Clostridium difficile Colonization in Oncologic Patients Undergoing Major Abdominopelvic Surgery: To Treat or Not to Treat? An Observational Study with Propensity Score Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Inclusion and Exclusion Criteria
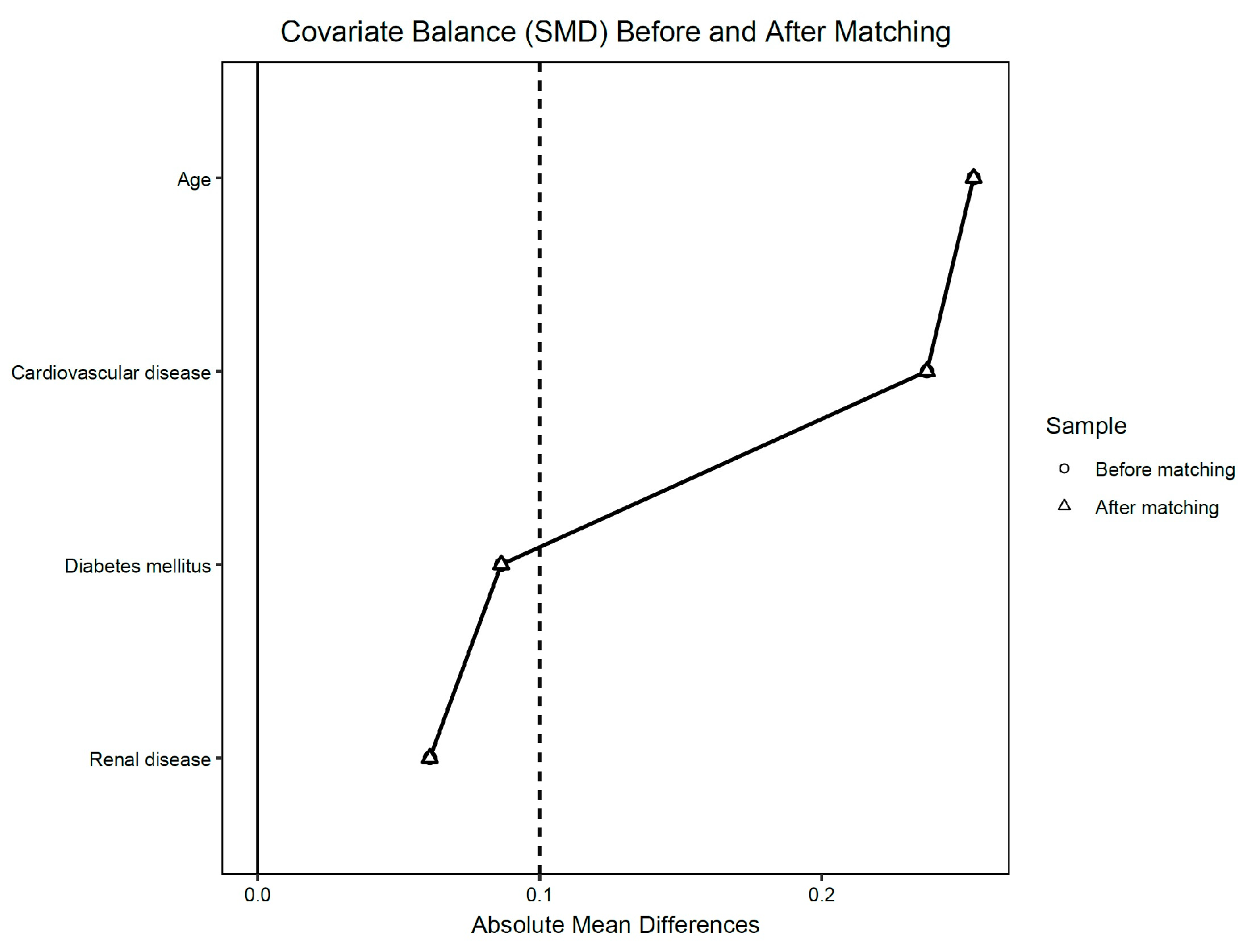
2.3. Data Analysis
3. Results
3.1. Cohort Characteristics
3.2. CDC Group
3.3. Preoperative Characteristics
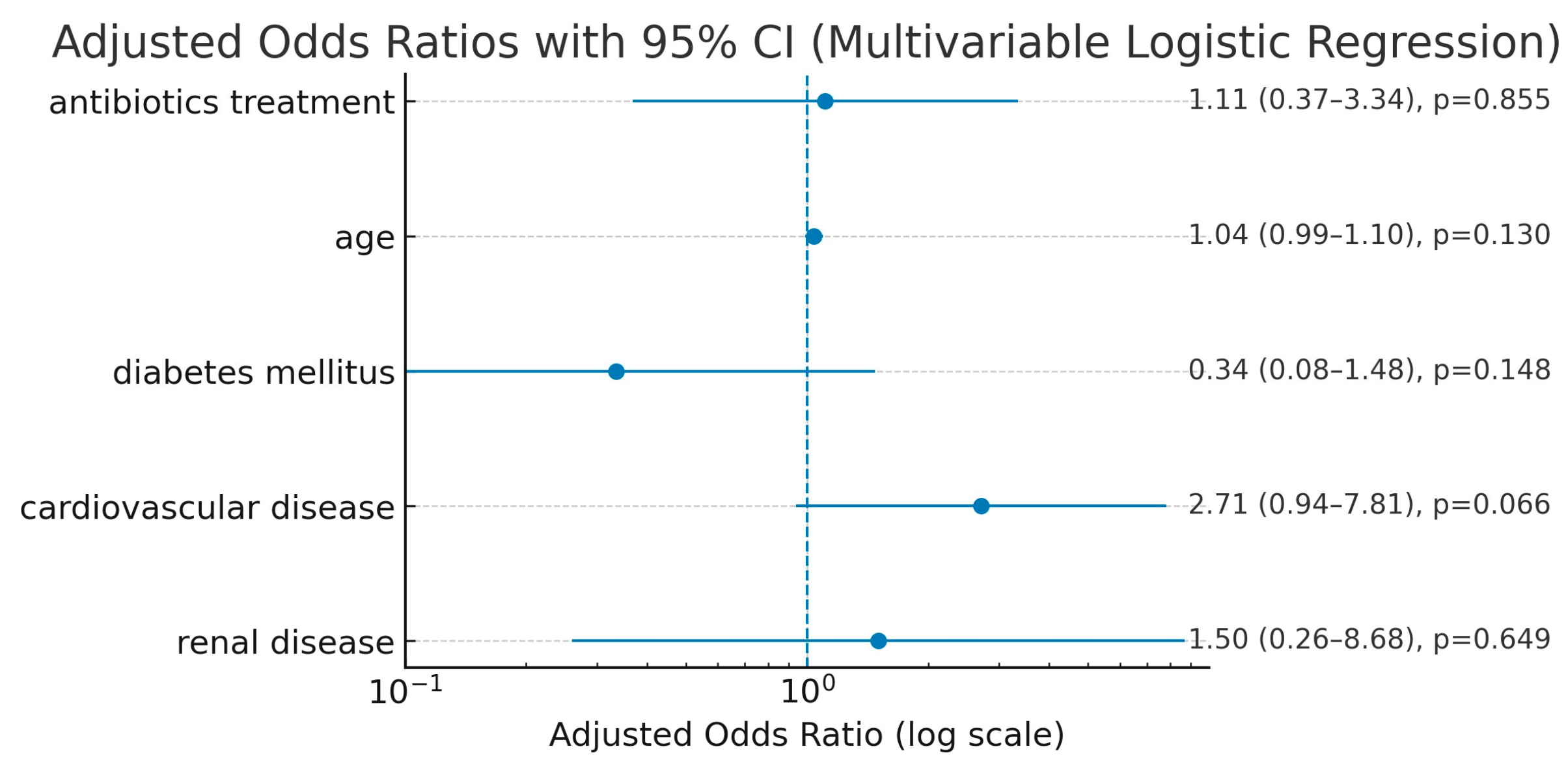
3.4. Postoperative Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under Curve |
| CDI | Clostridium difficile Infection (active infection) |
| CDC | Clostridium difficile Colonization |
| CI | Confidence Interval |
| CKD | Chronic Kidney Disease |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-Reactive Protein |
| GDH | Glutamate Dehydrogenase |
| HA-CDI | Hospital-Acquired Acute Clostridium difficile Infection |
| Hb | Hemoglobin |
| IHD | Ischemic Heart Disease |
| LOS | Length of Hospital Stay |
| MDROs | Multidrug-Resistant Organisms |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| OR | Odds Ratio |
| PAD | Peripheral Arterial Disease |
| PSM | Propensity Score Matching |
| QDS | Four times a day (Quater die sumendum) |
| ROC | Receiver Operating Characteristic |
| SSI | Surgical Site Infection |
| VRE | Vancomycin-Resistant Enterococcus |
References
- Lourenço, L.C.; Prosty, C.; Huttner, A.; Lee, T.C.; McDonald, E.G. Approach to Clostridioides difficile Diarrheal Infection. CMI Commun. 2025, 2, 105079. [Google Scholar] [CrossRef]
- CDC. C. diff: Facts for Clinicians. C. diff (Clostridioides difficile). Available online: https://www.cdc.gov/c-diff/hcp/clinical-overview/index.html (accessed on 2 August 2025).
- Puerta-Alcalde, P.; O’Keefe, J.; Woolstencroft, R.; Kaul, S.; López, N.; Cronin, K.; Lim, A.; Garcia-Pouton, N.; Álvarez, M.; Chee, L.; et al. Clostridioides difficile Infection and Recurrence in Cancer Patients (CIRCA): A Multicentre, International Study. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2025, 153, 107785. [Google Scholar] [CrossRef]
- Abughanimeh, O.; Qasrawi, A.; Kaddourah, O.; Al Momani, L.; Abu Ghanimeh, M. Clostridium difficile Infection in Oncology Patients: Epidemiology, Pathophysiology, Risk Factors, Diagnosis, and Treatment. Hosp. Pract. 1995 2018, 46, 266–277. [Google Scholar] [CrossRef]
- Kamthan, A.G.; Bruckner, H.W.; Hirschman, S.Z.; Agus, S.G. Clostridium difficile Diarrhea Induced by Cancer Chemotherapy. Arch. Intern. Med. 1992, 152, 1715–1717. [Google Scholar] [CrossRef]
- Hess, A.; Byerly, S.; Lenart, E.; Evans, C.; Kerwin, A.; Filiberto, D. Risk Factors for Clostridium difficile Infection in General Surgery Patients. Am. J. Surg. 2023, 225, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.L.; Morarasu, S.; Lunca, S.; Pruna, R.M.; Roata, C.E.; Dimofte, G.M. Impact of Clostridium difficile Infection Versus Colonization on Postoperative Outcomes After Oncological Colorectal Surgery: An Observational Single-Center Study With Propensity Score Analysis. J. Surg. Oncol. 2025, 131, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.J.; Taljaard, M.; Oake, N.; Wilson, K.; Roth, V.; van Walraven, C. The Effect of Hospital-Acquired Infection with Clostridium difficile on Length of Stay in Hospital. CMAJ Can. Med. Assoc. J. 2012, 184, 37–42. [Google Scholar] [CrossRef]
- Cohen, B.; Levine, M.; Kamboj, M. Clostridium difficile Infection Following Outpatient Cancer Surgery. Infect. Control Hosp. Epidemiol. 2019, 40, 1440–1441. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Stanhope, S.; Sugitani, T. Excess Length of Hospital Stay, Mortality and Cost Attributable to Clostridioides (Clostridium) difficile Infection and Recurrence: A Nationwide Analysis in Japan. Epidemiol. Infect. 2020, 148, e65. [Google Scholar] [CrossRef]
- Johnson, S.; Homann, S.R.; Bettin, K.M.; Quick, J.N.; Clabots, C.R.; Peterson, L.R.; Gerding, D.N. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann Intern Med. 1992, 117, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Salvati, F.; Catania, F.; Murri, R.; Fantoni, M.; Torti, C. Clostridioides difficile infection: An update. Infez Med. 2024, 32, 280–291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mada, P.K.; Alam, M.U. Clostridioides difficile Infection. [Updated 10 April 2024]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bae, J.; Park, E.Y.; Kim, U.; Kim, J.; Kim, J.H.; Chun, J.Y.; Choi, Y.J.; Lim, M.C. Impact of Clostridium difficile Infection on Chemotherapy in Patients With Primary Ovarian Cancer. J. Korean Med. Sci. 2025, 40, e179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ziegler, M.J.; Anesi, J.; Tolomeo, P.; Glaser, L.; Cowden, L.; Cressman, L.; Huang, E.; Patel, A.; Lautenbach, E.; Pegues, D.A.; et al. Screening and Targeted Prophylaxis for Clostridioides difficile Infection: STOP-CDI. Clin. Microbiol. Infect. 2025. Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Iacob, S.; Morarasu, S.; Roata, C.E.; Musina, A.-M.; Bargaoanu, R.; Ong, W.L.; Baboi, B.; Lunca, S.; Dimofte, G.M. Splenectomy Does Not Increase Early Morbidity in Patients Undergoing Total Gastrectomy: A Case-Control Study with Propensity Score Analysis. Chir. Buchar. Rom. 1990 2023, 118, 464–469. [Google Scholar] [CrossRef]
- Lunca, S.; Morarasu, S.; Zaharia, R.; Ivanov, A.-A.; Clancy, C.; O’Brien, L.; Ong, W.L.; Dimofte, G.-M. Can We Achieve More with Less? Parenchymal Sparing Surgery Versus Major Liver Resection for Colorectal Liver Metastases: An Observational Single‐Center Study with Propensity Score Analysis. Diagn 2025, 15, 1334. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Lebwohl, B.; Cuaresma, E.; Cadwell, K.; Green, P.H.R.; Freedberg, D.E. Gut Colonization with Vancomycin-Resistant Enterococcus and Risk for Subsequent Enteric Infection. Gut Pathog. 2018, 10, 28. [Google Scholar] [CrossRef]
- Sethi, A.K.; Al-Nassir, W.N.; Nerandzic, M.M.; Donskey, C.J. Skin and Environmental Contamination with Vancomycin-Resistant Enterococci in Patients Receiving Oral Metronidazole or Oral Vancomycin Treatment for Clostridium Difficile-Associated Disease. Infect. Control Hosp. Epidemiol. 2009, 30, 13–17. [Google Scholar] [CrossRef]
- Al-Nassir, W.N.; Sethi, A.K.; Li, Y.; Pultz, M.J.; Riggs, M.M.; Donskey, C.J. Both Oral Metronidazole and Oral Vancomycin Promote Persistent Overgrowth of Vancomycin-Resistant Enterococci during Treatment of Clostridium difficile-Associated Disease. Antimicrob. Agents Chemother. 2008, 52, 2403–2406. [Google Scholar] [CrossRef]
- Fishbein, S.R.S.; Hink, T.; Reske, K.A.; Cass, C.; Struttmann, E.; Iqbal, Z.H.; Seiler, S.; Kwon, J.H.; Burnham, C.A.; Dantas, G.; et al. Randomized Controlled Trial of Oral Vancomycin Treatment in Clostridioides difficile-Colonized Patients. mSphere 2021, 6, e00936-20. [Google Scholar] [CrossRef]
- Walker, A.S.; Eyre, D.W.; Wyllie, D.H.; Dingle, K.E.; Harding, R.M.; O’Connor, L.; Griffiths, D.; Vaughan, A.; Finney, J.; Wilcox, M.H.; et al. Characterisation of Clostridium difficile Hospital Ward-Based Transmission Using Extensive Epidemiological Data and Molecular Typing. PLoS Med. 2012, 9, e1001172. [Google Scholar] [CrossRef] [PubMed]
- Crobach, M.J.T.; Vernon, J.J.; Loo, V.G.; Kong, L.Y.; Péchiné, S.; Wilcox, M.H.; Kuijper, E.J. Understanding Clostridium Difficile Colonization. Clin. Microbiol. Rev. 2018, 31, e00021-17. [Google Scholar] [CrossRef]
- Steele, S.R.; McCormick, J.; Melton, G.B.; Paquette, I.; Rivadeneira, D.E.; Stewart, D.; Buie, W.D.; Rafferty, J. Practice Parameters for the Management of Clostridium difficile Infection. Dis. Colon Rectum 2015, 58, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Na, X.; Kelly, C. Probiotics in Clostridium difficile Infection. J. Clin. Gastroenterol. 2011, 45 (Suppl. S8), S154–S158. [Google Scholar] [CrossRef]
- Gaisawat, M.B.; MacPherson, C.W.; Tremblay, J.; Piano, A.; Iskandar, M.M.; Tompkins, T.A.; Kubow, S. Probiotic Supplementation in a Clostridium Difficile-Infected Gastrointestinal Model Is Associated with Restoring Metabolic Function of Microbiota. Microorganisms 2019, 8, 60. [Google Scholar] [CrossRef]
- Kukla, M.; Adrych, K.; Dobrowolska, A.; Mach, T.; Reguła, J.; Rydzewska, G. Guidelines for Clostridium difficile Infection in Adults. Przeglပd Gastroenterol. 2020, 15, 1–21. [Google Scholar] [CrossRef]
- Zacharioudakis, I.M.; Zervou, F.N.; Pliakos, E.E.; Ziakas, P.D.; Mylonakis, E. Colonization with toxinogenic C. difficile upon hospital admission, and risk of infection: A systematic review and meta-analysis. Am. J. Gastroenterol. 2015, 110, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.R.; Hecker, M.T.; O'Hagan, J.; Kutty, P.K.; Alhmidi, H.; Ng-Wong, Y.K.; Cadnum, J.L.; Jencson, A.L.; Gonzalez-Orta, M.; Saldana, C.; et al. Natural History of Clostridioides difficile Colonization and Infection Following New Acquisition of Carriage in Healthcare Settings: A Prospective Cohort Study. Clin. Infect. Dis. 2023, 77, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Di Bella, S.; McFarland, L.V.; Khanna, S.; Furuya-Kanamori, L.; Abuzeid, N.; Abu-Zidan, F.M.; Ansaloni, L.; Augustin, G.; Bala, M.; et al. 2019 update of the WSES guidelines for management of Clostridioides (Clostridium) difficile infection in surgical patients. World J. Emerg. Surg. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am. J. Gastroenterol. 2021, 116, 1124–1147. [Google Scholar] [CrossRef]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.T.D.; Ferraz, Á.A.B.; Barchi, L.C.; Boin, I.D.F.S.F. Antibiotic prophylaxis for abdominal surgery: When to recommend? Brazilian college of digestive surgery position paper. ABCD Arq. Bras. Cir. Dig. São Paulo 2023, 36, e1758. [Google Scholar] [CrossRef] [PubMed]

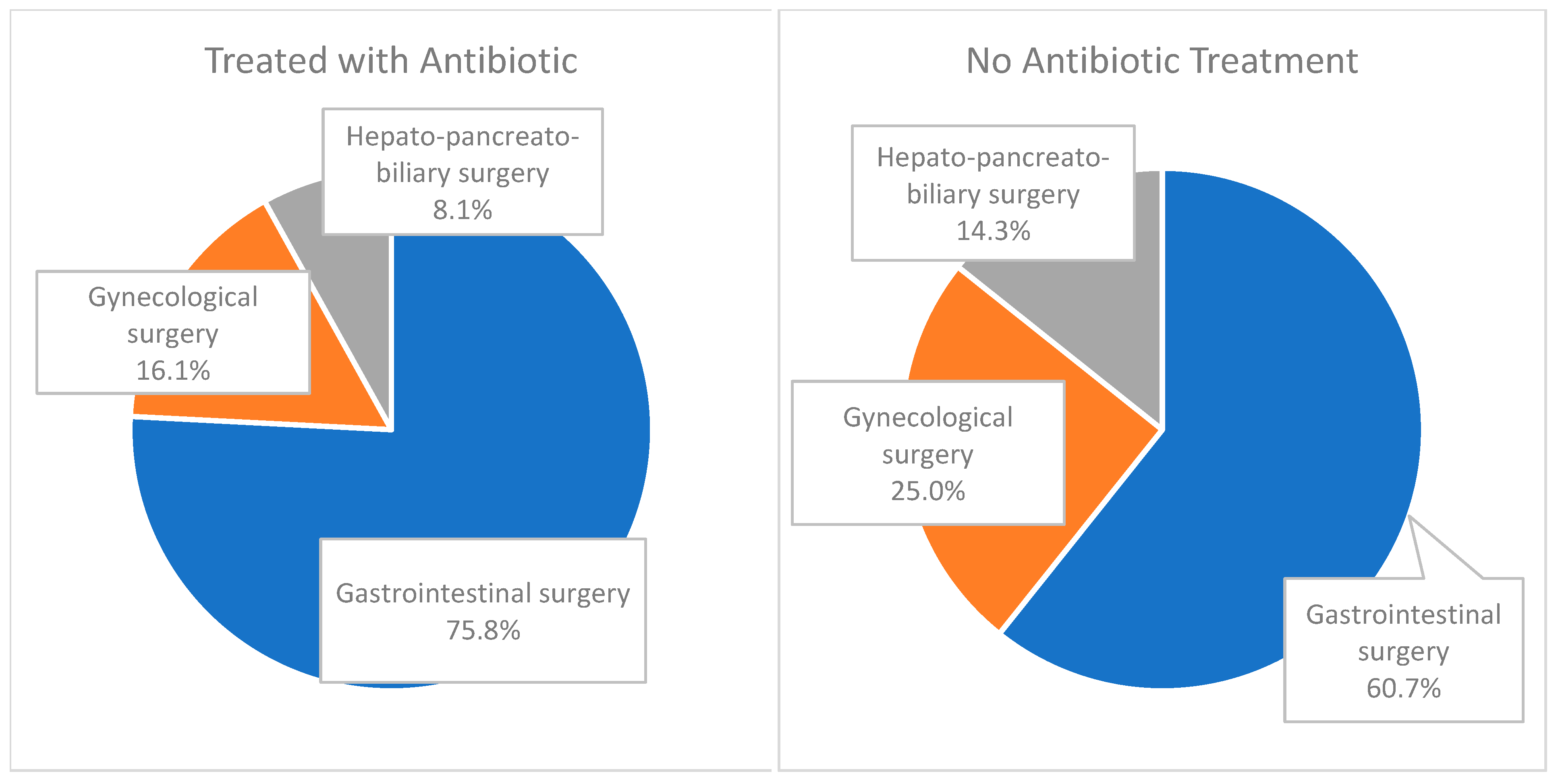
| Treated with Antibiotic n (%) | No Antibiotic Treatment n (%) | Total | |
|---|---|---|---|
| Total | 62 (100) | 28 (100) | 90 (100) |
| Gastrointestinal surgery | 47 (75.8) | 17 (60.7) | 64 (71.1) |
| Gynecological surgery | 10 (16.1) | 7 (25.0) | 17 (18.8) |
| Hepato-pancreato-biliary surgery | 5 (8.1) | 4 (14.3) | 9 (10.0) |
| BEFORE PSM | |||
|---|---|---|---|
| Treated with Antibiotic n (%)/Mean (SD) | No Antibiotic Treatment n (%)/Mean (SD) | p Value | |
| Total | 62 (100) | 28 (100) | |
| Gender (males) | 32 (51.6) | 9 (32.1) | p = 0.110 |
| Age, mean (SD) | 64.9 (12.8) | 67.9 (10.0) | p = 0.224 |
| Diabetes | 12 (19.4) | 3 (10.7) | p = 0.375 |
| IHD | 46 (74.2) | 15 (53.6) | p = 0.086 |
| COPD | 5 (8.1) | 0 (0) | p = 0.319 |
| CKD | 6 (9.7) | 1 (3.6) | p = 0.428 |
| PAD | 0 (0) | 0 (0) | - |
| Albumin (g/dL) | 4.2 (0.7) | 4.2 (0.5) | p = 0.512 |
| Hb (g/dL) | 11.3 (2.2) | 11.8 (2.1) | p = 0.318 |
| CRP (mg/dL) | 37.5 (62.0) | 49.8 (83.3) | p = 0.661 |
| Creatinine (mg/dL) | 0.9 (0.5) | 0.8 (0.1) | p = 0.376 |
| AFTER PSM | |||
|---|---|---|---|
| Treated with Antibiotic n (%)/Mean (SD) | No Antibiotic Treatment n (%)/Mean (SD) | p Value | |
| Total | 26 (100) | 26 (100) | |
| Gender (males) | 16 (61.5) | 9 (34.6) | p = 0.035 |
| Age, mean (SD) | 64 (11.5) | 64.1 (12.6) | p = 0.973 |
| Diabetes | 2 (7.6) | 2 (7.6) | p = 1.000 |
| IHD | 14 (53.8) | 13 (50.0) | p = 0.781 |
| COPD | 4 (15.3) | 0 (0) | p = 0.037 |
| CKD | 0 (0) | 0 (0) | - |
| PAD | 0 (0) | 0 (0) | - |
| Before PSM | |||
|---|---|---|---|
| Treated with Antibiotic n (%)/Mean (SD) | No Antibiotic Treatment n (%)/Mean (SD) | p Value | |
| Total | 62 (100) | 28 (100) | |
| Morbidity | 20 (32.2) | 6 (21.4) | p = 0.327 |
| Exacerbation | 0 (0) | 0(0) | - |
| Mortality | 2 (3.22) | 0 (0) | p = 1.000 |
| SSI | 6 (9.6) | 0 (0) | p = 0.171 |
| Anastomotic Leak | 1(1.6) | 0 (0) | - |
| LOS | 14.1 (8.9) | 10.4 (4.3) | p = 0.040 |
| Albumin (g/dL) | 2.9 (0.61) | 3.2 (0.7) | p = 0.546 |
| Hb (g/dL) | 10.2 (1.7) | 10.6 (1.6) | p = 0.425 |
| CRP (mg/dL) | 95.7 (61.5) | 61.5 (74.5) | p = 0.195 |
| NLR | 8.3 (9.7) | 7.2 (5.7) | p = 0.619 |
| Creatinine (mg/dL) | 0.9 (0.6) | 0.7 (0.2) | p = 0.127 |
| After PSM | |||
|---|---|---|---|
| Treated with Antibiotic n (%)/Mean (SD) | No Antibiotic Treatment n (%)/Mean (SD) | p Value | |
| Total | 26 (100) | 26 (100) | |
| Morbidity | 8 (30.7) | 6 (23.0) | 0.538 |
| Mortality | 1 (3.8) | 0 (0) | 0.317 |
| SSI | 3 (11.5) | 0 (0) | 0.077 |
| Anastomotic leak | 0 (0) | 0 (0) | - |
| LOS | 11.7(3.9) | 10.5(4.5) | 0.306 |
| Hb (g/dL) | 10.6(1.8) | 10.6(1.6) | 0.917 |
| CRP (mg/dL) | 83.0 (59.6) | 61.5 (74.5) | 0.431 |
| NLR | 6.4 (4.0) | 7.1 (5.9) | 0.597 |
| Creatinine (mg/dL) | 0.8 (0.2) | 0.7 (0.2) | 0.443 |
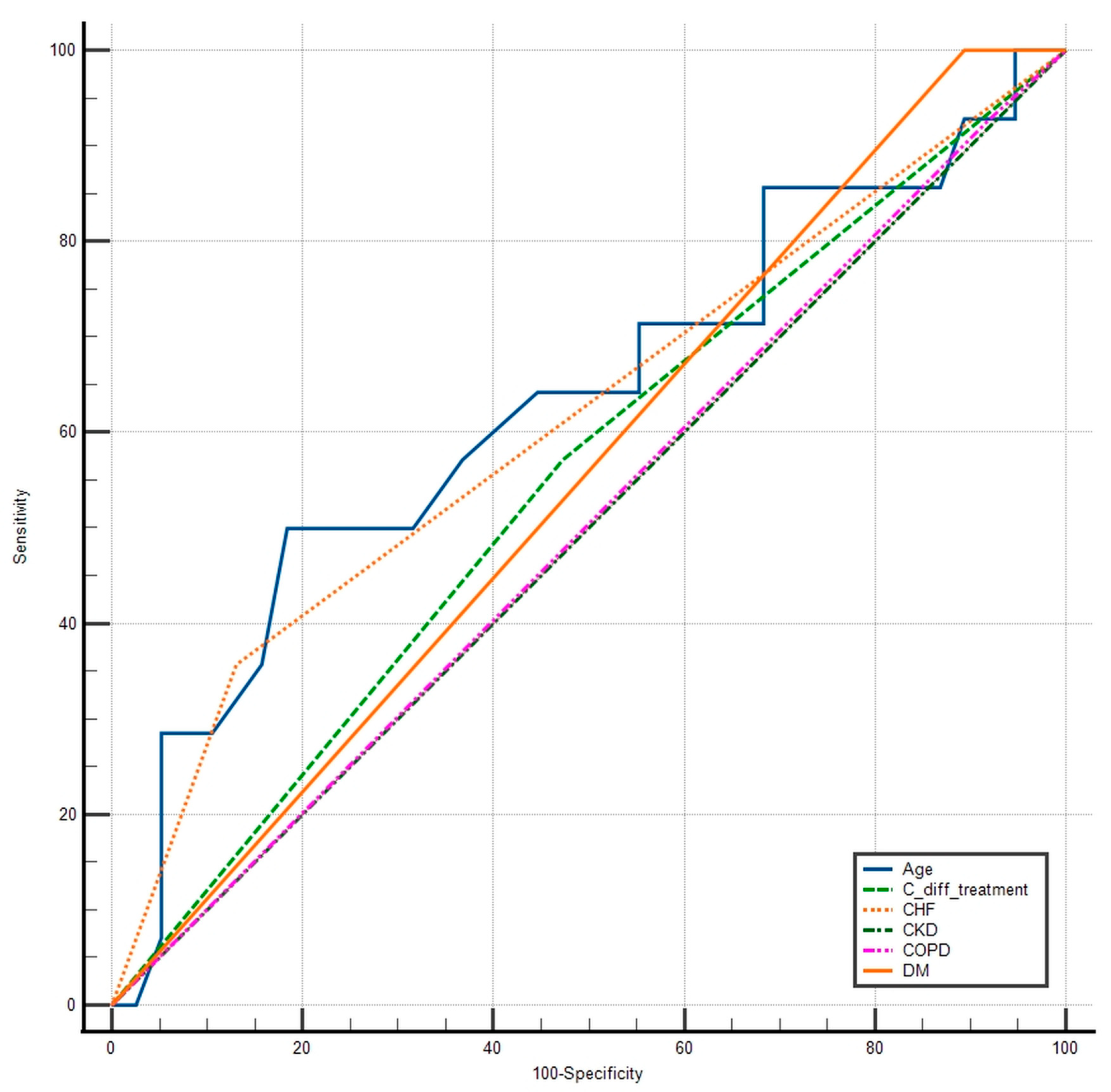
| Variable | AUC | SE | 95% CI |
|---|---|---|---|
| Age | 0.631 | 0.0957 | 0.486 to 0.760 |
| C. diff treatment | 0.549 | 0.0800 | 0.405 to 0.687 |
| IHD | 0.613 | 0.0720 | 0.468 to 0.745 |
| CKD | 0.500 | 0.000 | 0.358 to 0.642 |
| COPD | 0.504 | 0.0420 | 0.362 to 0.645 |
| DM | 0.553 | 0.0252 | 0.408 to 0.691 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunca, S.; Ong, W.L.; Zaharia, R.; Pruna, R.M.; Dimofte, G.M.; Morarasu, S. Clostridium difficile Colonization in Oncologic Patients Undergoing Major Abdominopelvic Surgery: To Treat or Not to Treat? An Observational Study with Propensity Score Analysis. Medicina 2025, 61, 1606. https://doi.org/10.3390/medicina61091606
Lunca S, Ong WL, Zaharia R, Pruna RM, Dimofte GM, Morarasu S. Clostridium difficile Colonization in Oncologic Patients Undergoing Major Abdominopelvic Surgery: To Treat or Not to Treat? An Observational Study with Propensity Score Analysis. Medicina. 2025; 61(9):1606. https://doi.org/10.3390/medicina61091606
Chicago/Turabian StyleLunca, Sorinel, Wee Liam Ong, Raluca Zaharia, Romulus Mihaita Pruna, Gabriel Mihail Dimofte, and Stefan Morarasu. 2025. "Clostridium difficile Colonization in Oncologic Patients Undergoing Major Abdominopelvic Surgery: To Treat or Not to Treat? An Observational Study with Propensity Score Analysis" Medicina 61, no. 9: 1606. https://doi.org/10.3390/medicina61091606
APA StyleLunca, S., Ong, W. L., Zaharia, R., Pruna, R. M., Dimofte, G. M., & Morarasu, S. (2025). Clostridium difficile Colonization in Oncologic Patients Undergoing Major Abdominopelvic Surgery: To Treat or Not to Treat? An Observational Study with Propensity Score Analysis. Medicina, 61(9), 1606. https://doi.org/10.3390/medicina61091606






