Urinary Urocortin as a Potential Non-Invasive Biomarker in Endometriosis: Exploratory Study with Histone H4
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion/Exclusion Criteria
2.3. Surgery, Sample Collection, and Staging
2.4. Biomarker Measurement
2.4.1. Urocortin Quantification
2.4.2. Histone H4 Quantification
2.4.3. CA-125
2.5. Statistical Analysis
3. Results
3.1. Baseline Parameters
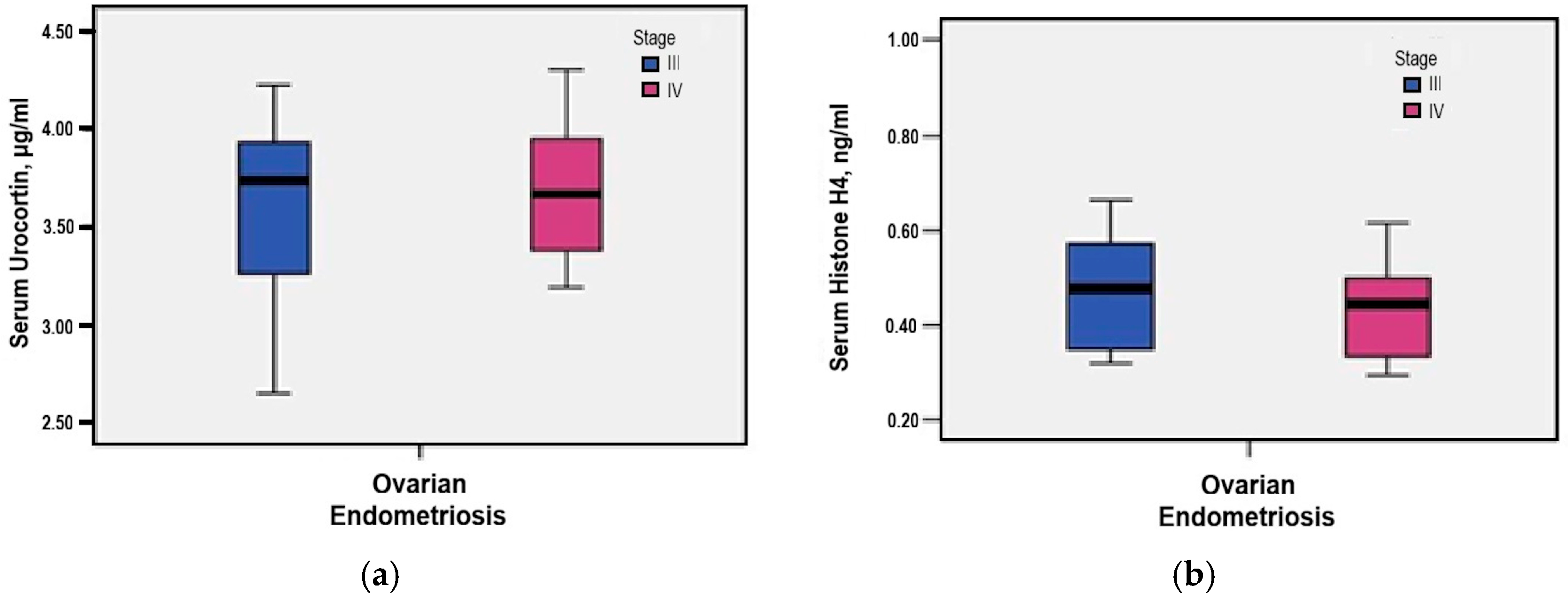
3.2. Surgical Staging and Disease Extension
3.3. Endometriosis Fertility Index (EFI) and Postoperative Reproductive Outcomes
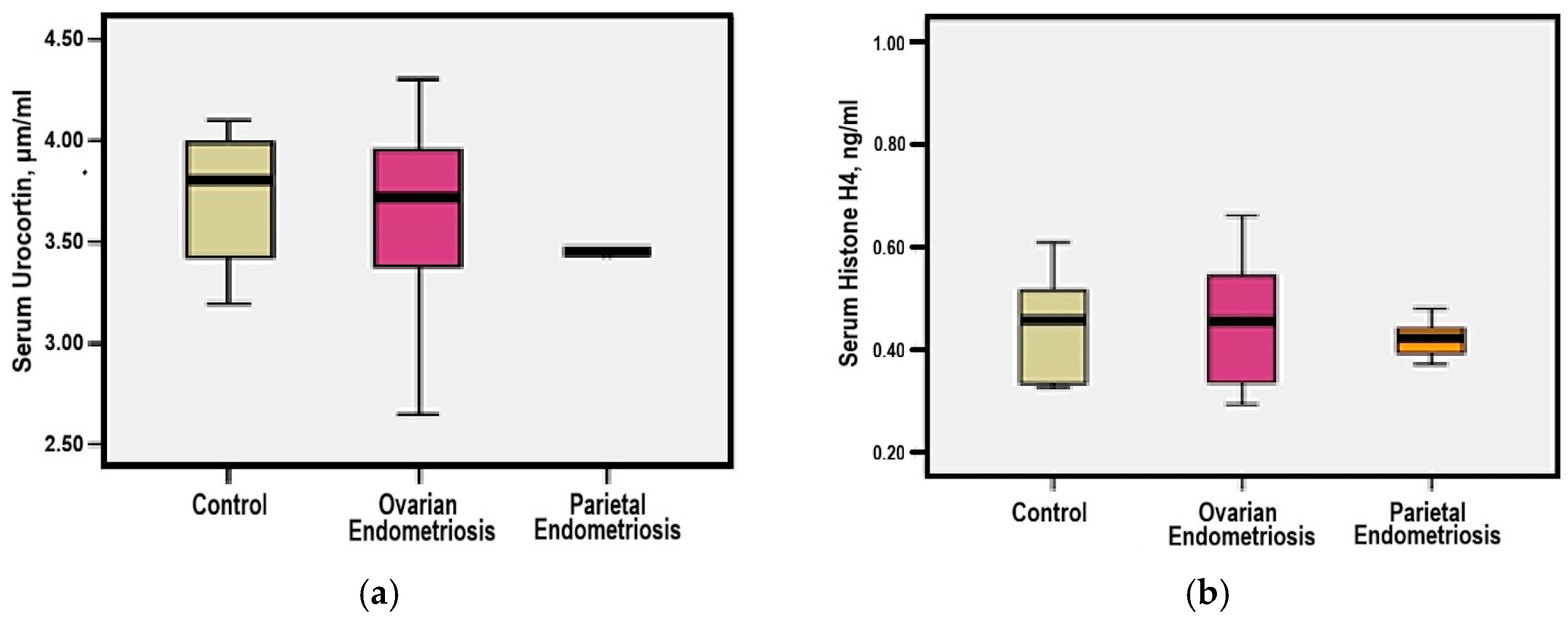
3.4. Serum Urocortin and Histone H4 Levels
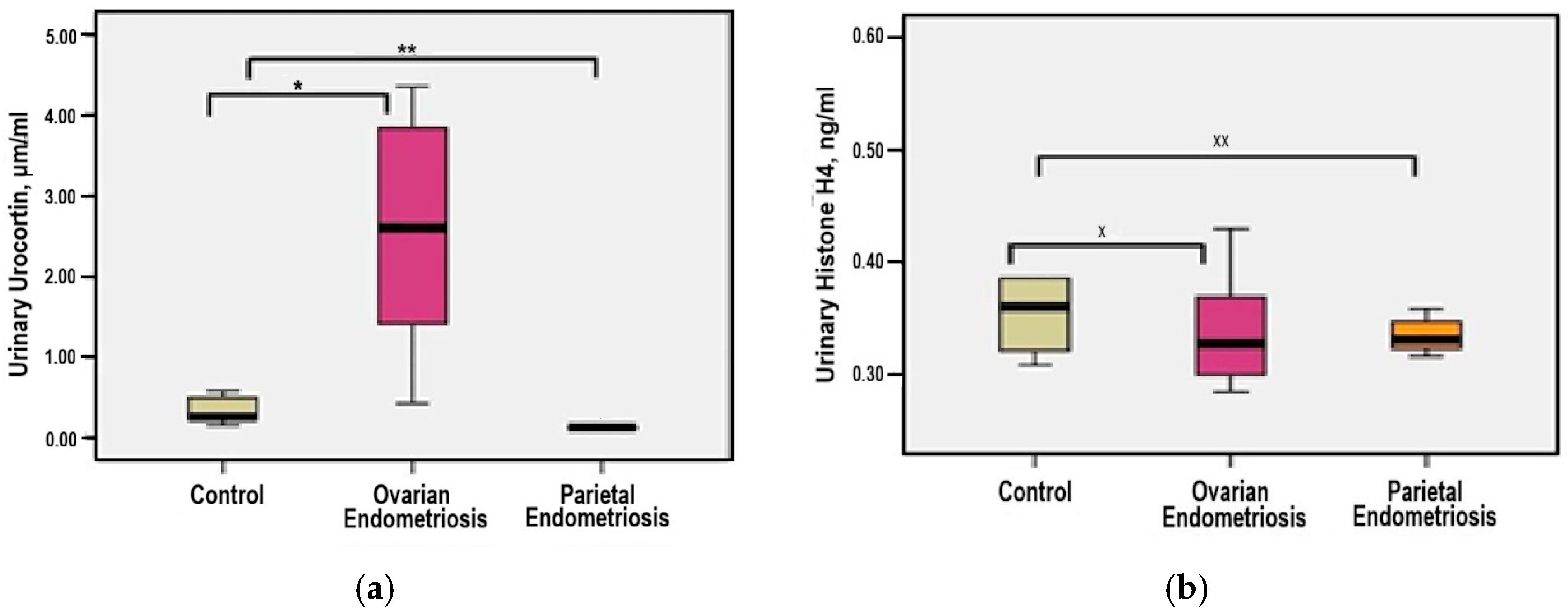
3.5. Urinary Urocortin and Histone H4 Levels
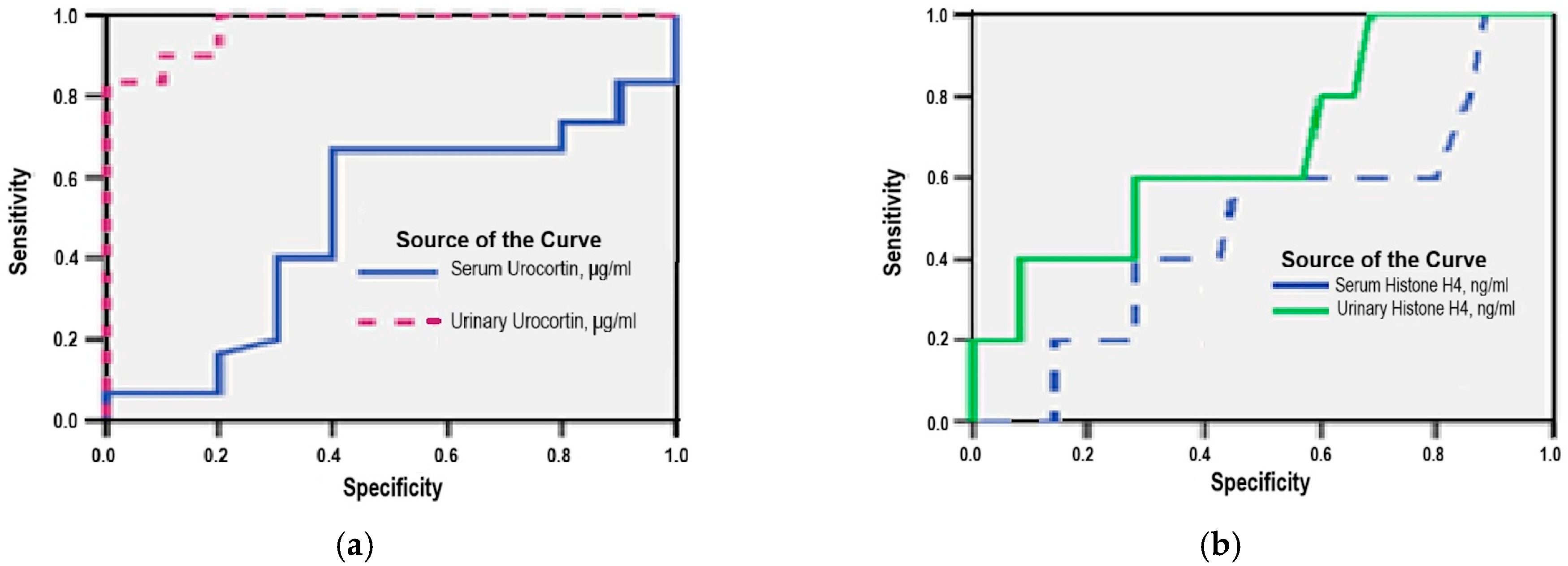
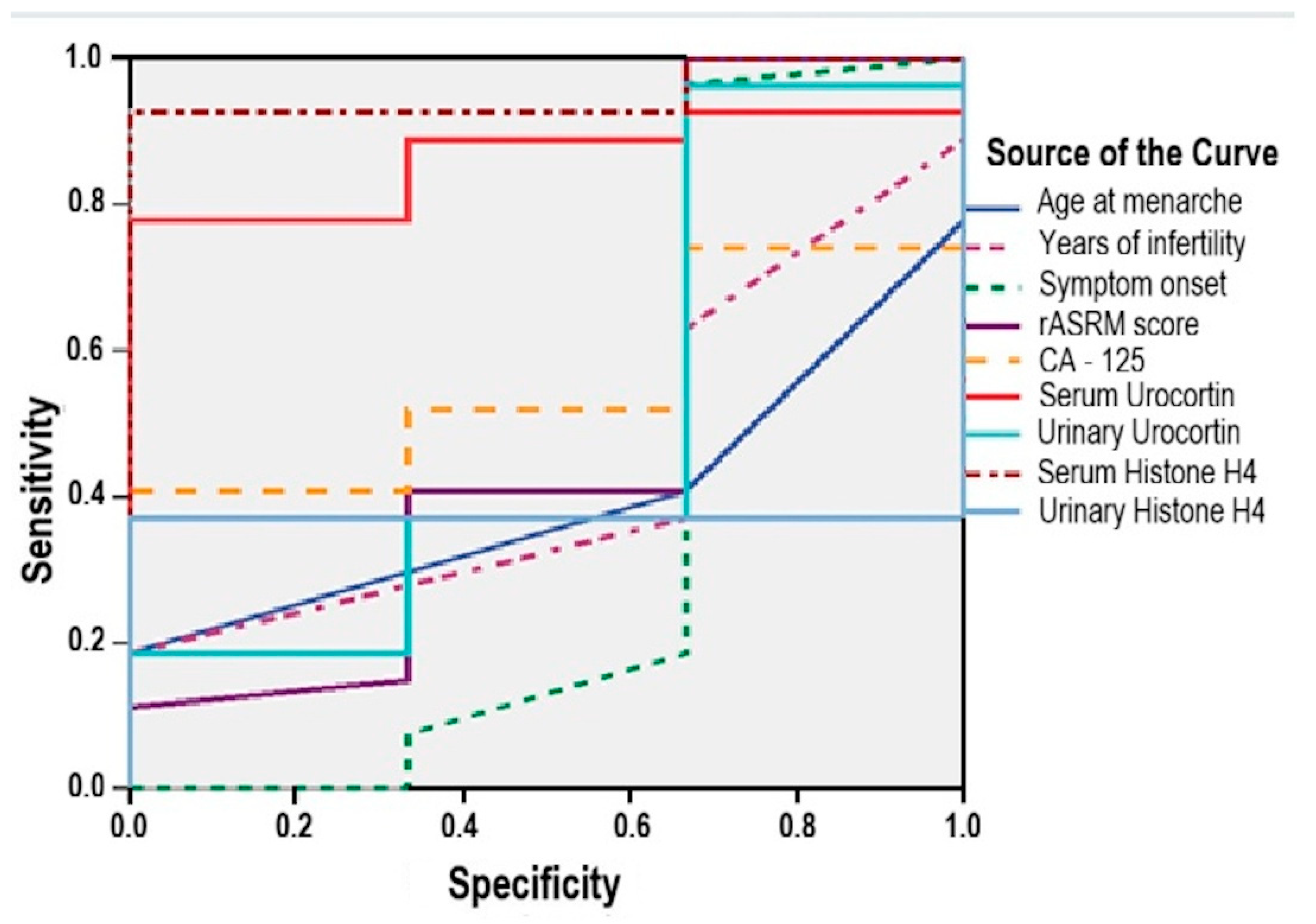
3.6. Diagnostic Accuracy of Serum and Urinary Urocortin and Histone H4
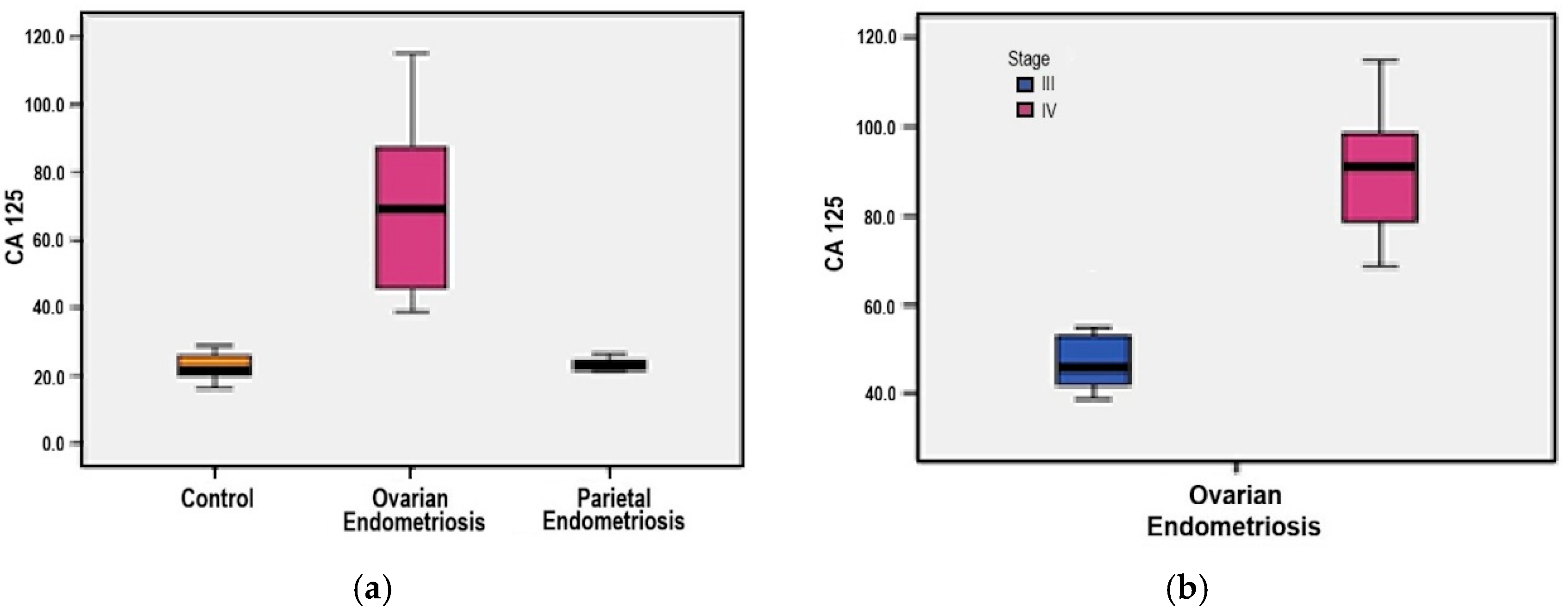
3.7. Serum CA-125 Levels
3.8. Multivariate Regression Analysis
3.8.1. Serum Urocortin
3.8.2. Urinary Urocortin
3.8.3. Serum Histone H4
3.8.4. Urinary Histone H4
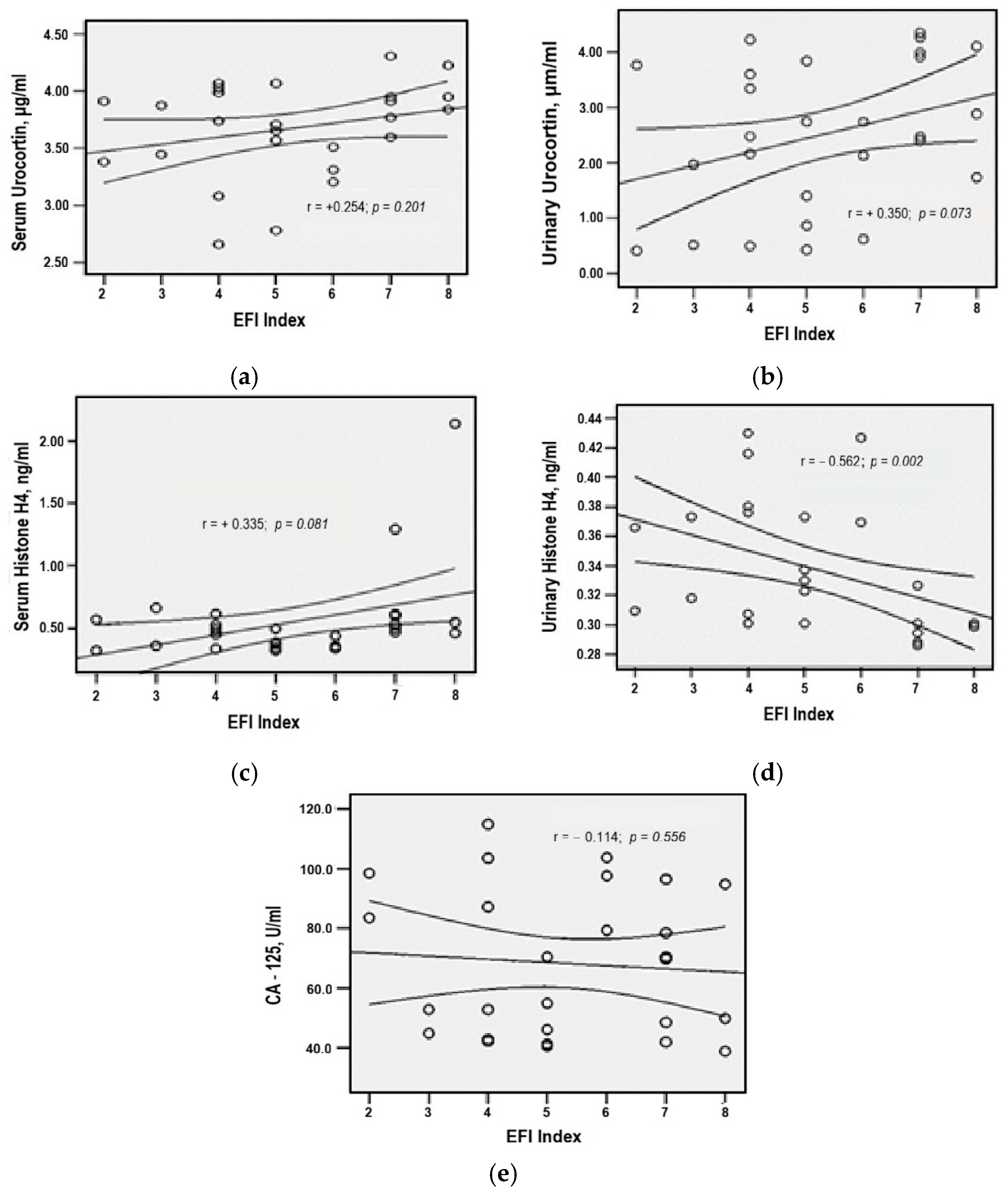
3.9. Correlation Patterns of Serum and Urinary Biomarkers in Ovarian Endometriosis
3.10. Predictive Biomarkers for Postoperative Conception in Ovarian Endometriosis
- -
- Serum Urocortin was a significant predictor (AUC = 0.864; 95% CI: 0.728–0.999; p = 0.041), with a sensitivity of 85% and specificity of 67% at a cut-off value above 3.25 μg/mL;
- -
- Serum Histone H4 showed even stronger performance (AUC = 0.951; 95% CI: 0.869–0.999; p = 0.012), yielding a sensitivity of 92.6% and specificity of 67% for a cut-off threshold above 0.32 ng/mL.
- -
- age at menarche (AUC = 0.395; 95% CI: 0.115–0.635; p = 0.557);
- -
- infertility duration (AUC = 0.438; 95% CI: 0.136–0.740; p = 0.730);
- -
- symptom duration (AUC = 0.370; 95%: 0.126–0.867; p = 0.468);
- -
- rASRM score (AUC = 0.512; 95% CI: 0.088–0.936; p = 0.945);
- -
- serum CA125 (AUC = 0.556; 95% CI: 0.328–0.783; p = 0.756);
- -
- urinary Urocortin (AUC = 0.506; 95% CI: 0.113–0.900; p = 0.972);
- -
- urinary Histone H4 (AUC = 0.370; 95% CI: 0.188–0.553; p = 0.468).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.A.; D’Hooghe, T.M.; Fazleabas, A.; Giudice, L.C.; Montgomery, G.W.; Petraglia, F.; Taylor, R.N. Defining future directions for endometriosis research: Workshop report from the 2011 World Congress of Endometriosis in Montpellier, France. Reprod. Sci. 2013, 20, 483–499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fassbender, A.; Vodolazkaia, A.; Saunders, P.; Lebovic, D.; Waelkens, E.; De Moor, B.; D’Hooghe, T. Biomarkers of endometriosis. Fertil. Steril. 2013, 99, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T. Biomarkers for Endometriosis; Springer International Publishing AG: Cham, Switzerland, 2017; ISBN 978-3-319-59856-7. [Google Scholar] [CrossRef]
- Krygere, L.; Jukna, P.; Jariene, K.; Drejeriene, E. Diagnostic Potential of Cytokine Biomarkers in Endometriosis: Challenges and Insights. Biomedicines 2024, 12, 2867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE Endometriosis Guideline Group. ESHRE guideline: Endometriosis. Hum. Reprod. 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Višnić, A.; Barišić, D.; Jurešić, G.Č.; Klarić, M.; Šepić, T.S. Concentration of total proteins in urine as a non-invasive biomarker of endometriosis. JBRA Assist. Reprod. 2023, 27, 624–628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Konrad, L.; Fruhmann Berger, L.M.; Maier, V.; Horné, F.; Neuheisel, L.M.; Laucks, E.V.; Riaz, M.A.; Oehmke, F.; Meinhold-Heerlein, I.; Zeppernick, F. Predictive Model for the Non-Invasive Diagnosis of Endometriosis Based on Clinical Parameters. J. Clin. Med. 2023, 12, 4231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Condous, G.; Gerges, B.; Thomassin-Naggara, I.; Becker, C.; Tomassetti, C.; Krentel, H.; van Herendael, B.J.; Malzoni, M.; Abrao, M.S.; Saridogan, E.; et al. Non-invasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An International Consensus Statement. Facts Views Vis. ObGyn 2024, 16, 127–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ravaggi, A.; Bergamaschi, C.; Galbiati, C.; Zanotti, L.; Fabricio, A.S.C.; Gion, M.; Cappelletto, E.; Leon, A.E.; Gennarelli, M.; Romagnolo, C.; et al. Circulating Serum Micro-RNA as Non-Invasive Diagnostic Biomarkers of Endometriosis. Biomedicines 2024, 12, 2393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liew, H.K.; Huang, L.C.; Yang, H.I.; Peng, H.F.; Li, K.W.; Tsai, A.P.; Chen, S.Y.; Kuo, J.S.; Pang, C.Y. Therapeutic effects of human urocortin-1, -2 and -3 in intracerebral hemorrhage of rats. Neuropeptides 2015, 52, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, E.W.; Grammatopoulos, D.K. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: Implications for physiology and pathophysiology. Endocr. Rev. 2006, 27, 260–286. [Google Scholar] [CrossRef] [PubMed]
- Florio, P.; Arcuri, F.; Ciarmela, P.; Runci, Y.; Romagnoli, R.; Cintorino, M.; Di Blasio, A.M.; Petraglia, F. Identification of urocortin mRNA and peptide in the human endometrium. J. Endocrinol. 2002, 173, R9–R14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Florio, P.; Vale, W.; Petraglia, F. Urocortins in human reproduction. Peptides 2004, 25, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Di Blasio, A.M.; Pecori Giraldi, F.; Viganò, P.; Petraglia, F.; Vignali, M.; Cavagnini, F. Expression of corticotropin-releasing hormone and its R1 receptor in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 1997, 82, 1594–1597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Makrigiannakis, A.; Zoumakis, E.; Margioris, A.N.; Theodoropoulos, P.; Stournaras, C.; Gravanis, A. The corticotropin-releasing hormone (CRH) in normal and tumoral epithelial cells of human endometrium. J. Clin. Endocrinol. Metab. 1995, 80, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Makrigiannakis, A.; Zoumakis, E.; Margioris, A.N.; Stournaras, C.; Chrousos, G.P.; Gravanis, A. Regulation of the promoter of the human corticotropin-releasing hormone gene in transfected human endometrial cells. Neuroendocrinology 1996, 64, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Makrigiannakis, A.; Margioris, A.N.; Chatzaki, E.; Zoumakis, E.; Chrousos, G.P.; Gravanis, A. The decidualizing effect of progesterone may involve direct transcriptional activation of corticotrophin-releasing hormone from human endometrial stromal cells. Mol. Hum. Reprod. 1999, 5, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Makrigiannakis, A.; Zoumakis, E.; Kalantaridou, S.; Coutifaris, C.; Margioris, A.N.; Coukos, G.; Rice, K.C.; Gravanis, A.; Chrousos, G.P. Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat. Immunol. 2001, 2, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Minas, V.; Loutradis, D.; Makrigiannakis, A. Factors controlling blastocyst implantation. Reprod. Biomed. Online 2005, 10, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Iavazzo, C.; Baka, S.; Malamitsi-Puchner, A. The role of urocortin in gynecological and obstetrical conditions. Arch. Gynecol. Obstet. 2009, 279, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Pergialiotis, V.; Tagkou, N.M.; Tsimpiktsioglou, A.; Klavdianou, O.; Neonaki, A.; Trompoukis, P. Urocortin Expression in Endometriosis: A Systematic Review. Int. J. Fertil. Steril. 2019, 13, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muramatsu, Y.; Sugino, N.; Suzuki, T.; Totsune, K.; Takahashi, K.; Tashiro, A.; Hongo, M.; Oki, Y.; Sasano, H. Urocortin and corticotropin-releasing factor receptor expression in normal cycling human ovaries. J. Clin. Endocrinol. Metab. 2001, 86, 1362–1369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Florio, P.; Reis, F.M.; Torres, P.B.; Calonaci, F.; Toti, P.; Bocchi, C.; Linton, E.A.; Petraglia, F. Plasma urocortin levels in the diagnosis of ovarian endometriosis. Obstet. Gynecol. 2007, 110, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, H.; Sun, W.; Guo, Z.; Lang, J. Elevated urine histone 4 levels in women with ovarian endometriosis revealed by discovery and parallel reaction monitoring proteomics. J. Proteom. 2019, 204, 103398. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, R.; Fang, T.; Huang, L.; Ouyang, N.; Wang, L.; Zhang, Q.; Yang, D. Endometriosis fertility index score maybe more accurate for predicting the outcomes of in vitro fertilisation than r-AFS classification in women with endometriosis. Reprod. Biol. Endocrinol. 2013, 11, 112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adamson, G.D.; Pasta, D.J. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil. Steril. 2010, 94, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Gift, A.G. Visual analogue scales: Measurement of subjective phenomena. Nurs. Res. 1989, 38, 286–288. [Google Scholar] [CrossRef]
- Andres, M.; Casagranda de Camargo, P.; Orlandi, C.; Silva, M.; Ferreira, A.; Azevedo, R.; Abrão, M. 12,366 Surgical Outcomes of the National Training Program (PROADI) for Minimally Invasive Surgery for Endometriosis in Brazil. J. Minim. Invasive Gynecol. 2024, 31, S152–S153. [Google Scholar] [CrossRef]
- Kiesel, L.; Sourouni, M. Diagnosis of endometriosis in the 21st century. Climacteric 2019, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- GiglioAyers, P.; Ezike, O.; Foley, C.E.; Brown, B.P. Demographic Correlates of Endometriosis Diagnosis Among United States Women Aged 15–50. J. Minim. Invasive Gynecol. 2024, 31, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Lessans, N.; Friedman, S.; Dior, U. 12,469 The Relationship Between Clinical Presentation and Endometriosis Types. J. Minim. Invasive Gynecol. 2024, 31, S165–S166. [Google Scholar] [CrossRef]
- Marín Sánchez, M.; Carratalá Pérez, O.; Martínez Gómez, A.; Oliva Sánchez, R.; Nieto Díaz, A. Evaluation of hopelessness in patients with endometriosis. Clín. Investig. Ginecol. Obstet. 2024, 51, 100936. [Google Scholar] [CrossRef]
- Vercellini, P.; Aimi, G.; De Giorgi, O.; Maddalena, S.; Carinelli, S.; Crosignani, P.G. Is cystic ovarian endometriosis an asymmetric disease? Br. J. Obs. Gynaecol. 1998, 105, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Busacca, M.; Aimi, G.; Bianchi, S.; Frontino, G.; Crosignani, P.G. Lateral distribution of recurrent ovarian endometriotic cysts. Fertil. Steril. 2002, 77, 848–849. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.; Olive, D.L.; Haney, A.F. Endometriosis: Pathogenetic implications of the anatomic distribution. Obstet. Gynecol. 1986, 67, 335–338. [Google Scholar] [PubMed]
- Demir, E.; Soyman, Z.; Kelekci, S. Outcomes between non-IVF and IVF treatment after laparoscopic conservative surgery of advanced endometriosis with Endometriosis Fertility Index score >3. Medicine 2022, 101, e30602. [Google Scholar] [CrossRef]
- Hobo, R.; Nakagawa, K.; Usui, C.; Sugiyama, R.; Ino, N.; Motoyama, H.; Kuribayashi, Y.; Inoue, M.; Sugiyama, R. The Endometriosis Fertility Index Is Useful for Predicting the Ability to Conceive Without Assisted Reproductive Technology Treatment after Laparoscopic Surgery, Regardless of Endometriosis. Gynecol. Obstet. Investig. 2018, 83, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Maheux-Lacroix, S.; Nesbitt-Hawes, E.; Deans, R.; Won, H.; Budden, A.; Adamson, D.; Abbott, J.A. Endometriosis fertility index predicts live births following surgical resection of moderate and severe endometriosis. Hum. Reprod. 2017, 32, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Leone Roberti Maggiore, U.; Chiappa, V.; Ceccaroni, M.; Roviglione, G.; Savelli, L.; Ferrero, S.; Raspagliesi, F.; Spanò Bascio, L. Epidemiology of infertility in women with endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2024, 92, 102454. [Google Scholar] [CrossRef] [PubMed]
- Vergetaki, A.; Jeschke, U.; Vrekoussis, T.; Taliouri, E.; Sabatini, L.; Papakonstanti, E.A.; Makrigiannakis, A. Differential expression of CRH, UCN, CRHR1 and CRHR2 in eutopic and ectopic endometrium of women with endometriosis. PLoS ONE 2013, 8, e62313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vergetaki, A.; Jeschke, U.; Vrekoussis, T.; Taliouri, E.; Sabatini, L.; Papakonstanti, E.A.; Makrigiannakis, A. Galectin-1 overexpression in endometriosis and its regulation by neuropeptides (CRH, UCN) indicating its important role in reproduction and inflammation. PLoS ONE 2014, 9, e114229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carrarelli, P.; Luddi, A.; Funghi, L.; Arcuri, F.; Batteux, F.; Dela Cruz, C.; Tosti, C.; Reis, F.M.; Chapron, C.; Petraglia, F. Urocortin and corticotrophin-releasing hormone receptor type 2 mRNA are highly expressed in deep infiltrating endometriotic lesions. Reprod. Biomed. Online 2016, 33, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Novembri, R.; Carrarelli, P.; Toti, P.; Rocha, A.L.; Borges, L.E.; Reis, F.M.; Piomboni, P.; Florio, P.; Petraglia, F. Urocortin 2 and urocortin 3 in endometriosis: Evidence for a possible role in inflammatory response. Mol. Hum. Reprod. 2011, 17, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Tokmak, A.; Ugur, M.; Tonguc, E.; Var, T.; Moraloğlu, O.; Ozaksit, G. The value of urocortin and Ca-125 in the diagnosis of endometrioma. Arch. Gynecol. Obstet. 2011, 283, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.E.; Ocal, I.; Yalcin, Y.; Selim, H.S.; Caltekin, M.D.; Aydogmus, H.; Kelekci, S. Evaluation of the Ki-67 Proliferation Index and Urocortin Expression in Women with Ovarian Endometriomas. Eurasian J. Med. 2017, 49, 107–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abramiuk, M.; Frankowska, K.; Kułak, K.; Tarkowski, R.; Mertowska, P.; Mertowski, S.; Grywalska, E. Possible Correlation between Urocortin 1 (Ucn1) and Immune Parameters in Patients with Endometriosis. Int. J. Mol. Sci. 2023, 24, 7787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chmaj-Wierzchowska, K.; Kampioni, M.; Wilczak, M.; Sajdak, S.; Opala, T. Novel markers in the diagnostics of endometriomas: Urocortin, ghrelin, and leptin or leukocytes, fibrinogen, and CA-125? Taiwan. J. Obstet. Gynecol. 2015, 54, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.M.; Rocha, A.L.; Del Puerto, H.L.; Petraglia, F.; Reis, F.M. Plasma urocortin-1 as a preoperative marker of endometriosis in symptomatic women. Gynecol. Endocrinol. 2018, 34, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Xiaomeng, X.; Ming, Z.; Jiezhi, M.; Xiaoling, F. Aberrant histone acetylation and methylation levels in woman with endometriosis. Arch. Gynecol. Obstet. 2013, 287, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.B.; Colón-Díaz, M.; García, M.; Gutierrez, S.; Colón, M.; Seto, E.; Laboy, J.; Flores, I. Endometriosis is characterized by a distinct pattern of histone 3 and histone 4 lysine modifications. Reprod. Sci. 2014, 21, 305–318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gong, Y.; Liu, M.; Zhang, Q.; Li, J.; Cai, H.; Ran, J.; Ma, L.; Ma, Y.; Quan, S. Lysine acetyltransferase 14 mediates TGF-β-induced fibrosis in ovarian endometrioma via co-operation with serum response factor. J. Transl. Med. 2024, 22, 561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oki, Y.; Sasano, H. Localization and physiological roles of Urocortin. Peptides 2024, 25, 1745–1749. [Google Scholar] [CrossRef]
- Pant, A.; Moar, K.K.; Arora, T.; Maurya, P.K. Biomarkers of endometriosis. Clin. Chim. Acta. 2023, 549, 117563. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bedrick, B.S.; Courtright, L.; Zhang, J.; Snow, M.; Amendola, I.L.S.; Nylander, E.; Cayton-Vaught, K.; Segars, J.; Singh, B. A Systematic Review of Epigenetics of Endometriosis. FS Rev. 2024, 5, 100070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, E.; Wang, X.; Chen, L. Regulated Cell Death in Endometriosis. Biomolecules 2024, 14, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2016, CD012179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J. Ovarian Res. 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, Z.; Chang, X.H.; Zhao, Y.; Zhu, H.L. Current biomarkers for the detection of endometriosis. Chin. Med. J. 2020, 133, 2346–2352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobayashi, H.; Imanaka, S.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H. Rethinking the pathogenesis of endometriosis: Complex interactions of genomic, epigenetic, and environmental factors. J. Obstet. Gynaecol. Res. 2024, 50, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Vissers, G.; Giacomozzi, M.; Verdurmen, W.; Peek, R.; Nap, A. The role of fibrosis in endometriosis: A systematic review. Hum. Reprod. Update 2024, 30, 706–750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Parameter | Group 1 Ovarian Endometriosis #30 | Group 2 Parietal Endometriosis #5 | Group 3 Control #5 |
|---|---|---|---|
| Mean age (years) | 33.60 ± 5.14 | 31.20 ± 3.90 | 30.40 ± 4.78 |
| Age of menarche (years) | ≈12 (11–15) | ≈12 (11–15) | ≈12 (11–15) |
| Infertility > 2 years | 19 (63.3%) | NA | NA |
| Symptom onset > 2 years | 93.3% | NA | NA |
| Mean symptom duration (years) | 5 ± 2 | 0.5 ± 0.4 | NA |
| Severity symptoms—Visual Analog Scale (#, %) | |||
| No Treatment | |||
| Dysmenorrhea—VAS ≥ 7 | 25 (83%) | 5 (100%) | NA |
| Dyspareunia—VAS 4–6 | 18 (60%) | 5 (100%) | NA |
| Chronic pelvic pain—VAS 4–6 | 28 (93.3%) | 4 (80%) | NA |
| Pharmacological Treatment | |||
| Dysmenorrhea—VAS 4–6 | 29 (96.7%) | 5 (100%) | NA |
| Dyspareunia—VAS 4–6 | 28 (93.3%) | 2 (40%) | NA |
| Chronic pelvic pain—VAS 4–6 | 16 (53.3%) | 0 (0%) | NA |
| 1-year follow—up | |||
| Dysmenorrhea—VAS 4–6 | 23 (76.7%) | 2 (40%) | NA |
| Dyspareunia—VAS < 4 | 21 (70%) | 5 (100%) | NA |
| Chronic pelvic pain—VAS < 4 | 29 (96.7%) | 5 (100%) | NA |
| Intra-operative aspects (#, %) | |||
| Endometrioma laterality: left | 16 (53.3%) | NA | NA |
| Endometrioma laterality: right | 6 (20%) | NA | NA |
| Endometrioma laterality: bilateral | 8 (26.7%) | NA | NA |
| Laparoscopic approach | 27 (90%) | NA | NA |
| Conversion to open surgery | 3 (10%) | NA | NA |
| Adhesiolysis performed | 29 (96.7%) | NA | NA |
| Recurrent lesion of endometriosis (#, %) | 5 (16.7%) | NA | NA |
| Biomarker | Index EFI < 55 #5 | Index EFI 5–7 #14 | Index EFI ≥ 8 #3 |
|---|---|---|---|
| CA–125 (U/mL) | 72.30 ± 28.12 | 67.11 ± 22.18 (p = 0.873) | 61.13 ± 29.67 (p = 0.784) (p = 0.927) |
| Serum Urocortin (μg/mL) | 3.62 ± 0.47 | 3.65 ± 0.38 (p = 0.079) | 4.00 ± 0.20 (p = 0.332) (p = 0.371) |
| Urinary Urocortin (μg/mL) | 2.30 ± 1.44 | 2.58 ± 1.37 (p = 0.872) | 2.91 ± 1.18 (p = 0.782) (p = 0.928) |
| Serum Histone H4 (ng/mL) | 0.46 ± 0.13 | 0.50 ± 0.25 (p = 0.961) | 1.05 ± 0.95 (p = 0.038) (p = 0.045) |
| Urinary Histone H4 (ng/mL) | 0.36 ± 0.05 | 0.33 ± 0.04 (p = 0.255) | 0.30 ± 0.01 (p = 0.111) (p = 0.513) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, B.; Caruntu, I.-D.; Simionescu, N.; Onofriescu, M.; Socolov, D.; Ilea, C.; Chifu, B.; Giusca, S.-E.; Timofte, A.D.; Tirnovanu, M.; et al. Urinary Urocortin as a Potential Non-Invasive Biomarker in Endometriosis: Exploratory Study with Histone H4. Medicina 2025, 61, 1671. https://doi.org/10.3390/medicina61091671
Toma B, Caruntu I-D, Simionescu N, Onofriescu M, Socolov D, Ilea C, Chifu B, Giusca S-E, Timofte AD, Tirnovanu M, et al. Urinary Urocortin as a Potential Non-Invasive Biomarker in Endometriosis: Exploratory Study with Histone H4. Medicina. 2025; 61(9):1671. https://doi.org/10.3390/medicina61091671
Chicago/Turabian StyleToma, Bogdan, Irina-Draga Caruntu, Natalia Simionescu, Mircea Onofriescu, Demetra Socolov, Ciprian Ilea, Bianca Chifu, Simona-Eliza Giusca, Andrei Daniel Timofte, Mihaela Tirnovanu, and et al. 2025. "Urinary Urocortin as a Potential Non-Invasive Biomarker in Endometriosis: Exploratory Study with Histone H4" Medicina 61, no. 9: 1671. https://doi.org/10.3390/medicina61091671
APA StyleToma, B., Caruntu, I.-D., Simionescu, N., Onofriescu, M., Socolov, D., Ilea, C., Chifu, B., Giusca, S.-E., Timofte, A. D., Tirnovanu, M., & Socolov, R. (2025). Urinary Urocortin as a Potential Non-Invasive Biomarker in Endometriosis: Exploratory Study with Histone H4. Medicina, 61(9), 1671. https://doi.org/10.3390/medicina61091671








