Driving Down Mortality: A 12-Year Retrospective Cohort Analysis of Mechanical Power and Driving Pressure in Ventilated ICU Patients
Abstract
1. Introduction
2. Materials and Methods
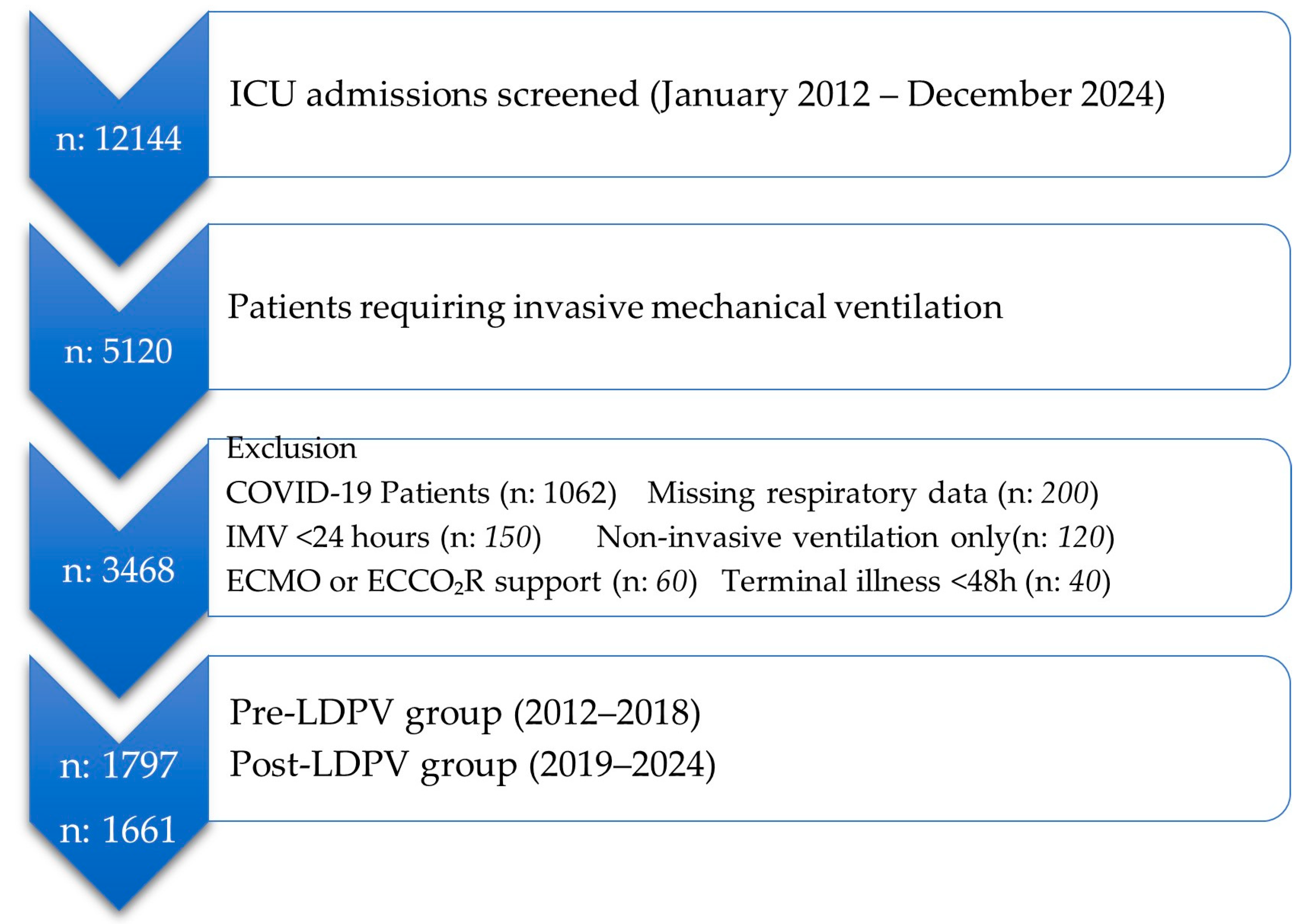
2.1. Study Design and Population
2.2. Study Periods
2.3. Data Collection
2.4. Calculation of Total Mechanical Power (MPtot)
2.5. Statistical Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APACHE II | Acute Physiology and Chronic Health Evaluation II |
| ARDS | Acute Respiratory Distress Syndrome |
| BMI | Body Mass Index |
| C | Respiratory System Compliance |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| ΔP | Driving Pressure |
| ECMO | Extracorporeal Membrane Oxygenation |
| ECCO2R | Extracorporeal Carbon Dioxide Removal |
| HR | Hazard Ratio |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| LDPV | Lung and Diaphragm Protective Ventilation |
| MP | Mechanical Power |
| MPtot | Total Mechanical Power |
| OR | Odds Ratio |
| PCV | Pressure-Controlled Ventilation |
| PBW | Predicted Body Weight |
| PEEP | Positive End-Expiratory Pressure |
| Ppeak | Peak Inspiratory Pressure |
| ROC | Receiver Operating Characteristic |
| RR | Respiratory Rate |
| SOFA | Sequential Organ Failure Assessment |
| TVe | Expiratory Tidal Volume |
| VCV | Volume-Controlled Ventilation |
| VFD | Ventilator-Free Days |
| VILI | Ventilator-Induced Lung Injury |
References
- Silva, P.L.; Ball, L.; Rocco, P.R.M.; Pelosi, P. Physiological and Pathophysiological Consequences of Mechanical Ventilation. Semin. Respir. Crit. Care Med. 2022, 43, 321–334. [Google Scholar] [CrossRef]
- Rali, A.S.; Tran, L.; Balakrishna, A.; Senussi, M.; Kapur, N.K.; Mektus, T.; Tedford, R.J.; Lindenfeld, J. Guide to Lung-Protective Ventilation in Cardiac Patients. J. Card. Fail. 2024, 30, 829–837. [Google Scholar] [CrossRef]
- Paudel, R.; Trinkle, C.A.; Waters, C.M.; Robinson, L.E.; Cassity, E.; Sturgill, J.L.; Broaddus, R.; Morris, P.E. Mechanical Power: A New Concept in Mechanical Ventilation. Am. J. Med. Sci. 2021, 362, 537–545. [Google Scholar] [CrossRef]
- Roca, O.; Goligher, E.C.; Amato, M.B.P. Driving pressure: Applying the concept at the bedside. Intensive Care Med. 2023, 49, 991–995. [Google Scholar] [CrossRef]
- Rezaiguia-Delclaux, S.; Ren, L.; Gruner, A.; Roman, C.; Genty, T.; Stéphan, F. Oxygenation versus driving pressure for determining the best positive end-expiratory pressure in acute respiratory distress syndrome. Crit. Care 2022, 26, 214. [Google Scholar] [CrossRef]
- Amato, M.B.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.S.; Mercat, A.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef]
- Serpa Neto, A.; Deliberato, R.O.; Johnson, A.E.W.; Bos, L.D.; Amorim, P.; Pereira, S.M.; Cazati, D.C.; Cordioli, R.L.; Correa, T.D.; Pollard, T.J.; et al. PROVE Network Investigators. Mechanical power of ventilation is associated with mortality in critically ill patients: An analysis of patients in two observational cohorts. Intensive Care Med. 2018, 44, 1914–1922. [Google Scholar] [CrossRef]
- Gattinoni, L.; Tonetti, T.; Cressoni, M.; Cadringher, P.; Herrmann, P.; Moerer, O.; Protti, A.; Gotti, M.; Chiurazzi, C.; Carlesso, E.; et al. Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 2016, 42, 1567–1575. [Google Scholar] [CrossRef]
- Becher, T.; van der Staay, M.; Schädler, D.; Frerichs, I.; Weiler, N. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med. 2019, 45, 1321–1323. [Google Scholar] [CrossRef]
- Li, S.; Chen, B.; Tong, C. Influence of the Driving Pressure on Mortality in ARDS Patients with or without Abdominal Obesity: A Retrospective Cohort Study. Contrast Media Mol. Imaging 2022, 2022, 1219666. [Google Scholar] [CrossRef]
- Wu, H.P.; Leu, S.W.; Lin, S.W.; Hung, C.Y.; Chen, N.H.; Hu, H.C.; Huang, C.-C.; Kao, K.-C. Role of Changes in Driving Pressure and Mechanical Power in Predicting Mortality in Patients with Acute Respiratory Distress Syndrome. Diagnostics 2023, 13, 1226. [Google Scholar] [CrossRef]
- Alzahrani, H.A.; Corcione, N.; Alghamdi, S.M.; Alhindi, A.O.; Albishi, O.A.; Mawlawi, M.M.; Nofal, W.O.; Ali, S.M.; Albadrani, S.A.; AlJuaid, M.A.; et al. Driving pressure in acute respiratory distress syndrome for developing a protective lung strategy: A systematic review. World J. Crit. Care Med. 2025, 14, 101377. [Google Scholar] [CrossRef]
- von Düring, S.; Liu, K.; Munshi, L.; Kim, S.J.; Urner, M.; Adhikari, N.K.J.; Parhar, K.K.S.; Fan, E. The Association Between Mechanical Power Within the First 24 Hours and ICU Mortality in Mechanically Ventilated Adult Patients With Acute Hypoxemic Respiratory Failure: A Registry-Based Cohort Study. Chest 2025. [Google Scholar] [CrossRef]
- van Meenen, D.M.P.; Algera, A.G.; Schuijt, M.T.U.; Simonis, F.D.; van der Hoeven, S.M.; Neto, A.S.; de Abreu, M.G.; Pelosi, P.; Paulus, F.; Schultz, M.J. Effect of mechanical power on mortality in invasively ventilated ICU patients without the acute respiratory distress syndrome: An analysis of three randomised clinical trials. Eur. J. Anaesthesiol. 2023, 40, 21–28. [Google Scholar] [CrossRef]
- Sahetya, S.K.; Mallow, C.; Sevransky, J.E.; Martin, G.S.; Girard, T.D.; Brower, R.G.; Checkley, W. Society of Critical Care Medicine Discovery Network Critical Illness Outcomes Study Investigators. Association between hospital mortality and inspiratory airway pressures in mechanically ventilated patients without acute respiratory distress syndrome: A prospective cohort study. Crit. Care 2019, 23, 367. [Google Scholar] [CrossRef]
- Mamun, M.U.R.; Ferdous Rahman, A.K.M.; Rahaman, M. Mean Airway Pressure As A Predictor of Mortality (Short Term) In Mechanically Ventilated ARDS Patients. Planet 2024, 7, 212–217. [Google Scholar]
- Serpa Neto, A.; Filho, R.R.; Cherpanath, T.; Determann, R.; Dongelmans, D.A.; Paulus, F.; Tuinman, P.R.; Pelosi, P.; de Abreu, M.G.; Schultz, M.J.; et al. PROVE Network Investigators. Associations between positive end-expiratory pressure and outcome of patients without ARDS at onset of ventilation: A systematic review and meta-analysis of randomized controlled trials. Ann. Intensive Care 2016, 6, 109. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Deng, X.; Liu, B.; Zhang, Y.; Zheng, Y.; Zheng, H.; Wang, Y.; Lai, Y.; Huang, W.; et al. Optimal Positive End Expiratory Pressure Levels in Ventilated Patients Without Acute Respiratory Distress Syndrome: A Bayesian Network Meta-Analysis and Systematic Review of Randomized Controlled Trials. Front. Med. 2021, 8, 730018. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Okazaki, S.R.; Fujita, Y.; Seki, N.; Kokei, Y.; Sekine, S.; Wada, S.; Norisue, Y.; Narita, C. Usefulness of low tidal volume ventilation strategy for patients with acute respiratory distress syndrome: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 9331. [Google Scholar] [CrossRef] [PubMed]
- Samanta, R.J.; Ercole, A.; Harris, S.; Summers, C. Low Tidal Volume Ventilation Is Poorly Implemented for Patients in North American and United Kingdom ICUs Using Electronic Health Records. Chest 2024, 165, 333–347. [Google Scholar] [CrossRef]
- Aglen, S.A.S.; Simonsen, H.F.; Sjøset, T.E.; Jammer, I. Respiratory Rate as a Predictor of Clinical Deterioration and Mortality: A Scoping Review. Acta Anaesthesiol. Scand. 2025, 69, e70113. [Google Scholar] [CrossRef] [PubMed]

| 2012–2018 Group (n: 1797) Median (IQR25–75) | 2019–2024 Group (n: 1661) Median (IQR25–75) | p Value | |
|---|---|---|---|
| Age (year) | 61 (44 to 75) | 59 (49 to 69) | 0.13 b |
| Gender (female) (n) (%) | 675 (37.5%) | 663 (39.9%) | 0.16 a |
| Height (m) | 170 (160 to 175) | 170 (162 to 175) | 0.55 b |
| Weight (kg) | 80 (70–85) | 70 (80 to 86) | 0.60 b |
| BMI (kg/m2) | 26.1 (24.2 to 29.4) | 26.2 (24.2 to 29.2) | 0.47 b |
| SOFA | 14.0 (10.0 to 15.0) | 13.0 (11.0 to 14.0) | 0.14 b |
| APACHE II | 18.0 (10.0 to 25.0) | 19.0 (13.0 to 23.0) | 0.83 b |
| Ventilatory Free Days | 7.2 (3.4 to 14.4) | 6.0 (3.0 to 10.8) | <0.0001 b* |
| ICU Length of Stay (day) | 11.0 (6.1 to 20.7) | 8.8 (5.5 to 14.4) | <0.0001 b* |
| ICU Mortality (%) | 858 (47.7%) | 682 (41.1%) | <0.0001 b* |
| Type of Admission n (%) | 2012–2018 Group (n: 1797) | 2019–2024 Group (n: 1661) | p Value |
|---|---|---|---|
| Pulmonary | 467 (26.2%) | 458 (27.6%) | 0.29 a |
| Neurologic | 469 (26.3%) | 423(25.5%) | 0.67 a |
| Gastrointestinal | 453 (25.4%) | 394 (23.7%) | 0.31 a |
| Cardiac | 277 (15.5%) | 219 (13.2%) | 0.06 a |
| Renal | 72 (4.0%) | 54 (3.3%) | 0.27 b |
| Metabolic | 25 (1.4%) | 19 (1.1%) | 0.62 b |
| Other | 22 (1.2%) | 35(2.1%) | 0.0.06 b |
| Comorbidities n (%) | 2012–2018 Group (n: 1797) | 2019–2024 Group (n: 1661) | p Value |
| COPD | 1513 (84.8%) | 1134 (68.5%) | 0.10 a |
| Diabetes Mellitus | 72 (4.0%) | 84 (5.1%) | 0.16 b |
| Hypertension | 72 (4.0%) | 90 (5.4%) | 0.06 b |
| Malignancy | 32 (1.8%) | 43(2.6%) | 0.13 b |
| Cerebrovascular disease | 36 (2.0%) | 51 (3.1%) | 0.06 b |
| Heart failure | 24 (1.3%) | 37 (2.2%) | 0.06 b |
| Coronary artery disease | 11 (0.6%) | 21 (1.3%) | 0.07 b |
| Liver failure | 13 (0.7%) | 24 (1.4%) | 0.058 b |
| Chronic kidney disease | 6 (0.3%) | 15 (0.9%) | 0.053 b |
| Other | 5 (0.3%) | 13 (0.8%) | 0.068 b |
| Respiratory Mechanics | 2012–2018 Group (n: 1797) Median (IQR25–75) | 2019–2024 Group (n: 1661) Median (IQR25–75) | p Value |
|---|---|---|---|
| Respiratory rate (per minute) | 14.0 (14.0 to 15.0) | 14.0 (14.0 to 14.0) | <0.0001 * |
| PEEP (cmH2O) | 7.0 (6.0 to 8.1) | 6.8 (6.0 to 7.8) | 0.0003 * |
| Expiratory Tidal Volume (mL) | 511 (452 to 576) | 477 (434 to 520) | <0.0001 * |
| Ppeak (cmH2O) | 21.5 (18.6 to 24.5) | 20.4 (17.9 to 23.1) | <0.0001 * |
| Compliance (mL/cmH2O) | 37.7 (30.0 to 46.3) | 39.9 (32.5 to 46.9) | <0.0001 * |
| Driving Pressure (cmH2O) | 14.3 (12.3 to 16.5) | 12.9 (11.4 to 14.7) | <0.0001 * |
| Mechanical Power (J/min) | 17.2 (14.1 to 21.3) | 14.2 (12.1 to 16.4) | <0.0001 * |
| 2012–2018 Group (n = 1797) | Non-Survived (n = 858) Median (IQR25–75) | Survived (n = 939) Median (IQR25–75) | p Value |
|---|---|---|---|
| Ppeak (cmH2O) | 20.2 (17.9 to 23.0) | 23.1 (20.3 to 26.2) | <0.0001 * |
| Expiratory Tidal Volume (mL) | 521 (465 to 592) | 499 (440 to 561) | <0.0001 * |
| PEEP (cmH2O) | 6.8 (5.7 to 7.9) | 7.4 (6.3 to 8.7) | <0.0001 * |
| Respiratory Rate (per minute) | 14.0 (14.0 to 17.0) | 14.0 (14.0 to 14.0) | <0.0001 * |
| Compliance (mL/cmH2O) | 40.4 (33.4 to 48.8) | 33.6 (27.2 to 42.9) | <0.0001 * |
| Driving Pressure (cmH2O) | 13.3 (11.7 to 15.3) | 15.5 (13.2 to 17.7) | <0.0001 * |
| Mechanical Power (J/min) | 16.2 (13.1 to 19.8) | 18.8 (15.3 to 23.3) | <0.0001 * |
| 2019–2024 Group (n = 1661) | Non-survived (n = 682) Median (IQR25–75) | Survived (n = 979) Median (IQR25–75) | p Value |
| Ppeak (cmH2O) | 19.7 (17.5 to 22.2) | 21.6 (19.2 to 24.7) | <0.0001 * |
| Expiratory Tidal Volume (mL) | 482 (442 to 526) | 469 (424 to 509) | <0.0001 * |
| PEEP (cmH2O) | 6.7 (5.8 to 7.5) | 7.1 (6.2 to 8.0) | <0.0001 * |
| Respiratory Rate (per minute) | 14.0 (14.0 to 14.0) | 14.0 (14.0 to 14.0) | 0.6066 |
| Compliance (mL/cmH2O) | 41.4 (34.7 to 48.5) | 36.5 (27.8 to 43.7) | <0.0001 * |
| Driving Pressure (cmH2O) | 12.5(11.0 to 14.2) | 13.7 (12.0 to 15.9) | <0.0001 * |
| Mechanical Power (J/min) | 13.8 (11.7 to 15.9) | 14.9 (12.6 to 17.2) | <0.0001 * |
| Fraction of Survival | 2012–2018 Group (n = 1797) | 2019–2024 Group (n = 1661) | ||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Cutoff | HR | 95% CI | Log Rank p | Cutoff | HR | 95% CI | Log Rank p |
| Mechanical Power (J/min) | ≥17.7 | 1.39 | 1.22–1.59 | <0.0001 * | ≥15.3 | 1.40 | 1.20–1.64 | <0.0001 * |
| Driving Pressure (cmH2O) | ≥15.2 | 1.66 | 1.45–1.90 | <0.0001 * | ≥13.2 | 1.37 | 1.18–1.59 | <0.0001 * |
| Ppeak (cmH2O) | ≥23.5 | 1.56 | 1.36–1.78 | <0.0001 * | ≥21.6 | 1.33 | 1.15–1.55 | 0.0002 * |
| PEEP (cmH2O) | ≥7.0 | 1.24 | 1.08–1.42 | 0.002 * | ≥7.4 | 1.17 | 1.00–1.73 | 0.048 * |
| TVe (mL) | ≥469 | 1.27 | 1.11–1.46 | 0.0007 * | ≥503 | 1.60 | 1.39–1.84 | <0.0001 * |
| Compliance (mL/cmH2O) | <33.6 | 1.67 | 1.46–1.91 | <0.0001 * | <34.0 | 1.75 | 1.54–2.00 | <0.0001 * |
| Respiratory Rate | ≥14/min | 1.51 | 1.33–1.73 | <0.0001 * | ≥14/min | 0.78 | 0.60–1.01 | 0.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahimi, P.; Aşar, S.; Soylu, N.B.; Yücel Yenice, T.; Canan, E.; Çukurova, Z. Driving Down Mortality: A 12-Year Retrospective Cohort Analysis of Mechanical Power and Driving Pressure in Ventilated ICU Patients. Medicina 2025, 61, 1668. https://doi.org/10.3390/medicina61091668
Rahimi P, Aşar S, Soylu NB, Yücel Yenice T, Canan E, Çukurova Z. Driving Down Mortality: A 12-Year Retrospective Cohort Analysis of Mechanical Power and Driving Pressure in Ventilated ICU Patients. Medicina. 2025; 61(9):1668. https://doi.org/10.3390/medicina61091668
Chicago/Turabian StyleRahimi, Payam, Sinan Aşar, Nuri Burkay Soylu, Tuğba Yücel Yenice, Emral Canan, and Zafer Çukurova. 2025. "Driving Down Mortality: A 12-Year Retrospective Cohort Analysis of Mechanical Power and Driving Pressure in Ventilated ICU Patients" Medicina 61, no. 9: 1668. https://doi.org/10.3390/medicina61091668
APA StyleRahimi, P., Aşar, S., Soylu, N. B., Yücel Yenice, T., Canan, E., & Çukurova, Z. (2025). Driving Down Mortality: A 12-Year Retrospective Cohort Analysis of Mechanical Power and Driving Pressure in Ventilated ICU Patients. Medicina, 61(9), 1668. https://doi.org/10.3390/medicina61091668





