Physiological Changes and Trimester-Specific Reference Intervals for Complete Blood Count Parameters in Korean Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CBC Results
2.3. Establishment of Reference Ranges
2.4. Statistics
3. Results
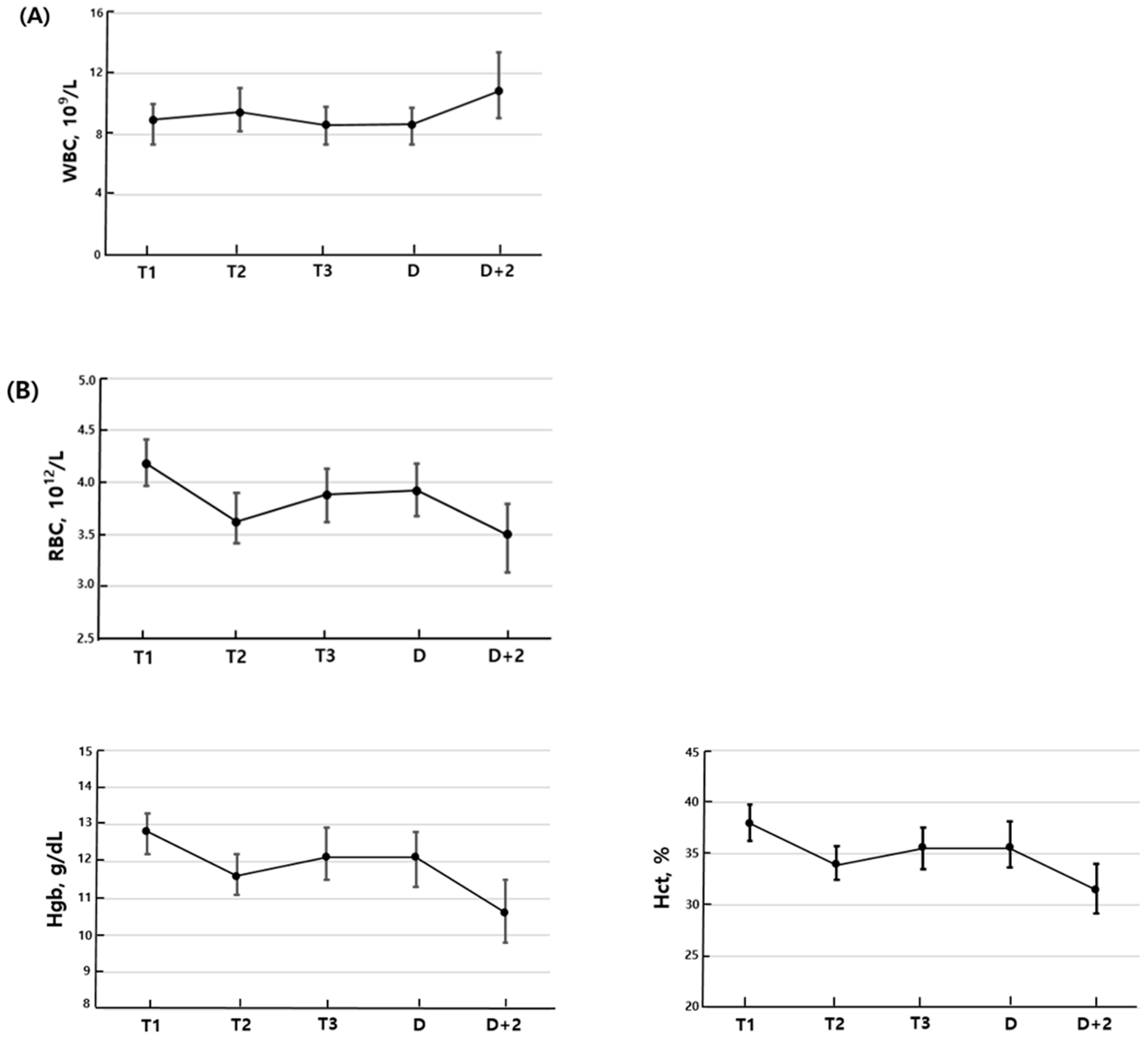
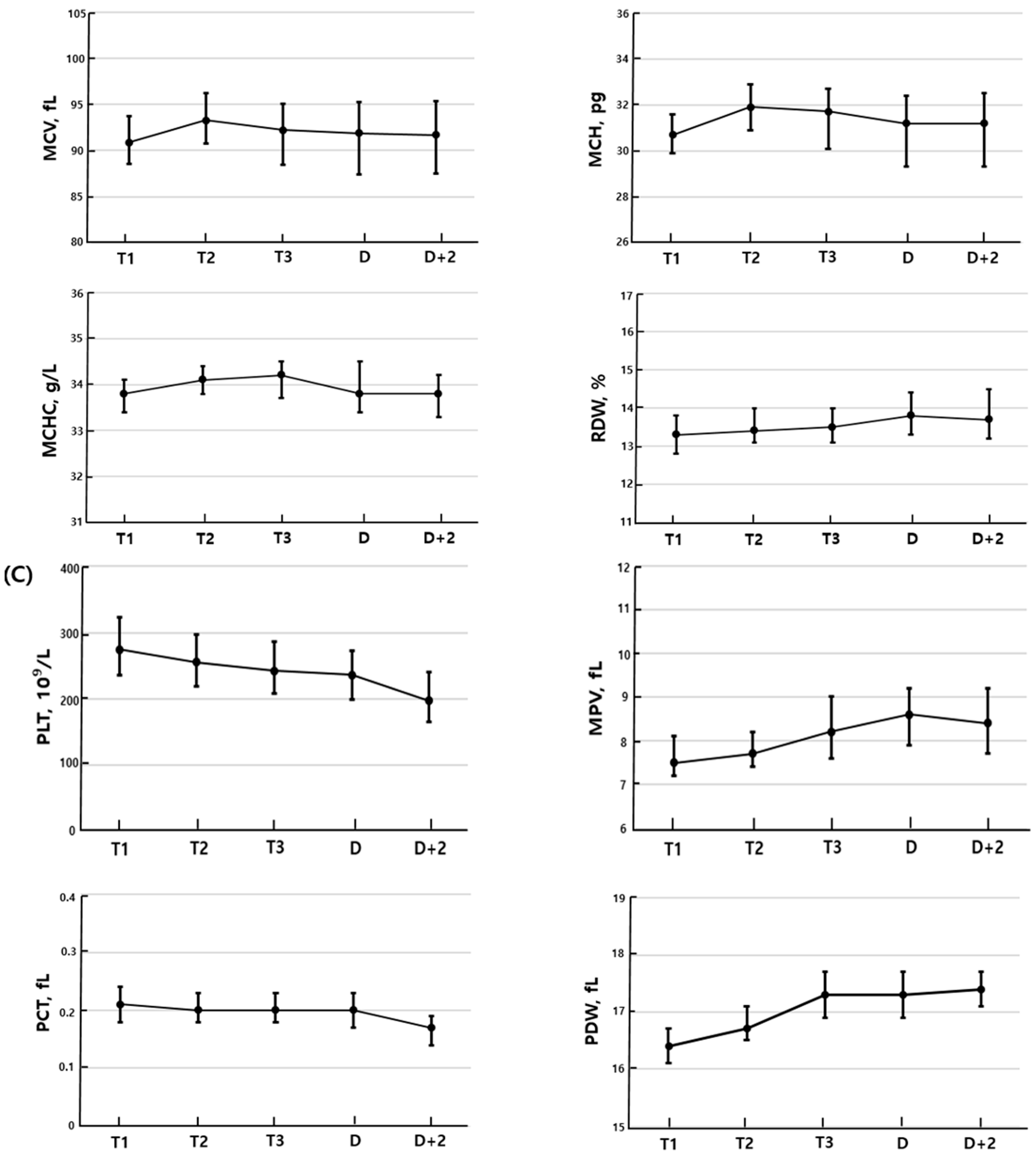
3.1. Longitudinal Changes Within Individual Patients
3.2. Reference Interval
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBC | Complete blood count |
| WBC | White blood cell |
| RBC | Red blood cells |
| Hgb | Hemoglobin |
| MCV | Mean corpuscular volume |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| RDW | Red blood cell distribution width |
| PLT | Platelet |
| PCT | Plateletcrit |
| MPV | Mean platelet volume |
| PDW | Platelet distribution width |
| CLSI | Clinical and laboratory standards institute |
| CIs | Confidence intervals |
| WHO | World Health Organization |
| CDC | Centers for Disease Control and Prevention |
References
- Torgersen, C.K.L.; Curran, C.A. A systematic approach to the physiologic adaptations of pregnancy. Crit. Care Nurs. Q. 2006, 29, 2–19. [Google Scholar] [CrossRef]
- Tan, E.K.; Tan, E.L. Alterations in physiology and anatomy during pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 791–802. [Google Scholar] [CrossRef]
- Allan, G.M.; Young, J. Complete blood count for screening? Can. Fam. Physician 2017, 63, 772. [Google Scholar] [PubMed]
- Dixon, L.R. The complete blood count: Physiologic basis and clinical usage. J. Perinat. Neonatal Nurs. 1997, 11, 1–18. [Google Scholar] [CrossRef]
- Ma, M.; Zhu, M.; Zhuo, B.; Li, L.; Chen, H.; Xu, L.; Wu, Z.; Cheng, F.; Xu, L.; Yan, J. Use of complete blood count for predicting preterm birth in asymptomatic pregnant women: A propensity score-matched analysis. J. Clin. Lab. Anal. 2020, 34, e23313. [Google Scholar] [CrossRef]
- Padoan, A. Laboratory tests to monitoring physiological pregnancy. J. Lab. Precis. Med. 2020, 5, 7. [Google Scholar] [CrossRef]
- Costantine, M.M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 2014, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Tripathi, A.K.; Mishra, S.; Amzarul, M.; Vaish, A.K. Physiological Changes in Hematological Parameters During Pregnancy. Indian. J. Hematol. Blood Transfus. 2012, 28, 144–146. [Google Scholar] [CrossRef]
- Li, A.; Yang, S.; Zhang, J.; Qiao, R. Establishment of reference intervals for complete blood count parameters during normal pregnancy in Beijing. J. Clin. Lab. Anal. 2017, 31, e22150. [Google Scholar] [CrossRef]
- Pham, H.N.; Huynh, N.X.; Pham, P.N.H.; Dang, D.N.Y.; Cao, L.T.; Huynh, D.M.; Thoi, H.T.T.; Le, O.H.; Cheanh Beaupha, S.M. Reference intervals of complete blood count and coagulation tests in Vietnamese pregnant women. BMC Pregnancy Childbirth 2023, 23, 788. [Google Scholar] [CrossRef]
- Horowitz, G.L.; Clinical and Laboratory Standards Institute. Defining Establishing and Verifying Reference Intervals in the Clinical Laboratory: Approved Guideline, 3rd ed.; CLSI document C28-A3c; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- Patel, P.B.; Patel, N.; Hedges, M.A.; Benson, A.E.; Tomer, A.; Lo, J.O.; Shatzel, J.J. Hematologic Complications of Pregnancy. Eur. J. Haematol. 2025, 114, 596–614. [Google Scholar] [CrossRef]
- Challis, J.R.G.; Matthews, S.G.; Gibb, W.; Lye, S.J. Fetal and Maternal Physiology, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2015; pp. 123–135. [Google Scholar]
- Zhu, J.; Li, Z.; Deng, Y.; Lan, L.; Yang, J. Comprehensive reference intervals for white blood cell counts during pregnancy. BMC Pregnancy Childbirth 2024, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy: Review articles. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Efrati, P.; Presentey, B.; Margalith, M.; Rozenszajn, L. Leukocytes of normal pregnant women. Obstet. Gynecol. 1964, 23, 429–432. [Google Scholar]
- Horowitz, K.M.; Ingardia, C.J.; Borgida, A.F. Anemia in pregnancy. Clin. Lab. Med. 2013, 33, 281–291. [Google Scholar] [CrossRef]
- Milman, N. Oral iron prophylaxis in pregnancy: Not too little and not too much! J. Pregnancy 2012, 2012, 514345. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, G.C.; Spano, F.; Giardina, I.; Brillo, E.; Clerici, G.; Roura, L.C. Iron deficiency anemia in pregnancy. Women’s Health 2015, 11, 891–900. [Google Scholar] [CrossRef]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; WHO/NMH/NHD/MNM/11.1; World Health Organization: Geneva, Switzerland, 2011.
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm. Rep. 1998, 47, 1–29. [Google Scholar]
- Jin, Y.; Lu, J.; Jin, H.; Fei, C.; Xie, X.; Zhang, J. Reference intervals for biochemical, haemostatic and haematological parameters in healthy Chinese women during early and late pregnancy. Clin. Chem. Lab. Med. 2018, 56, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.H.; Yoon, J.S. Comparison and evaluation of hematological indices for assessment of iron nutritional status in Korean pregnant women (III). Korean J. Nutr. 2000, 33, 532–539. [Google Scholar]
- Ciobanu, A.M.; Colibaba, S.; Cimpoca, B.; Peltecu, G.; Panaitescu, A.M. Thrombocytopenia in Pregnancy. Maedica (Bucur) 2016, 11, 55–60. [Google Scholar] [PubMed]
- Perepu, U.; Rosenstein, L. Maternal thrombocytopenia in pregnancy. Proc. Obstet. Gynecol. 2013, 3, 6. [Google Scholar] [CrossRef]
- Park, Y.H. Diagnosis and management of thrombocytopenia in pregnancy. Blood Res. 2022, 30, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Yoo, J.; Jekarl, D.W.; Chae, H.; Kim, M.; Park, Y.J.; Oh, E.J.; Kim, Y. Indirect method for estimation of reference intervals of inflammatory markers. Ann. Lab. Med. 2023, 43, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Bain, B.J. Ethnic and sex differences in the total and differential white cell count and platelet count. J. Clin. Pathol. 1996, 49, 664–666. [Google Scholar] [CrossRef]
- Eun, H.N.; Suyoung, K.; Seon, C.; Hanik, C. Complete blood count reference intervals and patterns of changes across Pediatric, Adult and Geriatric ages in Korea. Annals Lab. Med. 2018, 38, 503–511. [Google Scholar] [CrossRef]
- Lim, E.; Miyamura, J.; Chen, J.J. Racial/Ethnic-Specific reference intervals for Common Laboratory tests: A comparison among asians, blacks, hispanics, and White. Hawaii J. Med. Public Health 2015, 74, 302–310. [Google Scholar]
- Mustafa, M.I.; Ali, I.A.; Mohammed, M.A.; Taha, E.H.; Awad, K.M.; Musa, O.A. Reference intervals of complete blood count parameters in the adult western Sudanese population. BMC Res. Notes 2024, 17, 99. [Google Scholar] [CrossRef]
- Fiseha, M.; Mohammed, M.; Ebrahim, E.; Demsiss, W.; Tarekegn, M.; Angelo, A.; Negash, M.; Tamir, Z.; Tilahun, M.; Tsegaye, A. Common hematological parameters reference intervals for apparently healthy pregnant and non-pregnant women of South Wollo Zone, Amhara Regional State, Northeast Ethiopia. PLoS ONE 2022, 17, e0270685. [Google Scholar] [CrossRef]
- Bach, K.Q.; Nguyen, H.T.T.; Nguyen, T.H.; Nguyen, M.B.; Nguyen, T.A. Thalassemia in Viet Nam. Hemoglobin 2022, 46, 62–65. [Google Scholar] [CrossRef]
- Karateke, A.; Keskin Kurt, R.; Baloğlu, A. Relation of platelet distribution width (PDW) and platelet crit (PCT) to preeclampsia. Ginekol. Pol. 2015, 86, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Yang, J.; Zheng, T.Q.; Chen, Y.M. Association between platelet indices and risk of preeclampsia in pregnant women. J. Obstet. Gynaecol. 2022, 42, 2764–2770. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, K.H.; Kim, H.H.; Kim, M.J.; Kim, J.N. A Survey of the Opinions of Transfusion Specialists on Transfusion Policy of Thalassemia Patients in Korea. Korean J. Blood Transfus. 2017, 28, 282–289. [Google Scholar] [CrossRef]
- La Verde, M.; Luciano, M.; Fordellone, M.; Sampogna, G.; Lettieri, D.; Palma, M.; Torella, D.; Marrapodi, M.M.; Di Vincenzo, M.; Torella, M. Postpartum Depression and Inflammatory Biomarkers of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, and Monocyte-Lymphocyte Ratio: A Prospective Observational Study. Gynecol. Obstet. Investig. 2024, 89, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, P.; Sinha, P.; Gupta, B.; Firmal, P.; Rajaram, S. Neutrophil-to-lymphocyte ratio and platelet indices in pre-eclampsia. Int. J. Gynaecol. Obstet. 2019, 144, 16–20. [Google Scholar] [CrossRef] [PubMed]
| N (%) | Mean ± SD | Median | |
|---|---|---|---|
| Age | 35.2 ± 3.8 | 35 | |
| Parity | |||
| Primipara | 77 (64.2) | ||
| Multipara | 43 (35.8) | ||
| Method of pregnancy | |||
| Natural | 71 (59.2) | ||
| In vitro fertilization | 49 (40.8) | ||
| Delivery method | |||
| Vaginal | 31 (25.8) | ||
| Cesarean section | 89 (74.2) | ||
| Blood loss (mL) | 445.4 ± 284.6 | 400 | |
| Fetal weight | 3131.4 ± 383.9 | 3160 | |
| Placenta weight | 698.8 ± 153.4 | 670 |
| Control (N = 222) | First Trimester (N = 160) | Second Trimester (N = 382) | Third Trimester (N = 497) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Median | Range | Median | Range | p1 | Median | Range | p2 | Median | Range | p3 | ||||
| Low | High | Low | High | Low | High | Low | High | ||||||||
| WBC, 109/L | 5.45 | 4.00 | 8.66 | 8.86 | 4.57 | 13.56 | <0.001 | 9.39 | 4.82 | 15.07 | <0.001 | 8.53 | 4.02 | 13.54 | <0.001 |
| RBC, 1012/L | 4.33 | 3.63 | 4.96 | 4.19 | 3.40 | 4.98 | <0.001 | 3.61 | 2.93 | 4.33 | <0.001 | 3.91 | 3.03 | 4.76 | <0.001 |
| Hgb, g/dL | 13.3 | 11.5 | 14.9 | 12.9 | 11.2 | 14.5 | <0.001 | 11.6 | 9.5 | 13.8 | <0.001 | 12.3 | 9.7 | 14.8 | <0.001 |
| Hct, % | 39.5 | 33.7 | 44.8 | 38.1 | 31.5 | 44.6 | <0.001 | 33.9 | 27.5 | 40.8 | <0.001 | 35.8 | 28.6 | 43.3 | <0.001 |
| MCV, fL | 91.6 | 82.2 | 100.6 | 91.0 | 77.3 | 99.9 | 0.486 | 94.1 | 84.2 | 103.2 | <0.001 | 92.8 | 79.5 | 104.1 | <0.001 |
| MCH, pg | 30.9 | 27.0 | 34.3 | 30.8 | 27.3 | 34.3 | 0.567 | 32.3 | 28.3 | 36.1 | <0.001 | 31.7 | 26.3 | 35.8 | <0.001 |
| MCHC, g/L | 33.6 | 32.4 | 34.6 | 33.8 | 32.7 | 34.9 | <0.001 | 34.2 | 32.9 | 35.6 | <0.001 | 34.1 | 32.5 | 35.5 | 0.702 |
| RDW, % | 13.1 | 11.8 | 15.2 | 13.2 | 12.0 | 15.3 | 0.623 | 13.5 | 12.0 | 15.3 | 0.063 | 13.5 | 11.9 | 15.5 | 0.053 |
| PLT, 109/L | 259 | 157 | 435 | 273 | 148 | 422 | 0.280 | 247 | 120 | 389 | <0.001 | 235 | 106 | 372 | 0.005 |
| MPV, fL | 8.0 | 6.4 | 9.9 | 7.6 | 6.2 | 9.5 | <0.001 | 7.8 | 5.9 | 10.1 | 0.007 | 8.3 | 6.1 | 10.9 | <0.001 |
| PCT, fL | 0.21 | 0.13 | 0.34 | 0.21 | 0.13 | 0.32 | 0.175 | 0.20 | 0.11 | 0.29 | 0.002 | 0.20 | 0.12 | 0.27 | 0.374 |
| PDW, fL | 16.5 | 15.6 | 17.7 | 16.4 | 15.6 | 17.4 | 0.021 | 16.9 | 15.5 | 18.2 | <0.001 | 17.3 | 16.1 | 18.7 | <0.001 |
| Parameter | Control (N = 222) | First Trimester (N = 160) | Second Trimester (N = 382) | Third Trimester (N = 497) | ||||
|---|---|---|---|---|---|---|---|---|
| 2.5th (90% CI) | 97.5th (90% CI) | 2.5th (90% CI) | 97.5th (90% CI) | 2.5th (90% CI) | 97.5th (90% CI) | 2.5th (90% CI) | 97.5th (90% CI) | |
| WBC, 109/L | 4.06 (4.01–4.17) | 8.11 (7.88–8.62) | 5.11 (4.57–5.64) | 12.14 (11.55–13.56) | 6.11 (5.37–6.27) | 13.45 (13.18–13.9) | 5.62 (4.54–5.74) | 12.42 (12.34–13.04) |
| RBC, 1012/L | 3.76 (3.68–3.80) | 4.83 (4.78–4.96) | 3.69 (3.40–3.72) | 4.78 (4.72–4.98) | 3.09 (3.03–3.12) | 4.17 (4.11–4.30) | 3.26 (3.15–3.29) | 4.48 (4.46–4.71) |
| Hgb, g/dL | 11.7 (11.6–11.9) | 14.6 (14.5–14.9) | 11.3 (11.2–11.5) | 14.3 (14.2–14.5) | 10.1 (9.8–10.2) | 13.3 (13.2–13.7) | 10.1 (9.8–10.1) | 14.1 (14.1–14.4) |
| Hct, % | 34.3 (33.8–35.6) | 43.6 (43.1–44.7) | 32.8 (31.5–33.5) | 43.9 (42.5–44.6) | 29.2 (28.2–29.3) | 39.1 (38.6–39.6) | 30.2 (29.1–30.2) | 41.6 (40.9–42.6) |
| MCV, fL | 83.2 (82.4–84.3) | 98.6 (97.5–99.1) | 83.5 (81.0–84.2) | 98.5 (97.6–99.9) | 86.9 (85.4–87.7) | 100.8 (100.6–101.5) | 81.6 (80.9–81.8) | 100.8 (100.6–102.0) |
| MCH, pg | 27.5 (27.2–27.9) | 33.2 (32.9–34.2) | 28.1 (27.3–28.4) | 33.9 (33.3–34.3) | 28.8 (28.5–29.5) | 34.9 (34.5–35.2) | 26.9 (26.7–27.1) | 34.9 (34.8–35.5) |
| MCHC, g/L | 32.6 (32.5–32.7) | 34.5 (34.4–34.6) | 32.7 (32.7–32.9) | 34.6 (34.5–34.9) | 33.2 (33.1–33.3) | 35.3 (35.2–35.4) | 32.8 (32.7–32.8) | 35.1 (35.0–35.4) |
| RDW, % | 12.2 (12.2–12.3) | 14.8 (14.6–15.2) | 12.2 (12–12.3) | 14.8 (14.3–15.3) | 12.5 (12.4–12.6) | 14.9 (14.7–15.1) | 12.4 (12.3–12.4) | 15.2 (15.1–15.3) |
| PLT, 109/L | 177 (158–182) | 392 (367–422) | 184 (148–196) | 374 (370–422) | 164 (156–167) | 356 (345–378) | 145 (121–148) | 349 (348–362) |
| MPV, fL | 6.8 (6.5–7.0) | 9.7 (9.5–9.8) | 6.6 (6.2–6.7) | 9.1 (9.1–9.5) | 6.7 (6.5–6.7) | 9.5 (9.4–9.8) | 6.8 (6.3–6.8) | 10.3 (10.2–10.6) |
| PCT, fL | 0.15 (0.13–0.15) | 0.31 (0.3–0.33) | 0.15 (0.13–0.16) | 0.29 (0.27–0.32) | 0.14 (0.13–0.14) | 0.28 (0.27–0.29) | 0.13 (0.12–0.13) | 0.26 (0.26–0.27) |
| PDW, fL | 15.8 (15.7–15.9) | 17.5 (17.3–17.7) | 15.7 (15.6–15.8) | 17.2 (17.2–17.4) | 16.1 (15.9–16.1) | 18.0 (18.0–18.1) | 16.4 (16.3–16.4) | 18.5 (18.4–18.7) |
| This Study (Korean) | Li et al. [9] (Chinese) | Pham et al. [10] (Vietnamese) | Fiseha et al. [33] (Ethiopian) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | 2.5th | 97.5th | ||
| WBC, 109/L | Trimester 1 | 5.11 | 12.14 | 4.68 | 12.87 | 6.33 | 15.24 | 3.6 | 13.2 |
| Trimester 2 | 6.11 | 13.45 | 5.97 | 16.78 | 6.99 | 15.55 | 4.56 | 13.59 | |
| Trimester 3 | 5.62 | 12.42 | 5.53 | 19.56 | 6.22 | 14.14 | 4.56 | 13.62 | |
| RBC, 1012/L | Trimester 1 | 20 | 4.78 | 3.70 | 5.07 | 3.73 | 5.32 | 3.58 | 4.90 |
| Trimester 2 | 3.09 | 4.17 | 2.85 | 4.59 | 3.33 | 4.98 | 3.35 | 4.01 | |
| Trimester 3 | 3.26 | 4.48 | 2.75 | 4.64 | 3.54 | 4.98 | 3.76 | 4.99 | |
| Hgb, g/dL | Trimester 1 | 11.3 | 14.3 | 11.0 | 14.7 | 10.33 | 13.95 | 10.37 | 13.53 |
| Trimester 2 | 10.1 | 13.3 | 8.8 | 13.6 | 9.71 | 13.17 | 9.99 | 12.90 | |
| Trimester 3 | 10.1 | 14.1 | 8.4 | 14.1 | 9.80 | 13.97 | 10.68 | 13.71 | |
| Hct, % | Trimester 1 | 32.8 | 43.9 | 33 | 43 | 32.22 | 42.29 | 34.86 | 47.80 |
| Trimester 2 | 29.2 | 39.1 | 27 | 40 | 30.26 | 40.07 | 33.93 | 46.19 | |
| Trimester 3 | 30.2 | 41.6 | 26 | 42 | 31.11 | 42.70 | 32.33 | 45.98 | |
| MCV, fL | Trimester 1 | 83.5 | 98.5 | 76.8 | 95.2 | 66.13 | 95.85 | 86.67 | 103.03 |
| Trimester 2 | 86.9 | 100.8 | 78.3 | 99.7 | 69.14 | 97.79 | 86.10 | 103.58 | |
| Trimester 3 | 81.6 | 100.8 | 78.7 | 101.7 | 69.43 | 98.40 | 87.62 | 105.77 | |
| MCH, pg | Trimester 1 | 28.1 | 33.9 | 24.6 | 32.7 | 20.57 | 31.78 | 26.40 | 32.94 |
| Trimester 2 | 28.8 | 34.9 | 24.6 | 34.0 | 21.51 | 32.68 | 26.89 | 33.20 | |
| Trimester 3 | 26.9 | 34.9 | 25.1 | 34.6 | 30.70 | 33.94 | 27.51 | 33.99 | |
| MCHC, g/L | Trimester 1 | 32.7 | 34.6 | 32 | 35.5 | 31.13 | 33.85 | 30.30 | 33.66 |
| Trimester 2 | 33.2 | 35.3 | 31.9 | 35.1 | 30.99 | 34.13 | 30.13 | 33.2 | |
| Trimester 3 | 32.8 | 35.1 | 31.5 | 34.8 | 30.70 | 33.94 | 30.31 | 33.86 | |
| RDW, % | Trimester 1 | 12.2 | 14.8 | 11.9 | 16.8 | 12.27 | 17.78 | 12.44 | 15.99 |
| Trimester 2 | 12.5 | 14.9 | 12.3 | 17.2 | 12.69 | 16.17 | 12.52 | 17.0 | |
| Trimester 3 | 12.4 | 15.2 | 12.3 | 19.8 | 12.75 | 17.29 | 12.62 | 16.20 | |
| PLT, 109/L | Trimester 1 | 184 | 374 | 148 | 352 | 169.66 | 413.88 | 167.05 | 390.0 |
| Trimester 2 | 164 | 356 | 111 | 346 | 172.34 | 372.19 | 149.58 | 373.32 | |
| Trimester 3 | 145 | 349 | 80 | 309 | 151.30 | 417.14 | 124.60 | 356.90 | |
| MPV, fL | Trimester 1 | 6.6 | 9.1 | 8.5 | 11.9 | 6.65 | 9.85 | 6.73 | 9.80 |
| Trimester 2 | 6.7 | 9.5 | 7.0 | 11.8 | 6.48 | 9.70 | 7.05 | 10.25 | |
| Trimester 3 | 6.8 | 10.3 | 7.0 | 12.9 | 6.69 | 10.39 | 7.40 | 10.30 | |
| PCT, fL | Trimester 1 | 0.15 | 0.29 | 0.152 | 0.316 | ||||
| Trimester 2 | 0.14 | 0.28 | 0.110 | 0.321 | |||||
| Trimester 3 | 0.13 | 0.26 | 0.118 | 0.321 | |||||
| PDW, fL | Trimester 1 | 15.7 | 17.2 | 9.0 | 16.4 | 15.10 | 16.36 | ||
| Trimester 2 | 16.1 | 18.0 | 9.1 | 18.1 | 15.22 | 16.48 | |||
| Trimester 3 | 16.4 | 18.5 | 10.2 | 19.1 | 15.16 | 16.57 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, H.; Kwon, K.; Jung, S.; Kim, K. Physiological Changes and Trimester-Specific Reference Intervals for Complete Blood Count Parameters in Korean Pregnant Women. Medicina 2025, 61, 1665. https://doi.org/10.3390/medicina61091665
So H, Kwon K, Jung S, Kim K. Physiological Changes and Trimester-Specific Reference Intervals for Complete Blood Count Parameters in Korean Pregnant Women. Medicina. 2025; 61(9):1665. https://doi.org/10.3390/medicina61091665
Chicago/Turabian StyleSo, Heejin, Kyungsuk Kwon, Sukhyun Jung, and Kyeongmi Kim. 2025. "Physiological Changes and Trimester-Specific Reference Intervals for Complete Blood Count Parameters in Korean Pregnant Women" Medicina 61, no. 9: 1665. https://doi.org/10.3390/medicina61091665
APA StyleSo, H., Kwon, K., Jung, S., & Kim, K. (2025). Physiological Changes and Trimester-Specific Reference Intervals for Complete Blood Count Parameters in Korean Pregnant Women. Medicina, 61(9), 1665. https://doi.org/10.3390/medicina61091665





