Nutritional Ultrasound in the Morphofunctional Assessment of Malnutrition in Patients Undergoing Incremental Versus Conventional Hemodialysis: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphofunctional Assessment of Malnutrition Included
2.1.1. Anthropometric Measurements
2.1.2. BIA
2.1.3. Handgrip Strength (HGS)
2.1.4. Nutritional and Frailty Scales
2.1.5. Functional Physical Performance Variable
2.1.6. Nutritional Ultrasound (NUS)
- Anterior QRF ultrasound: The variables measured to assess muscle mass were anterior–posterior muscle thickness (Y-axis in mm), adjusted for height (Y-axis/h in mm/m2) transversal muscle thickness (X-axis in mm), supramuscular fat (SMF in mm) and cross-sectional muscle area of rectus femoris (CS-MARF in cm2). The CS-MARF was standardized by height [MARF (cm2)/(height × height) (m2)], which was named the muscle area rectus femoris index (MARFIh in cm2/m2).
- PPVF and abdominal subcutaneous fat: An imaginary line was drawn connecting the xiphoid process and the umbilicus. At the midpoint of this line, images of total transverse subcutaneous fat (SSCF in cm plus DSCF in cm) and PPVF were acquired, with the transducer positioned perpendicular to the longitudinal axis.
2.1.7. Analytic Variables
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schieppati, A.; Remuzzi, G. Chronic renal diseases as a public health problem: Epidemiology, social, and economic implications. Kidney Int. 2005, 68, S7–S10. [Google Scholar] [CrossRef]
- Soi, V.; Faber, M.D.; Paul, R. Incremental hemodialysis: What we know so far. Int. J. Nephrol. Renov. Dis. 2022, 15, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Murea, M.; Kalantar-Zadeh, K. Incremental and twice-weekly hemodialysis program in practice. Clin. J. Am. Soc. Nephrol. 2020, 16, 147–149. [Google Scholar] [CrossRef]
- Casino, F.G.; Basile, C. One-size-does-not-fit-all: The case of incremental hemodialysis. Kidney Dial. 2024, 4, 27–36. [Google Scholar] [CrossRef]
- Takkavatakarn, K.; Jintanapramote, K.; Phannajit, J.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Incremental versus conventional haemodialysis in end-stage kidney disease: A systematic review and meta-analysis. Clin. Kidney J. 2023, 17, sfad280. [Google Scholar] [CrossRef]
- Sahathevan, S.; Khor, B.H.; Ng, H.M.; Gafor, A.H.A.; Mat Daud, Z.A.; Mafra, D.; Karupaiah, T. Understanding development of malnutrition in hemodialysis patients: A narrative review. Nutrients 2020, 12, 3147. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. S1), S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.A.; Oliveira, D.; Mansur, H.N.; Fernandes, N.M.; Bastos, M.G. Sarcopenia in chronic kidney disease. J. Bras. Nefrol. 2015, 37, 98–105. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Bellido Guerrero, D.; Bretón Lesmes, I.; Tinahones Madueño, F.J. Nutritional ultrasound: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2023, 70 (Suppl. S1), 74–84. [Google Scholar] [CrossRef]
- Güner, M.; Girgin, S.; Ceylan, S.; Özcan, B.; Öztürk, Y.; Baş, A.O.; Koca, M.; Balcı, C.; Doğu, B.B.; Cankurtaran, M.; et al. The role of muscle ultrasonography to diagnose malnutrition and sarcopenia in maintenance hemodialysis. J. Ren. Nutr. 2024, 34, 330–336. [Google Scholar] [CrossRef] [PubMed]
- De la Flor, J.C.; García-Menéndez, E.; Romero-González, G.; Rodríguez Tudero, C.; Jiménez Mayor, E.; Florit Mengual, E.; Moral Berrio, E.; Soria Morales, B.; Cieza Terrones, M.; Cigarrán Guldris, S.; et al. Morphofunctional assessment of malnutrition and sarcopenia using nutritional ultrasonography in patients undergoing maintenance hemodialysis. Medicina 2025, 61, 1044. [Google Scholar] [CrossRef] [PubMed]
- Deira, J.; Suárez, M.A.; López, F.; García-Cabrera, E.; Gascón, A.; Torregrosa, E.; García, G.E.; Huertas, J.; de la Flor, J.C.; Puello, S.; et al. IHDIP: A controlled randomized trial to assess the security and effectiveness of the incremental hemodialysis in incident patients. BMC Nephrol. 2019, 20, 8. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Unruh, M.; Zager, P.G.; Kovesdy, C.P.; Bargman, J.M.; Chen, J.; Sankarasubbaiyan, S.; Shah, G.; Golper, T.; Sherman, R.A.; et al. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am. J. Kidney Dis. 2014, 64, 181–186. [Google Scholar] [CrossRef]
- International Society for the Advancement of Kinanthropometry. International Standards for Anthropometric Assessment—ScienceOpen. Available online: https://www.scienceopen.com/document?vid=ccde4859-d1c7-4ea6-92b8-37d48744f862 (accessed on 30 March 2025).
- Sakaguchi, Y.; Kaimori, J.-Y.; Isaka, Y. Plant-Dominant Low Protein Diet: A Potential Alternative Dietary Practice for Patients with Chronic Kidney Disease. Nutrients 2023, 15, 1002. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef]
- Reliability of the 7-Point Subjective Global Assessment Scale in Assessing Nutritional Status of Dialysis Patients. Available online: https://pubmed.ncbi.nlm.nih.gov/10682107/ (accessed on 6 April 2025).
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Short Physical Performance Battery (SPPB)|National Institute on Aging. Available online: https://www.nia.nih.gov/research/labs/leps/short-physical-performance-battery-sppb (accessed on 30 March 2025).
- Wathanavasin, W.; Banjongjit, A.; Avihingsanon, Y.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S.; Susantitaphong, P. Prevalence of Sarcopenia and Its Impact on Cardiovascular Events and Mortality among Dialysis Patients: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4077. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.P.; Almeida, L.S.; Neri, S.G.; Oliveira, J.S.; Wilkinson, T.J.; Ribeiro, H.S.; Lima, R.M. Prevalence of sarcopenia in patients with chronic kidney disease: A global systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 501–512. [Google Scholar] [CrossRef]
- Mariños Cotrina, B.W.; Rodríguez Brown Agurto, F.S.; Mendez Carbajal, D.F. Sarcopenia y fragilidad en sujetos sometidos a hemodiálisis en un centro de diálisis en el Perú. Rev. Nutr. Clín. Metab. 2019, 2, 57–64. [Google Scholar] [CrossRef]
- Caria, S.; Cupisti, A.; Sau, G.; Bolasco, P. The incremental treatment of ESRD: A low-protein diet combined with weekly hemodialysis may be beneficial for selected patients. BMC Nephrol. 2014, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Del Vecchio, L.; Aicardi, V. Nutritional Issues with Incremental Dialysis: The Role of Low-Protein Diets. Semin. Dial. 2017, 30, 246–250. [Google Scholar] [CrossRef]
- Bahşi, R.; Üstüner, E.; Atmış, V.; Coşarderelioğlu, Ç.; SürMeli, D.M.; Öztorun, H.S.; Turgut, T.; Yalçın, A.; Varlı, M.; Aras, S. Visceral fat thickness may be useful in determining sarcopenia. Aging Med. Healthc. 2021, 12, 20–25. [Google Scholar] [CrossRef]
- Tang, J.; Wang, L.; Luo, J.; Xi, D.; Huang, W.; Yang, S.; Ye, J.; Zhang, Y. Early albumin level and mortality in hemodialysis patients: A retrospective study. Ann. Palliat. Med. 2021, 10, 10697–10705. [Google Scholar] [CrossRef]
- Jung, S.; Lee, S.; Jang, H.N.; Chang, S.H.; Kim, H.J. Comparison between incremental and conventional hemodialysis in a single center. J. Am. Soc. Nephrol. 2022, 33, 727. [Google Scholar] [CrossRef]

| Variable | cHD | iHD | p Value |
|---|---|---|---|
| Demographic and body measurement parameters | |||
| Age (years), mean (SD) | 72.6 (15.8) | 75.5 (14.5) | 0.526 |
| Sex (Men), n (%) | 43 (70.5%) | 9 (69.2%) | 1.000 |
| Weight (kg) | 67.63 (12.71) | 70.9 (10.45) | 0.34 |
| Height (m) | 1.66 (0.1) | 1.66 (0.1) | 0.99 |
| BMI (kg/m2), mean (SD) | 24.78 (4.4) | 25.75 (3.27) | 0.37 |
| Body surface area (m2), mean (SD) | 1.73 (0.19) | 1.77 (0.16) | 0.50 |
| Waist circumference (cm), mean (SD) | 78.6 (12.9) | 73.9 (21.7) | 0.463 |
| Triceps skinfold (mm), mean (SD) | 12.3 (6.4) | 12.5 (4.9) | 0.934 |
| Suprailiac skinfold (mm), mean (SD) | 16.5 (7.4) | 18.0 (10.6) | 0.628 |
| COPD, n (%) | 17 (27.9%) | 6 (46.2%) | 0.206 |
| Ischemic heart disease, n (%) | 31 (50.8%) | 6 (46.2%) | 1.000 |
| Secondary hyperparathyroidism, n (%) | 60 (98.4%) | 13 (100.0%) | 1.000 |
| Oncologic disease, n (%) | 17 (27.9%) | 2 (15.4%) | 0.494 |
| Depression, n (%) | 6 (9.8%) | 0 (0.0%) | 0.583 |
| Gastrointestinal disorders, n (%) | 16 (26.2%) | 1 (7.7%) | 0.275 |
| Causes of CKD | |||
| Diabetic kidney disease, n (%) | 22 (36.1%) | 4 (30.8%) | 0.200 |
| ADPKD, n (%) | 4 (6.6%) | 0 (0%) | 0.547 |
| Glomerular disease, n (%) | 6 (15.4%) | 2 (15.4%) | 1.000 |
| Hemodialysis parameters | |||
| HD vintage (months), mean (SD) | 42.5 (34.0) | 21.1 (19.5) | 0.004 |
| Dry weight (kg), mean (SD) | 67.6 (12.7) | 70.9 (10.5) | 0.327 |
| IDWG (kg), mean (SD) | 2.18 (0.7) | 1.70 (0.6) | 0.026 |
| Kt/V urea, mean (SD) | 1.6 (0.2) | 1.7 (0.3) | 0.160 |
| KT (L), mean (SD) | 51.4 (6.4) | 54.9 (5.5) | 0.060 |
| nPCR (g urea/kg/day), mean (SD) | 1.0 (0.3) | 1.55 (0.4) | 0.0004 |
| QB (mL/min), mean (SD) | 337.2 (22.3) | 325.4 (13.5) | 0.07 |
| APF (mL/min) | 177.1 (34.0) | 152.8 (40.8) | 0.063 |
| VPF (mL/min) | 163.8 (21.2) | 155.3 (28.1) | 0.316 |
| SBP (mmHg) | 126.7 (25.8) | 142.2 (16.5) | 0.011 |
| DBP (mmHg) | 83.6 (59.3) | 68.9 (12.4) | 0.322 |
| Renal Residual Function | |||
| KrU (mL/min/1.73 m2), mean (SD) | 1.2 (0.8) | 4.1 (1.1) | <0.001 |
| 24 h urine output (mL) | 420 (280) | 1150 (420) | <0.001 |
| Proteinuria (mg), mean (SD) | 105 (80) | 815 (260) | <0.001 |
| Vascular access type | |||
| Arteriovenous fistula (%) | 37.7% | 62.3% | 1.000 |
| Tunneled catheter (%) | 62% | 38% | 1.000 |
| cHD | iHD | p Value | |
|---|---|---|---|
| Patients (N) | 61 | 13 | |
| Hemoglobin (g/L), mean (SD) | 11.27 (1.67) | 10.98 (1.1) | 0.44 |
| Fe (mg/dL), mean (SD) | 72.34 (34.06) | 58.08 (27.35) | 0.12 |
| TSI (%), mean (SD) | 33.46 (5.5) | 28.31 (12.61) | 0.21 |
| Transferrin (mg/dL), mean (SD) | 171.36 (30.7) | 160.08 (20.12) | 0.11 |
| Ferritin (ng/mL), mean (SD) | 741.9 (560.6) | 937.69 (357.53) | 0.12 |
| Calcium (mg/dL), mean (SD) | 8.84 (0.69) | 8.92 (0.74) | 0.71 |
| Phosphorus (mg/dL), mean (SD) | 4.26 (1.59) | 4.42 (1.15) | 0.67 |
| 25OHD (ng/mL), mean (SD) | 26.46 (14.55) | 20.88 (9.33) | 0.09 |
| PTH (pg/mL), mean (SD) | 254.08 (206.39) | 275.87 (220.2) | 0.75 |
| Magnesium (mg/dL), mean (SD) | 2.14 (0.29) | 2.35 (0.27) | 0.02 |
| Total Cholesterol (mg/dL), mean (SD) | 130.92 (32.3) | 125.0 (29.61) | 0.53 |
| HDL (mg/dL), mean (SD) | 49.11 (19.17) | 44.62 (11.13) | 0.26 |
| LDL (mg/dL), mean (SD) | 62.08 (25.68) | 59.85 (23.4) | 0.76 |
| non-HDL (mg/dL), mean (SD) | 81.8 (28.37) | 80.38 (23.89) | 0.85 |
| Triglycerides (mg/dL), mean (SD) | 122.44 (71.23) | 124.69 (55.18) | 0.90 |
| C-reactive protein (mg/L), mean (SD) | 2.37 (4.92) | 1.75 (4.04) | 0.63 |
| Lymphocytes (103/μL), mean (SD) | 1.09 (0.47) | 1.22 (0.61) | 0.49 |
| Sodium (mmol/L), mean (SD) | 137.93 (3.36) | 139.23 (3.37) | 0.22 |
| Potassium (mmol/L), mean (SD) | 4.58 (0.74) | 4.39 (1.01) | 0.53 |
| Chloride (mmol/L), mean (SD) | 101.1 (3.54) | 104.38 (3.38) | 0.005 |
| Bicarbonate (mEq/L), mean (SD) | 23.31 (2.31) | 23.05 (2.96) | 0.76 |
| Urea (mmol/mL), mean (SD) | 108.49 (43.81) | 169.38 (62.41) | 0.004 |
| BUN (mmol/mL), mean (SD) | 50.63 (20.45) | 79.05 (29.12) | 0.004 |
| Creatinine (g/dL), mean (SD) | 6.01 (2.02) | 6.2 (2.97) | 0.82 |
| Total proteins (g/dL), mean (SD) | 6.38 (0.62) | 6.59 (0.51) | 0.21 |
| Prealbumin (mg/dL), mean (SD) | 26.39 (6.24) | 26.86 (8.89) | 0.86 |
| Albumin (g/dL), mean (SD) | 3.2 (0.51) | 3.38 (0.47) | 0.24 |
| Cr/BSA (mg/dL/m2) | 3.49 (1.24) | 3.53 (1.68) | 0.93 |
| cHD | iHD | p Value | |
|---|---|---|---|
| Patients (N) | 61 | 13 | |
| Muscle strength | |||
| Handgrip strength (HGS) (kg), mean (SD) | 17.6 (8.8) | 21.1 (7.7) | 0.19 |
| Reduced HGS, n (%) | 41 (67.2) | 7 (53.9) | 0.4 |
| Functional performance | |||
| SPPB (points) | 8.6 (2.1) | 8.9 (2.2) | 0.2 |
| Low performance (SPPB ≤ 8), n (%) | 18 (29.5) | 3 (23.07) | 0.42 |
| Scales | |||
| MIS (points) | 8.1 (3.5) | 5.6 (4.9) | 0.04 |
| MIS > 8 points, n (%) | 47.5 | 7.7 | 0.008 |
| MST > 2 points, n (%) | 24.6 | 7.7 | 0.18 |
| 7–points SGA scale (Mild–moderate–severely malnourished), n (%) | 54.1 | 30.08 | 0.13 |
| PEW > 2 points, n (%) | 49.2 | 23.1 | 0.09 |
| FRAIL scale, (score ≥ 3 points), n (%) | 28 (45.9) | 1 (7.7) | 0.04 |
| SARCOPENIA (2019-EWGSOP2), n (%) | 23 (37.7) | 2 (15.4) | 0.12 |
| cHD | iHD | p Value | |
|---|---|---|---|
| Patients (N) | 61 | 13 | |
| ASMM (kg), mean (SD) | 18.1 (4.5) | 18.2 (4.3) | 0.95 |
| ASMMI (kg/m2), mean (SD) | 6.5 (1.2) | 6.6 (1.2) | 0.88 |
| PA (°), mean (SD) | 4.8 (1.5) | 4.8 (1.1) | 0.92 |
| VFA (cm2), mean (SD) | 92.6 (59.9) | 93.3 (36.9) | 0.97 |
| FFM (kg), mean (SD) | 47.3 (11.6) | 49.3 (9.4) | 0.57 |
| LBM (kg), mean (SD) | 44.8 (10.5) | 44.3 (10.8) | 0.88 |
| BFM (kg), mean (SD) | 20.6 (10.1) | 21.6 (7.3) | 0.73 |
| TBW (L), mean (SD) | 35.2 (8.1) | 34.8 (7.9) | 0.86 |
| ICW (L), mean (SD) | 22.2 (8.9) | 21.9 (3.9) | 0.9 |
| ECW (L), mean (SD) | 14.1 (3.9) | 14.4 (3.3) | 0.76 |
| cHD | iHD | p Value | |
|---|---|---|---|
| N | 61 | 13 | |
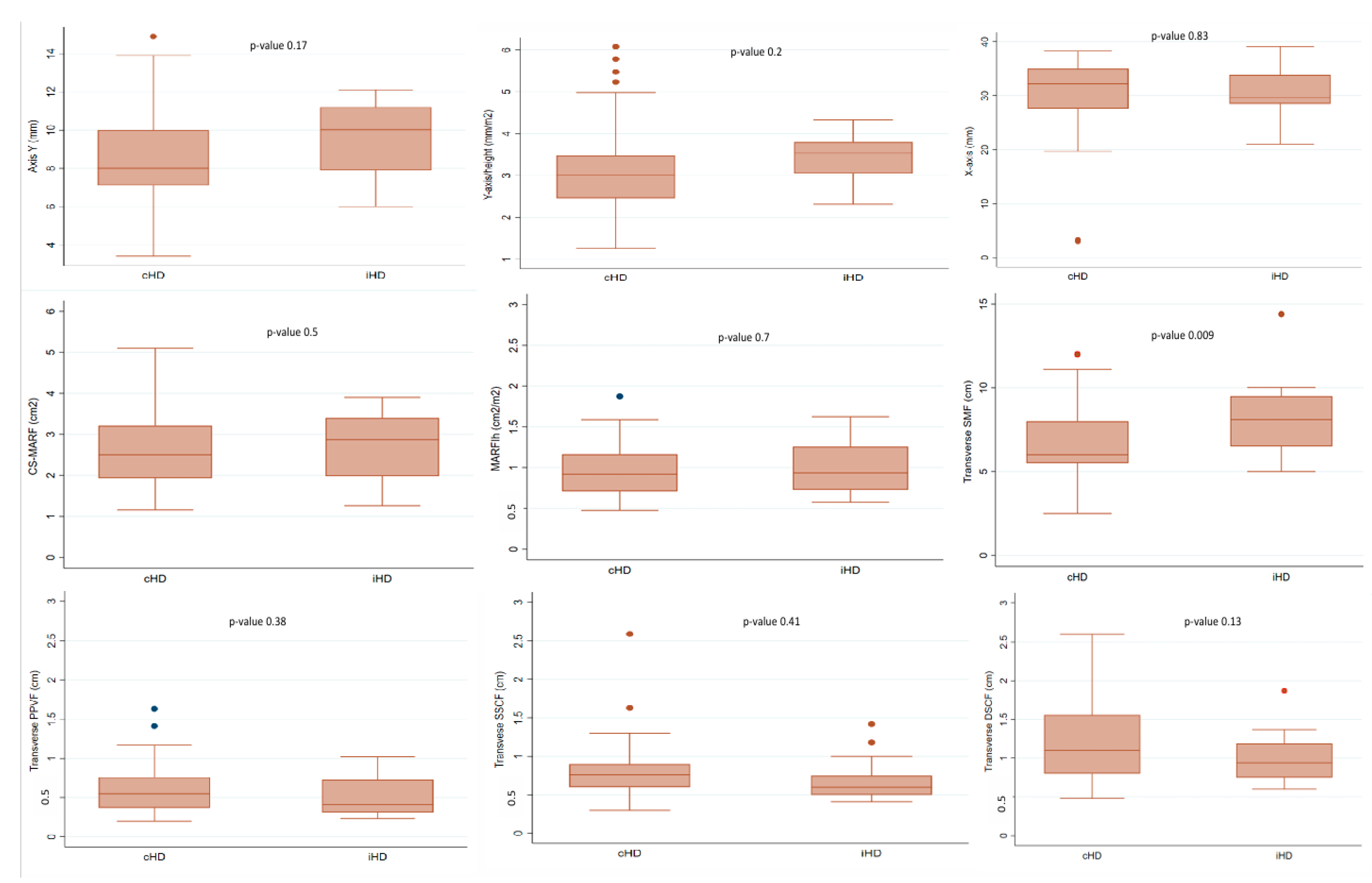
| Y-axis (mm), mean (SD) | 8.5 (2.3) | 9.5 (2) | 0.17 |
| X-axis (mm), mean (SD) | 30.4 (6.8) | 30.9 (5.3) | 0.83 |
| Y-axis/height (mm/m2), mean (SD) | 3.1 (1.0) | 3.4 (0.6) | 0.2 |
| Y-axis/BSA (mm/m2), mean (SD) | 2.9 (0.9) | 3.0 (0.6) | 0.5 |
| CS-MARF (cm2), mean (SD) | 2.6 (0.8) | 2.9 (0.6) | 0.1 |
| MARFIh (cm2/m2), mean (SD) | 0.96 (0.32) | 1.0 (0.31) | 0.66 |
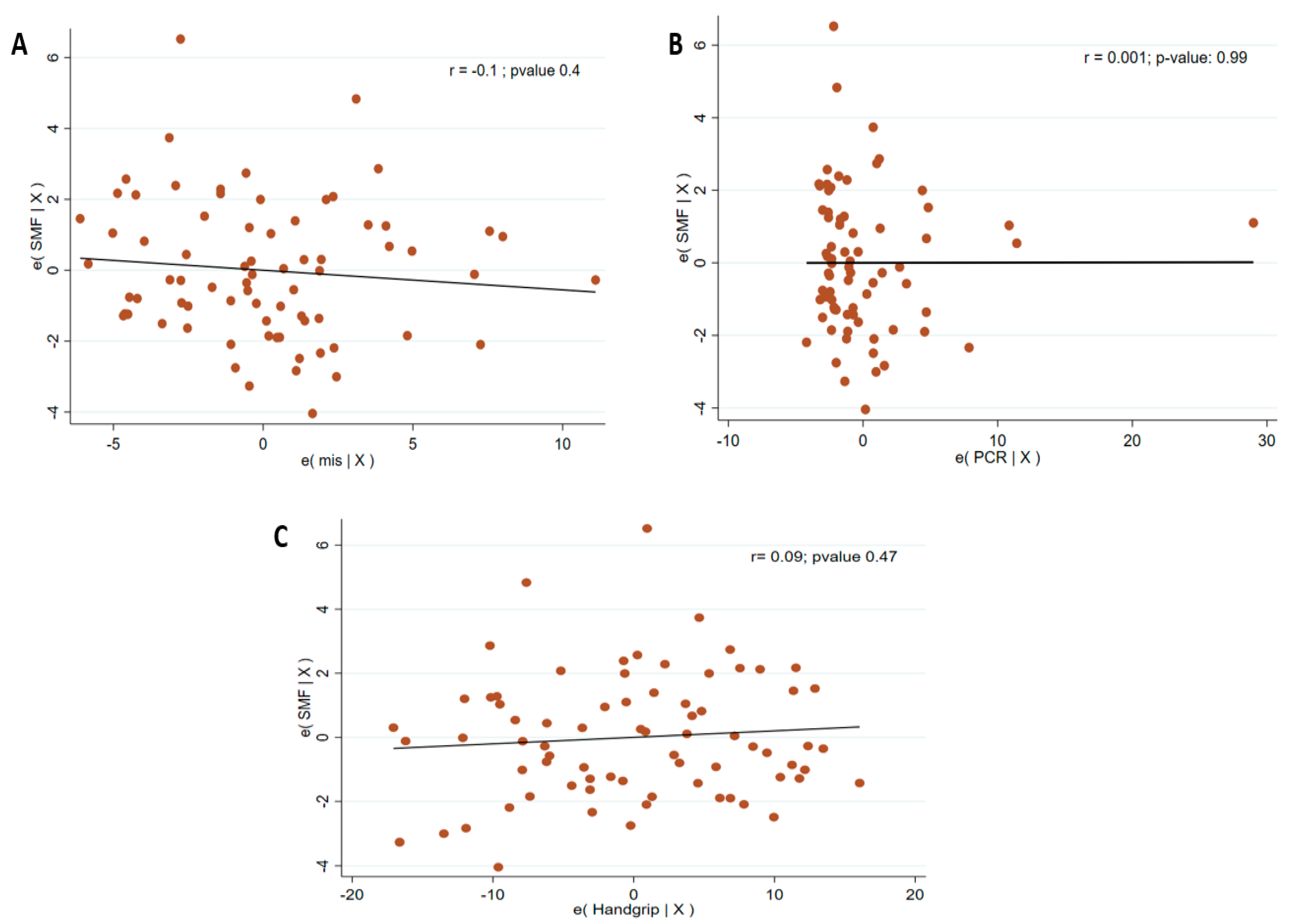
| Transverse SMF (cm) mean (SD) | 6.6 (2) | 8.3 (2.5) | 0.009 |
| Transverse PPVF (cm), mean (SD) | 0.6 (0.3) | 0.5 (0.3) | 0.38 |
| Transverse DSCF (cm) mean (SD) | 1.2 (0.5) | 1 (0.4) | 0.13 |
| Transverse SSCF (cm), mean (SD) | 0.8 (0.4) | 0.7 (0.3) | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez Mayor, E.; De La Flor, J.C.; Chandu Nanwani, A.; Rodríguez Tudero, C.; García-Menéndez, E.; Elias, R.; Chimoy, H.; Dominguez Davalos, M.; Cieza Terrones, M.; Valga, F.; et al. Nutritional Ultrasound in the Morphofunctional Assessment of Malnutrition in Patients Undergoing Incremental Versus Conventional Hemodialysis: A Comparative Study. Medicina 2025, 61, 1633. https://doi.org/10.3390/medicina61091633
Jiménez Mayor E, De La Flor JC, Chandu Nanwani A, Rodríguez Tudero C, García-Menéndez E, Elias R, Chimoy H, Dominguez Davalos M, Cieza Terrones M, Valga F, et al. Nutritional Ultrasound in the Morphofunctional Assessment of Malnutrition in Patients Undergoing Incremental Versus Conventional Hemodialysis: A Comparative Study. Medicina. 2025; 61(9):1633. https://doi.org/10.3390/medicina61091633
Chicago/Turabian StyleJiménez Mayor, Elena, José C. De La Flor, Avinash Chandu Nanwani, Celia Rodríguez Tudero, Estefanya García-Menéndez, Raul Elias, Hemily Chimoy, Marco Dominguez Davalos, Michael Cieza Terrones, Francisco Valga, and et al. 2025. "Nutritional Ultrasound in the Morphofunctional Assessment of Malnutrition in Patients Undergoing Incremental Versus Conventional Hemodialysis: A Comparative Study" Medicina 61, no. 9: 1633. https://doi.org/10.3390/medicina61091633
APA StyleJiménez Mayor, E., De La Flor, J. C., Chandu Nanwani, A., Rodríguez Tudero, C., García-Menéndez, E., Elias, R., Chimoy, H., Dominguez Davalos, M., Cieza Terrones, M., Valga, F., & Hernández Vaquero, J. (2025). Nutritional Ultrasound in the Morphofunctional Assessment of Malnutrition in Patients Undergoing Incremental Versus Conventional Hemodialysis: A Comparative Study. Medicina, 61(9), 1633. https://doi.org/10.3390/medicina61091633







