The Effect of Music Therapy on Psychological Outcomes for Neurological Conditions: A Systematic Review
Abstract
1. Introduction
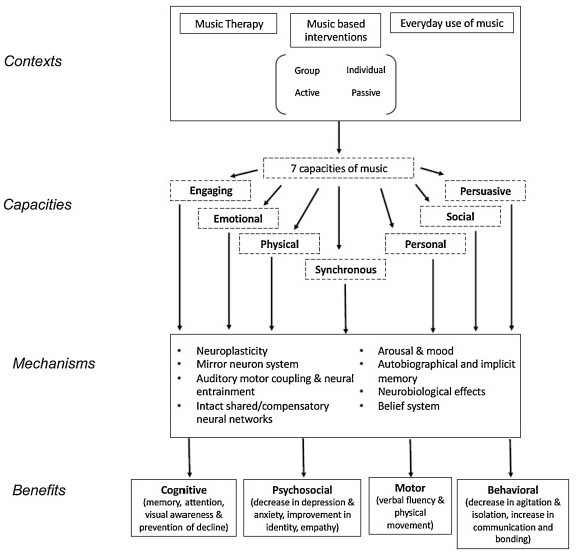
1.1. Music Therapy in Theory and Clinical Practice
1.2. Research in Music Therapy for Neurological Conditions
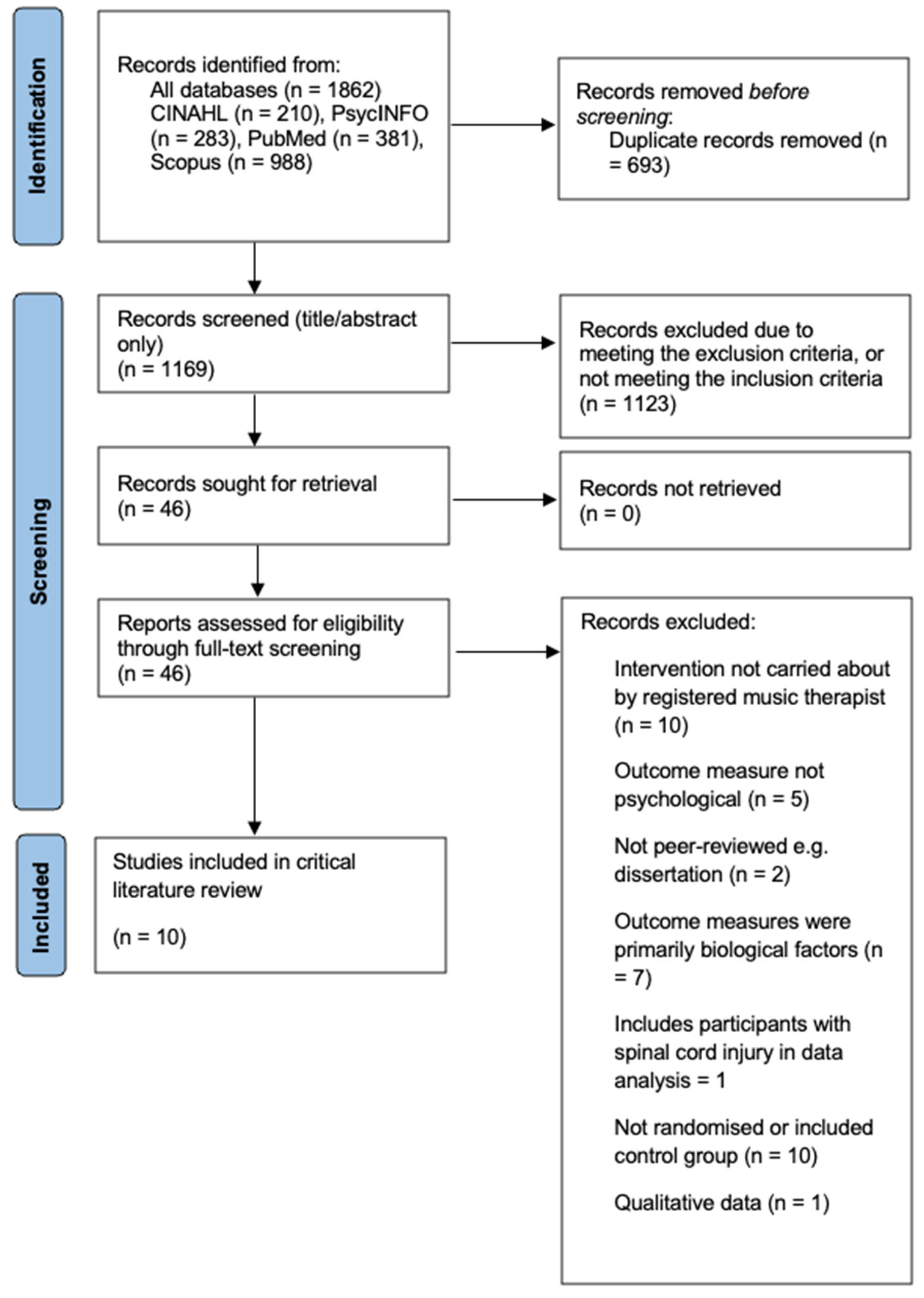
2. Method
2.1. Search Strategy
2.2. Search Terms
2.3. Data Synthesis and Quality Appraisal
2.4. SWiM Approach
3. Results
3.1. Characteristics of the Included Studies
3.2. Quality Appraisal and Risk of Bias
3.3. Randomisation
3.4. Deviations from Intended Interventions
| Study Design | Sample Size | Age (Mean Years) | Sex (Female) | Neurological Condition | Location and Research Setting | Outcome Measures | Music Therapy Intervention | Comparator | |
|---|---|---|---|---|---|---|---|---|---|
| Chou et al. (2024) [84] | RCT pilot study | 82 | 58 | 28% | Stroke | Taiwan, inpatient setting | BDI-II, MMSE, MRS, BI Timepoints: Before and after intervention | Neurologic Music Therapy—Therapeutic Singing, Melodic Intonation Therapy, Rhythmic Speech Cueing, Therapeutic Instrument Music Playing, Music Cognitive Training (from neurologic music therapy) (in addition to treatment as usual) Frequency: Four hours over four weeks (extra to neurorehabilitation as normal) | Conventional therapy (treatment as usual) |
| Haire et al. (2021 [55] | RCT | 30 | 55.9 | 47% | Stroke | Toronto, Canada, community setting | TMT-B, FDST, GSES, MAAC-R, SAM Timepoints: Two baseline assessments one-week apart. One post-intervention assessment. | Therapeutic Instrumental Music Performance (TIMP) Frequency: Three times a week for three weeks | The comparator groups consisted of TIMP plus cued motor imagery and TIMP plus motor imagery without external cues |
| Poćwierz-Marciniak & Bidzan (2017) [57] | RCT | 61 | 64 | 78.7% | Stroke | Gdynia, Poland, inpatient neurological rehabilitation hospital | SF-36, SA-SIP30, Cantril Ladder Timepoints: Before and after intervention | Cognitive Music Therapy, Guided Imagery and Music, 1:1 Frequency: Twice a week for five weeks | Standard care (physiotherapy, ergotherapy, psychological diagnosis, maintenance psychotherapy) |
| Raglio et al. (2017) [11] | RCT pilot | 38 | 72.7 | 58% | Stroke | Pavia, Italy, inpatient neurological rehabilitation hospital | HADS, MQOL-It Timepoints: Before and after intervention | Relational Active Music Therapy (RAMT) Frequency: Three sessions per week, 20 sessions total | Standard care (physiotherapy, occupational therapy) |
| Segura et al. (2024) [33] | RCT | 58 | 63.2 | 24% | Stroke | Barcelona, Spain, ex-inpatient neuro-rehabilitation | BRIEF, SART, Figural Memory subtest from the WMS-R, AVLT, Verbal Fluency test in Spanish, BDI-II, self- and informant-version of AES, POMS, SIS, TSRQ, IMI, Strategies Used to Promote Health Timepoints: Before and after intervention, with 3-month follow-up | Enriched Music-supported Therapy Frequency: Once a week music therapy, plus three weekly individual self-training session, for 10 weeks | Graded Repetitive Arm Supplementary Program (GRASP) only |
| Impellizzeri et al. (2020) [82] | RCT pilot study | 30 | 51 | 37% | Multiple Sclerosis | Messina, Italy, clinic centre setting | BRB-N, MSQOL-54, BDI, EAQ, MMF Timepoints: Before and after intervention | Neurologic Music Therapy—Associative mood and memory training, Music in psychosocial training and counselling (half of the treatment-as-usual time replaced with music therapy intervention) Frequency: Three times per week for 8 weeks | Treatment-as-usual (same number of sessions as the music therapy group) |
| Impellizzeri et al. (2024) [52] | Pilot Quasi-RCT | 40 | 62.45 | 30% | Parkinson’s disease | Messina, Italy, clinic centre setting | MoCA, HRSD, FAB, Stroop test, Visual search test Timepoints: Before and after intervention | Computer-Assisted Rehabilitation Environment (CAREN), Rhythmic Auditory Stimulation, Therapeutic Instrumental Music Performance Frequency: Three sessions per week for 8 weeks | Standard treatment with CAREN selected scenarios three times per week |
| Lee et al. (2024) [54] | RCT | 27 | 73.3 | 52% | Parkinson’s disease | Arizona, USA, Tremble Clefs therapeutic singing group | HY, GDS, VRQOL, VASM Timepoints: Before and after intervention (VASM only) | Therapeutic Group Singing (TGS), Straw Phonation Combined with Therapeutic Singing (SP + TGS) Frequency: Single session | Speaking-only control group |
| Siponkoski et al. (2020) [53] | Cross-over RCT | 40 | 41.3 | 41% | Traumatic Brain Injury | Helsinki, Finland, brain injury clinic setting | FAB, Number-Letter Task, Auditory N-back Task, Simon Task, SART, Similarities, Block Design, and Digit Span subtests of the WAIS-IV, Words Lists I and II subtests of the WMS-III Timepoints: Before and after intervention, follow-up (3 and 6 months) | Rhythmical Training, Structured Cognitive-motor Training, Assisted music playing Frequency: Twice per week, for 20 sessions | Standard care (physiotherapy, occupational therapy, neuropsychological rehabilitation, speech therapy) |
| Van Bruggen-Rufi et al. (2017) [56] | RCT | 63 | 54.4 | 68.3% | Huntington’s disease | Netherlands, set in four specialised Huntington’s disease care facilities | BOSH—social-cognitive functioning subscale and the mental rigidity and aggression subscale, PBA Timepoints: Before intervention, halfway (8th session), end of intervention (16th session), follow-up (12 weeks after intervention) | Followed protocol “music therapy for Huntington’s patients on improving and stimulating communication and self-expression” Frequency: One session per week, for 16 weeks | Recreational therapy (with treatment guide offered in same circumstances as music therapy group e.g., reading the newspaper, cooking, arts and crafts, handwork, puzzles/games) |
3.5. Missing Outcome Data
3.6. Measurement of the Outcome
3.7. Selection of the Reported Results
3.8. Data Synthesis and Key Findings
3.9. Within-Group Findings
3.10. Between-Group Findings
4. Certainty of Evidence Using GRADE
4.1. Risk of Bias
4.2. Inconsistency
4.3. Indirectness
4.4. Imprecision
4.5. Publication Bias
4.6. Overall Certainty of Evidence and Importance of Outcome
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Devlin, K.; Alshaikh, J.T.; Pantelyat, A. Music therapy and music-based interventions for movement disorders. Curr. Neurol. Neurosci. Rep. 2019, 19, 83–95. [Google Scholar] [CrossRef]
- De Witte, M.; Pinho, A.D.S.; Stams, G.J.; Moonen, X.; Bos, A.E.; Van Hooren, S. Music therapy for stress reduction: A systematic review and meta-analysis. Health Psychol. Rev. 2022, 16, 134–159. [Google Scholar] [CrossRef]
- Hurkmans, J.; de Bruijn, M.; Boonstra, A.M.; Jonkers, R.; Bastiaanse, R.; Arendzen, H.; Reinders-Messelink, H.A. Music in the treatment of neurological language and speech disorders: A systematic review. Aphasiology 2012, 26, 1–19. [Google Scholar] [CrossRef]
- Koelsch, S. A neuroscientific perspective on music therapy. Ann. N. Y. Acad. Sci. 2009, 1169, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Galińska, E. Music therapy in neurological rehabilitation settings. Psychiatr Pol. 2015, 49, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Robb, S.L.; Hanson-Abromeit, D.; May, L.; Hernandez-Ruiz, E.; Allison, M.; Beloat, A.; Daughtery, S.; Kurtz, R.; Ott, A.; Oyedele, O.O.; et al. Reporting quality of music intervention research in healthcare: A systematic review. Complement. Ther. Med. 2018, 38, 24–41. [Google Scholar] [CrossRef]
- Grau-Sánchez, J.; Jamey, K.; Paraskevopoulos, E.; Dalla Bella, S.; Gold, C.; Schlaug, G.; Belleville, S.; Rodríguez-Fornells, A.; Hackney, M.E.; Särkämö, T. Putting music to trial: Consensus on key methodological challenges investigating music-based rehabilitation. Ann. N. Y. Acad. Sci. 2022, 1518, 12–24. [Google Scholar] [CrossRef]
- Howlett, J.R.; Nelson, L.D.; Stein, M.B. Mental health consequences of traumatic brain injury. Biol. Psychiatry 2022, 91, 413–420. [Google Scholar] [CrossRef]
- McCaffrey, T. Evaluating music therapy in adult mental health services: Tuning into service user perspectives. Nord. J. Music. Ther. 2018, 27, 28–43. [Google Scholar] [CrossRef]
- Moore, K.S. A systematic review on the neural effects of music on emotion regulation: Implications for music therapy practice. J. Music. Ther. 2013, 50, 198–242. [Google Scholar] [CrossRef] [PubMed]
- Raglio, A.; Zaliani, A.; Baiardi, P.; Bossi, D.; Sguazzin, C.; Capodaglio, E.; Imbriani, C.; Gontero, G.; Imbriani, M. Active music therapy approach for stroke patients in the post-acute rehabilitation. Neurol. Sci. 2017, 38, 893–897. [Google Scholar] [CrossRef]
- Thompson, N.; Iyemere, K.; Underwood, B.R.; Odell-Miller, H. Investigating the impact of music therapy on two in-patient psychiatric wards for people living with dementia: Retrospective observational study. BJPsych Open 2023, 9, e42. [Google Scholar] [CrossRef]
- Wilson, L.; Horton, L.; Kunzmann, K.; Sahakian, B.J.; Newcombe, V.F.; Stamatakis, E.A.; von Steinbuechel, N.; Cunitz, K.; Covic, A.; Maas, A.; et al. Understanding the relationship between cognitive performance and function in daily life after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2021, 92, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Lena, F.; Modugno, N.; Greco, G.; Torre, M.; Cesarano, S.; Santilli, M.; Abdullahi, A.; Giovannico, G.; Etoom, M. Rehabilitation interventions for improving balance in Parkinson’s disease: A narrative review. Am. J. Phys. Med. Rehabil. 2023, 102, 270–274. [Google Scholar] [CrossRef]
- Brancatisano, O.; Baird, A.; Thompson, W.F. Why is music therapeutic for neurological disorders? The Therapeutic Music Capacities Model. Neurosci. Bobehav. Riv. 2020, 112, 600–615. [Google Scholar] [CrossRef]
- Leins, A.K.; Spintge, R. Music therapy in medical and neurological rehabilitation settings. In The Oxford Handbook of Music Psychology; Hallam, S., Cross, I., Thaut, M., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 526–535. [Google Scholar] [CrossRef]
- Machado Sotomayor, M.J.; Arufe-Giráldez, V.; Ruíz-Rico, G.; Navarro-Patón, R. Music therapy and Parkinson’s disease: A systematic review from 2015–2020. Int. J. Environ. Res. Public Health 2021, 18, 11618. [Google Scholar] [CrossRef]
- Magee, W.L. Why include music therapy in a neuro-rehabilitation team? ACNR 2019, 19, 10–12. [Google Scholar] [CrossRef]
- Mercier, L.J.; Langelier, D.M.; Buchanan, J.; Robinson, S.; Plamondon, S. Development and integration of a music therapy program in the neurologic inpatient setting: A qualitative study. Disabil. Rehabil. 2024, 47, 2304–2313. [Google Scholar] [CrossRef]
- Murtaugh, B.; Morrissey, A.M.; Fager, S.; Knight, H.E.; Rushing, J.; Weaver, J. Music, occupational, physical, and speech therapy interventions for patients in disorders of consciousness: An umbrella review. NeuroRehabilitation 2024, 54, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M.H. Assessment and the transformational design model (TDM). In Handbook of Neurologic Music Therapy; Thaut, M., Hoemberg, V., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 60–68. [Google Scholar]
- Breuer, E.; Lee, L.; De Silva, M.; Lund, C. Using theory of change to design and evaluate public health interventions: A systematic review. Implement. Sci. 2015, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- de L’Etoile, S.K. Processes of music therapy: Clinical and scientific rationales and models. In The Oxford handbook of music psychology, 2nd ed.; Hallam, S., Cross, I., Thaut, M., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 805–818. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N.; Roen, K.; Duffy, S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme Version; Lancaster University: Lancaster, UK, 2006. [Google Scholar] [CrossRef]
- Altenmüller, E.; Schlaug, G. Neurologic music therapy: The beneficial effects of music making on neurorehabilitation. Acoust. Sci. Technol. 2013, 34, 5–12. [Google Scholar] [CrossRef]
- Lam, H.L.; Li, W.T.V.; Laher, I.; Wong, R.Y. Effects of music therapy on patients with dementia—A systematic review. Geriatrics 2020, 5, 62–75. [Google Scholar] [CrossRef]
- Lanb, L.C.L.S.H.; Lanc, S.J.; Hsiehe, Y.P. Effectiveness of the Music Therapy in Dementia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Dement. Geriatr. Cogn. Disord. 2024, 54, 167–186. [Google Scholar] [CrossRef]
- Moreno-Morales, C.; Calero, R.; Moreno-Morales, P.; Pintado, C. Music therapy in the treatment of dementia: A systematic review and meta-analysis. Front. Med. 2020, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Dementia Quality Standard [NICE Guideline Quality Standard No. 184]. 2019. Available online: https://www.nice.org.uk/guidance/qs184 (accessed on 17 July 2025).
- NHS England. NHS Standard Contract for Specialised Rehabilitation for Patients with Highly Complex Needs (All Ages). 2014. Available online: https://www.england.nhs.uk/wp-content/uploads/2014/04/d02-rehab-pat-high-needs-0414.pdf (accessed on 17 July 2025).
- Thompson, N.; Odell-Miller, H. An audit of music therapy in acute National Health Service (NHS) settings for people with dementia in the UK and adaptations made due to COVID-19. Approaches Interdiscip. J. Music. Ther. 2024, 16, 1–16. [Google Scholar] [CrossRef]
- Segura, E.; Grau-Sánchez, J.; Cerda-Company, X.; Porto, M.F.; De la Cruz-Puebla, M.; Sanchez-Pinsach, D.; Cerquides, J.; Duarte, E.; Palumbo, A.; Turry, A.; et al. Enriched music-supported therapy for individuals with chronic stroke: A randomized controlled trial. J. Neurol. 2024, 271, 6606–6617. [Google Scholar] [CrossRef]
- Fusar-Poli, L.; Bieleninik, Ł.; Brondino, N.; Chen, X.J.; Gold, C. The effect of music therapy on cognitive functions in patients with dementia: A systematic review and meta-analysis. Aging Ment. Health 2018, 22, 1103–1112. [Google Scholar] [CrossRef]
- Zaatar, M.T.; Alhakim, K.; Enayeh, M.; Tamer, R. The transformative power of music: Insights into neuroplasticity, health, and disease. Brain Behav. Immun.-Health 2023, 35, 100716. [Google Scholar] [CrossRef]
- Chen, W.G.; Iversen, J.R.; Kao, M.H.; Loui, P.; Patel, A.D.; Zatorre, R.J.; Edwards, E. Music and Brain Circuitry: Strategies for Strengthening Evidence-Based Research for Music-Based Interventions. J. Neurosci. 2022, 42, 8498–8507. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Fernández-Company, J.F.; Pita, M.F.; Garcia-Rodriguez, M. Music therapy for adolescents with psychiatric disorders: An overview. Clin. Child. Psychol. Psychiatry 2022, 27, 895–910. [Google Scholar] [CrossRef]
- Tramontano, M.; De Angelis, S.; Mastrogiacomo, S.; Princi, A.A.; Ciancarelli, I.; Frizziero, A.; Iosa, M.; Paolucci, S.; Morone, G. Music-based techniques and related devices in neurorehabilitation: A scoping review. Expert. Rev. Med. Devices 2021, 18, 733–749. [Google Scholar] [CrossRef]
- Mishra, R.; Florez-Perdomo, W.A.; Shrivatava, A.; Chouksey, P.; Raj, S.; Moscote-Salazar, L.R.; Rahman, M.M.; Sutar, R.; Agrawal, A. Role of music therapy in traumatic brain injury: A systematic review and meta-analysis. World Neurosurg. 2021, 146, 197–204. [Google Scholar] [CrossRef]
- Odell-Miller, H. The role, function and identity of music therapists in the 21st century, including new research and thinking from a UK perspective. BJMT 2016, 30, 5–12. [Google Scholar] [CrossRef]
- Carr, C.E.; Tsiris, G.; Swijghuisen Reigersberg, M. Understanding the present, re-visioning the future: An initial mapping of music therapists in the United Kingdom. BJMT 2017, 31, 68–85. [Google Scholar] [CrossRef]
- Wood, J.; Sandford, S.; Bailey, E. ‘The whole is greater’. Developing music therapy services in the National Health Service: A case study revisited. BJMT 2016, 30, 36–46. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Stroke Rehabilitation in Adults [NICE Guideline No. 236]. 2023. Available online: https://www.nice.org.uk/guidance/ng236/chapter/Recommendations (accessed on 17 July 2025).
- Hariton, E.; Locascio, J.J. Randomised controlled trials—The gold standard for effectiveness research. BJOG 2018, 125, 1716. [Google Scholar] [CrossRef]
- García-Navarro, E.B.; Buzón-Pérez, A.; Cabillas-Romero, M. Effect of Music Therapy as a Non-Pharmacological Measure Applied to Alzheimer’s Disease Patients: A Systematic Review. Nurs. Rep. 2022, 12, 775–790. [Google Scholar] [CrossRef]
- Bleibel, M.; El Cheikh, A.; Sadier, N.S.; Abou-Abbas, L. The effect of music therapy on cognitive functions in patients with Alzheimer’s disease: A systematic review of randomized controlled trials. Alz Res. Ther. 2023, 15, 65. [Google Scholar] [CrossRef]
- Falzon, L.; Davidson, K.W.; Bruns, D. Evidence searching for evidence-based psychology practice. Prof. Psychol. Res. Pract. 2010, 41, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar] [CrossRef]
- Chiu, E.C.; Chen, Y.J.; Wu, W.C.; Chou, C.X.; Yu, M.Y. Psychometric comparisons of three depression measures for patients with stroke. AJOT 2022, 76, 7604205140. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa-Pacher, A. Research questions with PICO: A universal mnemonic. Publications 2022, 10, 21–30. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, 12–13. [Google Scholar] [CrossRef]
- Impellizzeri, F.; Maggio, M.G.; De Pasquale, P.; Bonanno, M.; Bonanno, L.; De Luca, R.; Paladina, G.; Alibrandi, A.; Milardi, D.; Thaut, M.; et al. Coupling neurologic music therapy with immersive virtual reality to improve executive functions in individuals with Parkinson’s disease: A Quasi-Randomized Clinical Trial. Clin. Park. Relat. Disord. 2024, 11, 100277. [Google Scholar] [CrossRef]
- Siponkoski, S.T.; Martínez-Molina, N.; Kuusela, L.; Laitinen, S.; Holma, M.; Ahlfors, M.; Jordan-Kilkki, P.; Ala-Kauhaluoma, K.; Melkas, S.; Pekkola, J.; et al. Music therapy enhances executive functions and prefrontal structural neuroplasticity after traumatic brain injury: Evidence from a randomized controlled trial. J. Neurotrauma 2020, 37, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Dvorak, A.L.; Manternach, J.N. Therapeutic Singing and Semi-Occluded Vocal Tract Exercises for Individuals with Parkinson’s Disease: A Randomized Controlled Trial of a Single Session Intervention. J. Music. Ther. 2024, 61, 132–167. [Google Scholar] [CrossRef]
- Haire, C.M.; Vuong, V.; Tremblay, L.; Patterson, K.K.; Chen, J.L.; Thaut, M.H. Effects of therapeutic instrumental music performance and motor imagery on chronic post-stroke cognition and affect: A randomized controlled trial. NeuroRehabilitation 2021, 48, 195–208. [Google Scholar] [CrossRef]
- van Bruggen-Rufi, M.C.; Vink, A.C.; Wolterbeek, R.; Achterberg, W.P.; Roos, R.A. The effect of music therapy in patients with Huntington’s disease: A randomized controlled trial. J. Huntington’s Dis. 2017, 6, 63–72. [Google Scholar] [CrossRef]
- Poćwierz-Marciniak, I.; Bidzan, M. The influence of music therapy on quality of life after a stroke. Health Psychol. Rep. 2017, 5, 173–185. [Google Scholar] [CrossRef]
- Blackburn, R.; Bradshaw, T. Music therapy for service users with dementia: A critical review of the literature. J. Psychiatr. Men. Health Nurs. 2014, 21, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Hoffecker, L. Grey Literature Searching for Systematic Reviews in the Health Sciences. Ser. Libr. 2020, 79, 252–260. [Google Scholar] [CrossRef]
- Kelly, J.; Sadeghieh, T.; Adeli, K. Peer review in scientific publications: Benefits, critiques, & a survival guide. J. Int. Fed. Clin. Chem. Lab. Med. 2014, 25, 227–243. [Google Scholar]
- Barrington, A. Perspectives on the development of the music therapy profession in the UK. Approaches 2015, 7, 118–122. [Google Scholar] [CrossRef]
- British Association for Music Therapy. Guidelines on Professional Titles for Music Therapists; British Association for Music Therapy: London, UK, 2020; Available online: https://www.bamt.org/music-therapy/what-is-a-music-therapist/guide-to-professional-practice (accessed on 17 July 2025).
- Chandler, G.; Maclean, E. “There has probably never been a more important time to be a music therapist”: Exploring how three music therapy practitioners working in adult mental health settings in the UK experienced the first year of the COVID-19 pandemic. Approaches 2024, 16, 288. [Google Scholar] [CrossRef]
- Helbach, J.; Pieper, D.; Mathes, T.; Rombey, T.; Zeeb, H.; Allers, K.; Hoffmann, F. Restrictions and their reporting in systematic reviews of effectiveness: An observational study. BMC Med. Res. Methodol. 2022, 22, 230. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, H.; Tsutani, K.; Yamada, M.; Park, H.; Okuizumi, H.; Tsuruoka, K.; Honda, T.; Okada, S.; Park, S.; Kitayuguchi, J.; et al. Effectiveness of music therapy: A summary of systematic reviews based on randomized controlled trials of music interventions. Patient Prefer. Adherence 2014, 8, 727–754. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Puljak, L. Language restrictions in systematic reviews should not be imposed in the search strategy but in the eligibility criteria if necessary. J. Clin. Epidemiol. 2021, 132, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Bond, C.; Lancaster, G.A.; Campbell, M.; Chan, C.; Eddy, S.; Hopewell, S.; Mellor, K.; Thabane, L.; Eldridge, S. Pilot and feasibility studies: Extending the conceptual framework. PFS 2023, 9, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.; Campbell, M.J.; Cooper, C.L.; Lancaster, G.A. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med. Res. Methodol. 2010, 10, 67. [Google Scholar] [CrossRef]
- Mayer-Benarous, H.; Benarous, X.; Vonthron, F.; Cohen, D. Music therapy for children with autistic spectrum disorder and/or other neurodevelopmental disorders: A systematic review. Front. Psychiatry 2021, 12, 643234. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; The Cochrane Collaboration: London, UK, 2019; pp. 205–228. [Google Scholar] [CrossRef]
- Nejadghaderi, S.A.; Balibegloo, M.; Rezaei, N. The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: A perspective on the pros and cons. Health Sci. Rep. 2024, 7, e2165. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Thomson, H.J.; Thomas, S. The effect direction plot: Visual display of non-standardised effects across multiple outcome domains. Res. Synth. Methods 2013, 4, 95–101. [Google Scholar] [CrossRef]
- Cohen, J. Quantitative methods in psychology: A power primer. Psychol. Bull. 1992, 112, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Bauchner, H.; Golub, R.M.; Fontanarosa, P.B. Reporting and interpretation of randomized clinical trials. JAMA 2019, 322, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Bhide, A.; Shah, P.S.; Acharya, G. A simplified guide to randomized controlled trials. AOGS 2018, 97, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Mustafa, R.A.; Schünemann, H.J.; Sultan, S.; Santesso, N. Rating the certainty in evidence in the absence of a single estimate of effect. BMJ EBM 2017, 22, 85–87. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Higgins, J.P.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H. Completing ‘summary of findings’ tables and grading the certainty of the evidence. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; The Cochrane Collaboration: London, UK, 2019; pp. 375–402. [Google Scholar] [CrossRef]
- Impellizzeri, F.; Leonardi, S.; Latella, D.; Maggio, M.G.; Foti Cuzzola, M.; Russo, M.; Sessa, E.; Bramanti, P.; De Luca, R.; Calabrò, R.S. An integrative cognitive rehabilitation using neurologic music therapy in multiple sclerosis: A pilot study. Medicine (Baltimore). 2020, 99, e18866. [Google Scholar] [CrossRef]
- Estellat, C.; Torgerson, D.J.; Ravaud, P. How to perform a critical analysis of a randomised controlled trial. Best. Pract. Res. Clin. Rheumatol. 2009, 23, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Chen, P.C.; Huang, Y.C.; Yang, T.H.; Wang, L.Y.; Chen, I.H.; Lee, H.J.; Lee, Y.Y. Neurological music therapy for poststroke depression, activity of daily living and cognitive function: A pilot randomized controlled study. Nord. J. Music. Ther. 2024, 33, 226–237. [Google Scholar] [CrossRef]
- Van Ginkel, J.R.; Linting, M.; Rippe, R.C.; Van Der Voort, A. Rebutting existing misconceptions about multiple imputation as a method for handling missing data. J. Pers. Assess. 2020, 102, 297–308. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. MICE: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Austin, P.C.; White, I.R.; Lee, D.S.; van Buuren, S. Missing data in clinical research: A tutorial on multiple imputation. Can. J. Cardiol. 2021, 37, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.; Arce, C.; Torrado, J.; Garrido, J.; De Francisco, C.; Arce, I. Factor structure and invariance of the POMS mood state questionnaire in Spanish. Span. J. Psychol. 2010, 13, 444–452. [Google Scholar] [CrossRef]
- Boutron, I.; Dutton, S.; Ravaud, P.; Altman, D.G. Reporting and interpretation of randomized controlled trials with statistically nonsignificant results for primary outcomes. JAMA 2010, 303, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.C.; Schönbrodt, F.D.; Gervais, W.M.; Hilgard, J. Correcting for bias in psychology: A comparison of meta-analytic methods. Adv. Meth Pract. Psychol. Sci. 2019, 2, 115–144. [Google Scholar] [CrossRef]
- van Bruggen-Rufi, M.; Vink, A.; Achterberg, W.; Roos, R. Music therapy in Huntington’s disease: A protocol for a multi-center randomized controlled trial. BMC Psychol. 2016, 4, 38. [Google Scholar] [CrossRef]
- Kraemer, H.C.; Mintz, J.; Noda, A.; Tinklenberg, J.; Yesavage, J.A. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch. Gen. Psychiatry 2006, 63, 484–489. [Google Scholar] [CrossRef]
- Beato, M. Recommendations for the design of randomized controlled trials in strength and conditioning. Common. Des. Data interpretation. Front. Sports Act. Living 2022, 4, 981836. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.A.; Muzzatti, B.; Bidoli, E.; Flaiban, C.; Bomben, F.; Piccinin, M.; Gipponi, K.M.; Mariutti, G.; Busato, S.; Mella, S. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support. Care Cancer 2020, 28, 3921–3926. [Google Scholar] [CrossRef]
- Costantini, M.; Musso, M.; Viterbori, P.; Bonci, F.; Del Mastro, L.; Garrone, O.; MVenturini, M.; Morasso, G. Detecting psychological distress in cancer patients: Validity of the Italian version of the Hospital Anxiety and Depression Scale. Support Care Cancer 1999, 7, 121–127. [Google Scholar] [CrossRef]
- Annell, S.; Sjöberg, A.; Sverke, M. Use and interpretation of test scores from limited cognitive test batteries: How g+ Gc can equal g. Scand. J. Psychol. 2014, 55, 399–408. [Google Scholar] [CrossRef]
- Essers, B.; Veerbeek, J.M.; Luft, A.R.; Verheyden, G. The feasibility of the adapted H-GRASP program for perceived and actual daily-life upper limb activity in the chronic phase post-stroke. Disabil. Rehabil. 2024, 46, 5815–5828. [Google Scholar] [CrossRef]
- Simpson, L.A.; Eng, J.J.; Chan, M. H-GRASP: The feasibility of an upper limb home exercise program monitored by phone for individuals post stroke. Disabil. Rehabil. 2017, 39, 874–882. [Google Scholar] [CrossRef]
- Russell, E.W.; Russell, S.L.; Hill, B.D. The fundamental psychometric status of neuropsychological batteries. Arch. Clin. Neuropsychol. 2005, 20, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Casaletto, K.B.; Heaton, R.K. Neuropsychological assessment: Past and future. JNS 2017, 23, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Neurologic Music Therapy; Thaut, M., Hoemberg, V., Eds.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- von Hippel, P.T. The heterogeneity statistic I 2 can be biased in small meta-analyses. BMC Med Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef]
- Königs, M.; Beurskens, E.A.; Snoep, L.; Scherder, E.J.; Oosterlaan, J. Effects of timing and intensity of neurorehabilitation on functional outcome after traumatic brain injury: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2018, 99, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Kern, P.; Tague, D.B. Music therapy practice status and trends worldwide: An international survey study. J. Music. Ther. 2017, 54, 255–286. [Google Scholar] [CrossRef]
- Dobran, S.A.; Gherman, A. Neurorehabilitation across continents: The WFNR-EFNR regional meeting in conjunction with the 19th congress of the society for the study of neuroprotection and neuroplasticity and the 19th international summer school of neurology in Baku, Azerbaijan. J. Med. Life 2024, 17, 825–829. [Google Scholar] [CrossRef]
- Nasios, G.; Messinis, L.; Dardiotis, E.; Sgantzos, M. Neurorehabilitation: Looking Back and Moving Forward. Healthcare 2023, 11, 1452. [Google Scholar] [CrossRef] [PubMed]
- Fernainy, P.; Cohen, A.A.; Murray, E.; Losina, E.; Lamontagne, F.; Sourial, N. Rethinking the pros and cons of randomized controlled trials and observational studies in the era of big data and advanced methods: A panel discussion. BMC Proc. 2024, 18, 1. [Google Scholar] [CrossRef] [PubMed]
| Outcome Variable | Definition for Included Studies |
|---|---|
| Cognitive function | Executive functions, memory, visuospatial abilities, attention, communication [52,53] |
| Mood | Depression, anxiety, anger, vigour, fatigue [11,33,54] |
| Emotion | Self-perceived emotional well-being, emotional awareness of self and others, sharing of emotions [33,52] |
| Self-efficacy | Sense of competence in managing new and challenging situations [55] |
| Behaviour | Communication and expressive skills, mental rigidity, aggression [56] |
| Affect | Valence, arousal, dominance [55] |
| Quality of life | An individual’s perception of their physical and mental state, and social position [57] |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| (a) Peer-reviewed original empirical RCTs | (a) Studies with dementia or spinal cord injury |
| (b) Adults with neurological conditions | (b) Studies with neurodevelopmental conditions |
| (c) MT delivered by a board-certified music therapist or a therapist skilled in delivering specific evidence-based NMT | (c) Study designs such as review, protocol, or feasibility studies |
| (d) Published between 1 January 2015–31 January 2025, | (d) Studies with qualitative or mixed methods data |
| (e) Published in the English language | (e) Studies containing non-psychological outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardener, S.H.; Mukaetova-Ladinska, E.B.; Perera, N.A. The Effect of Music Therapy on Psychological Outcomes for Neurological Conditions: A Systematic Review. Medicina 2025, 61, 1611. https://doi.org/10.3390/medicina61091611
Gardener SH, Mukaetova-Ladinska EB, Perera NA. The Effect of Music Therapy on Psychological Outcomes for Neurological Conditions: A Systematic Review. Medicina. 2025; 61(9):1611. https://doi.org/10.3390/medicina61091611
Chicago/Turabian StyleGardener, Sarah H., Elizabeta B. Mukaetova-Ladinska, and Nellinne Antoinette Perera. 2025. "The Effect of Music Therapy on Psychological Outcomes for Neurological Conditions: A Systematic Review" Medicina 61, no. 9: 1611. https://doi.org/10.3390/medicina61091611
APA StyleGardener, S. H., Mukaetova-Ladinska, E. B., & Perera, N. A. (2025). The Effect of Music Therapy on Psychological Outcomes for Neurological Conditions: A Systematic Review. Medicina, 61(9), 1611. https://doi.org/10.3390/medicina61091611






