Incidence of Uterine Cesarean Scar Niche After Cesarean Delivery and Assessment of Its Risk Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- Women who had undergone a cesarean section at least six months prior to the study.
- Availability for a transvaginal ultrasound (TVUS) evaluation.
- A single-layer uterine closure technique (due to potential confounding in niche healing).
- Uterine anomalies.
- Women with diagnosed chorioamnionitis or a postpartum pelvic infection.
2.2. Sample Size Calculation
2.3. Ultrasound Assessment
2.4. Statistical Analysis
3. Results
3.1. Incidence of Cesarean Scar Niche
3.2. Risk Factors of Cesarean Scar Niche
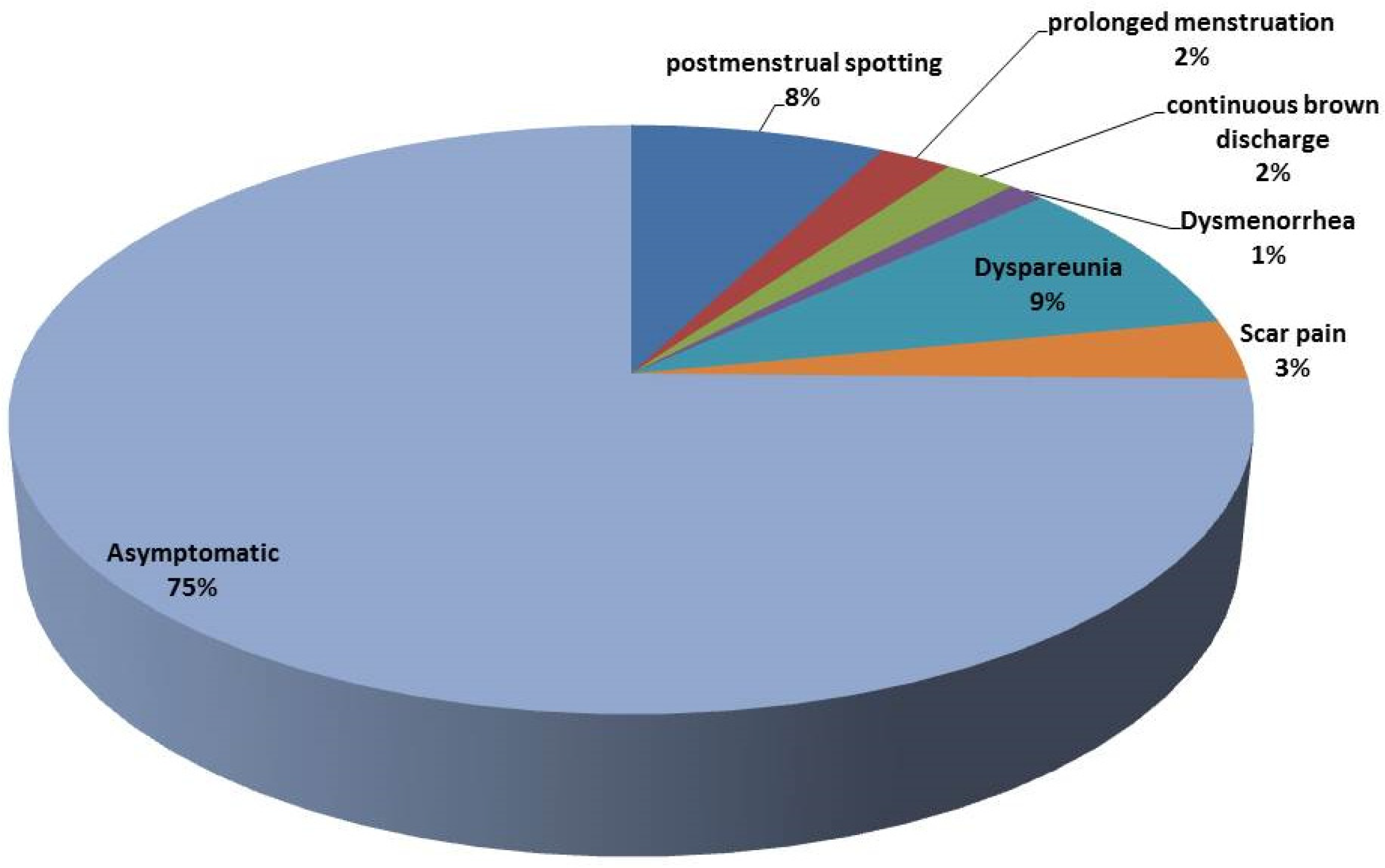
3.3. Complications
3.4. Correlation Between Factors Associated with CSs
4. Discussion
4.1. Clinical Implications
4.2. Preventive Considerations
- The adoption of double-layer closure techniques.
- The optimization of the maternal hematological status pre-operatively.
- The early detection of a postpartum fever or infection.
- Postpartum follow-up using TVUS or SHG at 6 months for high-risk patients.
4.3. Limitations
- Data on the suture technique and intra-operative variables were not collected.
- We did not assess hormonal profiles or breastfeeding intensity.
- Ultrasound measurements, though standardized, were not confirmed by MRI.
- Confounding variables may have influenced the platelet and breastfeeding associations.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.; Khan, N.; Rahman, A.; Alam, M.; Khan, A. Long-term effects of caesarean delivery on health and behavioural outcomes of the mother and child in Bangladesh. J. Health Popul. Nutr. 2022, 41, 45. [Google Scholar] [CrossRef]
- Zakherah, M.; Mohamed, A.A.; Rageh, A.M.; Abdel-aleem, M. Navigating uterine niche 360 degree: A narrative review. Middle East Fertil. Soc. J. 2024, 29, 29. [Google Scholar] [CrossRef]
- Armstrong, F.; Mulligan, K.; Dermott, R.M.; Bartels, H.C.; Carroll, S.; Robson, M.; Corcoran, S.; Parland, P.M.; Brien, D.O.; Brophy, D. Cesarean scar niche: An evolving concern in clinical practice. Int. J. Gynecol. Obstet. 2023, 161, 356–366. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Zhang, M.; Huang, F.; Wu, Y.; Hu, M.; Yang, Y.; Wei, W.; Pang, Q.; Wei, Z. The degree of risk factor and accumulation effect for large niche in individuals after cesarean section. BMC Pregnancy Childbirth 2024, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; El-Gendy, A.O.; Moniem Radi, N.A.; Mohamed, T. The bactericidal efficacy of femtosecond laser-based therapy on the most common infectious bacterial pathogens in chronic wounds: An in vitro study. Lasers Med. Sci. 2021, 36, 641–647. [Google Scholar] [CrossRef]
- Mahmoud Farhan, S.; Mahmoud Abd El-Baky, R.; Abdalla, M.; Osama El-Gendy, A.; Farag Azmy, A. Efficacy of Amikacin and Imipenem Against Multi-Drug Resistant Gram-Negative Bacteria Isolated from Wound Infections, Egypt. Iran. J. Med. Microbiol. 2023, 17, 218–229. [Google Scholar] [CrossRef]
- Taha, A.A.; Salem, S.A.M.; Faried, E.Z.E.A.; Habib, E.H.; Al-Fakharany, R.S.; Elgendy, M.O.; Abdelkader, H.; Al Fatease, A.; Elkady, M.S.E. Diagnostic Performance of Gynecologic Imaging Reporting and Data System (GI-RADS) in Preoperative Evaluation of Adnexal Masses. Medicina 2025, 61, 679. [Google Scholar] [CrossRef]
- Vervoort, A.; Van der Voet, L.; Hehenkamp, W.; Thurkow, A.; van Kesteren, P.; Quartero, H.; Kuchenbecker, W.; Bongers, M.; Geomini, P.; de Vleeschouwer, L. Hysteroscopic resection of a uterine caesarean scar defect (niche) in women with postmenstrual spotting: A randomised controlled trial. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Pędraszewski, P.; Wlaźlak, E.; Panek, W.; Surkont, G. Cesarean scar pregnancy—A new challenge for obstetricians. J. Ultrason. 2018, 18, 56. [Google Scholar] [CrossRef]
- Ahmed, E.; El-Gendy, A.O.; Mohamed, T. Antibacterial Effects of Laser in Treating Contaminated Wounds. Laser Innov. Res. Appl. 2024, 1, 37–69. [Google Scholar] [CrossRef]
- Patounakis, G.; Ozcan, M.C.; Chason, R.J.; Norian, J.M.; Payson, M.; DeCherney, A.H.; Yauger, B.J. Impact of a prior cesarean delivery on embryo transfer: A prospective study. Fertil. Steril. 2016, 106, 311–316. [Google Scholar] [CrossRef]
- Elgendy, M.O.; Khalaf, A.M.; El-Gendy, A.O.; Abdelrahman, M.; El Gendy, S.O.; Hamied, A.M.A.; Essam, O.; Al Amir, K.; Yousry, E.M.; Abdelrahim, M. An observational study on the management of COVID-19 patients in limited-resource hospitals. J. Clin. Nurs. Res. 2022, 6, 43–53. [Google Scholar] [CrossRef]
- Bamberg, C.; Hinkson, L.; Dudenhausen, J.W.; Bujak, V.; Kalache, K.D.; Henrich, W. Longitudinal transvaginal ultrasound evaluation of cesarean scar niche incidence and depth in the first two years after single-or double-layer uterotomy closure: A randomized controlled trial. Acta Obstet. Gynecol. Scand. 2017, 96, 1484–1489. [Google Scholar] [CrossRef]
- van der Voet, L.; Bij de Vaate, A.; Veersema, S.; Brölmann, H.; Huirne, J. Long-term complications of caesarean section. The niche in the scar: A prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 236–244. [Google Scholar] [CrossRef]
- Bij de Vaate, A.; Brölmann, H.; Van Der Voet, L.; Van Der Slikke, J.; Veersema, S.; Huirne, J. Ultrasound evaluation of the Cesarean scar: Relation between a niche and postmenstrual spotting. Ultrasound Obstet. Gynecol. 2011, 37, 93–99. [Google Scholar] [CrossRef]
- Rosa, F.; Perugin, G.; Schettini, D.; Romano, N.; Romeo, S.; Podestà, R.; Guastavino, A.; Casaleggio, A.; Gandolfo, N. Imaging findings of cesarean delivery complications: Cesarean scar disease and much more. Insights Imaging 2019, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, M.O.; El-Gendy, A.O.; Elgendy, S.O.; Abdelaty, L.N.; Abdelrahim, M.E.; Abdelrahman, M.A. Perceptions, Knowledge, and Experiences of Using Face Masks among Egyptian Healthcare Workers during the COVID-19 Pandemic: A Cross-Sectional Study. Healthcare 2023, 11, 838. [Google Scholar] [CrossRef]
- Martynov, S.A. Cesarean scar defects: Diagnosis and treatment in non-pregnant women. Gynecology 2020, 22, 6–10. [Google Scholar] [CrossRef]
- El Agwany, A.S. Gynecological and postpartum ultrasonography of cesarean uterine scar defects: A pictorial essay. J. Ultrasound 2020, 23, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Tulandi, T.; Cohen, A. Emerging manifestations of cesarean scar defect in reproductive-aged women. J. Minim. Invasive Gynecol. 2016, 23, 893–902. [Google Scholar] [CrossRef]
- Tanos, V.; Toney, Z.A. Uterine scar rupture-Prediction, prevention, diagnosis, and management. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 59, 115–131. [Google Scholar] [CrossRef]
- Antila, R.M.; Mäenpää, J.U.; Huhtala, H.S.; Tomás, E.I.; Staff, S.M. Association of cesarean scar defect with abnormal uterine bleeding: The results of a prospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 244, 134–140. [Google Scholar] [CrossRef]
- Rydahl, E.; Declercq, E.; Juhl, M.; Maimburg, R.D. Cesarean section on a rise—Does advanced maternal age explain the increase? A population register-based study. PLoS ONE 2019, 14, e0210655. [Google Scholar] [CrossRef] [PubMed]
- Khalil, E.M.; Madney, Y.M.; Hassan, M.; Fahmy, A.M.; Alshammari, S.O.; Alshammari, Q.A.; Abou-Taleb, H.A.; Taha, A.A.; Elgendy, M.O.; Ali, H.A. Maternal and Fetal Outcome of COVID-19 Infection among Pregnant Women. Medicina 2024, 60, 1676. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.A.; Bakr, R.B.; El-Gendy, A.O.; Kamel, G.M.; Azouz, A.A.; Bukhari, S.N.A. Discovery of a COX-2 selective inhibitor hit with anti-inflammatory activity and gastric ulcer protective effect. Future Med. Chem. 2017, 9, 1899–1912. [Google Scholar] [CrossRef]
- Dodd, J.M.; Turnbull, D.; McPhee, A.J.; Deussen, A.R.; Grivell, R.M.; Yelland, L.N.; Crowther, C.A.; Wittert, G.; Owens, J.A.; Robinson, J.S. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014, 348, g1285. [Google Scholar] [CrossRef] [PubMed]
- Fouad, S.G.; El-Beshry, G.M.; El-Kholy, A.-L.G.; Abbas, A.M. The association between body mass index and intra-abdominal adhesions in women undergoing previous cesarean sections. Ain Shams Med. J. 2022, 73, 327–337. [Google Scholar] [CrossRef]
- Dennis, A.T.; Ferguson, M.; Jackson, S. The prevalence of perioperative iron deficiency anaemia in women undergoing caesarean section—A retrospective cohort study. Perioper. Med. 2022, 11, 36. [Google Scholar] [CrossRef]
- Adam, I.; Salih, Y.; Hamdan, H.Z. Association of maternal anemia and cesarean delivery: A systematic review and meta-analysis. J. Clin. Med. 2023, 12, 490. [Google Scholar] [CrossRef]
- Xu, X.; Yan, J.-Y.; Chen, L.-C. Risk factors and maternal-fetal outcomes of pregnancies complicated by pre-eclampsia, following cesarean section after a trial vaginal birth. Chin. Med. J. 2021, 134, 2249–2251. [Google Scholar] [CrossRef]
- Zaki, A.; Elgendy, M.O.; Abdelrahman, M.A.; Ali, H.; Khalil, E.M.; Hassan, M.; Fahmy, A.M.; Gad, R.A.; Salem, H.F. The efficacy of using different antibiotics to prevent maternal surgical site infections in COVID-19-infected cases. Eur. Chem. Bull. 2023, 6, 1342–1348. [Google Scholar]
- Abdelrahman, M.A.; Zaki, A.; Salem, S.A.; Salem, H.F.; Ibrahim, A.R.; Hassan, A.; Elgendy, M.O. The Impact of Cefepime and Ampicillin/Sulbactam on Preventing Post-Cesarean Surgical Site Infections, Randomized Controlled Trail. Antibiotics 2023, 12, 1666. [Google Scholar] [CrossRef]
- Hassan Abd El-Ghany, S.S.; Azmy, A.F.; Osama EL-Gendy, A.; Abd El-Baky, R.M.; Mustafa, A.; Abourehab, M.A.; El-Beeh, M.E.; Ibrahem, R.A. Antimicrobial and Antibiofilm Activity of Monolaurin against Methicillin-Resistant Staphylococcus aureus Isolated from Wound Infections. Int. J. Microbiol. 2024, 2024, 7518368. [Google Scholar] [CrossRef] [PubMed]
- Antila-Långsjö, R.M.; Mäenpää, J.U.; Huhtala, H.S.; Tomás, E.I.; Staff, S.M. Cesarean scar defect: A prospective study on risk factors. Am. J. Obstet. Gynecol. 2018, 219, 458.e1–458.e8. [Google Scholar] [CrossRef]
- Tita, A.T.; Szychowski, J.M.; Boggess, K.; Saade, G.; Longo, S.; Clark, E.; Esplin, S.; Cleary, K.; Wapner, R.; Letson, K. Adjunctive azithromycin prophylaxis for cesarean delivery. N. Engl. J. Med. 2016, 375, 1231–1241. [Google Scholar] [CrossRef]
- Essa, N.M.; Salem, H.F.; Elgendy, M.O.; Gabr, A.; Omran, M.M.; Hassan, N.A.; Tashkandi, H.M.; Harakeh, S.; Boshra, M.S. Efficacy of metformin as adjuvant therapy in metastatic breast cancer treatment. J. Clin. Med. 2022, 11, 5505. [Google Scholar] [CrossRef]
- Taha, S.; Mohamed, W.R.; Elhemely, M.A.; El-Gendy, A.O.; Mohamed, T. Breast cancer and laser therapy. Laser Innov. Res. Appl. 2024, 2, 43–66. [Google Scholar] [CrossRef]
- Duong, D.T.T.; Binns, C.; Lee, A.; Zhao, Y.; Pham, N.M.; Hoa, D.T.P.; Ha, B.T.T. Intention to exclusively breastfeed is associated with lower rates of cesarean section for nonmedical reasons in a cohort of mothers in Vietnam. Int. J. Environ. Res. Public Health 2022, 19, 884. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2018, 99, 665–706. [Google Scholar] [CrossRef]
- Essa, N.; Elgendy, M.; Gabr, A.; Alharbi, A.; Tashkandi, H.; Salem, H.; Harakeh, S.; Boshra, M. The efficacy of metformin as adjuvant to chemotherapy on IGF levels in non-diabetic female patients with progressive and non-progressive metastatic breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5200–5210. [Google Scholar]
- Chen, Y.; Han, P.; Wang, Y.-J.; Li, Y.-X. Risk factors for incomplete healing of the uterine incision after cesarean section. Arch. Gynecol. Obstet. 2017, 296, 355–361. [Google Scholar] [CrossRef]
- Pan, H.; Zeng, M.; Xu, T.; Li, D.; Mol, B.W.; Sun, J.; Zhang, J. The prevalence and risk predictors of cesarean scar defect at 6 weeks postpartum in Shanghai, China: A prospective cohort study. Acta Obstet. Gynecol. Scand. 2019, 98, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Yazicioglu, F.; Gökdogan, A.; Kelekci, S.; Aygün, M.; Savan, K. Incomplete healing of the uterine incision after caesarean section: Is it preventable? Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 124, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Siraj, S.H.M.; Lional, K.M.; Tan, K.H.; Wright, A. Repair of the myometrial scar defect at repeat caesarean section: A modified surgical technique. BMC Pregnancy Childbirth 2021, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Bij de Vaate, A.; Van der Voet, L.; Naji, O.; Witmer, M.; Veersema, S.; Brölmann, H.; Bourne, T.; Huirne, J. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: Systematic review. Ultrasound Obstet. Gynecol. 2014, 43, 372–382. [Google Scholar] [CrossRef]
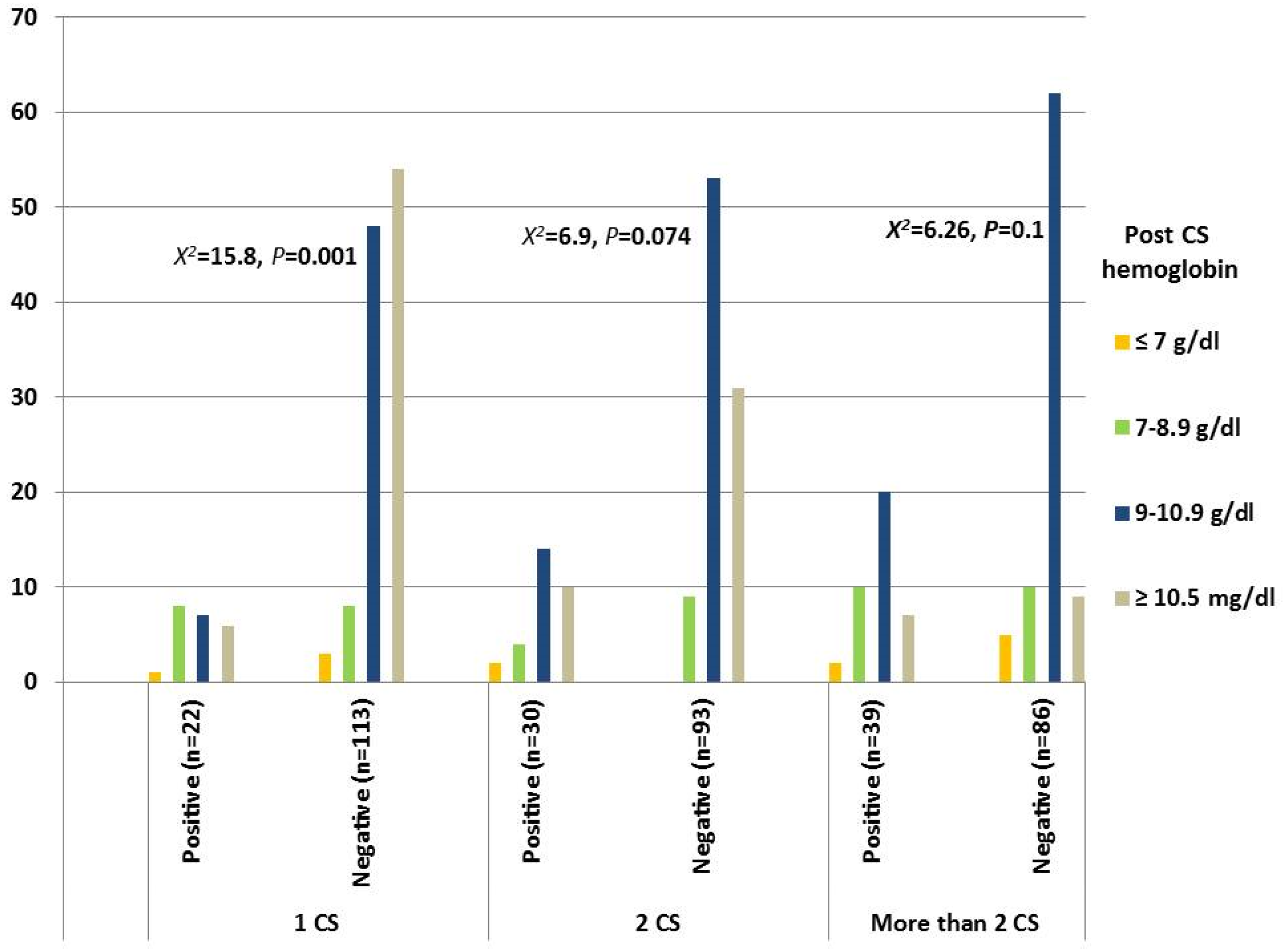
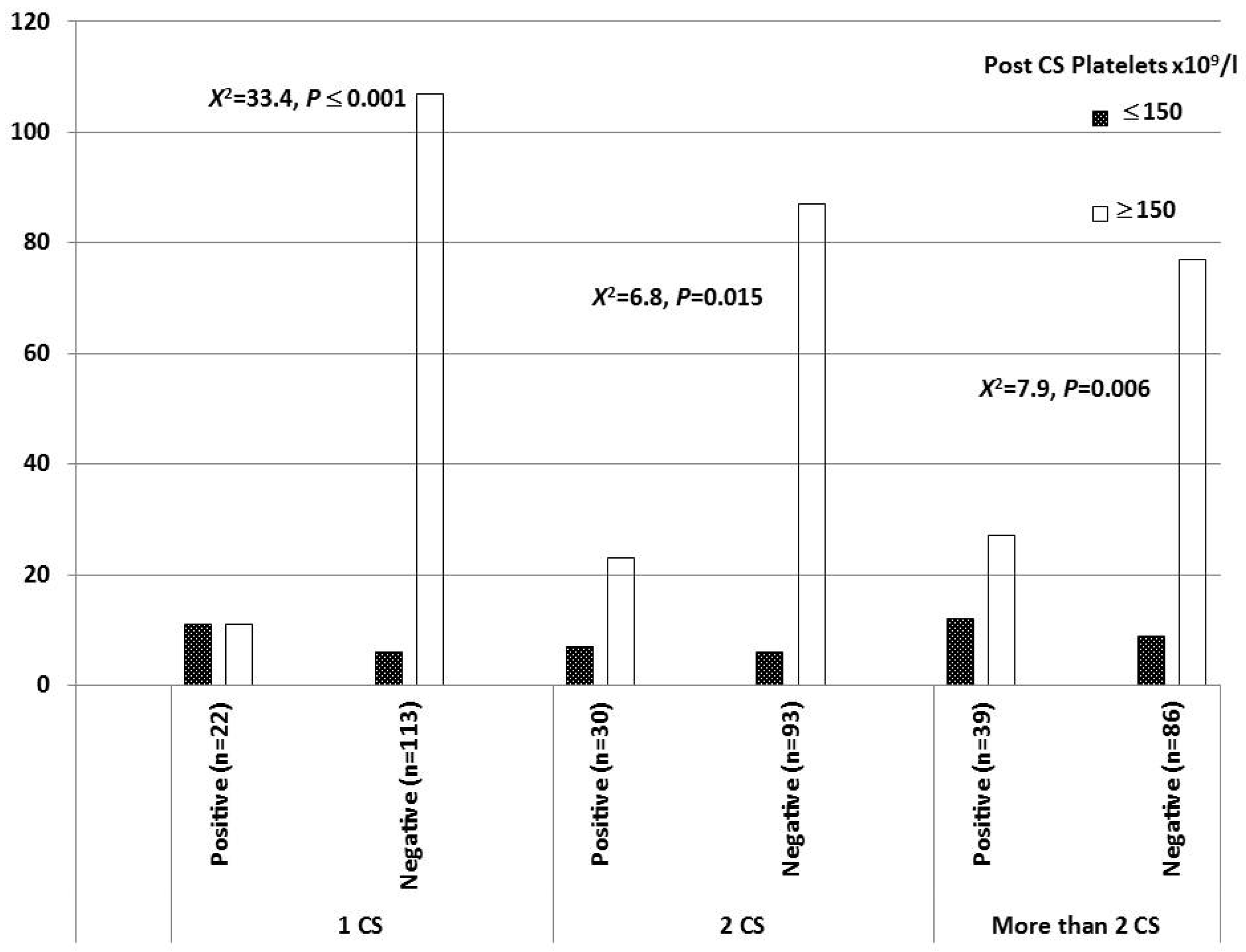
| CS Groups (n = 383) | CS Niche, n (%) | Statistic | |
|---|---|---|---|
| Positive | Negative | ||
| One Previous CS | 22 (16.3%) | 113 (83.7%) | X2 = 7.99, p = 0.018 |
| Two Previous CSs | 30 (24.4%) | 93 (75.6%) | |
| More than Two CSs | 39 (31.2%) | 86 (68.8%) | |
| Risk Factors | 1 CS | 2 CSs | More Than 2 CSs | Statistics | |||
|---|---|---|---|---|---|---|---|
| Positive n = 22 | Negative n = 113 | Positive n = 30 | Negative n = 93 | Positive n = 39 | Negative n = 86 | ||
| Maternal age | 25.77 ± 3.77 | 26.65 ± 3.92 | 29.2 ± 3.53 | 29.2 ± 3.89 | 33.76 ± 3.12 | 32.74 ± 3.95 | p = 0.001 |
| Prenatal BMI | 32.45 ± 3.23 | 32.14 ± 3.1 | 32.4 ± 3.28 | 33.16 ± 3.13 | 34.36 ± 2.65 | 32.72 ± 3.11 | p = 0.025 |
| Medical comorbidities | |||||||
| DM | 0 (0%) | 0 (0%) | 0 (0%) | 1(1.1%) | 1(2.6%) | 1(1.2%) | p = 0.558 |
| Gastrointestinal DM | 2 (0.1%) | 1(0.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 1(1.2%) | p = 0.24 |
| Preeclampsia | 3 (13.6%) | 4 (3.5%) | 0 (0%) | 2 (2.2%) | 1 (2.6%) | 2 (2.3%) | p = 0.49 |
| Anemia | 10 (45.5) | 23 (20.4%) | 8 (26.7%) | 20 (21.5%) | 12 (30.8%) | 15 (17.4%) | p = 0.015 |
| Predictors | p-Value | Odds Ratio | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Maternal Anemia | 0.276 | 1.66 | 0.668 | 4.125 |
| Post-CS Platelets (Less than 150 × 109/L) | ≤0.0001 | 4.946 | 2.305 | 10.61 |
| Post-Operative Fever | 0.082 | 2.072 | 0.912 | 4.708 |
| Uterus Position | ≤0.001 | |||
| Retroflexion | ≤0.001 | 3.125 | 1.832 | 5.332 |
| Midline Position | 0.218 | 1.393 | 0.53 | 3.664 |
| Breastfeeding | ≤0.0001 | 8.8 | 2.803 | 27.623 |
| Groups | Postpartum Fever | Breastfeeding | |||
|---|---|---|---|---|---|
| No | Yes | No | Yes | ||
| One Previous CS | Positive | 15 (68.2%) | 7 (31.8%) | 6 (27.3%) | 16 (72.7%) |
| Negative | 99 (87.6%) | 14 (12.4%) | 3 (2.7%) | 110 (97.3%) | |
| X2 = 5.29, p = 0.047 | X2 = 17.94, p ≤ 0.001 | ||||
| Two Previous CSs | Positive | 23 (76.7%) | 7 (23.3%) | 7 (23.3%) | 23(76.7%) |
| Negative | 87 (93.5%) | 6 (6.5%) | 3 (3.2%) | 90 (96.8%) | |
| X2 = 6.84, p = 0.015 | X2 = 12.38, p = 0.002 | ||||
| More than Two CSs | Positive | 31 (79.5%) | 8 (20.5%) | 8 (20.5%) | 31 (79.5%) |
| Negative | 80 (93.0%) | 6 (7%) | 2 (2.3%) | 84 (97.7%) | |
| X2 = 4.94, p = 0.035 | X2 = 12.1, p = 0.001 | ||||
| Groups | Uterus Position | p-Value | ||||
|---|---|---|---|---|---|---|
| Anteflexion | Retroflexion | Midline | ||||
| One CS | Positive | 10 (45.5%) | 10 (45.5%) | 2 (9.1%) | 0.043 | |
| Negative | 82 (72.6%) | 25 (22.1%) | 6 (5.3%) | |||
| Two CSs | Positive | 17 (56.7%) | 12 (40%) | 1 (3.3%) | 0.013 | |
| Negative | 72 (77.4%) | 14 (15.1%) | 7 (7.5%) | |||
| More than Two CSs | Positive | 24 (61.5%) | 12 (30.8%) | 3 (7.7%) | 0.017 | |
| Negative | 71 (82.6%) | 9 (10.5%) | 6 (7.0%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, A.K.; Abdel Moteleb, A.A.Y.; Elgendy, M.O.; Taha, A.A.K.; Salem, E.A.; Ibrahim, A.R.N.; Salem, S.A.M.; Farid, E.Z.E.; Khaled, W.M.E. Incidence of Uterine Cesarean Scar Niche After Cesarean Delivery and Assessment of Its Risk Factors. Medicina 2025, 61, 1621. https://doi.org/10.3390/medicina61091621
Khalifa AK, Abdel Moteleb AAY, Elgendy MO, Taha AAK, Salem EA, Ibrahim ARN, Salem SAM, Farid EZE, Khaled WME. Incidence of Uterine Cesarean Scar Niche After Cesarean Delivery and Assessment of Its Risk Factors. Medicina. 2025; 61(9):1621. https://doi.org/10.3390/medicina61091621
Chicago/Turabian StyleKhalifa, Ahmed Khedr, Ahmed Adel Yasseen Abdel Moteleb, Marwa O. Elgendy, Ahmed Abdel Khalek Taha, Eman A. Salem, Ahmed R. N. Ibrahim, Sara Abdallah Mohamed Salem, Eman Zein Elabein Farid, and Waleed Mohammed Elamin Khaled. 2025. "Incidence of Uterine Cesarean Scar Niche After Cesarean Delivery and Assessment of Its Risk Factors" Medicina 61, no. 9: 1621. https://doi.org/10.3390/medicina61091621
APA StyleKhalifa, A. K., Abdel Moteleb, A. A. Y., Elgendy, M. O., Taha, A. A. K., Salem, E. A., Ibrahim, A. R. N., Salem, S. A. M., Farid, E. Z. E., & Khaled, W. M. E. (2025). Incidence of Uterine Cesarean Scar Niche After Cesarean Delivery and Assessment of Its Risk Factors. Medicina, 61(9), 1621. https://doi.org/10.3390/medicina61091621






