The Impact of Sarcopenia, Myosteatosis, and Visceral Adiposity on Renal Transplantation Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Outcomes
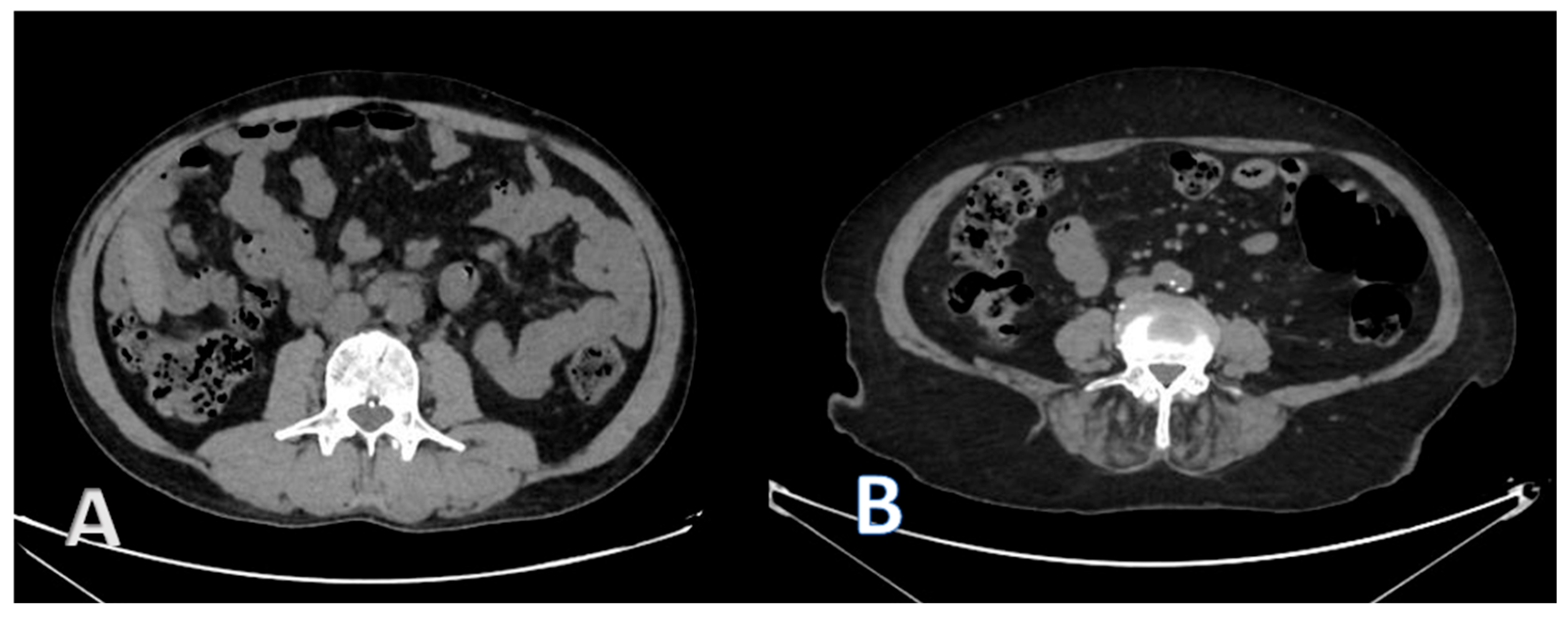
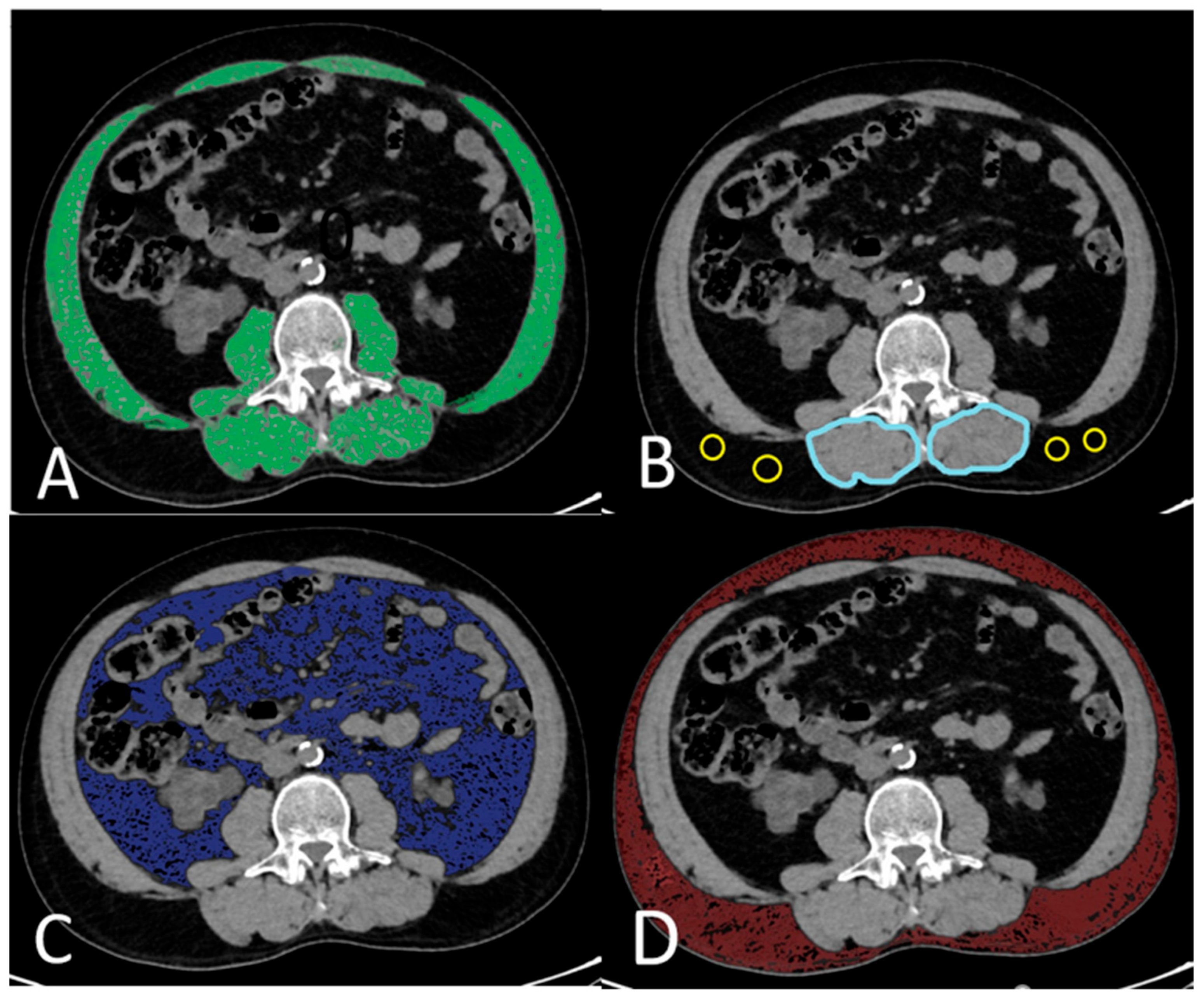
2.3. CT Image Acquisition and Analysis
2.4. Body Composition Parameters
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Visceral Adiposity
4.2. Sarcopenia
4.3. Myosteatosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RT | Renal transplantation |
| ESRF | End-stage renal failure |
| CT | Computed tomography |
| VSR | Visceral-to-subcutaneous adipose tissue ratio |
| SMI | Skeletal muscle index |
| IMAC | Intramuscular adipose tissue content |
| SMA | Skeletal muscle area |
| KTRs | Kidney transplant recipients |
| BMI | Body mass index |
| HU | Hounsfield unit |
| DM | Diabetes mellitus |
| HT | Hypertension |
| CVD | Cardiovascular diseases |
| SD | Standard deviation |
| IQR | Interquartile range |
| e-GFR | Estimated glomerular filtration rate |
| MS | Metabolic syndrome |
| VAT | Visceral adipose tissue |
References
- Kim, P.Y.; Shoghi, A.; Fananapazir, G. Renal transplantation: Immediate and late complications. Radiol. Clin. 2023, 61, 809–820. [Google Scholar] [CrossRef]
- El-Zoghby, Z.M.; Stegall, M.D.; Lager, D.J.; Kremers, W.K.; Amer, H.; Gloor, J.M.; Cosio, F.G.; Stegall, M.D. Identifying specific causes of kidney allograft loss. Am. J. Transplant. 2009, 9, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International clinical practice guidelines for sarcopenia (ICFSR): Screening, diagnosis and management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Gandolfini, I.; Regolisti, G.; Bazzocchi, A.; Maggiore, U.; Palmisano, A.; Piotti, G.; Fiaccadori, E.; Sabatino, A. Frailty and sarcopenia in older patients receiving kidney transplantation. Front. Nutr. 2019, 6, 169. [Google Scholar] [CrossRef]
- Correa-De-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the context of skeletal muscle function deficit: An interdisciplinary workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef]
- Deliège, P.-G.; Braconnier, A.; Chaix, F.; Renard, Y.; Petrache, A.; Guyot-Colosio, C.; Kazes, I.; Mokri, L.; Barbe, C.; Rieu, P. Skeletal muscle index as a prognostic marker for kidney transplantation in older patients. J. Ren. Nutr. 2021, 31, 286–295. [Google Scholar] [CrossRef]
- Druckmann, I.; Yashar, H.; Schwartz, D.; Schwartz, I.F.; Goykhman, Y.; Ben-Bassat, O.K.; Baruch, R.; Tzadok, R.; Shashar, M.; Cohen-Hagai, K.; et al. Presence of sarcopenia before kidney transplantation is associated with poor outcomes. Am. J. Nephrol. 2022, 53, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Karakizlis, H.; Trudel, N.; Brose, A.; Reinisch, A.; Reichert, M.; Hecker, A.; Bender, F.; Askevold, I.; Rainer, L.; Weimer, R.; et al. Sarcopenia of kidney transplant recipients as a predictive marker for reduced graft function and graft survival after kidney transplantation. Langenbeck’s Arch. Surg. 2023, 408, 103. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, W.; Zou, M.; Zeng, Q.; Feng, Y.; Luo, Z.; Gan, H. Diagnosis, prevalence, and outcomes of sarcopenia in kidney transplantation recipients: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Tabourin, T.; Pinar, U.; Cassagnes, L.; Boirie, Y.; Heng, A.-E.; Guandalino, M.; Guy, L. The role of CT-scan assessment of muscle mass in predicting postoperative surgical complications after renal transplantation. Int. Urol. Nephrol. 2022, 54, 517–523. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura , S.; Kobayashi, A.; Shirai, H.; Yagi, S.; Kamo, N.; Okajima, H.; Uemoto, S. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation 2017, 101, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes 2006, 55, 1537–1545. [Google Scholar] [CrossRef]
- Li, C.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Hamed, M.O.; Chen, Y.; Pasea, L.; Watson, C.J.; Torpey, N.; Bradley, J.A.; Pettigrew, G.; Saeb-Parsy, K. Early graft loss after kidney transplantation: Risk factors and consequences. Am. J. Transplant. 2015, 15, 1632–1643. [Google Scholar] [CrossRef]
- Bahl, D.; Haddad, Z.; Datoo, A.; Qazi, Y.A. Delayed graft function in kidney transplantation. Curr. Opin. Organ. Transplant. 2019, 24, 82–86. [Google Scholar] [CrossRef]
- Chavent, B.; Maillard, N.; Boutet, C.; Albertini, J.-N.; Duprey, A.; Favre, J.-P. Prognostic value of aortoiliac calcification score in kidney transplantation recipients. Ann. Vasc. Surg. 2017, 44, 245–252. [Google Scholar] [CrossRef]
- Benjamens, S.; Alghamdi, S.Z.; Rijkse, E.; Velde-Keyzer, C.A.T.; Berger, S.P.; Moers, C.; de Borst, M.H.; Slart, R.H.J.A.; Dor, F.J.M.F.; Minnee, R.C.; et al. Aorto-iliac artery calcification and graft outcomes in kidney transplant recipients. J. Clin. Med. 2021, 10, 325. [Google Scholar] [CrossRef]
- LaGuardia, H.; Zhang, R. Obesity and metabolic syndrome in kidney transplantation. Curr. Hypertens. Rep. 2013, 15, 215–223. [Google Scholar] [CrossRef]
- Abraham, T.M.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Fox, C.S. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation 2015, 132, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Ladeiras-Lopes, R.; Sampaio, F.; Bettencourt, N.; Fontes-Carvalho, R.; Ferreira, N.; Leite-Moreira, A.; Gama, V. The ratio between visceral and subcutaneous abdominal fat assessed by computed tomography is an independent predictor of mortality and cardiac events. Rev. Esp. Cardiol 2017, 70, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, S.; Yoon, Y.; Huh, K.; Kim, M.; Kim, S.; Kim, Y.; Han, W. Impact of the ratio of visceral to subcutaneous adipose tissue in donor nephrectomy patients. Transplant. Proc. 2017, 49, 940–943. [Google Scholar] [CrossRef]
- Gómez-Hernández, A.; Beneit, N.; Díaz-Castroverde, S.; Escribano, Ó. Differential role of adipose tissues in obesity and related metabolic and vascular complications. Int. J. Endocrinol. 2016, 2016, 1216783. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, Y.; Sadahira, T.; Araki, M.; Maruyama, Y.; Wada, K.; Tanimoto, R.; Kobayashi, Y.; Watanabe, M.; Watanabe, T.; Nasu, Y. Clinical impact of abdominal fat distribution measured by 3-D computed tomography volumetry on post-transplant renal function in recipients after living kidney transplantation: A retrospective study. Clin. Exp. Nephrol. 2019, 23, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Keddar, M.; Muylle, T.; Carrie, E.; Trefois, P.; Nachit, M.; Crott, R.; Christiaens, C.; Bammens, B.; Jadoul, M.; Goffin, E.; et al. Non-invasive quantification of fat deposits in skeletal muscle predicts cardiovascular outcome in kidney failure. Front. Physiol. 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Cheema, B.; Abas, H.; Smith, B.; O’Sullivan, A.J.; Chan, M.; Patwardhan, A.; Kelly, J.; Gillin, A.; Pang, G.; Lloyd, B.; et al. Investigation of skeletal muscle quantity and quality in end-stage renal disease. Nephrology 2010, 15, 454–463. [Google Scholar] [CrossRef]
- Morel, A.; Ouamri, Y.; Canouï-Poitrine, F.; Mulé, S.; Champy, C.M.; Ingels, A.; Audard, V.; Luciani, A.; Grimbert, P.; Matignon, M.; et al. Myosteatosis as an independent risk factor for mortality after kidney allograft transplantation: A retrospective cohort study. J. Cachexia Sarcopenia Muscle 2022, 13, 386–396. [Google Scholar] [CrossRef]
- Dolan, R.D.; Almasaudi, A.S.; Dieu, L.B.; Horgan, P.G.; McSorley, S.T.; McMillan, D.C. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 111–122. [Google Scholar] [CrossRef]
- Czigany, Z.; Kramp, W.; Lurje, I.; Miller, H.; Bednarsch, J.; Lang, S.A.; Ulmer, T.F.; Bruners, P.; Strnad, P.; Trautwein, C.; et al. The role of recipient myosteatosis in graft and patient survival after deceased donor liver transplantation. J. Cachexia Sarcopenia Muscle 2021, 12, 358–367. [Google Scholar] [CrossRef]
- Looijaard, W.G.P.M.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.J.; Twisk, J.W.R.; Straaten, H.M.O.-V.; Weijs, P.J.M. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit. Care 2016, 20, 386. [Google Scholar] [CrossRef]
- Larsen, B.; Bellettiere, J.; Allison, M.; McClelland, R.L.; Miljkovic, I.; Vella, C.A.; Ouyang, P.; De-Guzman, K.R.; Criqui, M.; Unkart, J. Muscle area and density and risk of all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. Metabolism 2020, 111, 154321. [Google Scholar] [CrossRef]
- Donato, B.; Almeida, R.; Raimundo, M.; Velho, S.; Primitivo, A.; Correia, F.; Falcão, L.; Teixeira, C.; Silva, S.; Almeida, E. Myosteatosis: An underrecognized risk factor for mortality in non-dialysis chronic kidney disease patients. J. Nephrol. 2024, 37, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Li, C.; Song, T. Myosteatosis is associated with poor survival after kidney transplantation: A large retrospective cohort validation. Abdom. Radiol. 2024, 49, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Cordeiro, A.C.; Prado, C.M.; Lindholm, B.; Stenvinkel, P.; Avesani, C.M. Myosteatosis is associated with adiposity, metabolic derangements and mortality in patients with chronic kidney disease. Eur. J. Clin. Nutr. 2025, 79, 475–483. [Google Scholar] [CrossRef] [PubMed]
| SMI | IMAC | VSR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 94) | Low (n = 24) | Normal (n = 70) | p | High (n = 35) | Normal (n = 59) | p | High (n = 28) | Normal (n = 66) | p | |
| Recipient age Mean (SD) | 42.69 (±12.47) | 41.29 (±12.08) | 43.17 (±12.66) | 0.527 * | 47.6 (±10.80) | 39.78 (±12.56) | 0.003 * | 46.25 (±10.03) | 41.18 (±13.15) | 0.071 * |
| Gender | ||||||||||
| Female n (%) | 43 (45.7%) | 10 (58.3%) | 33 (47.1%) | 0.642 ** | 15 (42.9%) | 28 (47.5%) | 0.665 ** | 13 (46.4%) | 30 (45.5%) | 0.931 ** |
| Male n (%) | 51 (54.3%) | 14 (41.7%) | 37 (52.9%) | 20 (57.1%) | 31 (52.5%) | 15 (53.6%) | 36 (54.5%) | |||
| BMI Median (IQR) | 23.59 (20.56–26.81) | 21.48 (19.32–23.25) | 24.45 (21.96–27.88) | 0.001 *** | 24.24 (22.56–27.78) | 22.65 (20.22–25.36) | 0.042 *** | 23.89 (21.81–27.29) | 23.15 (20.31–26.56) | 0.329 *** |
| Dialysis | ||||||||||
| Pre-emptive n (%) | 16 (17%) | 3 (12.5%) | 13 (18.6%) | 0.931 **** | 3 (8.6%) | 13 (22%) | 0.191 ** | 3 (10.7%) | 13 (19.7%) | 0.433 **** |
| Hemodialysis n (%) | 66 (70.2%) | 18 (75%) | 48 (68.6%) | 26 (74.3%) | 40 (67.8%) | 20 (71.4%) | 46 (69.7%) | |||
| Peritoneal Dialysis n (%) | 12 (12.8%) | 3 (12.5%) | 9 (12.9%) | 6 (10.2%) | 6 (17.1%) | 5 (17.9%) | 7 (10.6%) | |||
| Dialysis time (month) (n = 70) Median (IQR) | 36 (7.5–156) | 96 (13–132) | 36 (6–156) | 0.565 *** | 120 (36–180) | 15.5 (4–132) | 0.003 *** | 120 (5–156) | 36 (10–156) | 0.599 *** |
| Type of donation | ||||||||||
| Living n (%) | 57 (60.6%) | 11 (45.8%) | 46 (65.7%) | 0.085 ** | 16 (45.7%) | 41 (69.5%) | 0.023 ** | 13 (46.4%) | 44 (66.7%) | 0.066 ** |
| Cadaveric n (%) | 37 (39.4%) | 13 (54.2%) | 24 (34.3%) | 19 (54.3%) | 18 (30.5%) | 15 (53.6%) | 22 (33.3%) | |||
| Cold ischemia (min) Median (IQR) | 130 (110–840) | 430 (115–855) | 110 (130–810) | 0.572 *** | 660 (120–930) | 130 (110–690) | 0.03 *** | 645 (120–915) | 125 (110–720) | 0.036 *** |
| SMI | IMAC | VSR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 94) | Low (n = 24) | Normal (n = 70) | p | High (n = 35) | Normal (n = 59) | p | High (n = 28) | Normal (n = 66) | p | |
| Short-Term Graft Outcomes | ||||||||||
| Acute rejection n (%) | 26 (27.7%) | 6 (25.0%) | 20 (28.6%) | 0.736 * | 11 (31.4%) | 15 (25.4%) | 0.529 * | 6 (21.4%) | 20 (30.3%) | 0.379 * |
| Early graft failure n (%) | 6 (6.4%) | 2 (8.3%) | 4 (5.7%) | 0.651 * | 5 (14.3%) | 1 (1.7%) | 0.026 ** | 1 (3.6%) | 5 (7.6%) | 0.665 ** |
| Delayed graft function n (%) | 10 (10.6%) | 4 (16.7%) | 6 (8.6%) | 0.271 ** | 8 (22.9%) | 2 (3.4%) | 0.005 ** | 5 (17.9%) | 5 (7.6%) | 0.157 ** |
| Long-term Graft Outcomes | ||||||||||
| Graft function decline (n = 86) n (%) | 17 (19.8%) | 5 (22.7%) | 12 (18.8%) | 0.759 ** | 5 (17.9%) | 12 (20.7%) | 1 ** | 8 (30.8%) | 9 (15.0%) | 0.092 * |
| Death with a functioning graft n (%) | 5 (5.3%) | 0 (0.0%) | 5 (7.1%) | 0.324 ** | 2 (5.7%) | 3 (5.1%) | 1 ** | 2 (7.1%) | 3 (4.5%) | 0.632 ** |
| Death-censored graft failure n (%) | 10 (10.6%) | 3 (12.5%) | 7 (10.0%) | 0.712 ** | 7 (20.0%) | 3 (5.1%) | 0.036 ** | 3 (10.7%) | 7 (10.6%) | 1 ** |
| Overall graft failure n (%) | 15 (16.0%) | 3 (12.5%) | 12 (17.1%) | 0.753 ** | 9 (25.7%) | 6 (10.2%) | 0.047 ** | 5 (17.9%) | 10 (15.2%) | 0.763 ** |
| Mortality (1 year) (n = 78) n (%) | 4 (5.1%) | 1 (5.3%) | 3 (5.1%) | 1 ** | 4 (14.8%) | 0 (0.0%) | 0.012 ** | 1 (5.3%) | 3 (5.1%) | 1 ** |
| Mortality (Overall) n (%) | 10 (10.6%) | 2 (8.3%) | 8 (11.4%) | 1 ** | 6 (17.1%) | 4 (6.8%) | 0.166 ** | 4 (14.3%) | 6 (9.1%) | 0.478 ** |
| Early Graft Failure | ||||
| Univariate OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
| Low-SMI vs. Normal SMI | 1.5 (0.3–8.6) | 0.652 | - | |
| High-IMAC vs. Normal IMAC | 9.7 (1.1–86.5) | 0.042 | 1.23 (0.1–22.9) * | 0.888 |
| High VSR vs. Normal VSR | 2.2 (0.2–19.9) | 0.478 | - | |
| Delayed graft function | ||||
| Low-SMI vs. Normal SMI | 2.1 (0.5–8.3) | 0.275 | - | |
| High-IMAC vs. Normal IMAC | 8.4 (1.7–42.5) | 0.010 | 5.5 (0.9–34.2) ** | 0.065 |
| High VSR vs. Normal VSR | 2.7 (0.7–10.1) | 0.150 | 2.6 (0.5–13.3) ** | 0.256 |
| Death-censored graft failure | ||||
| Low-SMI vs. Normal SMI | 1.3 (0.3–5.4) | 0.732 | - | |
| High-IMAC vs. Normal IMAC | 4.7 (1.1–19.4) | 0.034 | 2.8 (0.5–17.2) *** | 0.271 |
| High VSR vs. Normal VSR | 1.1 (0.2–4.2) | 0.988 | - | |
| Overall graft failure | ||||
| Low-SMI vs. Normal SMI | 0.7 (0.2–2.7) | 0.594 | ||
| High-IMAC vs. Normal IMAC | 3.1 (0.9–9.5) | 0.054 | 2.2 (0.5–9.3) **** | 0.317 |
| High VSR vs. Normal VSR | 1.2 (0.4–4.0) | 0.743 | ||
| Mortality (1 year) (n = 78) | ||||
| Low-SMI vs. Normal SMI | 0.9 (0.1–9.8) | 0.980 | - | |
| High-IMAC vs. Normal IMAC | 280.9 (0.1–486.2) | 0.997 | - | |
| High VSR vs. Normal VSR | 0.8 (0.8–7.8) | 0.831 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olcucuoglu, E.; Ozkaya, U.E.; Polat, M.E.; Yılmaz, M.; Tastemur, S.; Okten, R.S.; Olcucuoglu, E. The Impact of Sarcopenia, Myosteatosis, and Visceral Adiposity on Renal Transplantation Outcomes. Medicina 2025, 61, 1608. https://doi.org/10.3390/medicina61091608
Olcucuoglu E, Ozkaya UE, Polat ME, Yılmaz M, Tastemur S, Okten RS, Olcucuoglu E. The Impact of Sarcopenia, Myosteatosis, and Visceral Adiposity on Renal Transplantation Outcomes. Medicina. 2025; 61(9):1608. https://doi.org/10.3390/medicina61091608
Chicago/Turabian StyleOlcucuoglu, Esin, Utku Eren Ozkaya, Muhammed Emin Polat, Mehmet Yılmaz, Sedat Tastemur, Rıza Sarper Okten, and Erkan Olcucuoglu. 2025. "The Impact of Sarcopenia, Myosteatosis, and Visceral Adiposity on Renal Transplantation Outcomes" Medicina 61, no. 9: 1608. https://doi.org/10.3390/medicina61091608
APA StyleOlcucuoglu, E., Ozkaya, U. E., Polat, M. E., Yılmaz, M., Tastemur, S., Okten, R. S., & Olcucuoglu, E. (2025). The Impact of Sarcopenia, Myosteatosis, and Visceral Adiposity on Renal Transplantation Outcomes. Medicina, 61(9), 1608. https://doi.org/10.3390/medicina61091608






