J. Affect. Disord. 2021, 287, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Anca, M.; Sebastian, A.; Cristina, R.; Zoltan, B.; Laura, B.; Adrian, B.; Septimiu, V.; Adina, S.; Smaranda, M. Predictors of Depression in Caucasian Patients with Amyotrophic Lateral Sclerosis in Romania. Brain Sci. 2020, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- McElhiney, M.C.; Rabkin, J.G.; Gordon, P.H.; Goetz, R.; Mitsumoto, H. Prevalence of fatigue and depression in ALS patients and change over time. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1146–1149. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134 Pt 9, 2456–2477. [Google Scholar] [CrossRef]
- Rabinowitz, J.A.; Ellis, J.D.; Strickland, J.C.; Hochheimer, M.; Zhou, Y.; Young, A.S.; Curtis, B.; Huhn, A.S. Patterns of demoralization and anhedonia during early substance use disorder treatment and associations with treatment attrition. J. Affect. Disord. 2023, 335, 248–255. [Google Scholar] [CrossRef]
- Roos, E.; Mariosa, D.; Ingre, C.; Lundholm, C.; Wirdefeldt, K.; Roos, P.M.; Fang, F. Depression in amyotrophic lateral sclerosis. Neurology 2016, 86, 2271–2277. [Google Scholar] [CrossRef]
- Jaafar, N.; Malek, E.; Ismail, H.; Salameh, J. Nonmotor Symptoms in Amyotrophic Lateral Sclerosis and Their Correlation with Disease Progression. J. Clin. Neuromuscul. Dis. 2021, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
| Patients (N = 44) | HC (N = 35) | p | |
|---|---|---|---|
| Age (mean ± SD) (years) o | 58.39 (±12.52) | 53.11 (±9.84) | 0.044 |
| Sex ratio (M:F) | 28:16 | 23:12 | 0.847 |
| BDI (mean ± SD) o | 10.75 (±8.59) | 5.49 (±3.04) | <0.001 |
| FAB (mean ± SD) o | 13.45 (±4.69) | 17.46 (±1.01) | <0.001 |
| ALSFRS-R scores o | |||
| Respiratory subscore (±SD) | 11.4 (±0.9) | NA | NA |
| Bulbar subscore (±SD) | 10.1 (±2.4) | NA | NA |
| Fine Motor subscore (±SD) | 8.8 (±2.7) | NA | NA |
| Gross Motor subscore (±SD) | 6.3 (±3.4) | NA | NA |
| Total score | 37.6 (±6.3) | NA | NA |
| ALS subtype ∞ | |||
| Bulbar | 9 (20.5%) | NA | NA |
| Spinal | 35 (79.6%) | NA | NA |
| ALS phenotype ∞ | |||
| Classic | 25 (56.8%) | NA | NA |
| LMN | 12 (27.3%) | NA | NA |
| Bulbar | 7 (15.9%) | NA | NA |
| Progression pattern ∞ | |||
| Horizontal | 23 (52.3%) | NA | NA |
| Vertical | 21 (47.7%) | NA | NA |
| ΔPR (range) | 0.5 (0.3–1.3) | NA | NA |
| Time from onset to diagnosis (months) * | 9.5 (5.75–24.25) * | NA | NA |
| N = 44 | N (%) * |
|---|---|
| Cognitive changes | 7 (15.9%) |
| memory impairment | 4.5% |
| attention/concentration deficit | 13.6% |
| Psychiatric symptoms | 28 (63.3%) |
| apathy | 18.2% |
| sadness | 56.8% |
| anxiety | 47.7% |
| perceptual problems | 2.3% |
| hallucinations | 0% |
| Sleep disorders/fatigue | 35 (79.6%) |
| tiredness | 2.3% |
| insomnia | 72.7% |
| vivid dreams | 65.9% |
| sleep-talking | 11.4% |
| Sensory symptoms | 37 (84%) |
| pain | 61.4% |
| discomfort in legs | 77.3% |
| swollen legs | 20.5% |
| Cardiovascular symptoms | 30 (68.2%) |
| dizziness/light-headedness | 68.2% |
| Falls | 50% |
| Urinary symptoms | 16 (36.4%) |
| urinary urgency | 22.7% |
| nocturia | 31.8% |
| Sexual symptoms | 27 (61.4%) |
| interest in sex | 56.8% |
| sexual dysfunction | 56.8% |
| Gastrointestinal symptoms | 36 (81.8%) |
| dribbling | 31.8% |
| difficulty swallowing | 34.1% |
| vomiting/nausea | 22.7% |
| constipation | 75% |
| bowel incontinence | 18.2% |
| incomplete bowel emptying | 29.5% |
| Miscellaneous | 31 (70.5%) |
| loss of/change in taste/smell | 25% |
| weight change | 31.8% |
| excessive sweating | 13.6% |
| double vision | 0% |
| Mean (±SD) | Mean (±SD) | p * | |
|---|---|---|---|
| Cognitive changes | |||
| YES | NO | ||
| ALSFRS-R Respiratory | 11.6 (±0.5) | 11.4 (±0.9) | 0.618 |
| ALSFRS-R Bulbar | 8.6 (±2.4) | 10.4 (±2.3) | 0.062 |
| ALSFRS-R Gross Motor | 6.6 (±4.0) | 6.2 (±3.4) | 0.819 |
| ALSFRS-R Fine Motor | 8.9 (±3.3) | 8.7 (±2.6) | 0.910 |
| ALSFRS-R Total | 36.6 (±6.2) | 37.8 (±6.4) | 0.647 |
| ΔPR | 1.0 (±1.9) | 0.9 (±0.9) | 0.753 |
| Time from onset to diagnosis | 29.6 (±20.0) | 13.5 (±12.0) | 0.006 |
| Psychiatric symptoms | |||
| YES | NO | ||
| ALSFRS-R Respiratory | 11.5 (±0.9) | 11.2 (±0.9) | 0.233 |
| ALSFRS-R Bulbar | 9.8 (±2.5) | 10.6 (±2.2) | 0.322 |
| ALSFRS-R Gross Motor | 6.6 (±3.7) | 5.8 (±3.0) | 0.431 |
| ALSFRS-R Fine Motor | 9.1 (±2.6) | 8.1 (±2.8) | 0.201 |
| ALSFRS-R Total | 38.2 (±6.3) | 36.5 (±6.5) | 0.393 |
| ΔPR | 0.9 (±1.1) | 1.0 (±0.9) | 0.607 |
| Time from onset to diagnosis | 18.7 (±15.8) | 11.5 (±11.2) | 0.117 |
| Sleep disorders | |||
| YES | NO | ||
| ALSFRS-R Respiratory | 11.3 (±0.9) | 12 (±0.0) | 0.030 |
| ALSFRS-R Bulbar | 9.7 (±2.5) | 11.4 (±0.9) | 0.053 |
| ALSFRS-R Gross Motor | 6.4 (±3.6) | 5.9 (±3.1) | 0.695 |
| ALSFRS-R Fine Motor | 8.3 (±2.7) | 10.7 (±1.4) | 0.014 |
| ALSFRS-R Total | 36.3 (±6.4) | 42.4 (±2.9) | 0.008 |
| ΔPR | 1.0 (±1.1) | 0.6 (±0.6) | 0.317 |
| Time from onset to diagnosis | 16.54 (±14.8) | 14.22 (±14.4) | 0.675 |
| Sensory symptoms | |||
| YES | NO | ||
| ALSFRS-R Respiratory | 11.3 (±1.0) | 11.9 (±0.4) | 0.164 |
| ALSFRS-R Bulbar | 10.0 (±2.5) | 10.9 (±1.6) | 0.355 |
| ALSFRS-R Gross Motor | 6.8 (±3.4) | 3.9 (±2.2) | 0.039 |
| ALSFRS-R Fine Motor | 8.5 (±2.8) | 10.0 (±1.5) | 0.181 |
| ALSFRS-R Total | 36.8 (±6.2) | 41.6 (±5.5) | 0.069 |
| ΔPR | 1.0 (±1.1) | 0.4 (±0.2) | 0.154 |
| Time from onset to diagnosis | 17.3 (±14.9) | 9.6 (±10.9) | 0.202 |
| Cardiovascular symptoms | |||
| YES | NO | ||
| ALSFRS-R Respiratory | 11.2 (±1.0) | 11.8 (±0.4) | 0.064 |
| ALSFRS-R Bulbar | 9.7 (±2.6) | 10.9 (±1.7) | 0.109 |
| ALSFRS-R Gross Motor | 5.8 (±3.6) | 7.3 (±2.9) | 0.194 |
| ALSFRS-R Fine Motor | 8.3 (±2.6) | 9.7 (±2.7) | 0.103 |
| ALSFRS-R Total | 36.2 (±5.8) | 40.6 (±6.4) | 0.027 |
| ΔPR | 0.9 (±0.9) | 0.9 (±1.3) | 0.817 |
| Time from onset to diagnosis | 18.9 (±15.2) | 9.9 (±11.5) | 0.055 |
| Urinary symptoms | |||
| YES | NO | ||
| ALSFRS-R Respiratory | 11.7 (±0.6) | 11.3 (±1.0) | 0.132 |
| ALSFRS-R Bulbar | 11.2 (±1.3) | 9.4 (±2.6) | 0.012 |
| ALSFRS-R Gross Motor | 5.6 (±3.2) | 6.8 (±3.5) | 0.289 |
| ALSFRS-R Fine Motor | 8.8 (±2.2) | 8.8 (±2.9) | 0.908 |
| ALSFRS-R Total | 38.8 (±4.9) | 36.9 (±7.0) | 0.338 |
| ΔPR | 0.5 (±0.5) | 1.2 (±1.2) | 0.045 |
| Time from onset to diagnosis | 19.1 (±14.7) | 14.4 (±14.5) | 0.309 |
| Sexual dysfunction | |||
| YES | NO | ||
| ALSFRS-R Respiratory | 11.5 (±0.8) | 11.3 (±1.1) | 0.518 |
| ALSFRS-R Bulbar | 9.8 (±2.6) | 10.6 (±1.8) | 0.273 |
| ALSFRS-R Gross Motor | 6.6 (±3.3) | 5.8 (±3.6) | 0.475 |
| ALSFRS-R Fine Motor | 8.8 (±2.7) | 8.7 (±2.8) | 0.932 |
| ALSFRS-R Total | 37.3 (±6.2) | 38.5 (±6.7) | 0.702 |
| ΔPR | 1.0 (±1.2) | 0.8 (±0.7) | 0.501 |
| Time from onset to diagnosis | 18.7 (±15.7) | 11.9 (±11.8) | 0.138 |
| Digestive symptoms | |||
| YES | NO | ||
| ALSFRS-R Respiratory | 11.3 (±0.9) | 12.0 (±0.0) | 0.044 |
| ALSFRS-R Bulbar | 9.7 (±2.5) | 11.8 (±0.7) | 0.026 |
| ALSFRS-R Gross Motor | 6.4 (±3.5) | 5.6 (±3.1) | 0.547 |
| ALSFRS-R Fine Motor | 8.4 (±2.8) | 10.1 (±1.8) | 0.109 |
| ALSFRS-R Total | 36.5 (±6.4) | 42.5 (±3.2) | 0.013 |
| ΔPR | 0.9 (±1.1) | 0.6 (±0.8) | 0.341 |
| Time from onset to diagnosis | 17.1 (±14.4) | 11.6 (15.5) | 0.347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moțățăianu, A.; Andone, S.; Maier, S.; Chinezu, R.; Roman, M.; Dumitreasă, M.; Bălașa, R.; Ormenișan, I. Beyond Motor Decline in ALS: Patient-Centered Insights into Non-Motor Manifestations. Medicina 2025, 61, 1694. https://doi.org/10.3390/medicina61091694
Moțățăianu A, Andone S, Maier S, Chinezu R, Roman M, Dumitreasă M, Bălașa R, Ormenișan I. Beyond Motor Decline in ALS: Patient-Centered Insights into Non-Motor Manifestations. Medicina. 2025; 61(9):1694. https://doi.org/10.3390/medicina61091694
Chicago/Turabian StyleMoțățăianu, Anca, Sebastian Andone, Smaranda Maier, Rareș Chinezu, Medeea Roman, Mihai Dumitreasă, Rodica Bălașa, and Ioana Ormenișan. 2025. "Beyond Motor Decline in ALS: Patient-Centered Insights into Non-Motor Manifestations" Medicina 61, no. 9: 1694. https://doi.org/10.3390/medicina61091694
APA StyleMoțățăianu, A., Andone, S., Maier, S., Chinezu, R., Roman, M., Dumitreasă, M., Bălașa, R., & Ormenișan, I. (2025). Beyond Motor Decline in ALS: Patient-Centered Insights into Non-Motor Manifestations. Medicina, 61(9), 1694. https://doi.org/10.3390/medicina61091694






