Non-Invasive Brain Stimulation for Amyotrophic Lateral Sclerosis: Current Evidence and Future Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Process and Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Data Collection Process and Items Selected
3. Pathophysiological Rationale for NIBS in ALS
3.1. Cortical Excitability Impairment
3.2. Microglial Activation and Inflammatory Pathways
3.3. Alteration of Neurotrophic Pathways
3.4. Neuromodulatory Network for Neurological Diseases
4. Overview of NIBS Modalities
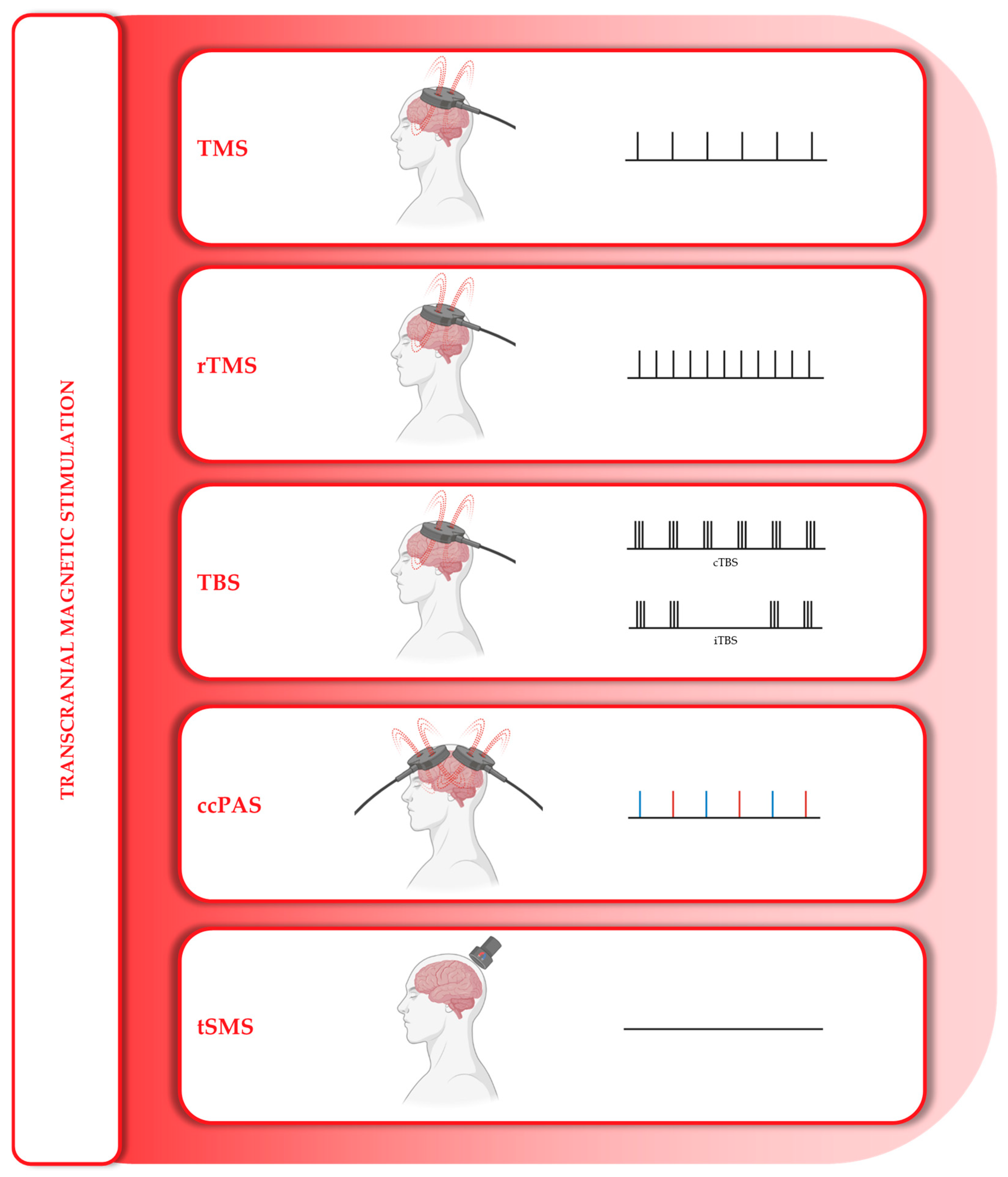
4.1. Magnetic Stimulation
4.2. Electrical Stimulation
4.3. Ultrasonic Stimulation
5. Clinical Evidence
| Technique | Protocol | Stimulation Site | Dosage | Sample | Assessments | Main Findings | |
|---|---|---|---|---|---|---|---|
| Magnetic Stimulation | |||||||
| Zheng W. et al. (2025) [122] | rTMS | 5 d/w, 4 w | DLPFC | 10 Hz | 80 ALS (c) | ALSFRS-R; ECAS; NfL; Zarit score | Significant short-term cognitive improvement and caregiver burden |
| Di Lazzaro V. et al. (2024) [92] | tSMS | 3 times daily, 3 + 6 m | Motor cortex | 0.12–0.20 T, 20 min × 2 | 40 ALS (c) | ALSFRS-R; MEP | Significantly improved tracheostomy-free survival (at 18 months) |
| Di Lazzaro V. et al. (2014) [129] | cTBS | 5 d/m, 5/10/12 m | Motor cortex (b) | 50 Hz | 3 ALS | ALSFRS-R | Slowing disease progression |
| Munneke M. et al. (2013) [121] | cTBS | 5 d | Motor cortex | 50 Hz | 10 ALS + 10 h | CMAP; RMT; SICI; ICF; EEG | Reduction in cortical excitability (MEP, RMT), which is not maintained at follow-up |
| Di Lazzaro V. et al. (2010) [120] | cTBS | 5 d/m, 26 m | Motor cortex (b) | 50 Hz | 1 ALS | ALSFRS-R; MEP; CMCT | Slowing disease progression |
| Di Lazzaro V. et al. (2009) [72] | cTBS | 5 d/m, 12 m | Motor cortex (b) | 50 Hz | 20 ALS (c) | ALSFRS-R; MRC; BDNF | No significant changes |
| Zanette G. et al. (2008) [119] | rTMS | 5 d/w, 2 w | Motor cortex (b) | 5 Hz | 10 ALS (c) | ALSFRS-R; MRC; FSS; SF-36; MVIC; Dynamometer (AP, ROM, ER) | Increased grip strength and an improvement in SF-36, which are not maintained at follow-up (2 weeks) |
| Di Lazzaro V. et al. (2006) [118] | cTBS | 5 d/m, 6 m | Motor cortex (b) | 50 Hz | 20 ALS (c) | ALSFRS-R; MRC; MVIC; BDNF | Slowing disease progression |
| Di Lazzaro V. et al. (2004) [117] | rTMS | 10 d/2 w every 4 m; 25/30 m | Motor cortex (b) | 1 Hz | 2 ALS | MRC; Norris | Slowing disease progression |
| 8 d/m; 2/3 m | 20 Hz | 2 ALS | Faster disease progression | ||||
| Angelucci F. et al. (2004) [71] | rTMS | 8 d | Motor cortex | 1 Hz, 20 Hz | 4 ALS + 10 h | BDNF | No significant changes in serum BDNF levels in ALS subjects. Significant reduction healthy subjects |
| Electrical Stimulation | |||||||
| Madhavan S. et al. (2025) [109] | tDCS | 3 d/w, 12 w × 2 | Motor cortex (an) | 2 mA, 20 min | 14 ALS (c) | ALSFRS-R | Slowing motor impairment |
| Benussi A. et al. (2023) [41] | tDCS | 5 d/w, 2 w × 2 | Motor cortex (an); cervical spine (cat) | 2 mA (m), 4 mA (s); 20 min | 31 ALS | ALSAQ-40; ALSFRS-R; CBI; EQ5D5L; EQ-VAS; ICF; MRC; NfL; SICI | Improvement in all parameters (confirmation of controlled phase results) |
| Sivaramakrishnan A. et al. (2019) [127] | tDCS | 3 d/w, 8 w | Motor cortex (an) | 2 mA, 20 min | 2 ALS | ALSFRS-R; 10MWT; TUG; 6MWD; FSS; BDI | No significant changes |
| Benussi A. et al. (2019) [128] | tDCS | 5 d/w, 2 w | Motor cortex (an); cervical spine (cat) | 2 mA (m), 4 mA (s); 20 min | 31 ALS (c) | ALSAQ-40; ALSFRS-R; CBI; EQ5D5L; EQ-VAS; ICF; MRC; NfL; SICI | Improvement in muscle strength, quality of life, survival and caregiver burden; but also, in neurophysiological parameters and serum reduction of NfL |
| Madhavan S. et al. (2018) [126] | tDCS | 3 d/w, 4 w | Left Motor cortex (an + cat + sham) | 2 mA, 20 min | 1 ALS | ALSFRS-R; 10MWT; TUG; 6MWD; MEP | Slight motor improvement (10MWT and TUG) |
| Di Lazzaro V. et al. (2013) [125] | tDCS | 1 d/m, 12 m | Motor cortex (cat) (b) | 1 mA, 20 min | 2 ALS | ALSFRS-R | No significant changes |
| Munneke M. et al. (2011) [124] | tDCS | 1 d/w, 3 w | Left Motor cortex (cat) | 1 mA, 7/11/15 min | 10 ALS + 10 h | MEP; CMAP; SICI; ICF | No significant changes |
| Quartarone A. et al. (2007) [123] | tDCS | 1 d | Motor cortex (an or cat) | 1 mA, 7 min | 8 ALS + 8 h | MEP; SICI; ICF | No significant changes |
- Quality of life: Regarding rTMS, Zanette and colleagues, and regarding tDCS, Benussi and colleagues, demonstrated an improvement in perceived quality of life (QoL), although this aspect is subject to individual coping strategies and the psychosocial dynamics in which the patient is immersed. Furthermore, Benussi et al. also demonstrated an increase in overall survival. Di Lazzaro and colleagues demonstrated an improvement in quality of life (QoL), defined as the period without tracheostomy, in their study of tSMS application in ALS [41,92,119,128].
- Motor assessment: The most promising results were presented by Zanette and colleagues regarding rTMS, which showed a significant increase in grip strength, but it should also be noted that Di Lazzaro’s group demonstrated a tendency towards slowing the progression of the disease, assessed mainly with the ALSFRS-R, both after low-frequency rTMS and, above all, after cTBS. Regarding tDCS stimulation, Benussi and colleagues demonstrated an improvement in muscle strength, confirming the trend found by Madhavan’s group [41,117,118,119,120,126,128,129].
- Cognitive assessment: This is an area that has rarely been investigated despite the motor component, so the results are very limited. The only satisfactory data comes from Zheng et al., who demonstrated short-term cognitive improvement following rTMS stimulation of the DLPFC (the only study to apply stimulation to this area). Sivaramakrishnan and his team considered evaluating the effects of stimulation on the BDI. Although the BDI predominantly represents a psychological domain, it showed no effect [122,127].
- Blood biomarkers: These have been considered since the first studies published in the literature, but while BDNF assessments have never yielded satisfactory results, tDCS stimulation performed by Benussi and colleagues appears to have induced a reduction in blood NfL levels, a result not observed by Zheng and colleagues with rTMS [41,71,72,118,122,128].
6. Mechanistic Insights from Human Studies
6.1. Changes in TMS-Derived Excitability Measures
6.2. Neuroimaging Correlates
6.3. Biomarkers
6.4. Links Between Physiological Changes and Clinical Outcomes
6.5. Links Between Genetic Background and NIBS Effectiveness
7. Safety and Tolerability
Safety and Tolerability in ALS Studies
8. Practical & Implementation Considerations
9. Limitations of Current Evidence Base
10. Future Directions
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MWD | Six-minute walk distance |
| 10MWT | Ten-minute walk test |
| 18F-FDG | [18F] Fluorodeoxyglucose |
| AD | Alzheimer disease |
| ALS | Amyotrophic lateral sclerosis |
| ALSAQ-40 | 40-item ALS assessment questionnaire |
| ALSFRS-R | Amyotrophic lateral sclerosis functional rating scale—revised |
| AP | Average power |
| ARTN | Artemin |
| BDI | Beck’s depression inventory |
| BDNF | Brain-derived neurotrophic factor |
| C9orf72 | Chromosome 9 open reading frame 72 (gene) |
| CBI | Caregiver burden inventory |
| ccPAS | Cortico-cortical paired associative stimulation |
| CMAP | Compound muscle action potential |
| CMCT | Central motor conduction time |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CSP | Cortical silent period |
| CST | Cortical-spinal tract |
| cTBS | Continuous theta burst stimulation |
| DLPFC | Dorsolateral prefrontal cortex |
| dMRI | Diffusion-weight magnetic resonance imaging |
| ECAS | Edinburgh cognitive and behavioural ALS screen |
| EEG | Electroencephalogram |
| EMG | Electromyogram |
| EQ-5D-5L | EuroQol group 5 dimensions 5 levels |
| EQ-VAS | EuroQol visual analogue scale |
| ER | Endurance ratio |
| FA | Fractional anisotropy |
| FDA | Food and drug administration |
| fMRI | Functional magnetic resonance imaging |
| FSS | Functional system score |
| FTD | Frontotemporal dementia |
| FUS | Fused in sarcoma |
| GABA | Gamma-aminobutyric acid |
| GDNF | Glial-derived neurotrophic factor |
| GenAI | Generative artificial intelligence |
| GFAP | Glial fibrillary acidic protein |
| HiFUS | High-intensity focused ultrasound stimulation |
| IBS | Invasive brain stimulation |
| ICF | Intracortical facilitation |
| IGF-1 | Insulin-growth factor 1 |
| ISI | Interstimulus intervals |
| iTBS | Intermittent theta burst stimulation |
| LICI | Long-interval intracortical inhibition |
| LiFUS | Low-intensity focused ultrasound stimulation |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| LMN | Lower motor neuron |
| M1 | Primary motor cortex |
| MDR | Medical device regulation |
| MEP | Motor evoked potential |
| MND | Motor neuron disease |
| MRC | Medical research council scale for muscle strength |
| MRI | Magnetic resonance imaging |
| MRS | Proton magnetic resonance spectroscopy |
| MT | Motor threshold |
| MVIC | Maximum voluntary isometric contraction |
| NAA | N-acetylaspartate |
| NfH | Neurofilament heavy chain |
| NfL | Neurofilament light chain |
| NfM | Neurofilament medium chain |
| Nfs | Neurofilaments |
| NGF | Nerve growth factor |
| NIBS | Non-invasive brain stimulation |
| NIRS | Near-infrared spectroscopy |
| NO | Nitric oxide |
| NT | Neurotrophins |
| NRTN | Neurturin |
| PBMC | Peripheral blood mononuclear cells |
| PD | Parkinson disease |
| PET | Positron emission tomography |
| PMCT | Peripheral motor conduction time |
| pNfH | Phosphorylated heavy chain of neurofilaments |
| PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| PSP | Progressive supranuclear palsy |
| PSPN | Persephin |
| QoL | Quality of life |
| RCTs | Randomized multicentre trials |
| RMT | Resting motor threshold |
| RoB2 | Revised Cochrane risk-of-bias tool |
| ROM | Range of motion |
| ROS | Reactive oxygen species |
| rTMS | Repetitive transcranial magnetic stimulation |
| SF-36 | 36-item short form health survey |
| SICI | Short-interval intracortical inhibition |
| SOD1 | Superoxide dismutase 1 |
| STDP | Spike-timing-dependent plasticity |
| tACS | Transcranial alternating current stimulation |
| TARDP | Transactive response DNA binding protein 43 |
| TBS | Theta burst stimulation |
| tDCS | Transcranial direct current stimulation |
| TDP-43 | Transactive response DNA binding protein 43 kDa |
| tES | Transcranial electrical stimulation |
| tFUS | Transcranial focused ultrasound stimulation |
| TMS | Transcranial magnetic stimulation |
| tPCS | Transcranial pulsed current stimulation |
| TPS | Transcranial pulse stimulation |
| tRNS | Transcranial random noise stimulation |
| tSMS | Transcranial static magnetic stimulation |
| TST | Triple-stimulation technique |
| tTIS | Transcranial temporal interference stimulation |
| TUG | Timed up-and-go test |
| TUS | Transcranial ultrasound stimulation |
| UMN | Upper motor neuron |
| VEGF | Vascular endothelial growth factor |
| VR | Virtual reality |
References
- Phukan, J.; Pender, N.P.; Hardiman, O. Cognitive Impairment in Amyotrophic Lateral Sclerosis. Lancet Neurol. 2007, 6, 994–1003. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic Lateral Sclerosis: A Clinical Review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Nijs, M.; Van Damme, P. The Genetics of Amyotrophic Lateral Sclerosis. Curr. Opin. Neurol. 2024, 37, 560–569. [Google Scholar] [CrossRef]
- Benussi, A.; Padovani, A.; Borroni, B. Phenotypic Heterogeneity of Monogenic Frontotemporal Dementia. Front. Aging Neurosci. 2015, 7, 171. [Google Scholar] [CrossRef]
- Miller, T.M.; Cudkowicz, M.E.; Genge, A.; Shaw, P.J.; Sobue, G.; Bucelli, R.C.; Chiò, A.; Van Damme, P.; Ludolph, A.C.; Glass, J.D.; et al. Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2022, 387, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic Lateral Sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, W.; Philips, T. The Changing Scene of Amyotrophic Lateral Sclerosis. Nat. Rev. Neurosci. 2013, 14, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Hardiman, O.; Benatar, M.; Brooks, B.R.; Chio, A.; de Carvalho, M.; Ince, P.G.; Lin, C.; Miller, R.G.; Mitsumoto, H.; et al. Controversies and Priorities in Amyotrophic Lateral Sclerosis. Lancet Neurol. 2013, 12, 310–322. [Google Scholar] [CrossRef]
- Mitsumoto, H.; Brooks, B.R.; Silani, V. Clinical Trials in Amyotrophic Lateral Sclerosis: Why so Many Negative Trials and How Can Trials Be Improved? Lancet Neurol. 2014, 13, 1127–1138. [Google Scholar] [CrossRef]
- Van Damme, P.; Al-Chalabi, A.; Andersen, P.M.; Chiò, A.; Couratier, P.; De Carvalho, M.; Hardiman, O.; Kuźma-Kozakiewicz, M.; Ludolph, A.; McDermott, C.J.; et al. European Academy of Neurology (EAN) Guideline on the Management of Amyotrophic Lateral Sclerosis in Collaboration with European Reference Network for Neuromuscular Diseases (ERN EURO-NMD). Eur. J. Neurol. 2024, 31, e16264. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Aoki, M.; Tsuji, S.; Itoyama, Y.; Sobue, G.; Togo, M.; Hamada, C.; Tanaka, M.; Akimoto, M.; Nakamura, K.; et al. Safety and Efficacy of Edaravone in Well Defined Patients with Amyotrophic Lateral Sclerosis: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2017, 16, 505–512. [Google Scholar] [CrossRef]
- Khamaysa, M.; Pradat, P.F. Status of ALS Treatment, Insights into Therapeutic Challenges and Dilemmas. J. Pers. Med. 2022, 12, 1601. [Google Scholar] [CrossRef] [PubMed]
- Hayden, D.; Lai, P.-Y.; Donahue, R.A.; Chen, H.-W.; Wang, J.; Mathai, N.; Lopes, G.; McCaffrey, A.; Scalia, J.; Luppino, S.; et al. Verdiperstat in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2025, 82, 333–343. [Google Scholar] [CrossRef]
- Eisen, A.; Braak, H.; Del Tredici, K.; Lemon, R.; Ludolph, A.C.; Kiernan, M.C. Cortical Influences Drive Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Cheah, B.C.; Yiannikas, C.; Kiernan, M.C. Cortical Excitability Distinguishes ALS from Mimic Disorders. Clin. Neurophysiol. 2011, 122, 1860–1866. [Google Scholar] [CrossRef]
- Cengiz, B.; Kuruoğlu, R. A New Parameter to Discriminate Amyotrophic Lateral Sclerosis Patients from Healthy Participants by Motor Cortical Excitability Changes. Muscle Nerve 2020, 61, 354–362. [Google Scholar] [CrossRef]
- Vucic, S.; Pavey, N.; Haidar, M.; Turner, B.J.; Kiernan, M.C. Cortical Hyperexcitability: Diagnostic and Pathogenic Biomarker of ALS. Neurosci. Lett. 2021, 759, 136039. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord, Roots and Peripheral Nerves: Basic Principles and Procedures for Routine Clinical and Research Application. An Updated Report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Miniussi, C.; Paulus, W.; Rossini, P.M. Transcranial Brain Stimulation, 1st ed.; Miniussi, C., Paulus, W., Rossini, P.M., Eds.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429189425. [Google Scholar]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-Invasive Magnetic Stimulation of Human Motor Cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Bella, R.; Benussi, A.; Bologna, M.; Borroni, B.; Capone, F.; Chen, K.-H.S.; Chen, R.; Chistyakov, A.V.; Classen, J.; et al. Diagnostic Contribution and Therapeutic Perspectives of Transcranial Magnetic Stimulation in Dementia. Clin. Neurophysiol. 2021, 132, 2568–2607. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Stanley Chen, K.H.; Kiernan, M.C.; Hallett, M.; Benninger, D.H.; Di Lazzaro, V.; Rossini, P.M.; Benussi, A.; Berardelli, A.; Currà, A.; et al. Clinical Diagnostic Utility of Transcranial Magnetic Stimulation in Neurological Disorders. Updated Report of an IFCN Committee. Clin. Neurophysiol. 2023, 150, 131–175. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Berardelli, A.; Rona, S.; Accornero, N.; Manfredi, M. Polarization of the Human Motor Cortex through the Scalp. Neuroreport 1998, 9, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.-J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.-H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041.e16. [Google Scholar] [CrossRef]
- Altomare, D.; Benussi, A.; Cantoni, V.; Premi, E.; Rivolta, J.; Cupidi, C.; Martorana, A.; Santarnecchi, E.; Padovani, A.; Koch, G.; et al. Home-Based Transcranial Alternating Current Stimulation (TACS) in Alzheimer’s Disease: Rationale and Study Design. Alzheimers Res. Ther. 2023, 15, 155. [Google Scholar] [CrossRef]
- Pilloni, G.; Vogel-Eyny, A.; Lustberg, M.; Best, P.; Malik, M.; Walton-Masters, L.; George, A.; Mirza, I.; Zhovtis, L.; Datta, A.; et al. Tolerability and Feasibility of At-Home Remotely Supervised Transcranial Direct Current Stimulation (RS-TDCS): Single-Center Evidence from 6,779 Sessions. Brain Stimul. 2022, 15, 707–716. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety, Ethical Considerations, and Application Guidelines for the Use of Transcranial Magnetic Stimulation in Clinical Practice and Research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low Intensity Transcranial Electric Stimulation: Safety, Ethical, Legal Regulatory and Application Guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, E.M. Risk and Safety of Repetitive Transcranial Magnetic Stimulation: Report and Suggested Guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1998, 108, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, F.; Senerchia, G.; Bonan, L.; Casali, S.; Cabona, C.; Cantone, M.; De Marchi, F.; Diamanti, L.; Doretti, A.; Fini, N.; et al. Cortical Excitability as a Prognostic and Phenotypic Stratification Biomarker in Amyotrophic Lateral Sclerosis. Ann. Neurol. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Benussi, A.; Premi, E.; Cantoni, V.; Compostella, S.; Magni, E.; Gilberti, N.; Vergani, V.; Delrio, I.; Gamba, M.; Spezi, R.; et al. Cortical Inhibitory Imbalance in Functional Paralysis. Front. Hum. Neurosci. 2020, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.M.; Benussi, A. Transcranial Magnetic Stimulation Across the Lifespan: Impact of Developmental and Degenerative Processes. Biol. Psychiatry 2023, 95, 581–591. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Borroni, B. The Role of Transcranial Magnetic Stimulation in the Diagnosis of Dementia. In Horizons in Neuroscience Research; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; Volume 44, pp. 55–104. ISBN 978-168507085-4&978-168507072-4. [Google Scholar]
- Bracca, V.; Cantoni, V.; Gadola, Y.; Rivolta, J.; Cosseddu, M.; Turrone, R.; Caratozzolo, S.; Di Luca, M.; Padovani, A.; Borroni, B.; et al. Neurophysiological Correlates of Altered Time Awareness in Alzheimer’s Disease and Frontotemporal Dementia. Neurol. Sci. 2023, 44, 3515–3522. [Google Scholar] [CrossRef]
- Benussi, A.; Pilotto, A.; Cantoni, V.; Ferrari, E.; Borroni, B.; Padovani, A. Neurophysiological Correlates of Motor and Cognitive Dysfunction in Prodromal and Overt Dementia with Lewy Bodies. J. Alzheimer’s Dis. 2022, 86, 579–588. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Grassi, M.; Libri, I.; Cotelli, M.S.; Tarantino, B.; Datta, A.; Thomas, C.; Huber, N.; Kärkkäinen, S.; et al. Cortico-Spinal TDCS in Amyotrophic Lateral Sclerosis: A Randomized, Double-Blind, Sham-Controlled Trial Followed by an Open-Label Phase. Brain Stimul. 2023, 16, 1666–1676. [Google Scholar] [CrossRef]
- Higashihara, M.; Pavey, N.; Menon, P.; van den Bos, M.; Shibuya, K.; Kuwabara, S.; Kiernan, M.C.; Koinuma, M.; Vucic, S. Reduction in Short Interval Intracortical Inhibition from the Early Stage Reflects the Pathophysiology in Amyotrophic Lateral Sclerosis: A Meta-Analysis Study. Eur. J. Neurol. 2024, 31, e16281. [Google Scholar] [CrossRef]
- Vucic, S.; Kiernan, M.C. Novel Threshold Tracking Techniques Suggest That Cortical Hyperexcitability Is an Early Feature of Motor Neuron Disease. Brain 2006, 129, 2436–2446. [Google Scholar] [CrossRef]
- Menon, P.; Kiernan, M.C.; Vucic, S. Cortical Hyperexcitability Precedes Lower Motor Neuron Dysfunction in ALS. Clin. Neurophysiol. 2015, 126, 803–809. [Google Scholar] [CrossRef]
- Menon, P.; Kiernan, M.C.; Vucic, S. Cortical Excitability Varies across Different Muscles. J. Neurophysiol. 2018, 120, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Cheah, B.C.; Kiernan, M.C. Defining the Mechanisms That Underlie Cortical Hyperexcitability in Amyotrophic Lateral Sclerosis. Exp. Neurol. 2009, 220, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Nicholson, G.A.; Kiernan, M.C. Cortical Hyperexcitability May Precede the Onset of Familial Amyotrophic Lateral Sclerosis. Brain 2008, 131, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Howells, J.; Trevillion, L.; Kiernan, M.C. Assessment of Cortical Excitability Using Threshold Tracking Techniques. Muscle Nerve 2006, 33, 477–486. [Google Scholar] [CrossRef]
- Vucic, S.; Ziemann, U.; Eisen, A.; Hallett, M.; Kiernan, M.C. Transcranial Magnetic Stimulation and Amyotrophic Lateral Sclerosis: Pathophysiological Insights. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1161–1170. [Google Scholar] [CrossRef]
- Geevasinga, N.; Menon, P.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Diagnostic Utility of Cortical Excitability Studies in Amyotrophic Lateral Sclerosis. Eur. J. Neurol. 2014, 21, 1451–1457. [Google Scholar] [CrossRef]
- Menon, P.; Geevasinga, N.; Yiannikas, C.; Howells, J.; Kiernan, M.C.; Vucic, S. Sensitivity and Specificity of Threshold Tracking Transcranial Magnetic Stimulation for Diagnosis of Amyotrophic Lateral Sclerosis: A Prospective Study. Lancet Neurol. 2015, 14, 478–484. [Google Scholar] [CrossRef]
- Menon, P.; Geevasinga, N.; van den Bos, M.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Cortical Hyperexcitability and Disease Spread in Amyotrophic Lateral Sclerosis. Eur. J. Neurol. 2017, 24, 816–824. [Google Scholar] [CrossRef]
- Vucic, S.; van den Bos, M.; Menon, P.; Howells, J.; Dharmadasa, T.; Kiernan, M.C. Utility of Threshold Tracking Transcranial Magnetic Stimulation in ALS. Clin. Neurophysiol. Pract. 2018, 3, 164–172. [Google Scholar] [CrossRef]
- Van Den Bos, M.A.J.; Higashihara, M.; Geevasinga, N.; Menon, P.; Kiernan, M.C.; Vucic, S. Imbalance of Cortical Facilitatory and Inhibitory Circuits Underlies Hyperexcitability in ALS. Neurology 2018, 91, E1669–E1676. [Google Scholar] [CrossRef]
- Pavey, N.; Hannaford, A.; Higashihara, M.; van den Bos, M.; Geevasinga, N.; Vucic, S.; Menon, P. Cortical Inexcitability in ALS: Correlating a Clinical Phenotype. J. Neurol. Neurosurg. Psychiatry 2024, 96, 219–225. [Google Scholar] [CrossRef]
- Nicholson, G.A.; Howells, J.; Kiernan, M.C.; Ng, K.; Vucic, S.; Geevasinga, N.; Kril, J.J.; Menon, P.; Yiannikas, C. Cortical Function in Asymptomatic Carriers and Patients with C9orf72 Amyotrophic Lateral Sclerosis. JAMA Neurol. 2015, 72, 1268. [Google Scholar] [CrossRef]
- Wittstock, M.; Wolters, A.; Benecke, R. Transcallosal Inhibition in Amyotrophic Lateral Sclerosis. Clin. Neurophysiol. 2007, 118, 301–307. [Google Scholar] [CrossRef]
- Zanette, G.; Tamburin, S.; Manganotti, P.; Refatti, N.; Forgione, A.; Rizzuto, N. Changes in Motor Cortex Inhibition over Time in Patients with Amyotrophic Lateral Sclerosis. J. Neurol. 2002, 249, 1723–1728. [Google Scholar] [CrossRef]
- Zanette, G.; Tamburin, S.; Manganotti, P.; Refatti, N.; Forgione, A.; Rizzuto, N. Different Mechanisms Contribute to Motor Cortex Hyperexcitability in Amyotrophic Lateral Sclerosis. Clin. Neurophysiol. 2002, 113, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.E.; Patani, R. The Microglial Component of Amyotrophic Lateral Sclerosis. Brain 2020, 143, 3526–3539. [Google Scholar] [CrossRef]
- Calma, A.D.; Pavey, N.; Menon, P.; Vucic, S. Neuroinflammation in Amyotrophic Lateral Sclerosis: Pathogenic Insights and Therapeutic Implications. Curr. Opin. Neurol. 2024, 37, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Papa, M.; De Luca, C.; Petta, F.; Alberghina, L.; Cirillo, G. Astrocyte–Neuron Interplay in Maladaptive Plasticity. Neurosci. Biobehav. Rev. 2014, 42, 35–54. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological After-Effects of Non-Invasive Brain Stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Pinto, N.; Serrenho, I.; Pato, M.V.; Baltazar, G. Contribution of Glial Cells to the Neuroprotective Effects Triggered by Repetitive Magnetic Stimulation: A Systematic Review. Neural Regen. Res. 2024, 19, 116–123. [Google Scholar] [CrossRef]
- Ruohonen, J.; Karhu, J. TDCS Possibly Stimulates Glial Cells. Clin. Neurophysiol. 2012, 123, 2006–2009. [Google Scholar] [CrossRef] [PubMed]
- Oishi, R.; Takeda, I.; Ode, Y.; Okada, Y.; Kato, D.; Nakashima, H.; Imagama, S.; Wake, H. Neuromodulation with Transcranial Direct Current Stimulation Contributes to Motor Function Recovery via Microglia in Spinal Cord Injury. Sci. Rep. 2024, 14, 18031. [Google Scholar] [CrossRef]
- Riolo, G.; Ricci, C.; De Angelis, N.; Marzocchi, C.; Guerrera, G.; Borsellino, G.; Giannini, F.; Battistini, S. BDNF and Pro-BDNF in Amyotrophic Lateral Sclerosis: A New Perspective for Biomarkers of Neurodegeneration. Brain Sci. 2022, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Rifai, O.M.; O’Shaughnessy, J.; Dando, O.R.; Munro, A.F.; Sewell, M.D.E.; Abrahams, S.; Waldron, F.M.; Sibley, C.R.; Gregory, J.M. Distinct Neuroinflammatory Signatures Exist across Genetic and Sporadic Amyotrophic Lateral Sclerosis Cohorts. Brain 2023, 146, 5124–5138. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; He, D. Repetitive Transcranial Magnetic Stimulation (RTMS) Fails to Increase Serum Brain-Derived Neurotrophic Factor (BDNF). Neurophysiol. Clin. 2019, 49, 295–300. [Google Scholar] [CrossRef]
- Jiménez-García, A.M.; Bonnel, G.; Álvarez-Mota, A.; Arias, N. Current Perspectives on Neuromodulation in ALS Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2024, 19, e0300671. [Google Scholar] [CrossRef]
- Angelucci, F.; Oliviero, A.; Pilato, F.; Saturno, E.; Dileone, M.; Versace, V.; Musumeci, G.; Batocchi, A.P.; Tonali, P.A.; Lazzaro, V. Di Transcranial Magnetic Stimulation and BDNF Plasma Levels in Amyotrophic Lateral Sclerosis. Neuroreport 2004, 15, 717–720. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Pilato, F.; Profice, P.; Ranieri, F.; Musumeci, G.; Florio, L.; Beghi, E.; Frisullo, G.; Capone, F.; Sabatelli, M.; et al. Motor Cortex Stimulation for ALS: A Double Blind Placebo-Controlled Study. Neurosci. Lett. 2009, 464, 18–21. [Google Scholar] [CrossRef]
- Siddiqi, S.H.; Kording, K.P.; Parvizi, J.; Fox, M.D. Causal Mapping of Human Brain Function. Nat. Rev. Neurosci. 2022, 23, 361–375. [Google Scholar] [CrossRef]
- Fox, M.D. Mapping Symptoms to Brain Networks with the Human Connectome. N. Engl. J. Med. 2018, 379, 2237–2245. [Google Scholar] [CrossRef]
- Laganiere, S.; Boes, A.D.; Fox, M.D. Network Localization of Hemichorea-Hemiballismus. Neurology 2016, 86, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Joutsa, J.; Horn, A.; Hsu, J.; Fox, M.D. Localizing Parkinsonism Based on Focal Brain Lesions. Brain 2018, 141, 2445–2456. [Google Scholar] [CrossRef]
- Corp, D.T.; Joutsa, J.; Darby, R.R.; Delnooz, C.C.S.; van de Warrenburg, B.P.C.; Cooke, D.; Prudente, C.N.; Ren, J.; Reich, M.M.; Batla, A.; et al. Network Localization of Cervical Dystonia Based on Causal Brain Lesions. Brain 2019, 142, 1660–1674. [Google Scholar] [CrossRef]
- Halliday, G.M.; McCann, H. The Progression of Pathology in Parkinson’s Disease. Ann. N. Y. Acad. Sci. 2010, 1184, 188–195. [Google Scholar] [CrossRef]
- Ogut, E.; Armagan, K.; Tufekci, D. The Guillain-Mollaret Triangle: A Key Player in Motor Coordination and Control with Implications for Neurological Disorders. Neurosurg. Rev. 2023, 46, 181. [Google Scholar] [CrossRef]
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of PTDP-43 Pathology in Amyotrophic Lateral Sclerosis. Ann. Neurol. 2013, 74, 20–38. [Google Scholar] [CrossRef]
- Grolez, G.; Moreau, C.; Danel-Brunaud, V.; Delmaire, C.; Lopes, R.; Pradat, P.F.; El Mendili, M.M.; Defebvre, L.; Devos, D. The Value of Magnetic Resonance Imaging as a Biomarker for Amyotrophic Lateral Sclerosis: A Systematic Review. BMC Neurol. 2016, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-Based Guidelines on the Therapeutic Use of Repetitive Transcranial Magnetic Stimulation (RTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Palacino, F.; Manganotti, P.; Benussi, A. Targeting Neural Oscillations for Cognitive Enhancement in Alzheimer’s Disease. Medicina 2025, 61, 547. [Google Scholar] [CrossRef]
- Carrarini, C.; Pappalettera, C.; Le Pera, D.; Rossini, P.M. Non-Invasive Brain Stimulation in Cognitive Sciences and Alzheimer’s Disease. Front. Hum. Neurosci. 2025, 18, 1500502. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Amedi, A.; Fregni, F.; Merabet, L.B. The Plastic Human Brain Cortex. Annu. Rev. Neurosci. 2005, 28, 377–401. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.M.; Thomson, R.H.; Rosenfeld, J.V.; Fitzgerald, P.B. Brain Neuromodulation Techniques. Neuroscientist 2016, 22, 406–421. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Ranieri, F.; Bączyk, M.; de Carvalho, M.; Dileone, M.; Dubbioso, R.; Fernandes, S.; Kozak, G.; Motolese, F.; Ziemann, U. Novel Approaches to Motoneuron Disease/ALS Treatment Using Non-Invasive Brain and Spinal Stimulation: IFCN Handbook Chapter. Clin. Neurophysiol. 2024, 158, 114–136. [Google Scholar] [CrossRef]
- Koch, G.; Altomare, D.; Benussi, A.; Bréchet, L.; Casula, E.P.; Dodich, A.; Pievani, M.; Santarnecchi, E.; Frisoni, G.B. The Emerging Field of Non-Invasive Brain Stimulation in Alzheimer’s Disease. Brain 2024, 147, 4003–4016. [Google Scholar] [CrossRef]
- Turrini, S.; Fiori, F.; Chiappini, E.; Lucero, B.; Santarnecchi, E.; Avenanti, A. Cortico-Cortical Paired Associative Stimulation (CcPAS) over Premotor-Motor Areas Affects Local Circuitries in the Human Motor Cortex via Hebbian Plasticity. Neuroimage 2023, 271, 120027. [Google Scholar] [CrossRef]
- Turrini, S.; Fiori, F.; Chiappini, E.; Santarnecchi, E.; Romei, V.; Avenanti, A. Gradual Enhancement of Corticomotor Excitability during Cortico-Cortical Paired Associative Stimulation. Sci. Rep. 2022, 12, 14670. [Google Scholar] [CrossRef]
- Tarasi, L.; Turrini, S.; Sel, A.; Avenanti, A.; Romei, V. Cortico-Cortical Paired-Associative Stimulation to Investigate the Plasticity of Cortico-Cortical Visual Networks in Humans. Curr. Opin. Behav. Sci. 2024, 56, 101359. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Ranieri, F.; Doretti, A.; Boscarino, M.; Maderna, L.; Colombo, E.; Soranna, D.; Zambon, A.; Ticozzi, N.; Musumeci, G.; et al. Transcranial Static Magnetic Stimulation for Amyotrophic Lateral Sclerosis: A Bicentric, Randomised, Double-Blind Placebo-Controlled Phase 2 Trial. Lancet Reg. Health—Eur. 2024, 45, 101019. [Google Scholar] [CrossRef]
- Oliviero, A.; Carrasco-López, M.C.; Campolo, M.; Perez-Borrego, Y.A.; Soto-León, V.; Gonzalez-Rosa, J.J.; Higuero, A.M.; Strange, B.A.; Abad-Rodriguez, J.; Foffani, G. Safety Study of Transcranial Static Magnetic Field Stimulation (TSMS) of the Human Cortex. Brain Stimul. 2015, 8, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Dileone, M.; Mordillo-Mateos, L.; Oliviero, A.; Foffani, G. Long-Lasting Effects of Transcranial Static Magnetic Field Stimulation on Motor Cortex Excitability. Brain Stimul. 2018, 11, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W. Transcranial Electrical Stimulation (TES—TDCS; TRNS, TACS) Methods. Neuropsychol. Rehabil. 2011, 21, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-Based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (TDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Paulus, W. Transcranial Alternating Current Stimulation (TACS). Front. Hum. Neurosci. 2013, 7, 317. [Google Scholar] [CrossRef]
- Antal, A.; Luber, B.; Brem, A.-K.; Bikson, M.; Brunoni, A.R.; Cohen Kadosh, R.; Dubljević, V.; Fecteau, S.; Ferreri, F.; Flöel, A.; et al. Non-Invasive Brain Stimulation and Neuroenhancement. Clin. Neurophysiol. Pract. 2022, 7, 146–165. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Cotelli, M.S.; Cotelli, M.; Brattini, C.; Datta, A.; Thomas, C.; Santarnecchi, E.; Pascual-Leone, A.; Borroni, B. Exposure to Gamma TACS in Alzheimer’s Disease: A Randomized, Double-Blind, Sham-Controlled, Crossover, Pilot Study. Brain Stimul. 2021, 14, 531–540. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Rivolta, J.; Zoppi, N.; Cotelli, M.S.; Bianchi, M.; Cotelli, M.; Borroni, B. Alpha TACS Improves Cognition and Modulates Neurotransmission in Dementia with Lewy Bodies. Mov. Disord. 2024, 39, 1993–2003. [Google Scholar] [CrossRef]
- Libri, I.; Cantoni, V.; Benussi, A.; Rivolta, J.; Ferrari, C.; Fancellu, R.; Synofzik, M.; Alberici, A.; Padovani, A.; Borroni, B. Comparing Cerebellar TDCS and Cerebellar TACS in Neurodegenerative Ataxias Using Wearable Sensors: A Randomized, Double-Blind, Sham-Controlled, Triple-Crossover Trial. Cerebellum 2023, 23, 570–578. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Grassi, M.; Brechet, L.; Michel, C.M.; Datta, A.; Thomas, C.; Gazzina, S.; Cotelli, M.S.; Bianchi, M.; et al. Increasing Brain Gamma Activity Improves Episodic Memory and Restores Cholinergic Dysfunction in Alzheimer’s Disease. Ann. Neurol. 2022, 92, 322–334. [Google Scholar] [CrossRef]
- Malekahmad, M.; Frazer, A.; Zoghi, M.; Jaberzadeh, S. Transcranial Pulsed Current Stimulation: A Scoping Review of the Current Literature on Scope, Nature, Underlying Mechanisms, and Gaps. Psychophysiology 2024, 61, e14521. [Google Scholar] [CrossRef]
- Jaberzadeh, S.; Bastani, A.; Zoghi, M.; Morgan, P.; Fitzgerald, P.B. Anodal Transcranial Pulsed Current Stimulation: The Effects of Pulse Duration on Corticospinal Excitability. PLoS ONE 2015, 10, e0131779. [Google Scholar] [CrossRef]
- Jaberzadeh, S.; Bastani, A.; Zoghi, M. Anodal Transcranial Pulsed Current Stimulation: A Novel Technique to Enhance Corticospinal Excitability. Clin. Neurophysiol. 2014, 125, 344–351. [Google Scholar] [CrossRef]
- Brancucci, A.; Rivolta, D.; Nitsche, M.A.; Manippa, V. The Effects of Transcranial Random Noise Stimulation on Motor Function: A Comprehensive Review of the Literature. Physiol. Behav. 2023, 261, 114073. [Google Scholar] [CrossRef] [PubMed]
- Neri, F.; Della Toffola, J.; Scoccia, A.; Benelli, A.; Lomi, F.; Cinti, A.; Smeralda, C.L.; Romanella, S.; Giannotta, A.; Rossi, S.; et al. Neuromodulation via TRNS Accelerates Learning and Enhances In-Game Performance at a Virtual-Reality First Person Shooter Game. Comput. Hum. Behav. 2025, 165, 108537. [Google Scholar] [CrossRef]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing Human Brain Excitability by Transcranial High-Frequency Random Noise Stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.; Deshmukh, S.; Cummings, M.; Doshi, A.; Rezania, K.; Freels, S.; Sawa, G. Home-Based Tele-tDCS in Amyotrophic Lateral Sclerosis: Feasibility, Safety, and Preliminary Efficacy. Ann. Clin. Transl. Neurol. 2025, 12, 1022–1033. [Google Scholar] [CrossRef]
- Guo, W.; He, Y.; Zhang, W.; Sun, Y.; Wang, J.; Liu, S.; Ming, D. A Novel Non-Invasive Brain Stimulation Technique: “Temporally Interfering Electrical Stimulation”. Front. Neurosci. 2023, 17, 1092539. [Google Scholar] [CrossRef]
- Violante, I.R.; Alania, K.; Cassarà, A.M.; Neufeld, E.; Acerbo, E.; Carron, R.; Williamson, A.; Kurtin, D.L.; Rhodes, E.; Hampshire, A.; et al. Non-Invasive Temporal Interference Electrical Stimulation of the Human Hippocampus. Nat. Neurosci. 2023, 26, 1994–2004. [Google Scholar] [CrossRef]
- Vassiliadis, P.; Beanato, E.; Popa, T.; Windel, F.; Morishita, T.; Neufeld, E.; Duque, J.; Derosiere, G.; Wessel, M.J.; Hummel, F.C. Non-Invasive Stimulation of the Human Striatum Disrupts Reinforcement Learning of Motor Skills. Nat. Hum. Behav. 2024, 8, 1581–1598. [Google Scholar] [CrossRef]
- Lamoš, M.; Bočková, M.; Missey, F.; Lubrano, C.; de Araújo e Silva, M.; Trajlínek, J.; Studnička, O.; Daniel, P.; Carron, R.; Jirsa, V.; et al. Noninvasive Temporal Interference Stimulation of the Subthalamic Nucleus in Parkinson’s Disease Reduces Beta Activity. Mov. Disord. 2025, 40, 1051–1060. [Google Scholar] [CrossRef]
- Murphy, K.R.; Nandi, T.; Kop, B.; Osada, T.; Lueckel, M.; N’Djin, W.A.; Caulfield, K.A.; Fomenko, A.; Siebner, H.R.; Ugawa, Y.; et al. A Practical Guide to Transcranial Ultrasonic Stimulation from the IFCN-Endorsed ITRUSST Consortium. Clin. Neurophysiol. 2025, 171, 192–226. [Google Scholar] [CrossRef]
- Manganotti, P.; Liccari, M.; Lombardo, T.M.I.; Della Toffola, J.; Cenacchi, V.; Catalan, M.; Busan, P. Effect of a Single Session of Transcranial Pulse Stimulation (TPS) on Resting Tremor in Patients with Parkinson’s Disease. Brain Res. 2025, 1850, 149405. [Google Scholar] [CrossRef] [PubMed]
- Matt, E.; Radjenovic, S.; Mitterwallner, M.; Beisteiner, R. Current State of Clinical Ultrasound Neuromodulation. Front. Neurosci. 2024, 18, 1420255. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Saturno, E.; Pilato, F.; Dileone, M.; Sabatelli, M.; Tonali, P.A. Motor Cortex Stimulation for Amyotrophic Lateral Sclerosis. Time for a Therapeutic Trial? Clin. Neurophysiol. 2004, 115, 1479–1485. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Dileone, M.; Pilato, F.; Profice, P.; Ranieri, F.; Musumeci, G.; Angelucci, F.; Sabatelli, M.; Tonali, P.A. Repetitive Transcranial Magnetic Stimulation for ALS. Neurosci. Lett. 2006, 408, 135–140. [Google Scholar] [CrossRef]
- Zanette, G.; Forgione, A.; Manganotti, P.; Fiaschi, A.; Tamburin, S. The Effect of Repetitive Transcranial Magnetic Stimulation on Motor Performance, Fatigue and Quality of Life in Amyotrophic Lateral Sclerosis. J. Neurol. Sci. 2008, 270, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Dileone, M.; Pilato, F.; Profice, P.; Cioni, B.; Meglio, M.; Papacci, F.; Sabatelli, M.; Musumeci, G.; Ranieri, F.; et al. Long-Term Motor Cortex Stimulation for Amyotrophic Lateral Sclerosis. Brain Stimul. 2010, 3, 22–27. [Google Scholar] [CrossRef]
- Munneke, M.A.M.; Rongen, J.J.; Overeem, S.; Schelhaas, H.J.; Zwarts, M.J.; Stegeman, D.F. Cumulative Effect of 5 Daily Sessions of Theta Burst Stimulation on Corticospinal Excitability in Amyotrophic Lateral Sclerosis. Muscle Nerve 2013, 48, 733–738. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, X.; Chen, J.; Luan, X.; Wang, J.; Zhang, L.; Liu, K.; Zhao, Y.; Xu, Z. The Effect of Repetitive Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex on the Amyotrophic Lateral Sclerosis Patients With Cognitive Impairment: A Double-Blinded, Randomized, and Sham Control Trial. CNS Neurosci. Ther. 2025, 31, e70316. [Google Scholar] [CrossRef]
- Quartarone, A.; Lang, N.; Rizzo, V.; Bagnato, S.; Morgante, F.; Sant’Angelo, A.; Crupi, D.; Battaglia, F.; Messina, C.; Girlanda, P. Motor Cortex Abnormalities in Amyotrophic Lateral Sclerosis with Transcranial Direct-current Stimulation. Muscle Nerve 2007, 35, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Munneke, M.A.M.; Stegeman, D.F.; Hengeveld, Y.A.; Rongen, J.J.; Schelhaas, H.J.; Zwarts, M.J. Transcranial Direct Current Stimulation Does Not Modulate Motor Cortex Excitability in Patients with Amyotrophic Lateral Sclerosis. Muscle Nerve 2011, 44, 109–114. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Ranieri, F.; Capone, F.; Musumeci, G.; Dileone, M. Direct Current Motor Cortex Stimulation for Amyotrophic Lateral Sclerosis: A Proof of Principle Study. Brain Stimul. 2013, 6, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.; Sivaramakrishnan, A.; Bond, S.; Jiang, Q.L. Safety and Feasibility of Transcranial Direct Current Stimulation in Amyotrophic Lateral Sclerosis—A Pilot Study with a Single Subject Experimental Design. Physiother. Theory Pract. 2019, 35, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, A.; Datta, A.; Bikson, M.; Madhavan, S. Remotely Supervised Transcranial Direct Current Stimulation: A Feasibility Study for Amyotrophic Lateral Sclerosis. NeuroRehabilitation 2019, 45, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Alberici, A.; Cotelli, M.S.; Dell’Era, V.; Cantoni, V.; Bonetta, E.; Manenti, R.; Filosto, M.; Morini, R.; Datta, A.; et al. Cortico-Spinal TDCS in ALS: A Randomized, Double-Blind, Sham-Controlled Trial. Brain Stimul. 2019, 12, 1332–1334. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Ranieri, F.; Capone, F.; Pilato, F.; Profice, P.; Pellegrino, G.; Musumeci, G.; Florio, L.; Dileone, M. Motor Cortex Stimulation for ALS: Open Label Extension Study of a Previous Small Trial. Brain Stimul. 2014, 7, 143–144. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Dharmadasa, T.; Pavey, N.; Tu, S.; Menon, P.; Huynh, W.; Mahoney, C.J.; Timmins, H.C.; Higashihara, M.; van den Bos, M.; Shibuya, K.; et al. Novel Approaches to Assessing Upper Motor Neuron Dysfunction in Motor Neuron Disease/Amyotrophic Lateral Sclerosis: IFCN Handbook Chapter. Clin. Neurophysiol. 2024, 163, 68–89. [Google Scholar] [CrossRef]
- Tokimura, R.; Murakami, T.; Ugawa, Y. Central Motor Conduction Time Reveals Upper Motor Neuron Involvement Masked by Lower Motor Neuron Impairment in a Significant Portion of Patients with Amyotrophic Lateral Sclerosis. Clin. Neurophysiol. 2020, 131, 1896–1901. [Google Scholar] [CrossRef]
- Grapperon, A.-M.; Verschueren, A.; Jouve, E.; Morizot-Koutlidis, R.; Lenglet, T.; Pradat, P.-F.; Salachas, F.; Bernard, E.; Delstanche, S.; Maertens de Noordhout, A.; et al. Assessing the Upper Motor Neuron in Amyotrophic Lateral Sclerosis Using the Triple Stimulation Technique: A Multicenter Prospective Study. Clin. Neurophysiol. 2021, 132, 2551–2557. [Google Scholar] [CrossRef]
- Tankisi, H.; Nielsen, C.S.-Z.; Howells, J.; Cengiz, B.; Samusyte, G.; Koltzenburg, M.; Blicher, J.U.; Møller, A.T.; Pugdahl, K.; Fuglsang-Frederiksen, A.; et al. Early Diagnosis of Amyotrophic Lateral Sclerosis by Threshold Tracking and Conventional Transcranial Magnetic Stimulation. Eur. J. Neurol. 2021, 28, 3030–3039. [Google Scholar] [CrossRef]
- Menon, P.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Regional Motor Cortex Dysfunction in Amyotrophic Lateral Sclerosis. Ann. Clin. Transl. Neurol. 2019, 6, 1373–1382. [Google Scholar] [CrossRef]
- Vucic, S.; Lin, C.S.-Y.; Cheah, B.C.; Murray, J.; Menon, P.; Krishnan, A.V.; Kiernan, M.C. Riluzole Exerts Central and Peripheral Modulating Effects in Amyotrophic Lateral Sclerosis. Brain 2013, 136, 1361–1370. [Google Scholar] [CrossRef]
- Agosta, F.; Galantucci, S.; Riva, N.; Chiò, A.; Messina, S.; Iannaccone, S.; Calvo, A.; Silani, V.; Copetti, M.; Falini, A.; et al. Intrahemispheric and Interhemispheric Structural Network Abnormalities in PLS and ALS. Hum. Brain Mapp. 2014, 35, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, R.; Prell, T.; Gaur, N.; Roediger, A.; Gaser, C.; Mayer, T.E.; Witte, O.W.; Grosskreutz, J. Patterns of Grey and White Matter Changes Differ between Bulbar and Limb Onset Amyotrophic Lateral Sclerosis. Neuroimage Clin. 2021, 30, 102674. [Google Scholar] [CrossRef] [PubMed]
- Foerster, B.R.; Pomper, M.G.; Callaghan, B.C.; Petrou, M.; Edden, R.A.E.; Mohamed, M.A.; Welsh, R.C.; Carlos, R.C.; Barker, P.B.; Feldman, E.L. An Imbalance Between Excitatory and Inhibitory Neurotransmitters in Amyotrophic Lateral Sclerosis Revealed by Use of 3-T Proton Magnetic Resonance Spectroscopy. JAMA Neurol. 2013, 70, 1009. [Google Scholar] [CrossRef]
- Caldwell, S.; Rothman, D.L. 1H Magnetic Resonance Spectroscopy to Understand the Biological Basis of ALS, Diagnose Patients Earlier, and Monitor Disease Progression. Front. Neurol. 2021, 12, 701170. [Google Scholar] [CrossRef] [PubMed]
- Christidi, F.; Karavasilis, E.; Argyropoulos, G.D.; Velonakis, G.; Zouvelou, V.; Murad, A.; Evdokimidis, I.; Rentzos, M.; Seimenis, I.; Bede, P. Neurometabolic Alterations in Motor Neuron Disease: Insights from Magnetic Resonance Spectroscopy. J. Integr. Neurosci. 2022, 21, 87. [Google Scholar] [CrossRef]
- Kocar, T.D.; Müller, H.-P.; Ludolph, A.C.; Kassubek, J. Feature Selection from Magnetic Resonance Imaging Data in ALS: A Systematic Review. Ther. Adv. Chronic. Dis. 2021, 12, 20406223211051002. [Google Scholar] [CrossRef]
- Agosta, F.; Canu, E.; Valsasina, P.; Riva, N.; Prelle, A.; Comi, G.; Filippi, M. Divergent Brain Network Connectivity in Amyotrophic Lateral Sclerosis. Neurobiol. Aging 2013, 34, 419–427. [Google Scholar] [CrossRef]
- Chenji, S.; Jha, S.; Lee, D.; Brown, M.; Seres, P.; Mah, D.; Kalra, S. Investigating Default Mode and Sensorimotor Network Connectivity in Amyotrophic Lateral Sclerosis. PLoS ONE 2016, 11, e0157443. [Google Scholar] [CrossRef]
- Pagani, M.; Chiò, A.; Valentini, M.C.; Öberg, J.; Nobili, F.; Calvo, A.; Moglia, C.; Bertuzzo, D.; Morbelli, S.; De Carli, F.; et al. Functional Pattern of Brain FDG-PET in Amyotrophic Lateral Sclerosis. Neurology 2014, 83, 1067–1074. [Google Scholar] [CrossRef]
- Zürcher, N.R.; Loggia, M.L.; Lawson, R.; Chonde, D.B.; Izquierdo-Garcia, D.; Yasek, J.E.; Akeju, O.; Catana, C.; Rosen, B.R.; Cudkowicz, M.E.; et al. Increased in Vivo Glial Activation in Patients with Amyotrophic Lateral Sclerosis: Assessed with [11C]-PBR28. Neuroimage Clin. 2015, 7, 409–414. [Google Scholar] [CrossRef]
- Tu, S.; Kiernan, M.C. Amyotrophic Lateral Sclerosis. In Advances in Magnetic Resonance Technology and Applications; Academic Press: Cambridge, MA, USA, 2023; pp. 363–385. [Google Scholar]
- Petzold, A. Neurofilament Phosphoforms: Surrogate Markers for Axonal Injury, Degeneration and Loss. J. Neurol. Sci. 2005, 233, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Macdonald-Wallis, C.; Gray, E.; Pearce, N.; Petzold, A.; Norgren, N.; Giovannoni, G.; Fratta, P.; Sidle, K.; Fish, M.; et al. Neurofilament Light Chain. Neurology 2015, 84, 2247–2257. [Google Scholar] [CrossRef]
- Steinacker, P.; Feneberg, E.; Weishaupt, J.; Brettschneider, J.; Tumani, H.; Andersen, P.M.; von Arnim, C.A.F.; Böhm, S.; Kassubek, J.; Kubisch, C.; et al. Neurofilaments in the Diagnosis of Motoneuron Diseases: A Prospective Study on 455 Patients. J. Neurol. Neurosurg. Psychiatry 2015, 87, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Y.; Dong, S.; Yang, W.; Qian, T.; Liu, X.; Cheng, Q.; Wang, J.; Chen, X. Role of Blood Neurofilaments in the Prognosis of Amyotrophic Lateral Sclerosis: A Meta-Analysis. Front. Neurol. 2021, 12, 712245. [Google Scholar] [CrossRef]
- Verde, F.; Steinacker, P.; Weishaupt, J.H.; Kassubek, J.; Oeckl, P.; Halbgebauer, S.; Tumani, H.; von Arnim, C.A.F.; Dorst, J.; Feneberg, E.; et al. Neurofilament Light Chain in Serum for the Diagnosis of Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 157–164. [Google Scholar] [CrossRef]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Lombardi, V.; Malaspina, A. Neurofilament Light: A Candidate Biomarker of Presymptomatic Amyotrophic Lateral Sclerosis and Phenoconversion. Ann. Neurol. 2018, 84, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Bucelli, R.C.; Andrews, J.A.; Otto, M.; Farahany, N.A.; Harrington, E.A.; Chen, W.; Mitchell, A.A.; et al. Design of a Randomized, Placebo-Controlled, Phase 3 Trial of Tofersen Initiated in Clinically Presymptomatic SOD1 Variant Carriers: The ATLAS Study. Neurotherapeutics 2022, 19, 1248–1258. [Google Scholar] [CrossRef]
- Benatar, M.; Robertson, J.; Andersen, P.M. Amyotrophic Lateral Sclerosis Caused by SOD1 Variants: From Genetic Discovery to Disease Prevention. Lancet Neurol. 2025, 24, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Moens, T.G.; Da Cruz, S.; Neumann, M.; Shelkovnikova, T.A.; Shneider, N.A.; Van Den Bosch, L. Amyotrophic Lateral Sclerosis Caused by FUS Mutations: Advances with Broad Implications. Lancet Neurol. 2025, 24, 166–178. [Google Scholar] [CrossRef]
- Mizielinska, S.; Hautbergue, G.M.; Gendron, T.F.; van Blitterswijk, M.; Hardiman, O.; Ravits, J.; Isaacs, A.M.; Rademakers, R. Amyotrophic Lateral Sclerosis Caused by Hexanucleotide Repeat Expansions in C9orf72: From Genetics to Therapeutics. Lancet Neurol. 2025, 24, 261–274. [Google Scholar] [CrossRef]
- Balendra, R.; Sreedharan, J.; Hallegger, M.; Luisier, R.; Lashuel, H.A.; Gregory, J.M.; Patani, R. Amyotrophic Lateral Sclerosis Caused by TARDBP Mutations: From Genetics to TDP-43 Proteinopathy. Lancet Neurol. 2025, 24, 456–470. [Google Scholar] [CrossRef]
- Saba, L.; Viscomi, M.T.; Caioli, S.; Pignataro, A.; Bisicchia, E.; Pieri, M.; Molinari, M.; Ammassari-Teule, M.; Zona, C. Altered Functionality, Morphology, and Vesicular Glutamate Transporter Expression of Cortical Motor Neurons from a Presymptomatic Mouse Model of Amyotrophic Lateral Sclerosis. Cereb. Cortex 2016, 26, 1512–1528. [Google Scholar] [CrossRef]
- Geevasinga, N.; Menon, P.; Özdinler, P.H.; Kiernan, M.C.; Vucic, S. Pathophysiological and Diagnostic Implications of Cortical Dysfunction in ALS. Nat. Rev. Neurol. 2016, 12, 651–661. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and Recommendations for TMS Use in Healthy Subjects and Patient Populations, with Updates on Training, Ethical and Regulatory Issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Pellegrino, G.; Ranieri, F.; Florio, L.; Musumeci, G.; Caulo, M.; Ferretti, A.; Capone, F. Effects of Repetitive TMS of the Motor Cortex on Disease Progression and on Glutamate and GABA Levels in ALS: A Proof of Principle Study. Brain Stimul. 2017, 10, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Pellegrino, G.; Capone, F.; Florio, L.; Dileone, M.; Cioni, B.; Ranieri, F. Reduction of Disease Progression in a Patient with Amyotrophic Lateral Sclerosis after Several Years of Epidural Motor Cortex Stimulation. Brain Stimul. 2017, 10, 324–325. [Google Scholar] [CrossRef]
- Voelker, R. Brain Stimulation Approved for Obsessive-Compulsive Disorder. JAMA 2018, 320, 1098. [Google Scholar] [CrossRef] [PubMed]
- Cocores, A.N.; Smirnoff, L.; Greco, G.; Herrera, R.; Monteith, T.S. Update on Neuromodulation for Migraine and Other Primary Headache Disorders: Recent Advances and New Indications. Curr. Pain Headache Rep. 2025, 29, 47. [Google Scholar] [CrossRef]
- Boes, A.D.; Kelly, M.S.; Trapp, N.T.; Stern, A.P.; Press, D.Z.; Pascual-Leone, A. Noninvasive Brain Stimulation: Challenges and Opportunities for a New Clinical Specialty. J. Neuropsychiatry Clin. Neurosci. 2018, 30, 173–179. [Google Scholar] [CrossRef]
- Antal, A.; Ganho-Ávila, A.; Assecondi, S.; Barbour, T.; Bjekić, J.; Blumberger, D.M.; Bolognini, N.; Brunelin, J.; Chanes, L.; Dale, M.; et al. The Consequences of the New European Reclassification of Non-Invasive Brain Stimulation Devices and the Medical Device Regulations Pose an Existential Threat to Research and Treatment: An Invited Opinion Paper. Clin. Neurophysiol. 2024, 163, 280–291. [Google Scholar] [CrossRef]
- Gordon, P.H.; Cheng, B.; Salachas, F.; Pradat, P.-F.; Bruneteau, G.; Corcia, P.; Lacomblez, L.; Meininger, V. Progression in ALS Is Not Linear but Is Curvilinear. J. Neurol. 2010, 257, 1713–1717. [Google Scholar] [CrossRef]
- Consonni, M.; Dalla Bella, E.; Contarino, V.E.; Bersano, E.; Lauria, G. Cortical Thinning Trajectories across Disease Stages and Cognitive Impairment in Amyotrophic Lateral Sclerosis. Cortex 2020, 131, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Hannaford, A.; Byth, K.; Pavey, N.; Henderson, R.D.; Mathers, S.; Needham, M.; Schultz, D.; Menon, P.; Kiernan, M.C.; Vucic, S. Clinical and Neurophysiological Biomarkers of Disease Progression in Amyotrophic Lateral Sclerosis. Muscle Nerve 2023, 67, 17–24. [Google Scholar] [CrossRef]
- Baker, M.R. ALS—Dying Forward, Backward or Outward? Nat. Rev. Neurol. 2014, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Gazzina, S.; Premi, E.; Cosseddu, M.; Archetti, S.; Dell’Era, V.; Cantoni, V.; Cotelli, M.S.; Alberici, A.; Micheli, A.; et al. Clinical and Biomarker Changes in Presymptomatic Genetic Frontotemporal Dementia. Neurobiol. Aging 2019, 76, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Benelli, A.; Memoli, C.; Neri, F.; Romanella, S.M.; Cinti, A.; Giannotta, A.; Lomi, F.; Scoccia, A.; Pandit, S.; Zambetta, R.M.; et al. Reduction of Cognitive Fatigue and Improved Performance at a VR-Based Driving Simulator Using TRNS. iScience 2024, 27, 110536. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cosseddu, M.; Cantoni, V.; Cotelli, M.S.; Cotelli, M.; Manenti, R.; Benussi, L.; Brattini, C.; Alberici, A.; et al. Transcranial Stimulation in Frontotemporal Dementia: A Randomized, Double-blind, Sham-controlled Trial. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12033. [Google Scholar] [CrossRef]
- Ceccanti, M.; Onesti, E.; Rubino, A.; Cambieri, C.; Tartaglia, G.; Miscioscia, A.; Frasca, V.; Inghilleri, M. Modulation of Human Corticospinal Excitability by Paired Associative Stimulation in Patients with Amyotrophic Lateral Sclerosis and Effects of Riluzole. Brain Stimul. 2018, 11, 775–781. [Google Scholar] [CrossRef] [PubMed]





| Study | Bias Arising from the Randomization Process | Bias Arising from Period and Carryover Effects (Crossover Trials Only) | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Outcome Data | Bias in the Measurement of the Outcome | Bias in the Selection of the Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| Zheng W. et al. (2025) [122] | Low Risk | Not applicable | Some Concerns | Some Concerns | Low Risk | Low Risk | High Risk |
| Madhavan S. et al. (2025) [109] | High Risk | Not applicable | Low Risk | High Risk | Low Risk | Some Concerns | High Risk |
| Di Lazzaro V. et al. (2024) [92] | Low Risk | Not applicable | Some Concerns | Some Concerns | Low Risk | Some Concerns | High Risk |
| Benussi A. et al. (2023) [41] | Low Risk | Not applicable | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Sivaramakrishnan A. et al. (2019) [127] | High Risk | Not applicable | High Risk | Low Risk | High Risk | High Risk | High Risk |
| Benussi A. et al. (2019) [128] | Low Risk | Not applicable | Low Risk | High Risk | Low Risk | Low Risk | High Risk |
| Madhavan S. et al. (2018) [126] | High Risk | Not applicable | High Risk | Low Risk | High Risk | High Risk | High Risk |
| Di Lazzaro V. et al. (2014) [129] | High Risk | Not applicable | High Risk | High Risk | High Risk | High Risk | High Risk |
| Munneke M. et al. (2013) [121] | High Risk | Not applicable | Some Concerns | Low Risk | Low Risk | Some Concerns | High Risk |
| Di Lazzaro V. et al. (2013) [125] | High Risk | Not applicable | High Risk | Low Risk | Some Concerns | High Risk | High Risk |
| Munneke M. et al. (2011) [124] | High Risk | Not applicable | Some Concerns | Low Risk | Low Risk | Some Concerns | High Risk |
| Di Lazzaro V. et al. (2010) [120] | High Risk | Not applicable | High Risk | Low Risk | Some Concerns | High Risk | High Risk |
| Di Lazzaro V. et al. (2009) [72] | Low Risk | Not applicable | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Zanette G. et al. (2008) [119] | Low Risk | Not applicable | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk |
| Quartarone A. et al. (2007) [123] | Some Concerns | Low Risk | Low Risk | Low Risk | Low Risk | Low Risk | Some Concerns |
| Di Lazzaro V. et al. (2006) [118] | Low Risk | Not applicable | Some Concerns | Some Concerns | Low Risk | Some Concerns | High Risk |
| Di Lazzaro V. et al. (2004) [117] | High Risk | Not applicable | High Risk | Low Risk | Some Concerns | Some Concerns | High Risk |
| Angelucci F. et al. (2004) [71] | High Risk | Some Concerns | Some Concerns | Low Risk | Low Risk | Some Concerns | High Risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Toffola, J.; Ricci, E.; Quagliotto, M.; Manganotti, P.; Benussi, A. Non-Invasive Brain Stimulation for Amyotrophic Lateral Sclerosis: Current Evidence and Future Perspectives. Medicina 2025, 61, 1685. https://doi.org/10.3390/medicina61091685
Della Toffola J, Ricci E, Quagliotto M, Manganotti P, Benussi A. Non-Invasive Brain Stimulation for Amyotrophic Lateral Sclerosis: Current Evidence and Future Perspectives. Medicina. 2025; 61(9):1685. https://doi.org/10.3390/medicina61091685
Chicago/Turabian StyleDella Toffola, Jacopo, Edoardo Ricci, Magda Quagliotto, Paolo Manganotti, and Alberto Benussi. 2025. "Non-Invasive Brain Stimulation for Amyotrophic Lateral Sclerosis: Current Evidence and Future Perspectives" Medicina 61, no. 9: 1685. https://doi.org/10.3390/medicina61091685
APA StyleDella Toffola, J., Ricci, E., Quagliotto, M., Manganotti, P., & Benussi, A. (2025). Non-Invasive Brain Stimulation for Amyotrophic Lateral Sclerosis: Current Evidence and Future Perspectives. Medicina, 61(9), 1685. https://doi.org/10.3390/medicina61091685






