Narrow-Band Imaging for the Detection of Early Gastric Cancer Among High-Risk Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Study Endpoints—Definitions
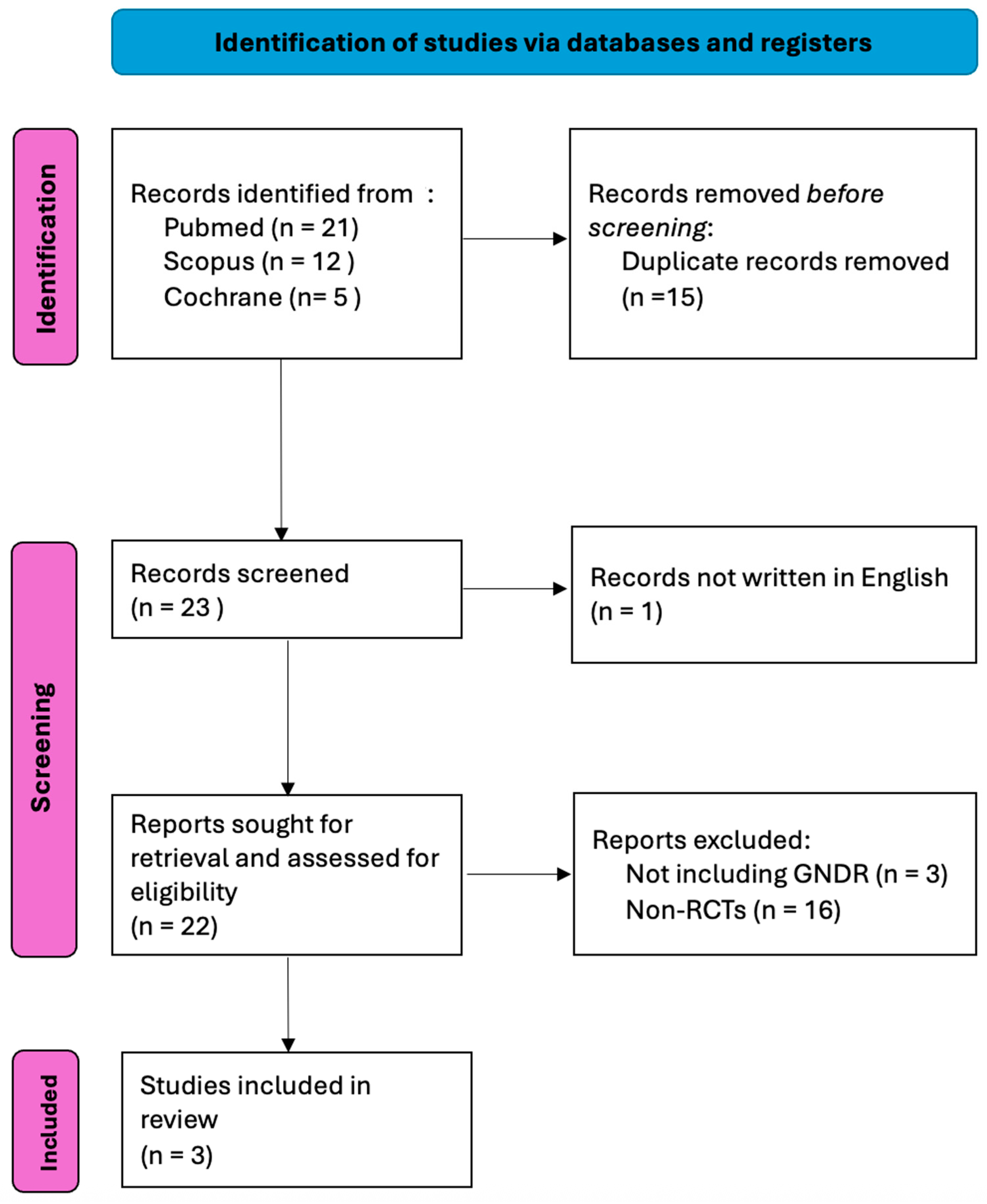
2.3. Search Methodology
2.4. Data Extraction Process
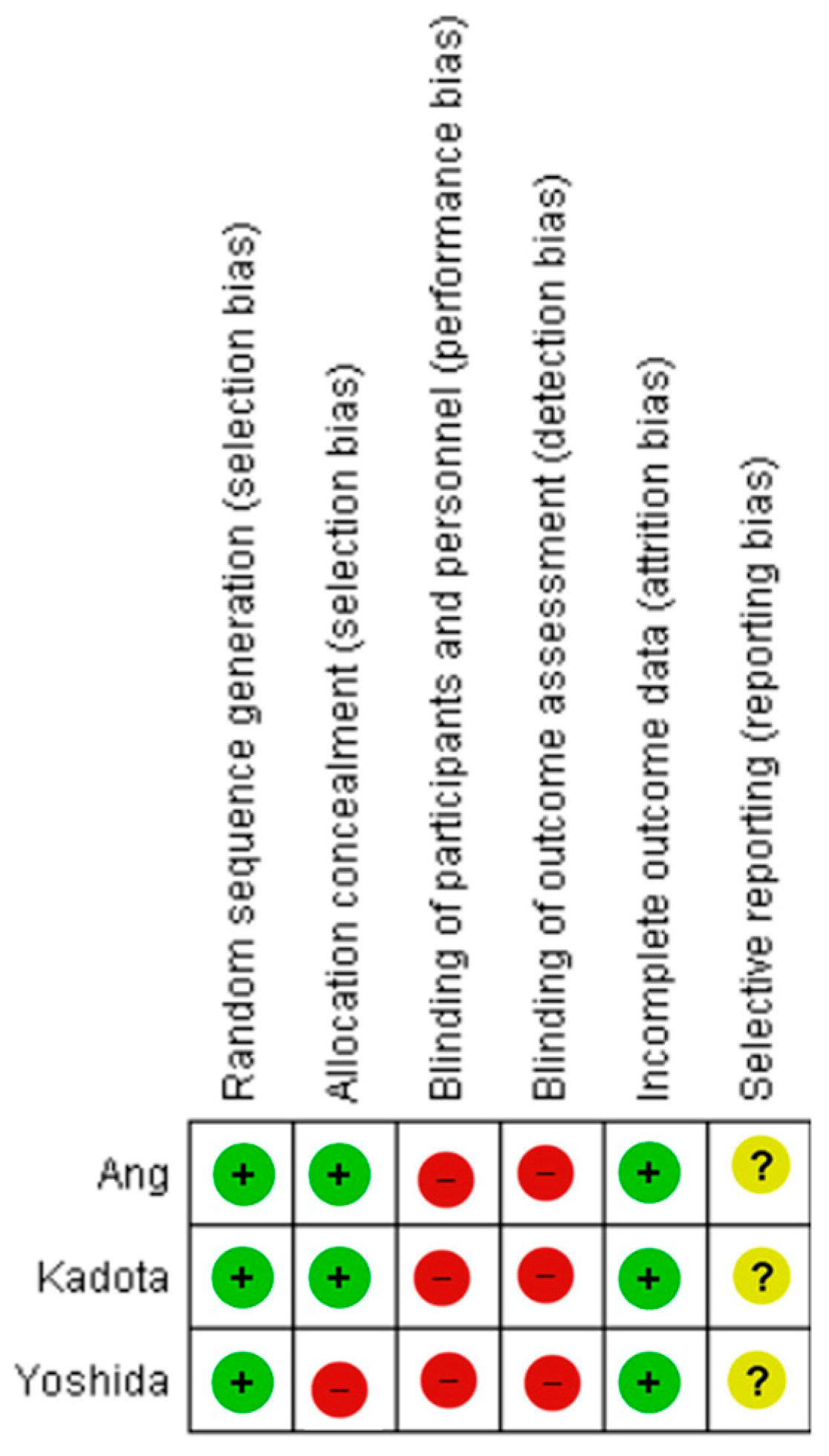
2.5. Risk of Bias Assessment
2.6. Analysis Methods
2.7. Grading the Evidence Strength
3. Results
3.1. Overview of Included Studies
3.2. Evidence Quality Overview
3.3. Quality of Evidence According to GRADE
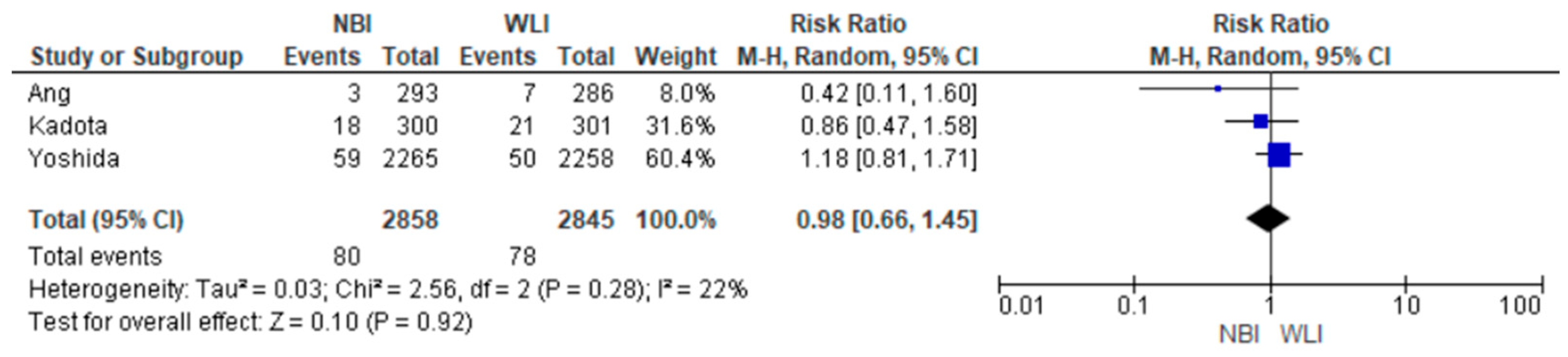
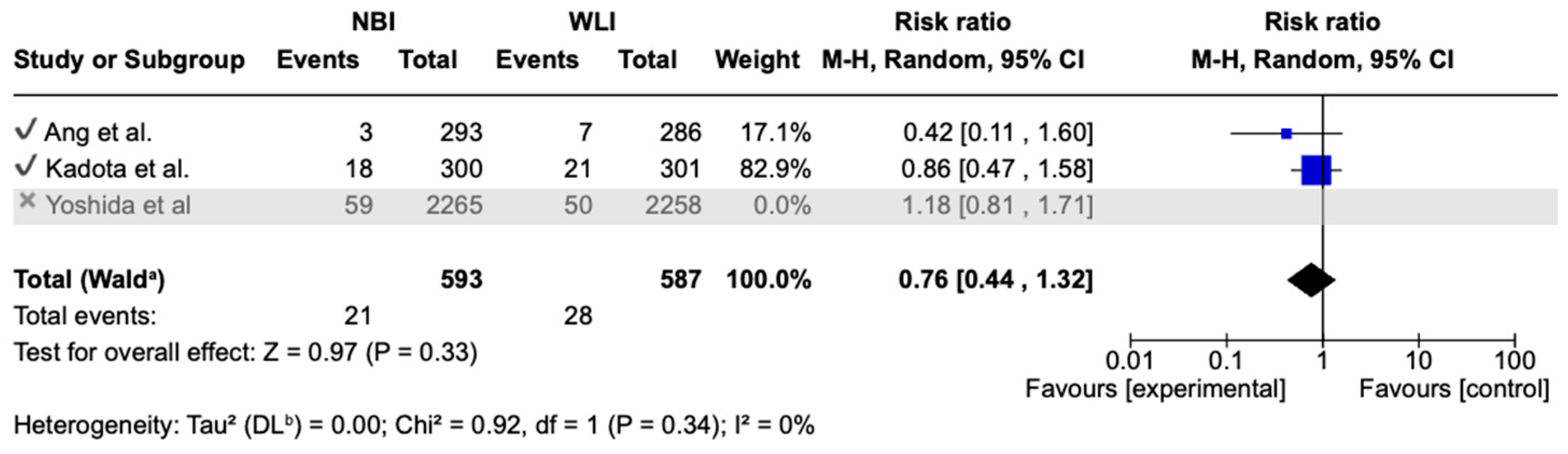
3.4. Primary Endpoint
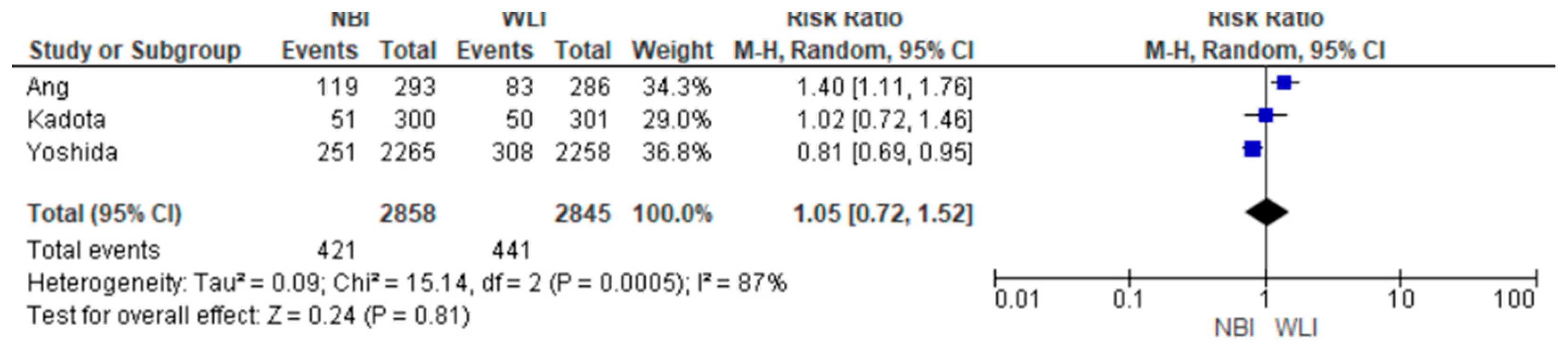
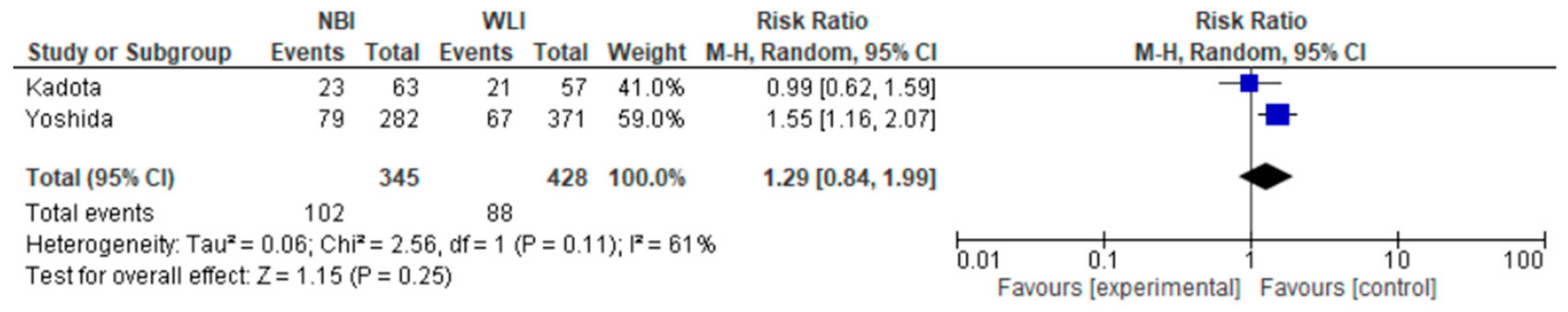
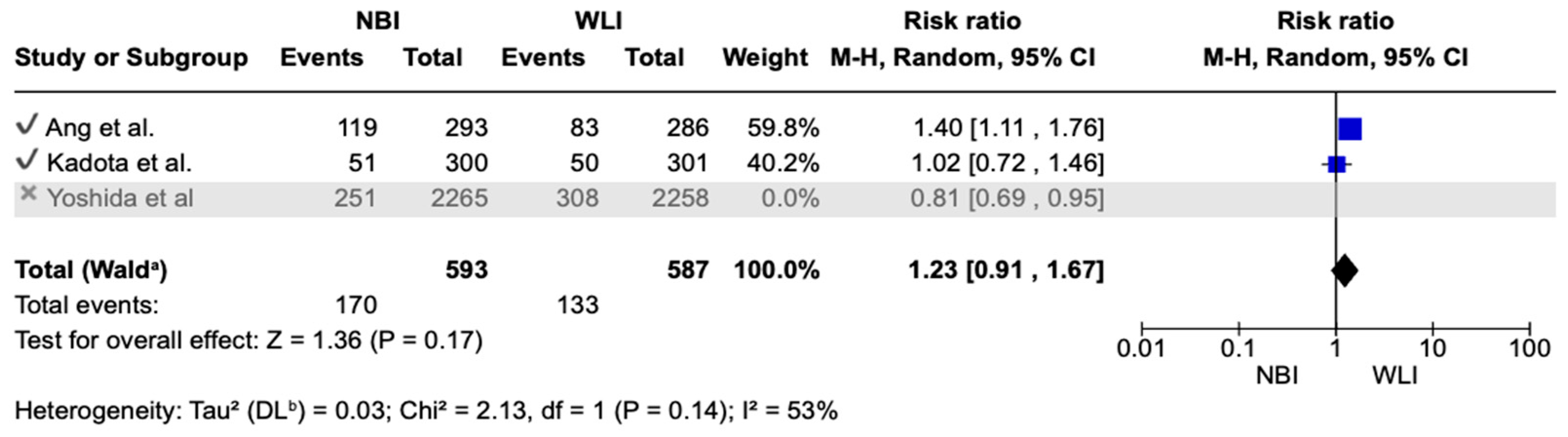
3.5. Secondary Endpoints
- Focal gastric lesion detection rates were also not statistically significant between the two groups [14.73% vs. 15.50%; RR (95%CI) = 1.05 (0.72–1.52); p = 0.81; Ι2 = 87%] (Figure 4).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLI | Blue Laser Imaging |
| ECG | Early Gastric Cancer |
| EMR | Endoscopic Mucosal Resection |
| ESD | Endoscopic Submucosal Dissection |
| FGLDR | Focal Gastric Lesion Detection Rate |
| GA | Gastric Atrophy |
| GNDR | Gastric Neoplasm Detection Rate |
| IM | Intestinal Metaplasia |
| LCI | Linked Color Imaging |
| ME | Magnifying Endoscopy |
| NBI | Narrow-Band Imaging |
| NF | Near Focus Magnification |
| PPV | Positive Predictive Value |
| RCT | Randomized Control Trial |
| VCE | Virtual Chromoendoscopy |
| WLI | Wight Light Imaging |
Appendix A
| Bias | Authors’ Judgement | Support for Judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned in a 1:1:1 ratio to 3G-NBI (primary 3G-NBI and secondary WLI), TXI (primary TXI and secondaryWLI), and WLI (primary and secondaryWLI) groups.” |
| Allocation concealment (selection bias) | Low risk | “The WLI group was set as the calibration arm by the minimization method with a random component balancing the groups regarding institution, age (<70 and >70 years), indication for endoscopy (surveillance and preoperative), and organ of previously treated or known lesion (stomach and oesophagus).” |
| Blinding of participants and personnel (performance bias) | High risk | “The endoscopists did not attempt to mask allocation to the study group.” |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of investigators to the intervention method was not implemented, which may have introduced bias in outcome assessment due to the potential influence of unblinded observation. |
| Incomplete outcome data (attrition bias) | Low risk | The per-protocol analysis was performed following the exclusion of comparable numbers of patients from each of the three allocation arms, based on consistent exclusion criteria. “After randomization, 5 patients were excluded: 2 were ineligible, one violated the study protocol, and 2 had esophageal stenosis.” |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was accessible, and the key pre-specified outcomes of interest to this review were reported in accordance with the original study plan. |
| Bias | Authors’ Judgement | Support for Judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low risk | “Patients were randomized in a 1: 1 ratio in blocks of 20 to undergo either NBI or HD-WLE for UGI endoscopy.” |
| Allocation concealment (selection bias) | Low risk | “Individual random sequence was placed in an opaque envelope and kept by an independent research assistant who was not involved in this study.” |
| Blinding of participants and personnel (performance bias) | High risk | The authors did not specify whether participants were informed about their allocation group. Regarding personnel blinding, the authors made the following statement: “Once informed consent was obtained, the research assistant would disclose the assigned imaging technique (NBI or HD-WLE) to the responsible endoscopist immediately before the procedure.” |
| Blinding of outcome assessment (detection bias) | High risk | The investigators were not blinded to the intervention method, which may have introduced bias in outcome assessment due to the lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Per protocol analysis performed after exclusion of balanced proportion of patients with similar reasons between the two allocation groups. “A total of 600 patients were initially enrolled, but as 21 patients were actually younger than 50 years of age and thus outside recruitment criteria, they were excluded, leaving 579 patients for analysis.” |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available, and the primary pre-specified outcomes relevant to this review—with the exception of the positive predictive value—were reported as originally planned. |
| Bias | Authors’ Judgement | Support for Judgement |
|---|---|---|
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned in a 1:1 ratio to the WLI group (primary WLI followed by secondary 2G-NBI) or the 2G-NBI group (primary 2G-NBI followed by secondary WLI). A centralised randomisation process was conducted using a computerised minimisation procedure on the Medical Research Support Web site (Kyoto, Japan).” |
| Allocation concealment (selection bias) | High risk | Authors do not report the exact method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of study group assignments was not implemented for either the endoscopists or the patients. |
| Blinding of outcome assessment (detection bias) | High risk | Investigators were aware of the intervention used, which may have introduced bias in the assessment of outcomes due to the lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Per protocol analysis performed after exclusion of balanced proportion of patients with similar reasons between the two allocation groups. “After randomisation, 51 patients were excluded: 21 patients who had inadequate preparation; 15 patients who violated the study protocol; 4 patients who had intragastric haemorrhage; 4 patients who had oesophageal stenosis; and 7 patients who had other reasons.” |
| Selective reporting (reporting bias) | Unclear risk | A publicly accessible protocol was provided, and the main outcomes of interest to this review were reported as originally specified. |
| Participants (Studies) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Other Considerations | Overall Certainty of Evidence | Study Event Rates (%) | Relative Effect (95% CI) | Anticipated Absolute Effects | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk with WLI | Risk with NBI | Risk with WLI | Risk Difference with NBI | |||||||||
| GNDR 5703 (3 RCTs) | serious | serious | not serious | not serious | undetected | none | ⊕⊕⊝⊝ Low | 441/2845 (15.5%) | 421/2858 (14.7%) | RR 1.05 (0.72 to 1.52) | 155 per 1000 | 8 more per 1000 (from 43 fewer to 81 more) |
| FGLDR 5703 (3 RCTs) | serious | serious | not serious | not serious | undetected | none | ⊕⊕⊝⊝ Low | 78/2845 (2.7%) | 80/2858 (2.8%) | RR 0.98 (0.66 to 1.45) | 27 per 1000 | 1 fewer per 1000 (from 9 fewer to 12 more) |
| PPV 773 (2 RCTs) | serious | serious | not serious | not serious | undetected | none | ⊕⊕⊝⊝ Low | 88/428 (20.6%) | 102/345 (29.6%) | RR 1.29 (0.84 to 1.99) | 206 per 1000 | 60 more per 1000 (from 33 fewer to 204 more) |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Neugut, A.I. Epidemiology of gastric cancer. World J. Gastroenterol. 2006, 12, 354. [Google Scholar] [CrossRef]
- Dinis-Ribeiro, M.; Libânio, D.; Uchima, H.; Spaander, M.C.; Bornschein, J.; Matysiak-Budnik, T.; Tziatzios, G.; Santos-Antunes, J.; Areia, M.; Chapelle, N.; et al. Management of epithelial precancerous conditions and early neoplasia of the stomach (MAPS III): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG) and European Society of Pathology (ESP) Guideline update 2025. Endoscopy 2025, 57, 504–554. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Pimenta-Melo, A.R.; Monteiro-Soares, M.; Libânio, D.; Dinis-Ribeiro, M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1041–1049. [Google Scholar] [CrossRef]
- Ezoe, Y.; Muto, M.; Uedo, N.; Doyama, H.; Yao, K.; Oda, I.; Kaneko, K.; Kawahara, Y.; Yokoi, C.; Sugiura, Y.; et al. Magnifying Narrowband Imaging Is More Accurate Than Conventional White-Light Imaging in Diagnosis of Gastric Mucosal Cancer. Gastroenterology 2011, 141, 2017–2025.e3. [Google Scholar] [CrossRef]
- Panteris, V.; Nikolopoulou, S.; Lountou, A.; Triantafillidis, J.K. Diagnostic capabilities of high-definition white light endoscopy for the diagnosis of gastric intestinal metaplasia and correlation with histologic and clinical data. Eur. J. Gastroenterol. Hepatol. 2014, 26, 594–601. [Google Scholar] [CrossRef]
- Nelson, D.B.; Block, K.P.; Bosco, J.J.; Burdick, J.S.; Curtis, W.D.; Faigel, D.O.; Greenwald, D.A.; Kelsey, P.B.; Rajan, E.; Slivka, A.; et al. High resolution and high-magnification endoscopy: September 2000. Gastrointest. Endosc. 2000, 52, 864–866. [Google Scholar] [CrossRef]
- Kiesslich, R.; Jung, M. Magnification Endoscopy: Does It Improve Mucosal Surface Analysis for the Diagnosis of Gastrointestinal Neoplasias? Endoscopy 2002, 34, 819–822. [Google Scholar] [CrossRef]
- Goda, K.; Dobashi, A.; Yoshimura, N.; Aihara, H.; Kato, M.; Sumiyama, K.; Toyoizumi, H.; Kato, T.; Saijo, H.; Ikegami, M.; et al. Dual-focus versus conventional magnification endoscopy for the diagnosis of superficial squamous neoplasms in the pharynx and esophagus: A randomized trial. Endoscopy 2016, 48, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Kobayashi, M.; Kozu, T. Development and clinical application of a narrow band imaging (NBI) system with built-in narrow-band RGB filters. Stomach Intest. 2001, 36, 1283–1287. [Google Scholar]
- Picot, J.; Rose, M.; Cooper, K.; Pickett, K.; Lord, J.; Halrris, P.; Whyte, S.; Böhning, D.; Shepherd, J. Virtual chromoendoscopy for the real-time assessment of colorectal polyps in vivo: A systematic review and economic evaluation. Southampton (UK): NIHR Journals Library. Health Technol. Assess. 2017, 21, 1–308. [Google Scholar] [CrossRef]
- Glover, B.; Teare, J.; Patel, N. A Review of New and Emerging Techniques For Optical Diagnosis of Colonic Polyps. J. Clin. Gastroenterol. 2019, 53, 495–506. [Google Scholar] [CrossRef]
- Li, H.-Y.; Ge, Z.-Z.; Fujishiro, M.; Li, X.-B. Current Clinical Applications of Magnifying Endoscopy with Narrow Band Imaging in the Stomach. Diagn. Ther. Endosc. 2012, 2012, 271914. [Google Scholar] [CrossRef] [PubMed]
- Gono, K.; Obi, T.; Yamaguchi, M.; Ohyama, N.; Machida, H.; Sano, Y.; Yoshida, S.; Hamamoto, Y.; Endo, T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J. Biomed. Opt. 2004, 9, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Takaki, Y.; Matsui, T.; Iwashita, A.; Anagnostopoulos, G.K.; Kaye, P.; Ragunath, K. Clinical Application of Magnification Endoscopy and Narrow-Band Imaging in the Upper Gastrointestinal Tract: New Imaging Techniques for Detecting and Characterizing Gastrointestinal Neoplasia. Gastrointest. Endosc. Clin. N. Am. 2008, 18, 415–433. [Google Scholar] [CrossRef]
- Sumiyama, K.; Kaise, M.; Nakayoshi, T.; Kato, M.; Mashiko, T.; Uchiyama, Y.; Goda, K.; Hino, S.; Nakamura, Y.; Matsuda, K.; et al. Combined use of a magnifying endoscope with a narrow band imaging system and a multibending endoscope for en bloc EMR of early stage gastric cancer. Gastrointest. Endosc. 2004, 60, 79–84. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y.; Jang, J.; Kim, G.H.; Bang, B.W.; Park, J.C.; Choi, H.S.; Cho, J.; Research Group for Endoscopic Instruments and Stents of Korean Society of Gastrointestinal Endoscopy. Narrowband imaging with near-focus magnification for discriminating the gastric tumor margin before endoscopic resection: A prospective randomized multicenter trial. J. Gastroenterol. Hepatol. 2020, 35, 1930–1937. [Google Scholar] [CrossRef]
- Kakushima, N.; Yoshida, N.; Doyama, H.; Yano, T.; Horimatsu, T.; Uedo, N.; Yamamoto, Y.; Kanzaki, H.; Hori, S.; Yao, K.; et al. Near-focus magnification and second-generation narrow-band imaging for early gastric cancer in a randomized trial. J. Gastroenterol. 2020, 55, 1127–1137. [Google Scholar] [CrossRef]
- Gastrointestinal Tract (Upper) Cancers—Recognition and Referral 2024. Available online: https://cks.nice.org.uk/topics/gastrointestinal-tract-upper-cancers-recognition-referral/ (accessed on 1 September 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, N. Diagnosis and Management of High Risk Group for Gastric Cancer. Gut Liver 2015, 9, 5–17. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Oxman, A. Grade Handbook. Introduction to GRADE Handbook. 2024. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 1 September 2024).
- Kadota, T.; Abe, S.; Uedo, N.; Doyama, H.; Furue, Y.; Muto, M.; Nonaka, S.; Takamaru, H.; Murano, T.; Nakajo, K.; et al. Comparison of Effective Imaging Modalities for Detecting Gastric Neoplasms: A Randomized 3-Arm Phase II Trial. Am. J. Gastroenterol. 2024, 119, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.L.; Pittayanon, R.; Lau, J.Y.W.; Rerknimitr, R.; Ho, S.H.; Singh, R.; Kwek, A.B.E.; Ang, D.S.W.; Chiu, P.W.Y.; Luk, S.; et al. A multicenter randomized comparison between high-definition white light endoscopy and narrow band imaging for detection of gastric lesions. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Doyama, H.; Yano, T.; Horimatsu, T.; Uedo, N.; Yamamoto, Y.; Kakushima, N.; Kanzaki, H.; Hori, S.; Yao, K.; et al. Early gastric cancer detection in high-risk patients: A multicentre randomised controlled trial on the effect of second-generation narrow band imaging. Gut 2021, 70, 67–75. [Google Scholar] [CrossRef]
- Dinis-Ribeiro, M.; Areia, M.; de Vries, A.C.; Marcos-Pinto, R.; Monteiro-Soares, M.; O’cOnnor, A.; Pereira, C.; Pimentel-Nunes, P.; Correia, R.; Ensari, A.; et al. Management of precancerous conditions and lesions in the stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012, 44, 74–94. [Google Scholar] [CrossRef]
- Yao, K.; Doyama, H.; Gotoda, T.; Ishikawa, H.; Nagahama, T.; Yokoi, C.; Oda, I.; Machida, H.; Uchita, K.; Tabuchi, M. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: A prospective multicenter feasibility study. Gastric Cancer 2014, 17, 669–679. [Google Scholar] [CrossRef]
- Nakajima, T.; Oda, I.; Gotoda, T.; Hamanaka, H.; Eguchi, T.; Yokoi, C.; Saito, D. Metachronous gastric cancers after endoscopic resection: How effective is annual endoscopic surveillance? Gastric Cancer 2006, 9, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.Y.; Cho, J.W.; Oh, W.G.; Ko, S.J.; Han, S.H.; Baek, H.K.; Lee, Y.J.; Kim, J.W.; Jung, G.M.; Cho, Y.K. Clinicopathological characteristics of synchronous and metachronous gastric neoplasms after endoscopic submucosal dissection. Korean J. Intern. Med. 2013, 28, 687–693. [Google Scholar] [CrossRef]
- Ławniczak, M. Synchronous and metachronous neoplasms in gastric cancer patients: A 23-year study. World J. Gastroenterol. 2014, 20, 7480–7487. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Jun, J.K.; Suh, M.; Park, B.; Noh, D.K.; Song, S.H.; Jung, K.W.; Lee, H.-Y.; Choi, I.J.; Park, E.-C. Effect of endoscopy screening on stage at gastric cancer diagnosis: Results of the National Cancer Screening Programme in Korea. Br. J. Cancer 2015, 112, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C.; Ogoshi, K.; Okamoto, M.; Shabana, M.; Kishimoto, T.; Fukao, A.; Lee, J.-S. A Community-Based, Case-Control Study Evaluating Mortality Reduction from Gastric Cancer by Endoscopic Screening in Japan. PLoS ONE 2013, 8, e79088. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, M.; Yoshida, N.; Tsuji, S.; Masunaga, T.; Hirai, H.; Miyajima, S.; Dejima, A.; Nakashima, T.; Wakita, S.; Takemura, K.; et al. Optimal number of endoscopic biopsies for diagnosis of early gastric cancer. Endosc. Int. Open 2019, 7, E1683–E1690. [Google Scholar] [CrossRef]
- Kanesaka, T.; Uedo, N.; Doyama, H.; Yoshida, N.; Nagahama, T.; Ohtsu, K.; Uchita, K.; Kojima, K.; Ueo, T.; Takahashi, H.; et al. Diagnosis of histological type of early gastric cancer by magnifying narrow-band imaging: A multicenter prospective study. DEN Open 2022, 2, e61. [Google Scholar] [CrossRef]
- Beg, S.; Ragunath, K.; Wyman, A.; Banks, M.; Trudgill, N.; Pritchard, M.D.; Riley, S.; Anderson, J.; Griffiths, H.; Bhandari, P.; et al. Quality standards in upper gastrointestinal endoscopy: A position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut 2017, 66, 1886–1899. [Google Scholar] [CrossRef]
- Neale, J.R.; James, S.; Callaghan, J.; Patel, P. Premedication with N-acetylcysteine and simethicone improves mucosal visualization during gastroscopy: A randomized, controlled, endoscopist-blinded study. Eur. J. Gastroenterol. Hepatol. 2013, 25, 778–783. [Google Scholar] [CrossRef]
- Li, H.-Y.; Dai, J.; Xue, H.-B.; Zhao, Y.-J.; Chen, X.-Y.; Gao, Y.-J.; Song, Y.; Ge, Z.-Z.; Li, X.-B. Application of magnifying endoscopy with narrow-band imaging in diagnosing gastric lesions: A prospective study. Gastrointest. Endosc. 2012, 76, 1124–1132. [Google Scholar] [CrossRef]
- Le, H.; Wang, L.; Zhang, L.; Chen, P.; Xu, B.; Peng, D.; Yang, M.; Tan, Y.; Cai, C.; Li, H.; et al. Magnifying endoscopy in detecting early gastric cancer: A network meta-analysis of prospective studies. Medicine 2021, 100, e23934. [Google Scholar] [CrossRef]
- Ono, S.; Kawada, K.; Dohi, O.; Kitamura, S.; Koike, T.; Hori, S.; Kanzaki, H.; Murao, T.; Yagi, N.; Sasaki, F.; et al. Linked Color Imaging Focused on Neoplasm Detection in the Upper Gastrointestinal Tract: A Randomized Trial. Ann. Intern. Med. 2021, 174, 18–24. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, X.; Meng, Q.; Jin, H.; Zhu, Z.; Wang, Z.; Qian, W.; Zhang, L.; Liu, Y.; Min, M.; et al. Linked Color Imaging Can Improve Detection Rate of Early Gastric Cancer in a High-Risk Population: A Multi-Center Randomized Controlled Clinical Trial. Dig. Dis. Sci. 2021, 66, 1212–1219. [Google Scholar] [CrossRef]
- Dohi, O.; Yagi, N.; Naito, Y.; Fukui, A.; Gen, Y.; Iwai, N.; Ueda, T.; Yoshida, N.; Kamada, K.; Uchiyama, K.; et al. Blue laser imaging-bright improves the real-time detection rate of early gastric cancer: A randomized controlled study. Gastrointest. Endosc. 2019, 89, 47–57. [Google Scholar] [CrossRef]
- Min, M.; Sun, X.; Bai, J.; Zhang, Q.; Yang, X.; Guo, Q.; Wang, R.; Wang, B.; Lv, Z.; Pan, J.; et al. Diagnostic accuracy of linked colour imaging versus white light imaging for early gastric cancers: A prospective, multicentre, randomized controlled trial study. Ann. Med. 2022, 54, 3305–3313. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, F.; Chen, Z.-Y.; Wang, Z.; Zhi, F.-C.; Liu, S.-D.; Bai, Y. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: A meta-analysis. Gastric Cancer 2016, 19, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kaise, M.; Yonezawa, J.; Toyoizumi, H.; Yoshimura, N.; Yoshida, Y.; Kawamura, M.; Tajiri, H. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: A prospective study. Gastrointest. Endosc. 2010, 72, 523–529. [Google Scholar] [CrossRef]
- Ezoe, Y.; Muto, M.; Horimatsu, T.; Minashi, K.; Yano, T.; Sano, Y.; Chiba, T.; Ohtsu, A. Magnifying narrow-band imaging versus magnifying white-light imaging for the differential diagnosis of gastric small depressive lesions: A prospective study. Gastrointest. Endosc. 2010, 71, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, J.; Lin, X.-C.; Wei, N.; Lin, W.; Chang, H.; Du, X.-M. Evaluating the Diagnoses of Gastric Antral Lesions Using Magnifying Endoscopy with Narrow-Band Imaging in a Chinese Population. Dig. Dis. Sci. 2014, 59, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, M.; Esposito, G.; Libânio, D.; Pimentel-Nunes, P.; Dinis-Ribeiro, M. Image-enhanced endoscopy for gastric preneoplastic conditions and neoplastic lesions: A systematic review and meta-analysis. Endoscopy 2020, 52, 1048–1065. [Google Scholar] [CrossRef]
- Yamada, S.; Doyama, H.; Yao, K.; Uedo, N.; Ezoe, Y.; Oda, I.; Kaneko, K.; Kawahara, Y.; Yokoi, C.; Sugiura, Y.; et al. An efficient diagnostic strategy for small, depressed early gastric cancer with magnifying narrow-band imaging: A post-hoc analysis of a prospective randomized controlled trial. Gastrointest. Endosc. 2014, 79, 55–63. [Google Scholar] [CrossRef]
| Author, Year | Recruitment Period | Site | Patients | Mean Age (Years) | Female (N) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | NBI | WLI | NBI | WLI | NBI | WLI | |||
| Ang et al., 2015 [27] | 2012–2013 | Asia-Pacific region | 579 | 293 | 286 | 62.6 | 62.3 | 165 | 178 |
| Yoshida et al., 2021 [28] | 2014–2017 | Japan | 4523 | 2265 | 2258 | 70.6 | 70.6 | 491 | 505 |
| Kadota et al., 2024 [26] | 2021–2022 | Japan | 901 | 300 | 301 | 73 | 73 | 72 | 73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manti, M.; Gkolfakis, P.; Kamperidis, N.; Toskas, A.; Papaefthymiou, A.; Tziatzios, G.; Misra, R.; Arebi, N. Narrow-Band Imaging for the Detection of Early Gastric Cancer Among High-Risk Patients: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 1613. https://doi.org/10.3390/medicina61091613
Manti M, Gkolfakis P, Kamperidis N, Toskas A, Papaefthymiou A, Tziatzios G, Misra R, Arebi N. Narrow-Band Imaging for the Detection of Early Gastric Cancer Among High-Risk Patients: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(9):1613. https://doi.org/10.3390/medicina61091613
Chicago/Turabian StyleManti, Magdalini, Paraskevas Gkolfakis, Nikolaos Kamperidis, Alexandros Toskas, Apostolis Papaefthymiou, Georgios Tziatzios, Ravi Misra, and Naila Arebi. 2025. "Narrow-Band Imaging for the Detection of Early Gastric Cancer Among High-Risk Patients: A Systematic Review and Meta-Analysis" Medicina 61, no. 9: 1613. https://doi.org/10.3390/medicina61091613
APA StyleManti, M., Gkolfakis, P., Kamperidis, N., Toskas, A., Papaefthymiou, A., Tziatzios, G., Misra, R., & Arebi, N. (2025). Narrow-Band Imaging for the Detection of Early Gastric Cancer Among High-Risk Patients: A Systematic Review and Meta-Analysis. Medicina, 61(9), 1613. https://doi.org/10.3390/medicina61091613