Amplitude of the V1-R Wave Predicts Survival After Transcatheter Aortic Valve Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. TAVR Procedure
2.3. Electrocardiography
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic valve stenosis |
| CVA | Cerebrovascular accident |
| ECG | Electrocardiography |
| PAH | Pulmonary arterial hypertension |
| PHT | Pulmonary hypertension |
| sPAP | Systolic pulmonary artery pressure |
| TAVR | Transcatheter aortic valve replacement |
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Martín, M.; Cuevas, J.; Cigarrán, H.; Calvo, J.; Morís, C. Transcatheter aortic valve implantation and subclinical and clinical leaflet thrombosis: Multimodality imaging for diagnosis and risk stratification. Eur. Cardiol. Rev. 2021, 16, e35. [Google Scholar] [CrossRef]
- Rouleau, S.G.; Brady, W.J.; Koyfman, A.; Long, B. Transcatheter aortic valve replacement complications: A narrative review for emergency clinicians. Am. J. Emerg. Med. 2022, 56, 77–86. [Google Scholar] [CrossRef]
- Cheng, X.L.; He, J.G.; Liu, Z.H.; Gu, Q.; Ni, X.H.; Zhao, Z.H.; Luo, Q.; Xiong, C.M. The value of the electrocardiogram for evaluating prognosis in patients with idiopathic pulmonary arterial hypertension. Lung 2017, 195, 139–146. [Google Scholar] [CrossRef]
- Sato, S.; Ogawa, A.; Matsubara, H. Change in R wave in lead V1 predicts survival of patients with pulmonary arterial hypertension. Pulm. Circ. 2018, 8, 2045894018776496. [Google Scholar] [CrossRef]
- Thornton, G.D.; Vassiliou, V.S.; Musa, T.A.; Aziminia, N.; Craig, N.; Dattani, A.; Davies, R.H.; Captur, G.; Moon, J.C.; Dweck, M.R.; et al. Myocardial scar and remodelling predict long-term mortality in severe aortic stenosis beyond 10 years. Eur. Heart J. 2024, 45, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Tzou, W.S.; Zado, E.S.; Lin, D.; Callans, D.J.; Dixit, S.; Cooper, J.M.; Bala, R.; Garcia, F.; Hutchinson, M.D.; Riley, M.P.; et al. Sinus rhythm ECG criteria associated with basal-lateral ventricular tachycardia substrate in patients with nonischemic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2011, 22, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Malouf, J.F.; Enriquez-Sarano, M.; Pellikka, P.A.; Oh, J.K.; Bailey, K.R.; Chandrasekaran, K.; Mullany, C.J.; Tajik, A.J. Severe pulmonary hypertension in patients with severe aortic valve stenosis: Clinical profile and prognostic implications. J. Am. Coll. Cardiol. 2002, 40, 789–795. [Google Scholar] [CrossRef]
- Kapoor, N.; Varadarajan, P.; Pai, R.G. Echocardiographic predictors of pulmonary hypertension in patients with severe aortic stenosis. Eur. J. Echocardiogr. 2008, 9, 31–33. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.M.; Shahian, D.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.L.T.; DeLong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 2—Isolated valve surgery. Ann. Thorac. Surg. 2009, 88, S23–S42. [Google Scholar] [CrossRef]
- Mansfield, A.; Inness, E.L.; McIlroy, W.E. Chapter 13—Stroke. In Handbook of Clinical Neurology; Day, B.L., Lord, S.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–228. [Google Scholar]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: Developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESE) and the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [PubMed]
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, ehae176. [Google Scholar]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, R.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Marini, M.; Stabile, E. European guidelines for the management of peripheral arterial and aortic diseases: What’s new? G. Ital. Cardiol. 2024, 25, 855–858. [Google Scholar]
- Halpin, D.M.G.; Criner, G.J.; Papi, A.; Singh, D.; Anzueto, A.; Martinez, F.J.; Agusti, A.A.; Vogelmeier, C.F. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease: The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2021, 203, 24–36. [Google Scholar] [CrossRef]
- Melby, S.J.; Moon, M.R.; Lindman, B.R.; Bailey, M.S.; Hill, L.L.; Damiano, R.J., Jr. Impact of pulmonary hypertension on outcomes after aortic valve replacement for aortic valve stenosis. J. Thorac. Cardiovasc. Surg. 2011, 141, 1424–1430. [Google Scholar] [CrossRef]
- Rocha, R.V.; Friedrich, J.O.; Hong, K.; Lee, J.; Cheema, A.; Bagai, A.; Verma, S.; Yanagawa, B. Aortic valve replacement with pulmonary hypertension: Meta-analysis of 70 676 patients. J. Card. Surg. 2019, 34, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Iliuta, L.; Rac-Albu, M.; Rac-Albu, M.-E.; Andronesi, A. Impact of Pulmonary Hypertension on Mortality after Surgery for Aortic Stenosis. Medicina 2022, 58, 1231. [Google Scholar] [CrossRef]
- Barbash, I.M.; Escarcega, R.O.; Minha, S.; Ben-Dor, I.; Torguson, R.; Goldstein, S.A.; Wang, Z.; Okubagzi, P.; Satler, L.F.; Pichard, A.D.; et al. Prevalence and impact of pulmonary hypertension on patients with aortic stenosis who underwent transcatheter aortic valve replacement. Am. J. Cardiol. 2015, 115, 1435–1542. [Google Scholar] [CrossRef]
- Luçon, A.; Oger, E.; Bedossa, M.; Boulmier, D.; Verhoye, J.P.; Eltchaninoff, H.; Iung, B.; Leguerrier, A.; Laskar, M.; Leprince, P.; et al. Prognostic implications of pulmonary hypertension in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation: Study from the FRANCE 2 Registry. Circ. Cardiovasc. Interv. 2014, 7, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Turco, A.; Klersy, C.; Scelsi, L.; Raineri, C.; Crescio, V.; Viscardi, A.; Grazioli, V.; Sciortino, A.; Visconti, L.O.; et al. Changes in surface electrocardiogram in patients with chronic thromboembolic pulmonary hypertension undergoing pulmonary endarterectomy: Correlations with hemodynamic and echocardiographic improvements after surgery. J. Electrocardiol. 2016, 49, 223–230. [Google Scholar] [CrossRef]
- Testa, L.; Latib, A.; De Marco, F.; De Carlo, M.; Fiorina, C.; Montone, R.; Agnifili, M.; Barbanti, M.; Petronio, A.S.; Biondi Zoccai, G.; et al. Persistence of severe pulmonary hypertension after transcatheter aortic valve replacement: Incidence and prognostic impact. Circ. Cardiovasc. Interv. 2016, 9, e003563. [Google Scholar] [CrossRef]
- Ogawa, A.; Ejiri, K.; Matsubara, H. Long-term patient survival with idiopathic/heritable pulmonary arterial hypertension treated at a single center in Japan. Life Sci. 2014, 118, 414–419. [Google Scholar] [CrossRef]
- Shibasaki, I.; Fukuda, T.; Ogawa, H.; Tsuchiya, G.; Takei, Y.; Seki, M.; Kato, T.; Kanazawa, Y.; Saito, S.; Kuwata, T.; et al. Mid-term results of surgical aortic valve replacement with bioprostheses in hemodialysis patients. IJC Heart Vasc. 2022, 40, 101030. [Google Scholar] [CrossRef]
- Takagi, H.; Hari, Y.; Nakashima, K.; Kuno, T.; Ando, T. ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Meta-analysis for impact of statin on mortality after transcatheter aortic valve implantation. Am. J. Cardiol. 2019, 124, 920–925. [Google Scholar] [CrossRef]
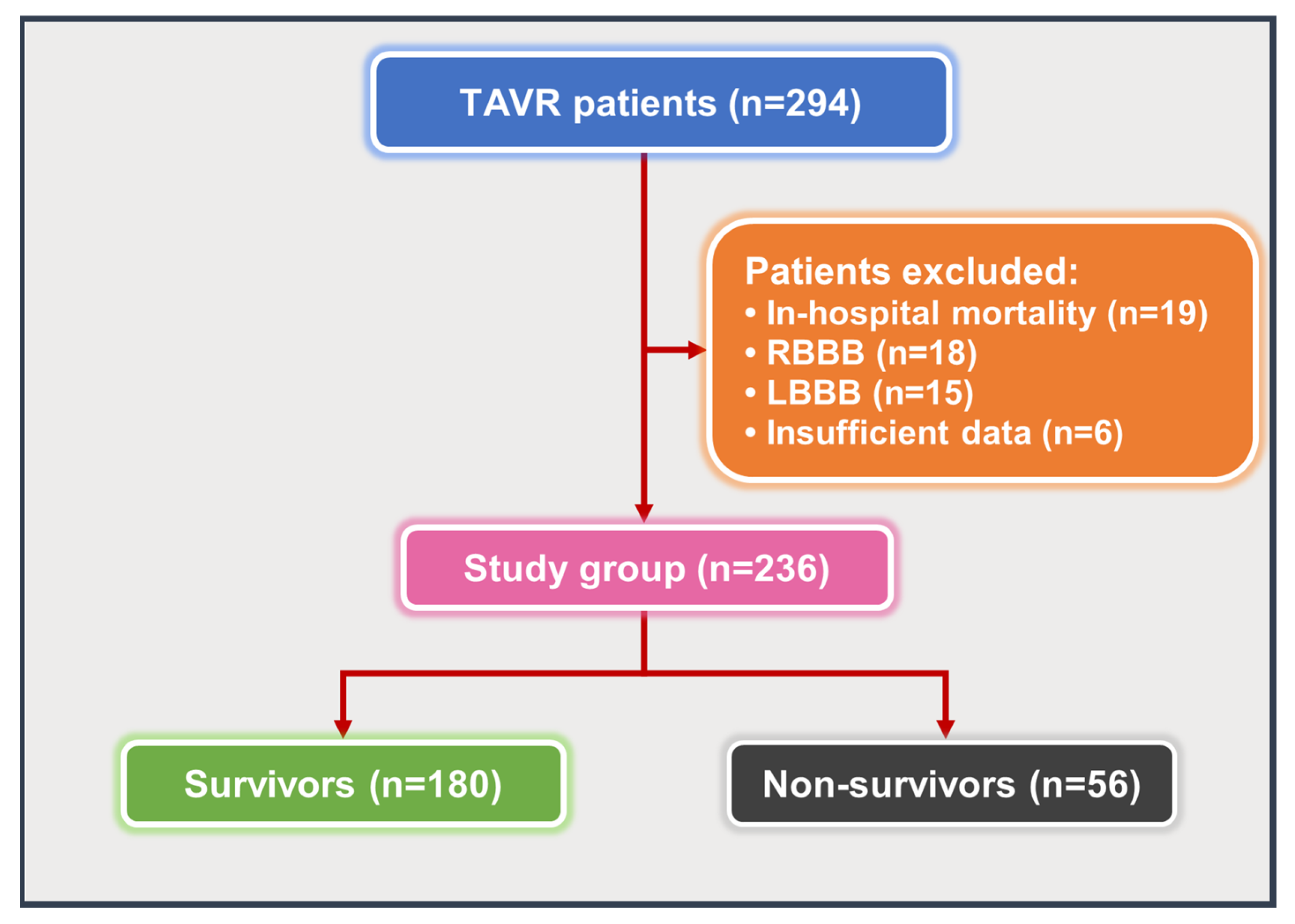
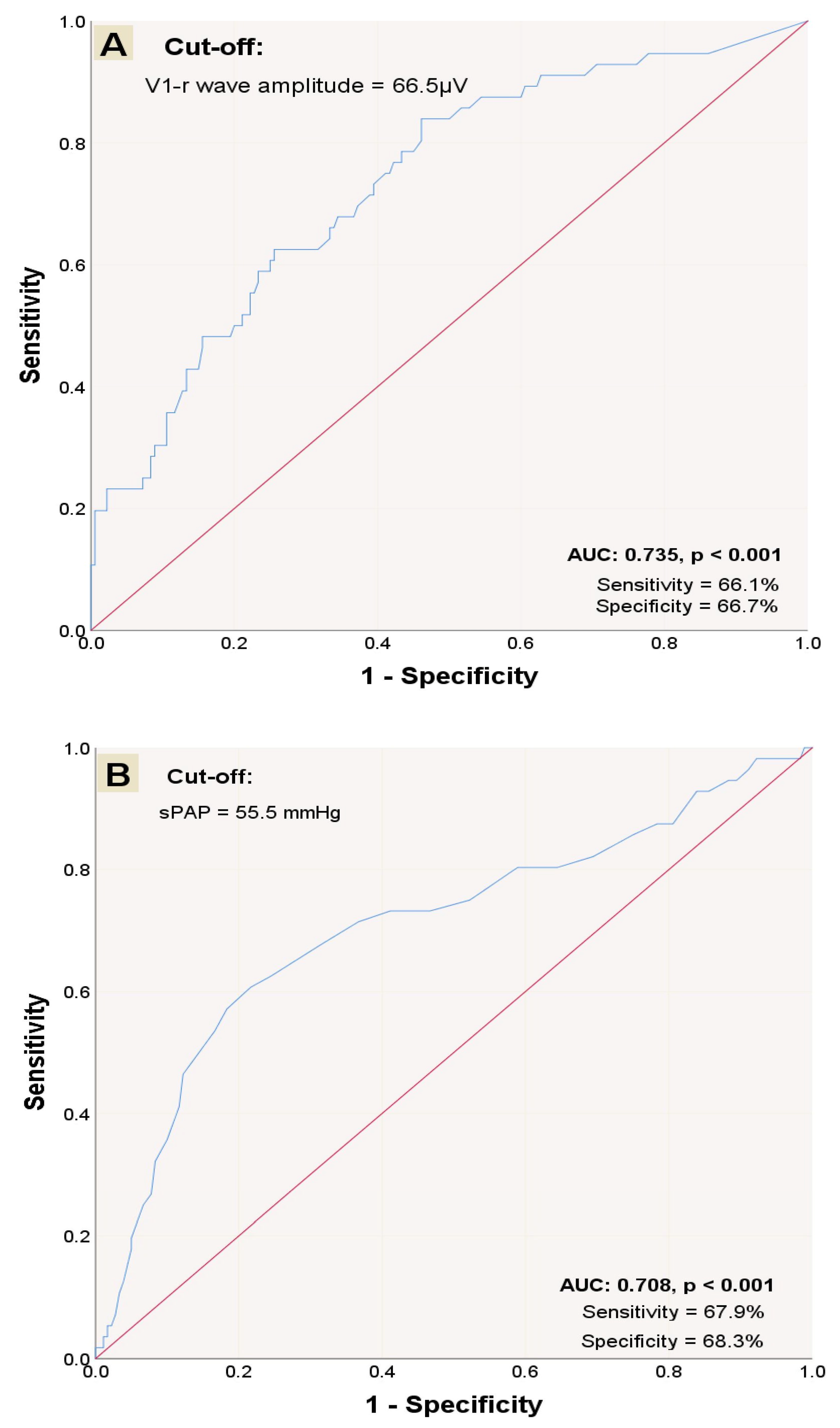
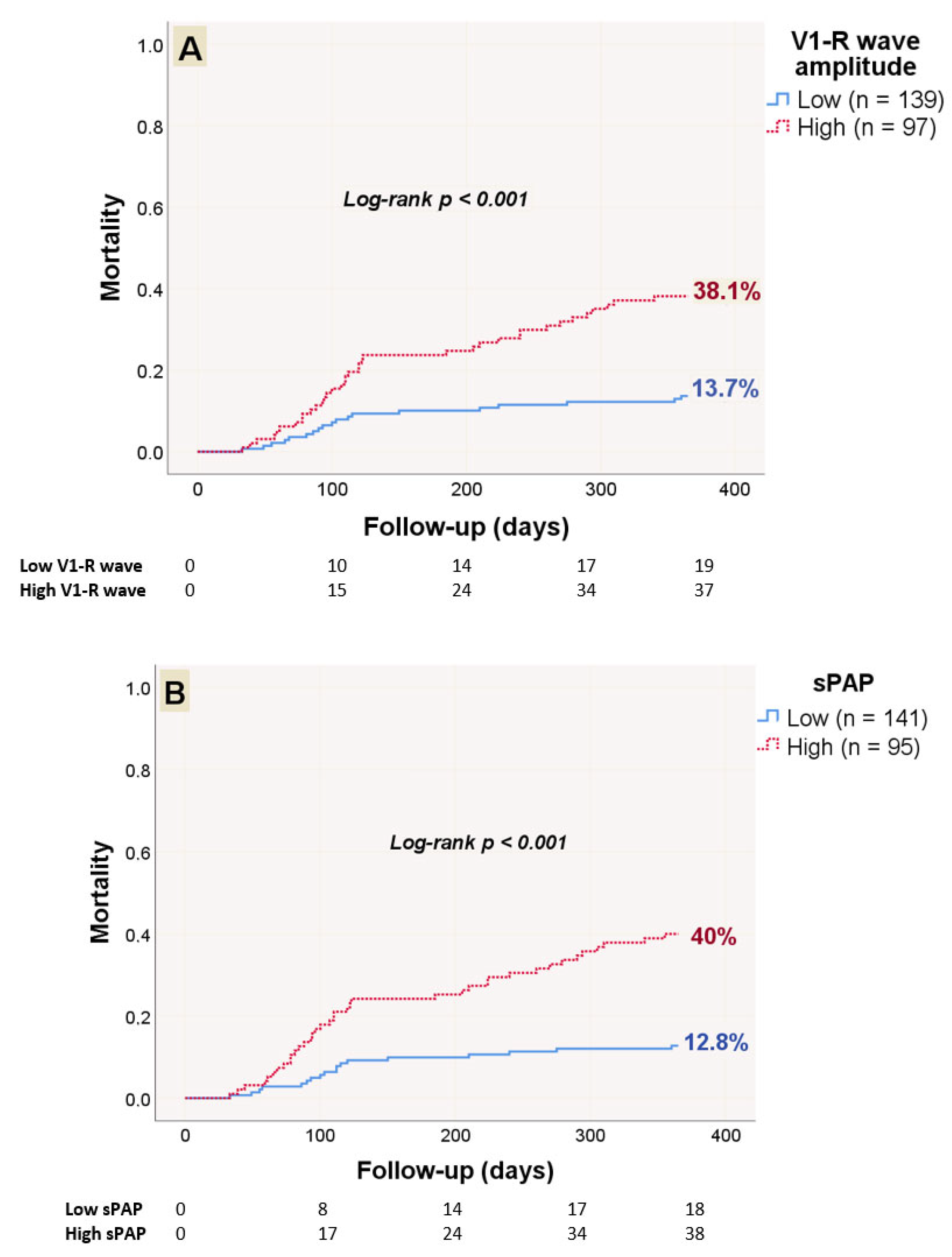
| Variables | Total Population (n = 236) | Survivors (n = 180) | Non-Survivors (n = 56) | p-Value |
|---|---|---|---|---|
| Age, (years) | 78.34 ± 6.81 | 77.87 ± 6.75 | 79.88 ± 6.83 | 0.054 |
| Female sex, n (%) | 128 (54.2) | 100 (55.6) | 28 (50) | 0.466 |
| BMI, (kg/m2) | 27.98 ± 4.29 | 28.11 ± 4.41 | 27.54 ± 3.86 | 0.397 |
| STS score | 7.46 [6.16–10.3] | 7.3 [5.83–10] | 8.24 [6.5–11.75] | 0.091 |
| CKD grade ≥ 3, n (%) | 116 (49.2) | 84 (46.7) | 32 (57.1) | 0.171 |
| CAD, n (%) | 165 (69.9) | 123 (68.3) | 42 (75.0) | 0.342 |
| DM, n (%) | 108 (45.8) | 83 (46.1) | 25 (44.6) | 0.847 |
| Hypertension, n (%) | 179 (75.8) | 139 (77.2) | 40 (71.4) | 0.376 |
| CVA, n (%) | 25 (10.6) | 14 (7.8) | 11 (19.6) | 0.012 |
| PAD, n (%) | 66 (28) | 46 (25.6) | 20 (35.7) | 0.139 |
| COPD, n (%) | 117 (49.6) | 88 (48.9) | 29 (51.8) | 0.705 |
| Hyperlipidemia, n (%) | 158 (66.9) | 128 (71.1) | 30 (53.6) | 0.015 |
| AF, n (%) | 58 (24.6) | 40 (22.2) | 18 (32.1) | 0.132 |
| AC, n (%) | 56 (23.7) | 38 (21.1) | 18 (32.1) | 0.090 |
| Antiplatelet, n (%) | 163 (69.1) | 129(71.7) | 34 (60.7) | 0.121 |
| BB usage, n (%) | 149 (63.1) | 120 (66.7) | 29 (51.8) | 0.044 |
| RAS inhibitor usage, n (%) | 159 (67.4) | 124 (68.9) | 35 (62.5) | 0.373 |
| Statin usage, n (%) | 103 (43.6) | 87 (48.3) | 16 (28.6) | 0.009 |
| SE valve, n (%) | 171 (72.5) | 134 (74.4) | 37 (66.1) | 0.221 |
| Contrast, (mL) | 244.72 ± 76.28 | 239.42 ± 75.43 | 261.79 ± 77.19 | 0.055 |
| Hemoglobin, (gr/dL) | 11.46 ± 1.88 | 11.57 ± 1.8 | 11.11 ± 2.09 | 0.112 |
| EF (%) | 60 [50–60] | 60 [50–60] | 55 [45–60] | 0.186 |
| Mean AVG, (mmHg) | 49.21 ± 9.95 | 49.38 ± 10.27 | 48.66 ± 8.9 | 0.637 |
| sPAP, (mmHg) | 53.4 ± 10.8 | 51.6 ± 9.9 | 59.2 ± 11.5 | <0.001 |
| V1-R wave amplitude, (µV) | 53 [30–104.5] | 44 [25–79.5] | 99 (54–148] | <0.001 |
| aVR-R wave amplitude, (µV) | 46 [22–80.75] | 45 [21.25- 76] | 50 [23.75–107] | 0.217 |
| QRS wide, (ms) | 95.08 ± 12.9 | 95.48 ± 12.94 | 93.80 ± 12.78 | 0.396 |
| Variables | Low V1-R (n = 139) | High V1-R (n = 97) | p-Value |
|---|---|---|---|
| Age, (years) | 78.6 ± 6.6 | 78 ± 7.1 | 0.519 |
| Female sex, n (%) | 80 (57.6) | 48 (49.5) | 0.221 |
| BMI, (kg/m2) | 28 ± 4.2 | 27.9 ± 4.4 | 0.845 |
| STS score | 7.6 [6.3–10.4] | 7.3 [5.8–10.3] | 0.464 |
| CKD grade ≥ 3, n (%) | 70 (50.4) | 46 (47.4) | 0.657 |
| CAD, n (%) | 100 (71.9) | 65 (67) | 0.416 |
| DM, n (%) | 65 (46.8) | 43 (44.3) | 0.712 |
| Hypertension, n (%) | 115 (82.7) | 64 (66) | 0.003 |
| CVA, n (%) | 12 (8.6) | 13 (13.4) | 0.241 |
| PAD, n (%) | 44 (31.7) | 22 (22.7) | 0.131 |
| COPD, n (%) | 73 (52.5) | 44 (45.4) | 0.279 |
| Hyperlipidemia, n (%) | 93 (66.9) | 64 (66) | 0.882 |
| AF, n (%) | 36 (25.9) | 22 (22.7) | 0.572 |
| AC, n (%) | 34 (24.5) | 22 (22.7) | 0.752 |
| Antiplatelet, n (%) | 96 (69.1) | 67 (69.1) | 0.999 |
| BB usage, n (%) | 91 (65.5) | 58 (59.8) | 0.374 |
| RAS inhibitor usage, n (%) | 100 (71.9) | 59 (60.8) | 0.073 |
| Statin usage, n (%) | 58 (41.7) | 45 (46.4) | 0.477 |
| SE valve, n (%) | 104 (74.8) | 67 (69.1) | 0.331 |
| Contrast, (mL) | 244.6 ± 78.2 | 244.9 ± 73.9 | 0.970 |
| Hemoglobin, (gr/dL) | 11.47 ± 2 | 11.45 ± 1.7 | 0.940 |
| EF (%) | 60 [48–60] | 60 [50–60] | 0.684 |
| Mean AVG, (mmHg) | 48.9 ± 10.4 | 49.6 ± 9.22 | 0.594 |
| sPAP, (mmHg) | 51.6 ± 10 | 55.9 ± 11.5 | 0.003 |
| QRS width, (ms) | 95.3 ± 12.7 | 94.7 ± 13.2 | 0.738 |
| Variables | Univariable HR (95% CI) | p-Value | Multivariable HR (95% CI) | p-Value |
|---|---|---|---|---|
| Age | 1.040 (0.999–1.082) | 0.056 | ||
| Female sex | 0.821 (0.486–1.387) | 0.461 | ||
| BMI | 0.976 (0.917–1.039) | 0.446 | ||
| STS score | 1.045 (0.992–1.100) | 0.100 | ||
| CKD grade ≥ 3 | 1.455 (0.857–2.470) | 0.165 | ||
| CAD | 1.359 (0.742–2.489) | 0.320 | ||
| Diabetes mellitus | 0.989 (0.584–1.676) | 0.969 | ||
| Hypertension | 0.737 (0.375–1.450) | 0.377 | ||
| CVA | 2.483 (1.283–4.805) | 0.007 | 2.336 (1.195–4.566) | 0.013 |
| PAD | 1.509 (0.873–2.606) | 0.140 | ||
| COPD | 1.068 (0.632–1.803) | 0.807 | ||
| Atrial fibrillation | 1.484 (0.847–2.600) | 0.168 | ||
| Contrast volume | 1.003 (1.000–1.006) | 0.067 | ||
| Self-expandable valve | 0.708 (0.407–1.231) | 0.221 | ||
| LV-EF | 0.985 (0.962–1.008) | 0.193 | ||
| AV mean gradient | 0.993 (0.968–1.020) | 0.616 | ||
| Baseline hemoglobin | 0.887 (0.765–1.028) | 0.112 | ||
| High sPAP | 3.645 (2.079–6.391) | <0.001 | 2.920 (1.639–5.202) | <0.001 |
| Hyperlipidemia | 0.539 (0.319–0.911) | 0.021 | ||
| Statin usage | 0.482 (0.270–0.861) | 0.014 | 0.478(0.266–0.856) | 0.013 |
| Beta-blocker usage | 0.600 (0.355–1.014) | 0.056 | ||
| High V1-R amplitude | 3.180 (1.828–5.533) | <0.001 | 2.376 (1.327–4.254) | 0.004 |
| QRS wide | 0.992 (0.971–1.012) | 0.429 | ||
| aVR-R amplitude | 1.003 (1.0003–1.007) | 0.034 | 1.001 (0.998–1.005) | 0.479 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ince, O.; Donmez, E.; Ozcan, S.; Gulsen, K.; Ziyrek, M.; Ozdemir, E.; Deniz, M.F.; Sahin, I.; Okuyan, E. Amplitude of the V1-R Wave Predicts Survival After Transcatheter Aortic Valve Replacement. Medicina 2025, 61, 1618. https://doi.org/10.3390/medicina61091618
Ince O, Donmez E, Ozcan S, Gulsen K, Ziyrek M, Ozdemir E, Deniz MF, Sahin I, Okuyan E. Amplitude of the V1-R Wave Predicts Survival After Transcatheter Aortic Valve Replacement. Medicina. 2025; 61(9):1618. https://doi.org/10.3390/medicina61091618
Chicago/Turabian StyleInce, Orhan, Esra Donmez, Sevgi Ozcan, Kamil Gulsen, Murat Ziyrek, Emrah Ozdemir, Muhammed Furkan Deniz, Irfan Sahin, and Ertugrul Okuyan. 2025. "Amplitude of the V1-R Wave Predicts Survival After Transcatheter Aortic Valve Replacement" Medicina 61, no. 9: 1618. https://doi.org/10.3390/medicina61091618
APA StyleInce, O., Donmez, E., Ozcan, S., Gulsen, K., Ziyrek, M., Ozdemir, E., Deniz, M. F., Sahin, I., & Okuyan, E. (2025). Amplitude of the V1-R Wave Predicts Survival After Transcatheter Aortic Valve Replacement. Medicina, 61(9), 1618. https://doi.org/10.3390/medicina61091618







