Indocyanine Green Angiography in the Evaluation of Surgical Amputation Level in Patients with Arterial Ulcers Due to Thromboangiitis Obliterans
Abstract
1. Introduction
2. Method
2.1. Ethical Considerations
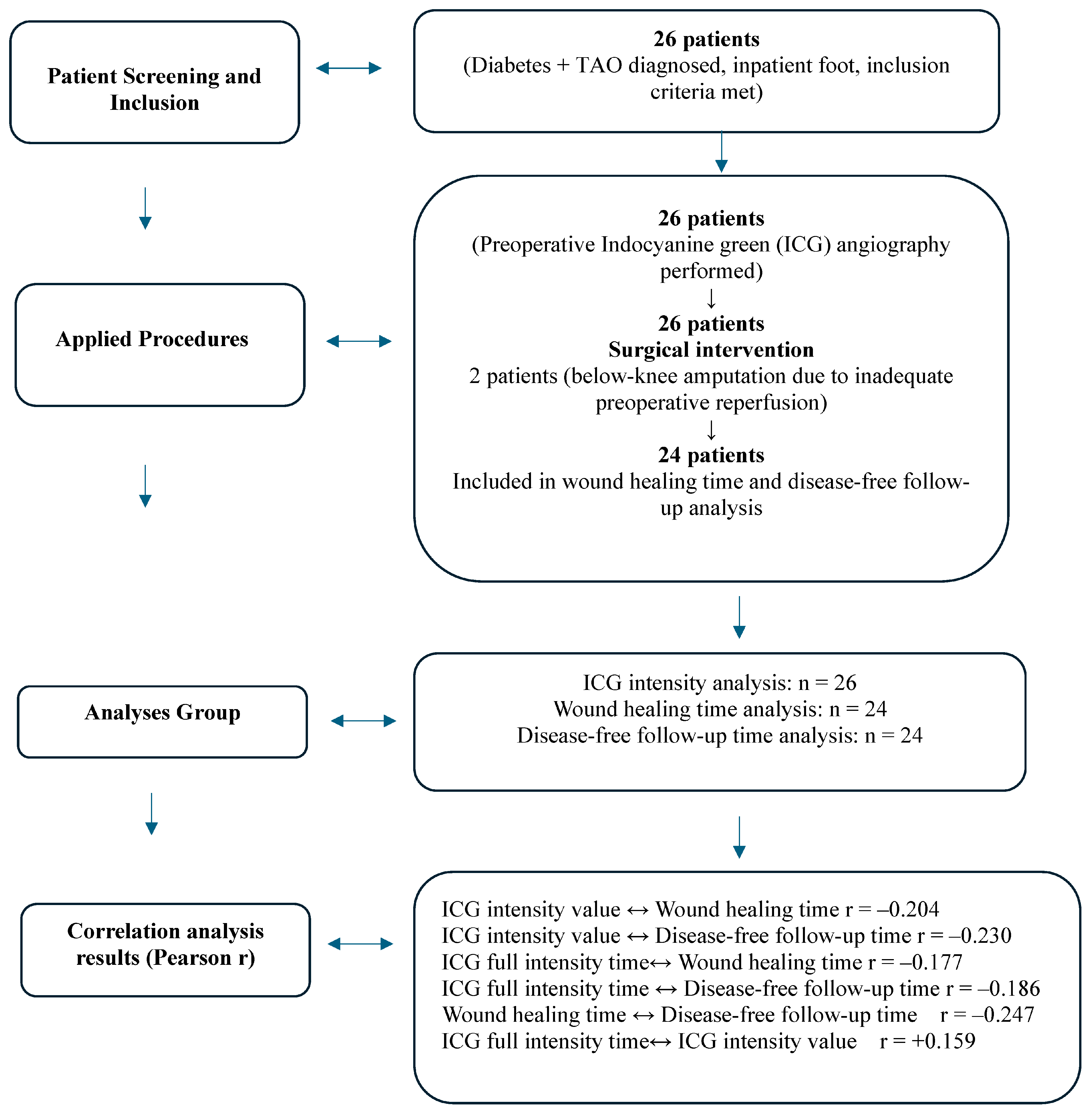
2.2. Research Implementation Process
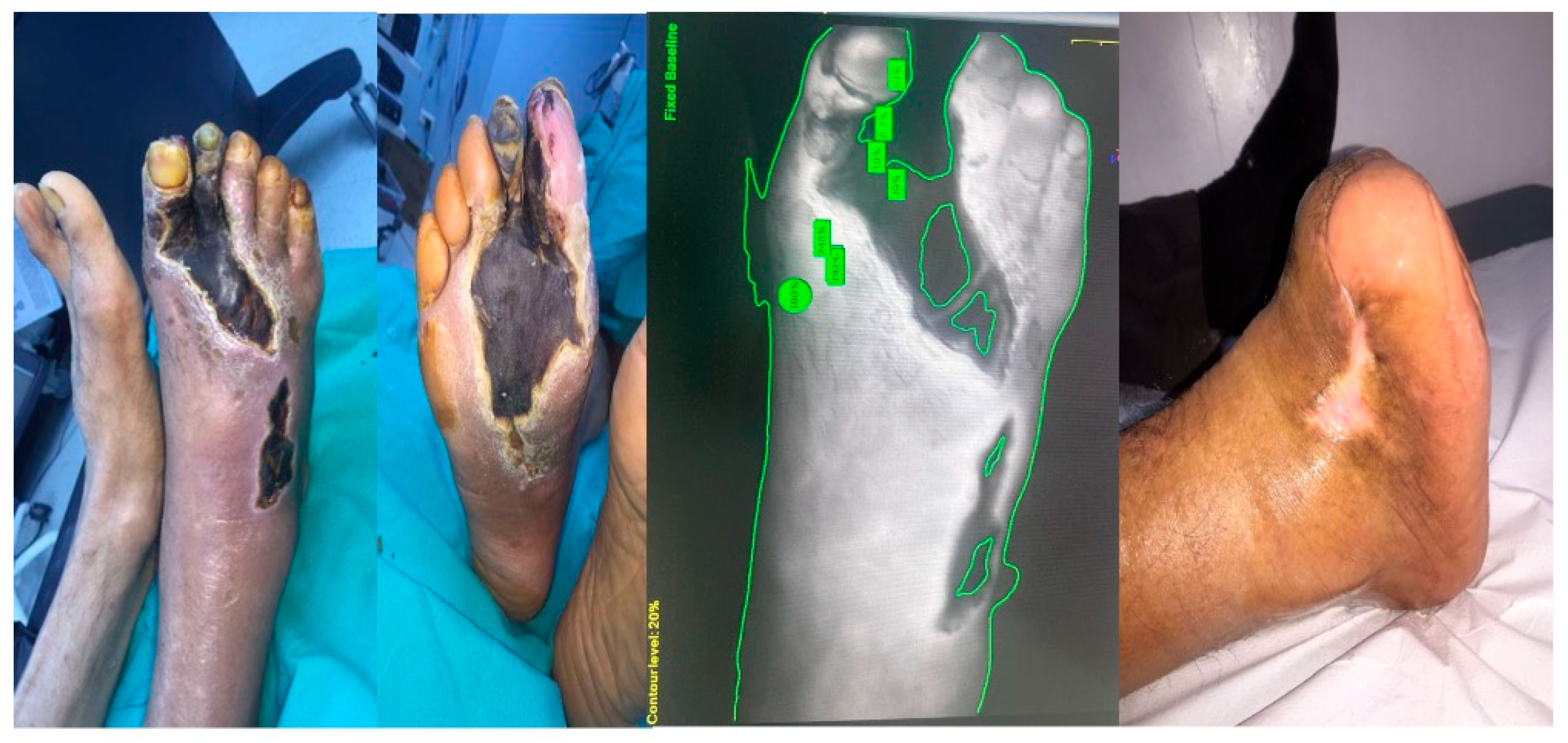
2.3. ICG Fluorescence Imaging
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Wang, Y.; Shi, W.; Shen, K.; Tao, K.; Ling, R.; Huang, Y.; Fu, X.; Hu, D. Diagnosis and Treatment of Diabetic Foot Ulcer Complicated with Lower Extremity Vasculopathy: Consensus Recommendation from The Chinese Medical Association (Cma), Chinese Medical Doctor Association (Cmda). Diabetes Metab. Res. Rev. 2024, 40, E3776. [Google Scholar] [CrossRef] [PubMed]
- Schaper, N.C.; Van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Fitridge, R.; Game, F.; Monteiro-Soares, M.; Senneville, E.; IWGDF Editorial Board. Practical Guidelines On the Prevention and Management of Diabetes-Related Foot Disease (Iwgdf 2023 Update). Diabetes Metab. Res. Rev. 2024, 40, E3657. [Google Scholar] [CrossRef]
- Olin, J.W. Thromboangiitis Obliterans (Buerger’s Disease). N. Engl. J. Med. 2000, 343, 864–869. [Google Scholar] [CrossRef]
- Dargon, P.T.; Landry, G.J. Buerger’s Disease. Ann. Vasc. Surg. 2012, 26, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Dimmick, S.J.; Goh, A.C.; Cauzza, E.; Steinbach, L.S.; Baumgartner, I.; Stauffer, E.; Voegelin, E.; Anderson, S.E. Imaging Appearances of Buerger’s Disease Complications İn The Upper And Lower Limbs. Clin. Radiol. 2012, 67, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, B.; Poredos, P.; Kozak, M.; Pecsvarady, Z.; Catalano, M.; Al Salman, M.M.; Altarazi, L.; Ali, A.A.; Bashar, A.H.; Bozkurt, K.; et al. Diagnostic Criteria for Buerger’s Disease: International Consensus of Vas-European Independent Foundation İn Angiology/Vascular Medicine. Int. Angiol. 2023, 42, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Shionoya, S. Diagnostic Criteria Of Buerger’s Disease. Int. J. Cardiol. 1998, 66 (Suppl. 1), S243–S245. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Tiwari, R.; Kumar Prabhuswamy, V. Thromboangiitis Obliterans (Buerger’s Disease)-Current Practices. Int. J. Inflam. 2013, 2013, 156905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mavioğlu, L.; Mungan, U.; Özeke, Ö.; Ertan, Ç.; Özatik, M.A. Buerger’s Disease (Thromboangiitis Obliterans) with an Atypical Presentation: A Case Report. Turk. J. Thorac. Cardiovasc. Surg. 2013, 21, 1039–1042. [Google Scholar] [CrossRef]
- Mills, J.L.; Taylor, L.M., Jr.; Porter, J.M. Buerger’s disease in the modern era. Am. J. Surg. 1987, 154, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.; Creager, M. Thromboangiitis Obliterans. Circulation 2010, 121, 1858–1861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghirardini, F.; Martini, R. Current Opinion on Diagnosis of Peripheral Artery Disease in Diabetic Patients. Medicina 2024, 60, 1179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Misra, S.; Shishehbor, M.H.; Takahashi, E.A.; Aronow, H.D.; Brewster, L.P.; Bunte, M.C.; Kim, E.S.H.; Lindner, J.R.; Rich, K.; American Heart Association Council on Peripheral Vascular Disease; et al. Perfusion Assessment in Critical Limb Ischemia: Principles for Understanding and the Development of Evidence and Evaluation of Devices: A Scientific Statement from the American Heart Association. Circulation 2019, 140, E657–E672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on The Management of Chronic Limb-Threatening İschemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109. [Google Scholar] [CrossRef]
- Leenstra, B.; Wijnand, J.; Verhoeven, B.; Koning, O.; Teraa, M.; Verhaar, M.C.; De Borst, G.J. Applicability of Transcutaneous Oxygen Tension Measurement in The Assessment of Chronic Limb-Threatening Ischemia. Angiology 2020, 71, 208–216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Catella, J.; Long, A.; Mazzolai, L. What Is Currently The Role of Tcpo2 in The Choice of The Amputation Level of Lower Limbs? A Comprehensive Review. J. Clin. Med. 2021, 10, 1413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Met, R.; Bipat, S.; Legemate, D.A.; Reekers, J.A.; Koelemay, M.J. Diagnostic performance of computed tomography angiography in peripheral arterial disease: A systematic review and meta-analysis. JAMA 2009, 301, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.A.; Boyle, E.M.; Clarke Moloney, M.; Hodnett, P.A.; Scanlon, T.; Grace, P.A.; Walsh, S.R. Contrast-enhanced magnetic resonance angiography in diabetic patients with infra-genicular peripheral arterial disease: A systematic review. Int. J. Surg. 2013, 11, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Spittell, J.A. Tromboangiitis Obliterans: An Autoimmune Disorder? N. Engl. Med. 1983, 308, 1157–1158. [Google Scholar] [CrossRef] [PubMed]
- Karakoyun, R.; Köksoy, C.; Şener, Z.; Gündüz, U.; Karakaş, B.; Karakoyun, M. Comparison of Quality of Life in Patients with Peripheral Arterial Disease Caused by Atherosclerosis Obliterans or Buerger’s Disease. Cardiovasc. J. Afr. 2014, 25, 124–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Van Den Hoven, P.; Ooms, S.; Van Manen, L.; Van Der Bogt Kea Van Schaik, J.; Hamming, J.F.; Vahrmeijer, A.L.; Van Der Vorst Jr Mieog, J.S.D. A Systematic Review of The Use of Near-İnfrared Fluorescence İmaging in Patients with Peripheral Artery Disease. J. Vasc. Surg. 2019, 70, 286–297.E1. [Google Scholar] [CrossRef] [PubMed]
- Mothes, H.; Dönicke, T.; Friedel, R.; Simon, M.; Markgraf, E.; Bach, O. Clinical use of indocyanine green fluorescence video angiography to assess tissue perfusion in microsurgery. J. Trauma Acute Care Surg. 2004, 57, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Jang, S.Y.; Kim, S.K.; Kim, N.; Kim, K.; Kim, D.K. The İncidence, Prevalence, And Survival Rate of Thromboangiitis Obliterans in Korea: A Retrospective Population-Based Study. Cardiovasc. Diagn. Ther. 2020, 10, 1238–1244. [Google Scholar] [CrossRef]
- Fiessinger, J.N.; Frank, M. Maladie de Buerger [Thromboangııtıs Oblıterans (Buerger’s Dısease)]. Rev. Prat. 2015, 65, 1079–1083. [Google Scholar]
- Chen, Q.; Chen, J.; Li, J.; Cheng, Y.; Zhang, R.; Liu, Z. Recent Advances of Oxidative Stress in Thromboangiitis Obliterans: Biomolecular Mechanisms, Biomarkers, Sources and Clinical Applications. Thromb. Res. 2023, 230, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kawarada, O.; Kume, T.; Ayabe, S.; Nakaya, T.; Nakai, M.; Nishimura, K.; Noguchi, T.; Yokoi, Y.; Ogawa, H.; Yasuda, S. Endovascular Therapy Outcomes and Intravascular Ultrasound Findings in Thromboangiitis Obliterans (Buerger’s Disease). J. Endovasc. Ther. 2017, 24, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Firat, A.; Igus, B. Endovascular Recanalization of Thromboangiitis Obliterans (Buerger’s Disease) in Twenty-Eight Consecutive Patients And Combined Antegrade-Retrograde Intervention in Eight Patients. Cardiovasc. Interv. Radiol. 2019, 42, 820–828. [Google Scholar] [CrossRef]
- Wilke, B.K.; Schultz, D.S.; Huayllani, M.T.; Boczar, D.; Spaulding, A.C.; Sherman, C.E.; Murray, P.M.; Forte, A.J. Intraoperative Indocyanine Green Fluorescence Angiography Is Sensitive for Predicting Postoperative Wound Complications in Soft-Tissue Sarcoma Surgery. J. Am. Acad. Orthop. Surg. 2021, 29, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Roenneberg, C.; Wendorff, H.; Holzbach, T.; Giunta, R.E.; Eckstein, H.H. Early Postoperative Detection of Tissue Necrosis İn Amputation Stumps with İndocyanine Green Fluorescence Angiography. Vasc. Endovasc. Surg. 2010, 44, 269–273. [Google Scholar] [CrossRef]
| Variables | Mean ± SD (Min–Max) | n (%) |
|---|---|---|
| Age (years) | 62.31 ± 9.97 | |
| Gender | ||
| Female | 3 (11.5) | |
| Male | 23 (88.5) | |
| Smoking history | ||
| Yes | 26 (100.0) | |
| Presence of Diabetes | ||
| Yes | 26 (100.0) | |
| Wound classification—WAGNER | ||
| Wagner Type 3 | 1 (3.8) | |
| Wagner Type 4 | 25 (96.2) | |
| Wound classification—PEDIS | ||
| PEDIS Type 2 | 3 (11.5) | |
| PEDIS Type 3 | 23 (88.5) | |
| Peripheral Endovascular Angiography | ||
| Performed | 23 (88.5) | |
| Not performed | 3 (11.5) | |
| Success status of angiography | ||
| Successful | 16 (61.5) | |
| Not successful | 7 (26.9) | |
| Surgery performed | ||
| Debridement | 1 (3.8) | |
| Minor Amputation | 9 (34.6) | |
| Minor Amputation and Debridement | 6 (23.1) | |
| Forefoot amputation | 10 (38.5) | |
| Postoperative Flap Necrosis | ||
| Yes | 4 (15.4) | |
| No | 22 (84.6) | |
| Wound Complications | ||
| None | 15 (57.7) | |
| Delayed wound healing | 7 (26.9) | |
| Flap necrosis | 4 (15.4) | |
| Repeated surgery/Re-amputation | ||
| Yes | 7 (26.9) | |
| No | 19 (73.1) | |
| Variables | Mean ± SD (Min–Max) | n (%) |
|---|---|---|
| ICG Intensity Value | 165.58 ± 27.6 (123–236) | 26 (100.0) |
| ICG Full Intensity Time (s) | 27.46 ± 6.4 (17–45) | 26 (100.0) |
| Time to Wound Healing (days) | 147.12 ± 107.36 (30–365) | 24 (100.0) |
| Disease-free follow-up duration (days) | 469.17 ± 206.48 (30–835) | 24 (100.0) |
| ICG Full Intensity Time (s) | Time to Wound Healing (Days) | Disease-Free Follow-Up Duration | ICG Intensity Value | |
|---|---|---|---|---|
| ICG Full Intensity Time (s) | 1 | |||
| Time to wound healing (days) | −0.177 | 1 | ||
| Disease-free follow-up duration | −0.186 | −247 | 1 | |
| ICG intensity value | 0.159 | −0.204 | −0.23 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akyüz, S.; Güven, H.E.; Yılmaz, K.B. Indocyanine Green Angiography in the Evaluation of Surgical Amputation Level in Patients with Arterial Ulcers Due to Thromboangiitis Obliterans. Medicina 2025, 61, 1678. https://doi.org/10.3390/medicina61091678
Akyüz S, Güven HE, Yılmaz KB. Indocyanine Green Angiography in the Evaluation of Surgical Amputation Level in Patients with Arterial Ulcers Due to Thromboangiitis Obliterans. Medicina. 2025; 61(9):1678. https://doi.org/10.3390/medicina61091678
Chicago/Turabian StyleAkyüz, Simay, Hikmet Erhan Güven, and Kerim Bora Yılmaz. 2025. "Indocyanine Green Angiography in the Evaluation of Surgical Amputation Level in Patients with Arterial Ulcers Due to Thromboangiitis Obliterans" Medicina 61, no. 9: 1678. https://doi.org/10.3390/medicina61091678
APA StyleAkyüz, S., Güven, H. E., & Yılmaz, K. B. (2025). Indocyanine Green Angiography in the Evaluation of Surgical Amputation Level in Patients with Arterial Ulcers Due to Thromboangiitis Obliterans. Medicina, 61(9), 1678. https://doi.org/10.3390/medicina61091678






