Risk Factors and Development of a Predictive Model for In-Hospital Mortality in Hemodynamically Stable Older Adults with Urinary Tract Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Population
2.2. Data Collection
2.3. Model Development and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical and Laboratory Differences Between Survivors and Non-Survivors
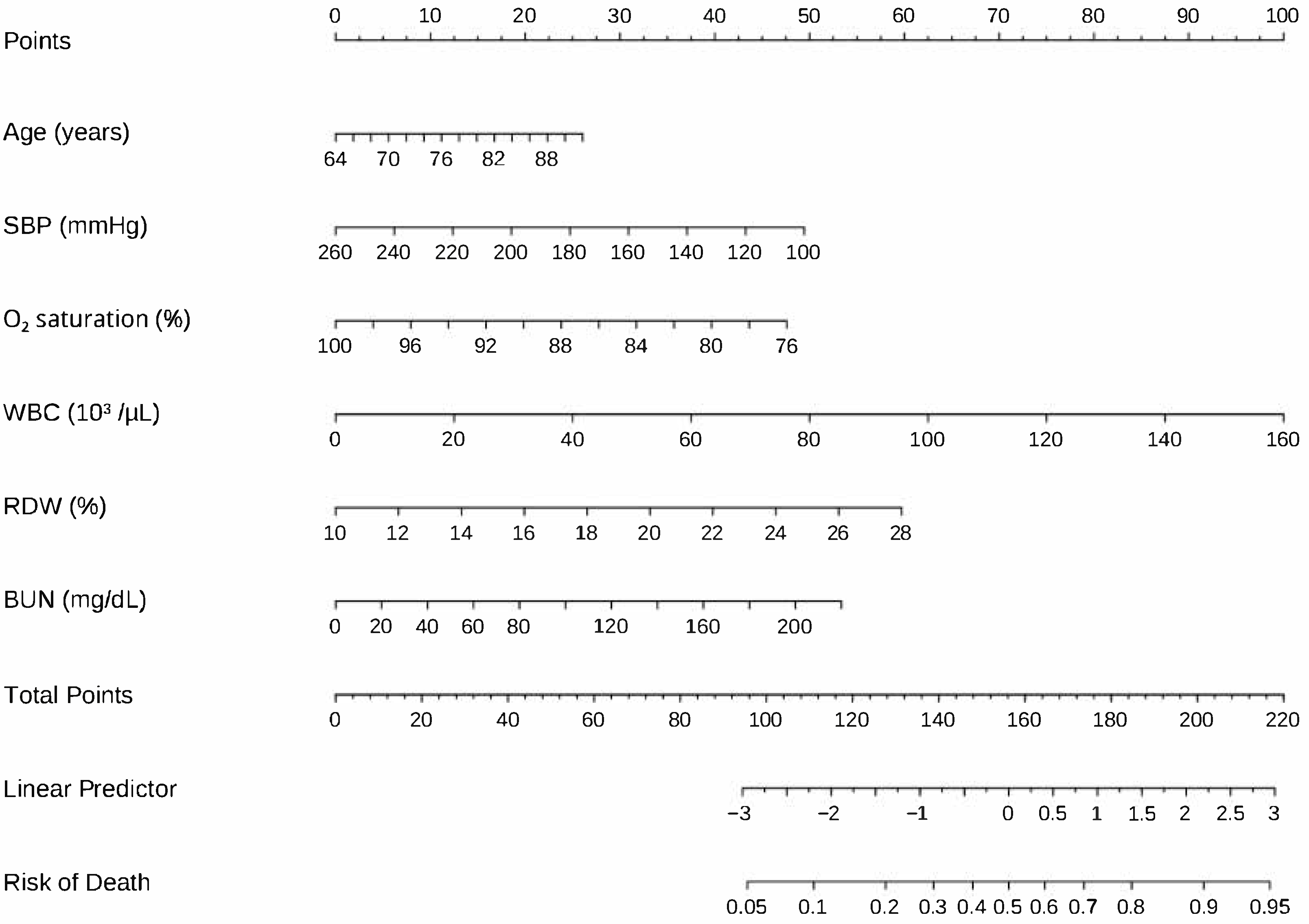
3.3. Variable Selection and Model Development
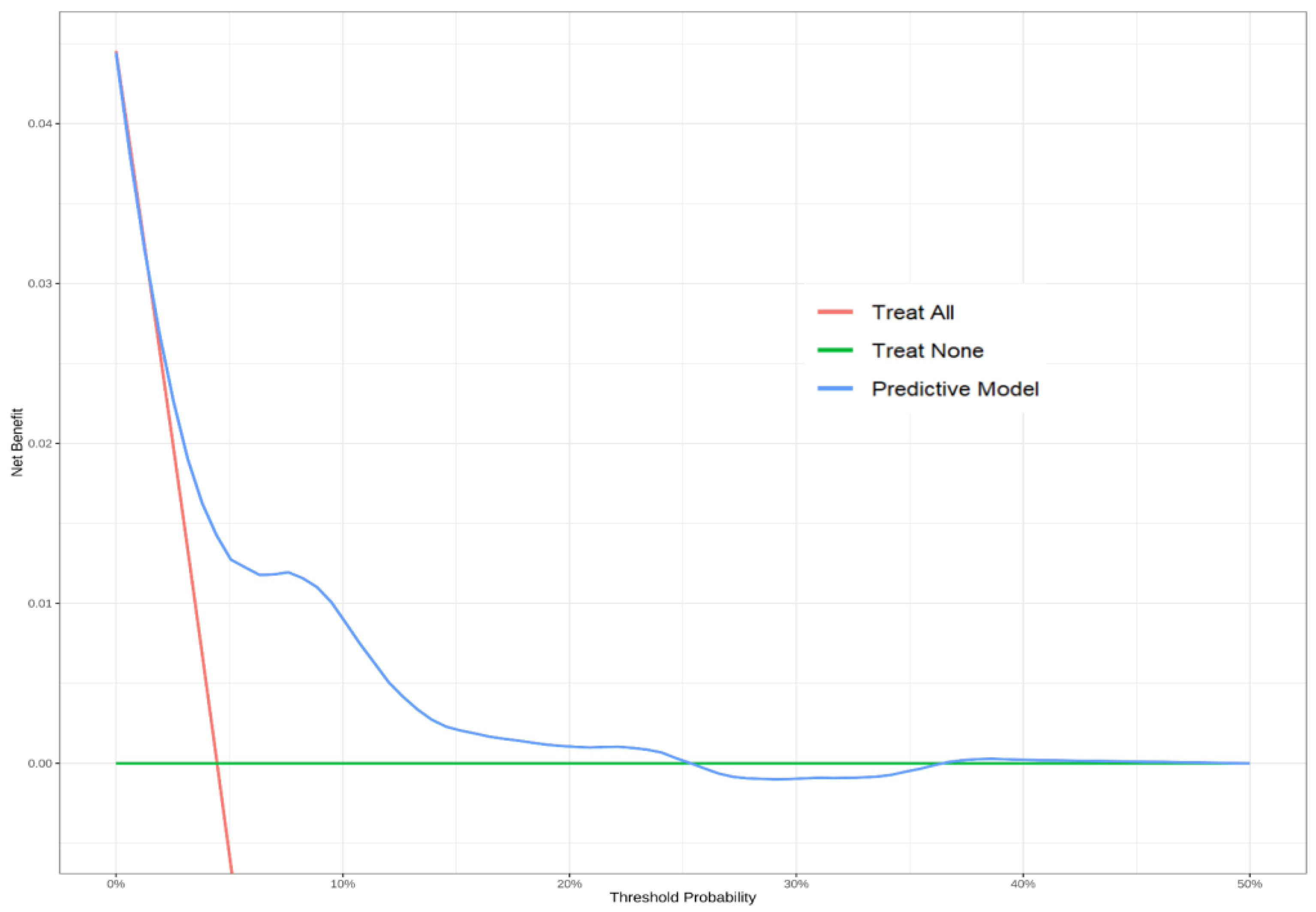
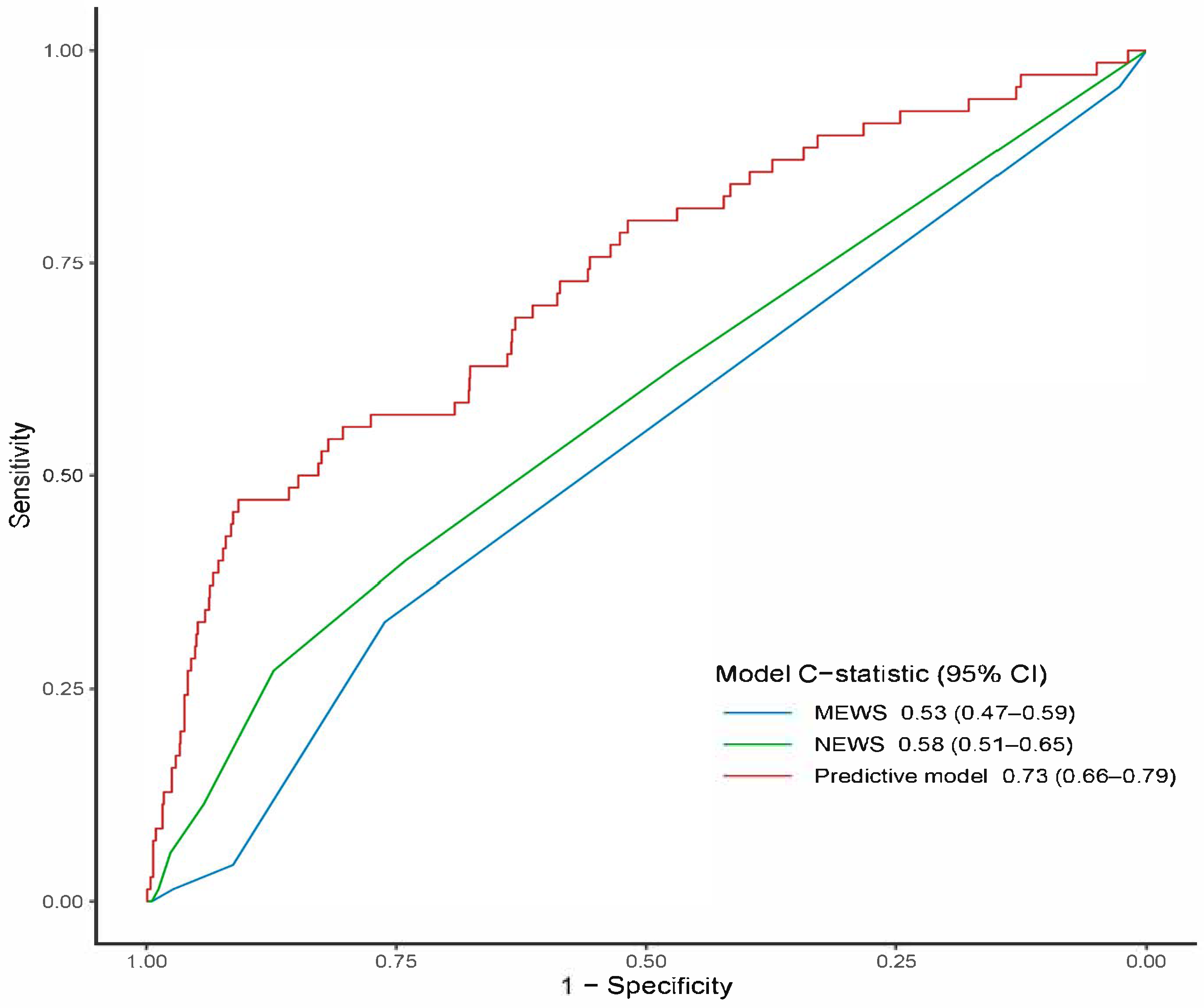
3.4. Model Performance and Bootstrap Internal Validation
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Zhao, J.; Wang, L.; Han, C.; Yan, R.; Zhu, P.; Qian, T.; Yu, S.; Zhu, X.; He, W. Epidemiological trends and predictions of urinary tract infections in the global burden of disease study 2021. Sci Rep. 2025, 15, 4702. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.E. Clinical Presentations and Epidemiology of Urinary Tract Infections. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-C.; Ma, C.-Y.; Tsai, Y.-C. Interpretable Machine Learning Models for Predicting Critical Outcomes in Patients with Suspected Urinary Tract Infection with Positive Urine Culture. Diagnostics 2024, 14, 1974. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Huang, Y.-H.; Niu, K.-Y.; Tsai, Y.-C.; Chen, C.-B.; Yen, C.-C. Development of a Predictive Nomogram for Sepsis in Patients with Urolithiasis-Related Obstructive Pyelonephritis. Medicina 2024, 60, 1113. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Nathanson, B.H.; Sulham, K.; Shorr, A.F. Descriptive epidemiology and outcomes of emergency department visits with complicated urinary tract infections in the United States, 2016-2018. J. Am. Coll. Emerg. Physicians Open 2022, 3, e12694. [Google Scholar] [CrossRef]
- Sabih, A.; Leslie, S.W. Complicated Urinary Tract Infections. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103-20. [Google Scholar] [CrossRef]
- Santo, L.; Kang, K. National hospital ambulatory medical care survey: 2019 national summary tables. 2023. [Google Scholar] [CrossRef]
- Theodorakis, N.; Feretzakis, G.; Hitas, C.; Kreouzi, M.; Kalantzi, S.; Spyridaki, A.; Kollia, Z.; Verykios, V.S.; Nikolaou, M. Immunosenescence: How Aging Increases Susceptibility to Bacterial Infections and Virulence Factors. Microorganisms 2024, 12, 2052. [Google Scholar] [CrossRef] [PubMed]
- Laborde, C.; Bador, J.; Hacquin, A.; Barben, J.; Putot, S.; Manckoundia, P.; Putot, A. Atypical Presentation of Bacteremic Urinary Tract Infection in Older Patients: Frequency and Prognostic Impact. Diagnostics 2021, 11, 523. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Li, J.-J.; Hsiao, C.-H.; Yen, C.-C. Clinical Characteristics and In-Hospital Outcomes in Patients with Iliopsoas Abscess: A Multicenter Study. J. Clin. Med. 2023, 12, 2760. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.C.; Yeh, H.; Ho, C.F.; Hsiao, C.H.; Niu, K.Y.; Yeh, C.C.; Lu, J.X.; Wu, C.C.; Chang, Y.C.; Ng, C.J. Risk factors for 30-day mortality in patients with head and neck cancer bleeding in the emergency department. Am. J. Emerg. Med. 2022, 58, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.C.; Ho, C.F.; Wu, C.C.; Tsao, Y.N.; Chaou, C.H.; Chen, S.Y.; Ng, C.J.; Yeh, H. In-Hospital and Long-Term Outcomes in Patients with Head and Neck Cancer Bleeding. Medicina 2022, 58, 177. [Google Scholar] [CrossRef]
- Yen, C.C.; Chen, S.Y.; Chaou, C.H.; Wang, C.K.; Yeh, H.T.; Ng, C.J. Prognostic Value of Cardiac Troponin and Risk Assessment in Pediatric Supraventricular Tachycardia. J. Clin. Med. 2021, 10, 3638. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.Y.; Chen, T.H.; Lee, Y.C.; Hsiao, M.C.; Hung, P.H.; Wang, M.C. Risk factors for uroseptic shock in hospitalized patients aged over 80 years with urinary tract infection. Ann. Transl. Med. 2020, 8, 477. [Google Scholar] [CrossRef]
- Johnson, A.; Bulgarelli, L.; Pollard, T.; Horng, S.; Celi, L.A.; Mark, R. MIMIC-IV, Version 2.2. PhysioNet, 2023. Available online: https://doi.org/10.13026/6mm1-ek67 (accessed on 1 January 2025).
- Johnson, A.; Bulgarelli, L.; Pollard, T.; Celi, L.A.; Mark, R.; Horng, S. MIMIC-IV-ED, Version 2.2. PhysioNet. 2023. Available online: https://doi.org/10.13026/5ntk-km72 (accessed on 1 January 2025).
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Germanos, G.; Light, P.; Zoorob, R.; Salemi, J.; Khan, F.; Hansen, M.; Gupta, K.; Trautner, B.; Grigoryan, L. Validating Use of Electronic Health Data to Identify Patients with Urinary Tract Infections in Outpatient Settings. Antibiotics 2020, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhou, J.; Lee, J.W.; Tan, M.; Li, S.; Rajnthern, L.S.O.; Chee, M.L.; Chakraborty, B.; Wong, A.-K.I.; Dagan, A.; et al. Benchmarking emergency department prediction models with machine learning and public electronic health records. Sci. Data 2022, 9, 658. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; McDermott, M.B.A.; Chauhan, G.; Ghassemi, M.; Hughes, M.C.; Naumann, T. MIMICExtract: A Data Extraction, Preprocessing, and Representation Pipeline for MIMIC-III. In Proceedings of the ACM Conference on Health, Inference, and Learning (ACM CHIL ’20), Toronto, ON, Canada, 2–4 April 2020. [Google Scholar]
- Azur, M.J.; Stuart, E.A.; Frangakis, C.; Leaf, P.J. Multiple imputation by chained equations: What is it and how does it work? Int. J. Methods Psychiatr. Res. 2011, 20, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.M.; Hu, C.; Roe, D.J.; Chen, Z.; Halonen, M.; Guerra, S. Least absolute shrinkage and selection operator type methods for the identification of serum biomarkers of overweight and obesity: Simulation and application. BMC Med Res. Methodol. 2016, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression shrinkage and selection via the lasso: A retrospective. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2011, 73, 273–282. [Google Scholar] [CrossRef]
- Christodoulou, E.; van Smeden, M.; Edlinger, M.; Timmerman, D.; Wanitschek, M.; Steyerberg, E.W.; Van Calster, B. Adaptive sample size determination for the development of clinical prediction models. Diagn. Progn. Res. 2021, 5, 6. [Google Scholar] [CrossRef]
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admissions. QJM 2001, 94, 521–526. [Google Scholar] [CrossRef]
- Smith, G.B.; Redfern, O.C.; Pimentel, M.A.; Gerry, S.; Collins, G.S.; Malycha, J.; Prytherch, D.; Schmidt, P.E.; Watkinson, P.J. The National Early Warning Score 2 (NEWS2). Clin. Med. 2019, 19, 260. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liang, R.; Liu, S.; Dong, W.; Gao, J.; Hua, T.; Xiao, W.; Li, H.; Zhu, H.; Hu, J.; et al. Nomogram predictive model for in-hospital mortality risk in elderly ICU patients with urosepsis. BMC Infect. Dis. 2024, 24, 442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, F.; Xu, F.; Wang, Z.; Ren, Y.; Han, D.; Lyu, J.; Yin, H. Construction and Evaluation of a Sepsis Risk Prediction Model for Urinary Tract Infection. Front. Med. 2021, 8, 671184. [Google Scholar] [CrossRef]
- Sugizaki, Y.; Utsumi, T.; Ishitsuka, N.; Noro, T.; Suzuki, Y.; Iijima, S.; Somoto, T.; Oka, R.; Endo, T.; Kamiya, N.; et al. Predicting Urosepsis in Ureteral Calculi: External Validation of Hu’s Nomogram and Identification of Novel Risk Factors. Diagnostics 2025, 15, 1104. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Ding, W.; Xuan, N.; Tian, B.; Huang, T.; Zhang, Z.; Cui, W.; Huang, H.; Zhang, G. National Early Warning Score Does Not Accurately Predict Mortality for Patients With Infection Outside the Intensive Care Unit: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 704358. [Google Scholar] [CrossRef]
- Blair, P.W.; Mehta, R.; Oppong, C.K.; Tin, S.; Ko, E.; Tsalik, E.L.; Chenoweth, J.; Rozo, M.; Adams, N.; Beckett, C.; et al. Screening tools for predicting mortality of adults with suspected sepsis: An international sepsis cohort validation study. BMJ Open 2023, 13, e067840. [Google Scholar] [CrossRef]
- Huabbangyang, T.; Klaiangthong, R.; Jaibergban, F.; Wanphen, C.; Faikhao, T.; Banjongkit, P.; Kuchapan, R. Pre-hospital Prognostic Factors of 30-Day Survival in Sepsis Patients; a Retrospective Cohort Study. Arch. Acad. Emerg. Med. 2023, 11, e48. [Google Scholar] [PubMed]
- Yang, T.H.; Shao, S.C.; Lee, Y.C.; Hsiao, C.H.; Yen, C.C. Risk factors for peri-intubation cardiac arrest: A systematic review and meta-analysis. Biomed. J. 2023, 47, 100656. [Google Scholar] [CrossRef] [PubMed]
- Warmerdam, M.; Baris, L.; van Liebergen, M.; Ansems, A.; Esteve Cuevas, L.; Willeboer, M.; Rijpsma, D.; Shetty, A.L.; de Groot, B. The association between systolic blood pressure and in-hospital mortality in older emergency department patients who are hospitalised with a suspected infection. Emerg. Med. J. 2018, 35, 619–622. [Google Scholar] [CrossRef]
- Dankl, D.; Rezar, R.; Mamandipoor, B.; Zhou, Z.; Wernly, S.; Wernly, B.; Osmani, V. Red Cell Distribution Width Is Independently Associated with Mortality in Sepsis. Med Princ. Pract. 2022, 31, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Alakare, J.; Kemp, K.; Strandberg, T.; Castrén, M.; Tolonen, J.; Harjola, V.-P. Red cell distribution width and mortality in older patients with frailty in the emergency department. BMC Emerg. Med. 2023, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Woo, S.H.; Kim, D.H.; Seol, S.H.; Lee, J.Y.; Lee, W.J.; Hong, S.; Cha, K.; Youn, C.S.; Park, S. Efficacy of blood urea nitrogen and the neutrophil-to-lymphocyte ratio as predictors of mortality among elderly patients with genitourinary tract infections: A retrospective multicentre study. J. Infect. Chemother. 2021, 27, 312–318. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Harrell, F.E. Prediction models need appropriate internal, internal–external, and external validation. J. Clin. Epidemiol. 2016, 69, 245–247. [Google Scholar] [CrossRef]
- Blomaard, L.C.; Speksnijder, C.; Lucke, J.A.; de Gelder, J.; Anten, S.; Schuit, S.C.E.; Steyerberg, E.W.; Gussekloo, J.; de Groot, B.; Mooijaart, S.P. Geriatric Screening, Triage Urgency, and 30-Day Mortality in Older Emergency Department Patients. J. Am. Geriatr. Soc. 2020, 68, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Hsiao, C.H.; Yen, C.C. Prognostic value of cardiac troponin in elderly patients with paroxysmal supraventricular tachycardia: A multicenter study. Am. J. Emerg. Med. 2023, 69, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, S.; Lucarelli, G.; Lasorsa, F.; Damiano, R.; Autorino, R.; Aveta, A.; Spena, G.; Perdonà, S.; Russo, P.; Giulioni, C.; et al. Urobiome and Inflammation: A Systematic Review on Microbial Imbalances and Diagnostic Tools for Urinary Disorders. Urology 2025, 200, 206–215. [Google Scholar] [CrossRef]
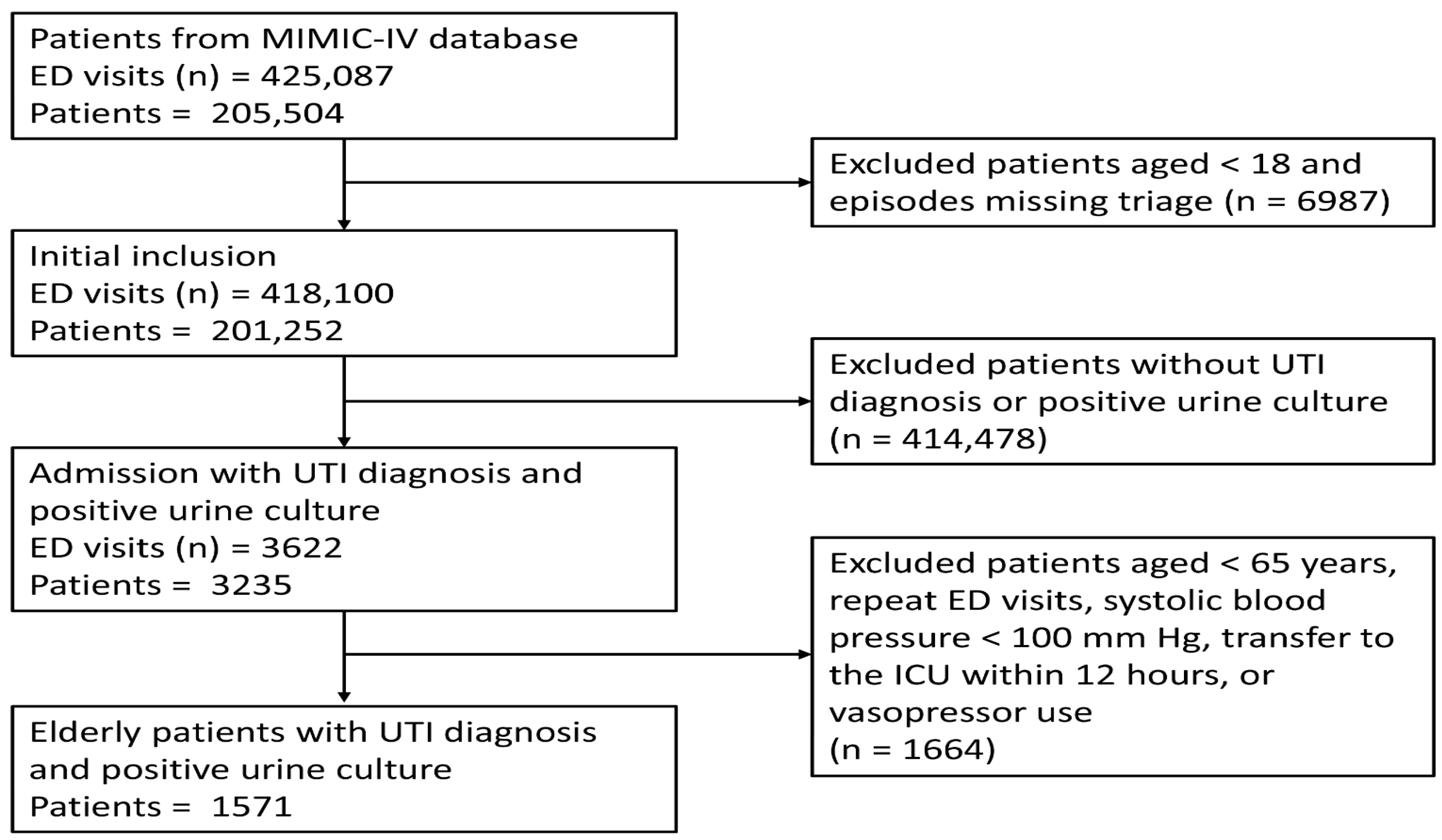
| Variables | Total (n = 1571) | Non-Survivors (n = 70) | Survivors (n = 1501) | p Value |
|---|---|---|---|---|
| Age (years), median [IQR] | 79.0 [72.0–85.0] | 82.0 [74.0–88.0] | 79.0 [72.0–85.0] | 0.014 * |
| Sex (male), n (%) | 524 (33.4) | 25 (35.7) | 499 (33.2) | 0.765 |
| Race, n (%) | 0.504 | |||
| Asian | 42 (2.7) | 0 (0.0) | 42 (2.8) | |
| Black | 201 (12.8) | 10 (14.3) | 191 (12.7) | |
| Hispanic | 64 (4.1) | 3 (4.3) | 61 (4.1) | |
| Other | 91 (5.8) | 2 (2.9) | 89 (5.9) | |
| White | 1173 (74.7) | 55 (78.6) | 1118 (74.5) | |
| Insurance, n (%) | 0.489 | |||
| Medicaid | 23 (1.5) | 2 (2.9) | 21 (1.4) | |
| Medicare | 1125 (71.6) | 47 (67.1) | 1078 (71.8) | |
| Other | 423 (26.9) | 21 (30.0) | 402 (26.8) | |
| Emergency Severity Index, n (%) | 0.782 | |||
| Level 1 | 136 (8.7) | 8 (11.4) | 128 (8.5) | |
| Level 2 | 824 (52.5) | 37 (52.9) | 787 (52.4) | |
| Level 3 | 605 (38.5) | 25 (35.7) | 580 (38.6) | |
| Level 4 | 6 (0.4) | 0 (0.0) | 6 (0.4) | |
| Vital signs at triage, median [IQR] | ||||
| body temperature (°C) | 37.6 [37.2–37.8] | 37.5 [37.2–37.7] | 37.6 [37.3–37.8] | 0.795 |
| Systolic blood pressure (mmHg) | 134.0 [120.0–152.0] | 122.5 [115.0–138.0] | 135.0 [121.0–152.0] | <0.001 * |
| Diastolic blood pressure (mmHg) | 71.0 [61.0–81.0] | 68.0 [61.0–78.0] | 71.0 [61.0–81.0] | 0.153 |
| Heart rate (beats/min) | 81.0 [70.0–91.5] | 84.0 [72.0–95.0] | 81.0 [70.0–91.0] | 0.387 |
| Respiratory rate (beats/min) | 18.0 [16.0–19.0] | 18.0 [16.2–20.0] | 18.0 [16.0–19.0] | 0.200 |
| Oxygen saturation (%) | 98.0 [96.0–99.0] | 97.5 [95.0–99.0] | 98.0 [96.0–99.0] | 0.164 |
| Comorbidities, n (%) | ||||
| Hypertension | 957 (60.9) | 52 (74.3) | 905 (60.3) | 0.026 * |
| Diabetes mellitus | 490 (31.2) | 29 (41.4) | 461 (30.7) | 0.078 |
| Congestive heart failure | 472 (30.0) | 34 (48.6) | 438 (29.2) | 0.001 * |
| Prior stroke | 221 (14.1) | 7 (10.0) | 214 (14.3) | 0.409 |
| Liver disease | 104 (6.6) | 7 (10.0) | 97 (6.5) | 0.359 |
| Chronic kidney disease | 471 (30.0) | 26 (37.1) | 445 (29.6) | 0.228 |
| Malignancy | 221 (14.1) | 15 (21.4) | 206 (13.7) | 0.102 |
| Charlson comorbidity index | 6.0 [4.0–9.0] | 8.0 [4.0–11.0] | 6.0 [4.0–9.0] | 0.002 * |
| Laboratory data (serum), median [IQR] | ||||
| WBC (103/uL) | 8.4 [6.2–11.1] | 9.9 [7.1–14.3] | 8.2 [6.2–11.1] | 0.002 * |
| Hemoglobin (g/dL) | 10.6 [9.1–12.0] | 9.9 [8.5–11.6] | 10.6 [9.1–12.0] | 0.052 |
| RDW (%) | 14.7 [13.6–16.3] | 15.7 [14.1–18.0] | 14.7 [13.6–16.2] | <0.001 * |
| Platelet count (103/uL) | 214.0 [162.0–278.0] | 205.0 [153.5–262.0] | 215.0 [163.0–278.0] | 0.338 |
| PT (s) | 13.1 [11.8–17.3] | 14.3 [12.4–16.4] | 13.1 [11.8–17.6] | 0.175 |
| Sodium (mEq/L) | 139.0 [136.0–142.0] | 138.0 [135.0–142.8] | 139.0 [136.0–142.0] | 0.304 |
| Potassium (mEq/L) | 4.1 [3.8–4.5] | 4.2 [3.8–4.6] | 4.1 [3.8–4.5] | 0.522 |
| AST (U/L) | 24.5 [18.0–37.0] | 30.0 [21.0–42.0] | 24.0 [18.0–37.0] | 0.073 |
| ALT (U/L) | 18.0 [12.0–29.0] | 19.5 [13.0–34.5] | 18.0 [12.0–28.0] | 0.130 |
| Total bilirubin (mg/dL) | 0.5 [0.3–0.8] | 0.5 [0.4–1.0] | 0.5 [0.3–0.7] | 0.054 |
| Glucose (mg/dL) | 110.0 [93.0–140.0] | 111.0 [94.0–143.0] | 110.0 [93.0–140.0] | 0.906 |
| BUN (mg/dL) | 23.0 [16.0–35.2] | 31.0 [20.2–44.5] | 23.0 [16.0–35.0] | 0.001 * |
| Creatinine (mg/dL) | 1.0 [0.8–1.6] | 1.3 [0.8–1.7] | 1.0 [0.8–1.5] | 0.048 * |
| Urinalysis, median [IQR] | ||||
| WBC (/HPF) | 20.0 [6.0–57.0] | 16.5 [3.8–57.8] | 20.0 [6.0–57.0] | 0.406 |
| RBC (/HPF) | 4.0 [2.0–14.0] | 6.0 [1.2–13.5] | 4.0 [2.0–14.0] | 0.992 |
| Urine culture, n (%) | <0.001 * | |||
| Escherichia coli | 583 (37.1%) | 15 (21.4%) | 568 (37.8%) | |
| Enterococcus sp. | 247 (15.7%) | 10 (14.3%) | 237 (15.8%) | |
| Yeast | 135 (8.6%) | 17 (24.3%) | 118 (7.9%) | |
| Klebsiella sp. | 163 (10.4%) | 8 (11.4%) | 155 (10.3%) | |
| Proteus sp. | 81 (5.2%) | 4 (5.7%) | 77 (5.1%) | |
| Pseudomonas sp. | 77 (4.9%) | 4 (5.7%) | 73 (4.9%) | |
| Other microorganisms | 285 (18.1%) | 12 (17.1%) | 273 (18.2%) | |
| Early warning score, median [IQR] | ||||
| MEWS | 1.0 [1.0–1.0] | 1.0 [1.0–2.0] | 1.0 [1.0–1.0] | 0.296 |
| NEWS | 1.0 [0.0–2.0] | 1.0 [0.0–3.0] | 1.0 [0.0–2.0] | 0.012 * |
| Outcome, n (%) | ||||
| ICU admission | 227 (14.4) | 52 (74.3) | 175 (11.7) | <0.001 * |
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age (per 10-year increase) | 1.47 (1.08–2.01) | 0.015 * | 1.62 (1.17–2.27) | 0.004 * |
| Systolic blood pressure (per 10 mm Hg decrease) | 1.24 (1.10–1.41) | 0.001 * | 1.16 (1.03–1.32) | 0.020 * |
| Oxygen saturation (per 5 percentage-point decrease) | 1.75 (1.16–2.56) | 0.005 * | 1.65 (1.08–2.44) | 0.015 * |
| Hypertension (yes vs. no) | 1.90 (1.12–3.37) | 0.021 * | 1.43 (0.75–2.77) | 0.284 |
| Congestive heart failure (yes vs. no) | 2.29 (1.41–3.71) | 0.001 * | 1.36 (0.75–2.49) | 0.315 |
| WBC (per 1 × 103 µL−1 increase) | 1.03 (1.01–1.06) | 0.007 * | 1.03 (1.01–1.06) | 0.008 * |
| RDW (per 1 percentage-point increase) | 1.20 (1.10–1.31) | <0.001 * | 1.15 (1.03–1.26) | 0.008 * |
| BUN (per 1 mg dL−1 increase) | 1.02 (1.01–1.02) | <0.001 * | 1.01 (1.00–1.02) | 0.013 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, T.-H.; Lu, W.; Chen, C.-B.; Seak, C.-J.; Yen, C.-C. Risk Factors and Development of a Predictive Model for In-Hospital Mortality in Hemodynamically Stable Older Adults with Urinary Tract Infection. Medicina 2025, 61, 1625. https://doi.org/10.3390/medicina61091625
Cheng T-H, Lu W, Chen C-B, Seak C-J, Yen C-C. Risk Factors and Development of a Predictive Model for In-Hospital Mortality in Hemodynamically Stable Older Adults with Urinary Tract Infection. Medicina. 2025; 61(9):1625. https://doi.org/10.3390/medicina61091625
Chicago/Turabian StyleCheng, Tzu-Heng, Wei Lu, Chen-Bin Chen, Chen-June Seak, and Chieh-Ching Yen. 2025. "Risk Factors and Development of a Predictive Model for In-Hospital Mortality in Hemodynamically Stable Older Adults with Urinary Tract Infection" Medicina 61, no. 9: 1625. https://doi.org/10.3390/medicina61091625
APA StyleCheng, T.-H., Lu, W., Chen, C.-B., Seak, C.-J., & Yen, C.-C. (2025). Risk Factors and Development of a Predictive Model for In-Hospital Mortality in Hemodynamically Stable Older Adults with Urinary Tract Infection. Medicina, 61(9), 1625. https://doi.org/10.3390/medicina61091625







