Is Body Mass Index a Prognostic Factor in Metastatic HER2-Positive Breast Cancer? A Real-World Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Inclusion Criteria
2.2. Data Collection, Study Variables, and Outcome Definitions
2.3. Ethical Considerations
2.4. Statistical Analysis
3. Results
3.1. Baseline Clinical, Demographic, and Treatment Characteristics
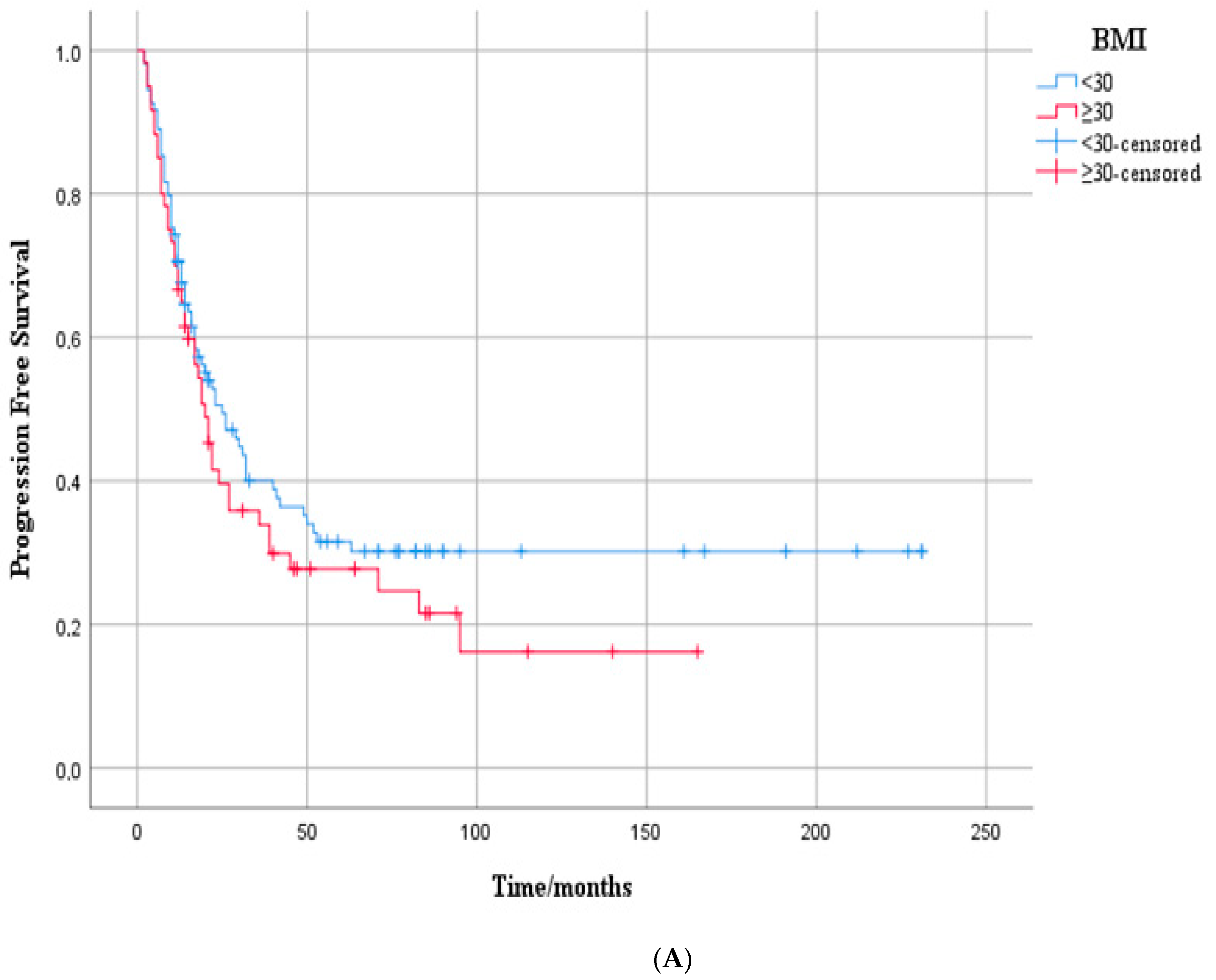
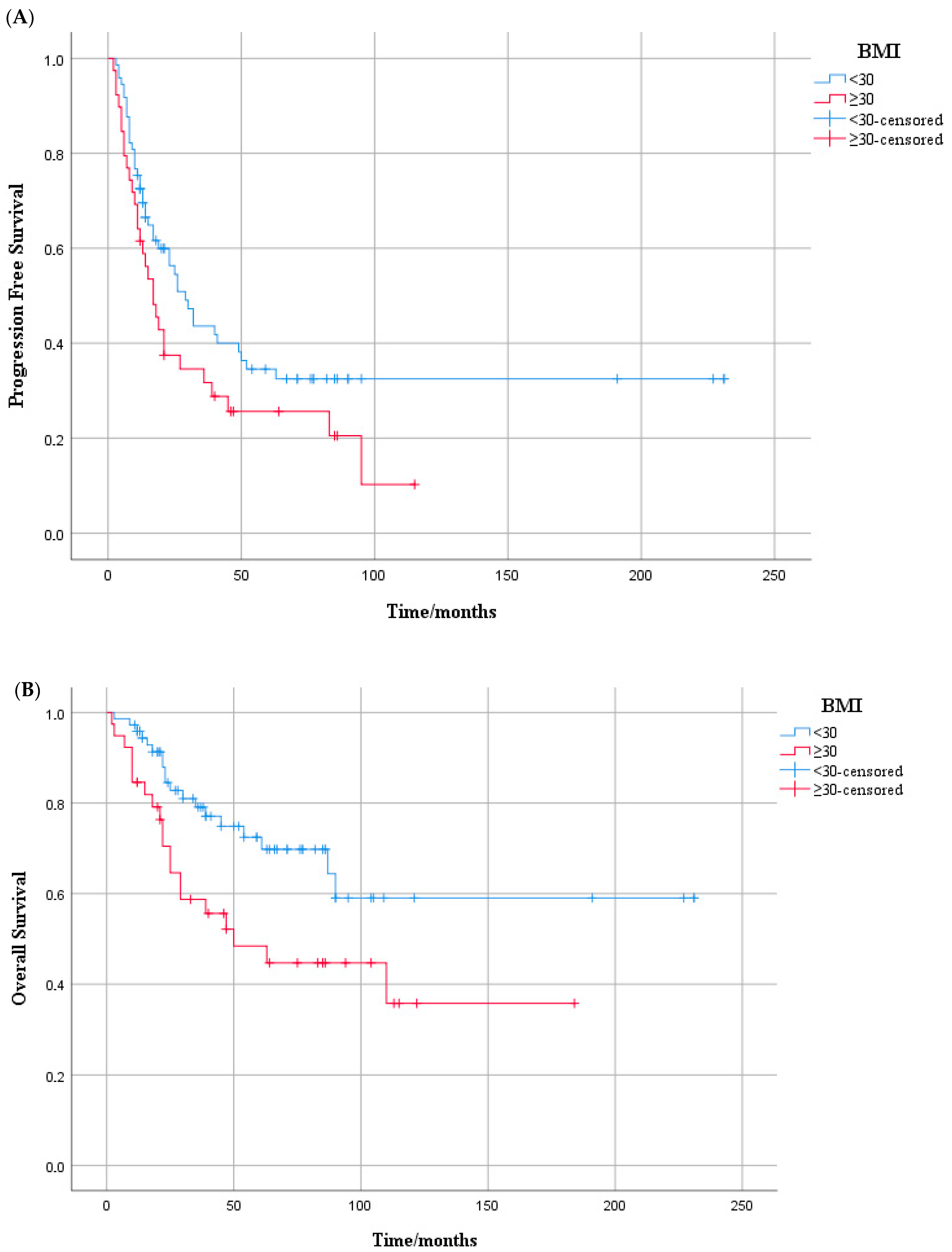
3.2. Progression-Free Survival Outcome
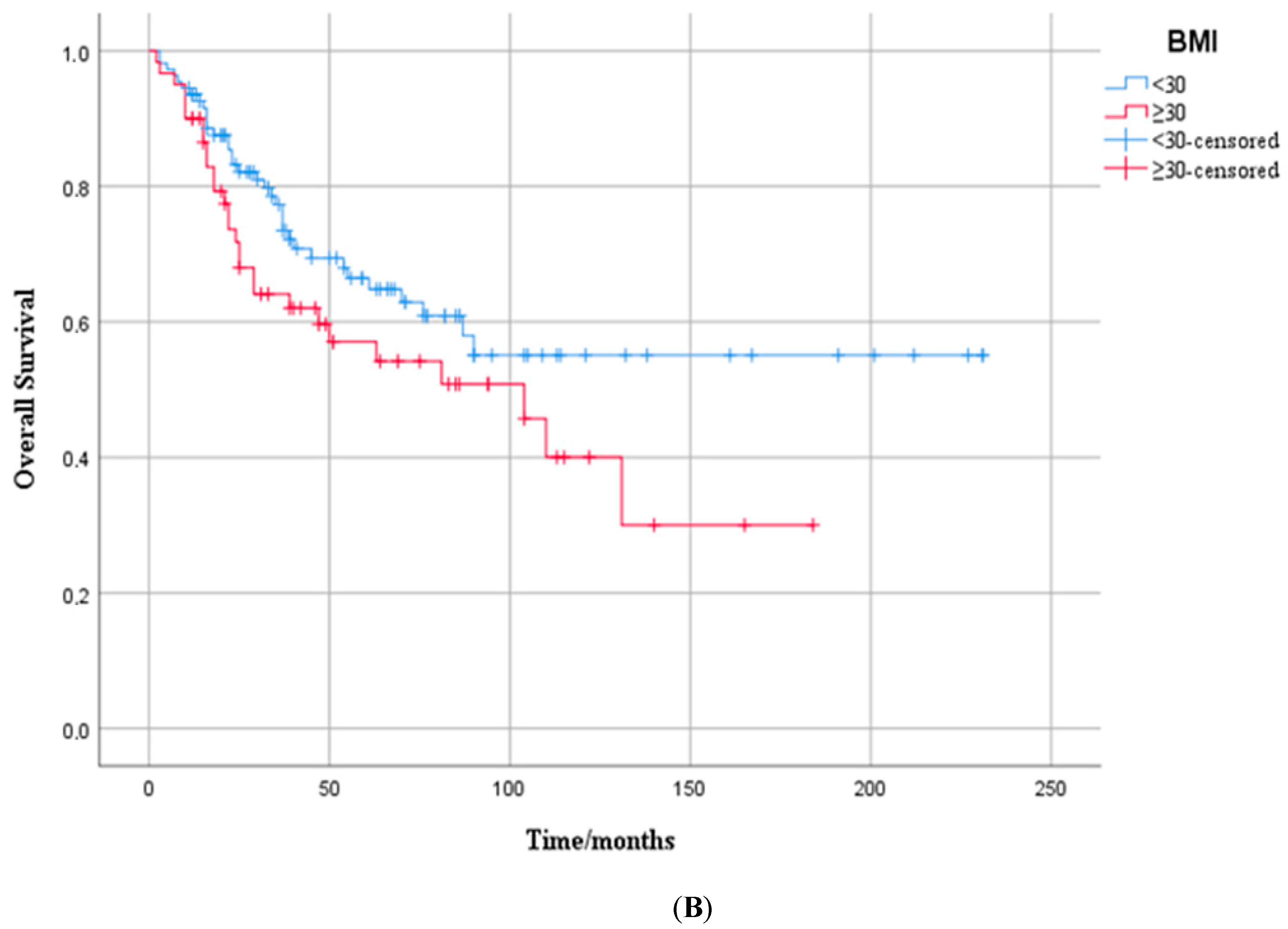
3.3. Overall Survival Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| BMI | Body mass index |
| CI | Confidence interval |
| ECOG-PS | Eastern Cooperative Oncology Group performance status |
| ER | Estrogen receptor |
| ERα | Estrogen receptor alpha |
| HER2 | Human epidermal growth factor receptor 2 |
| HR (hormone receptor) | ER and/or PR status |
| HR (hazard ratio) | Effect estimate from Cox models |
| IHC | Immunohistochemistry |
| IDC | Invasive ductal carcinoma |
| ILC | Invasive lobular carcinoma |
| ISH | In situ hybridization |
| KM | Kaplan–Meier |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| OS | Overall survival |
| PFS | Progression-free survival |
| PR | Progesterone receptor |
| REDD1 | Regulated in Development and DNA Damage Responses 1 |
| SISH | Silver in situ hybridization |
| SPSS | Statistical Package for the Social Sciences |
| T-DM1 | Ado-trastuzumab emtansine |
| TH | Trastuzumab + taxane |
| THP | Trastuzumab + pertuzumab + taxane |
| WHO | World Health Organization |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 July 2025).
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; World Health Organization Technical Report Series 894; World Health Organization: Geneva, Switzerland, 2000; pp. i–xii, 1–253. [Google Scholar]
- Berclaz, G.; Li, S.; Price, K.; Coates, A.; Castiglione-Gertsch, M.; Rudenstam, C.-M.; Holmberg, S.B.; Lindtner, J.; Erien, D.; Collins, J.; et al. Body mass index as a prognostic feature in operable breast cancer: The International Breast Cancer Study Group experience. Ann. Oncol. 2004, 15, 875–884. [Google Scholar] [CrossRef]
- Ewertz, M.; Jensen, M.-B.; Gunnarsdóttir, K.Á.; Højris, I.; Jakobsen, E.H.; Nielsen, D.; Stenbygaard, L.E.; Tange, U.B.; Cold, S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011, 29, 25–31. [Google Scholar] [CrossRef]
- Cecchini, R.S.; Swain, S.M.; Costantino, J.P.; Rastogi, P.; Jeong, J.-H.; Anderson, S.J.; Tang, G.; Geyer, C.E., Jr.; Lembersky, B.C.; Romond, E.H.; et al. Body mass index at diagnosis and breast cancer survival prognosis in clinical trial populations from NRG Oncology/NSABP B-30, B-31, B-34, and B-38. Cancer Epidemiol. Biomark. Prev. 2016, 25, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Dartois, L.; Fagherazzi, G.; Baglietto, L.; Boutron-Ruault, M.C.; Delaloge, S.; Mesrine, S.; Clavel-Chapelon, F. Proportion of premenopausal and postmenopausal breast cancers attributable to known risk factors: Estimates from the E3N-EPIC cohort. Int. J. Cancer 2016, 138, 2415–2427. [Google Scholar] [CrossRef]
- Pan, H.; Deng, L.L.; Cui, J.Q.; Shi, L.; Yang, Y.C.; Luo, J.H.; Qin, D.; Wang, L. Association between serum leptin levels and breast cancer risk: An updated systematic review and meta-analysis. Medicine 2018, 97, e11345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Lehuédé, C.; Laurent, V.; Dirat, B.; Dauvillier, S.; Bochet, L.; Le Gonidec, S.; Escourrou, G.; Valet, P.; Muller, C. Adipose tissue and breast epithelial cells: A dangerous dynamic duo in breast cancer. Cancer Lett. 2012, 324, 142–151. [Google Scholar] [CrossRef]
- Andò, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, Leptin and Breast Cancer: Epidemiological Evidence and Proposed Mechanisms. Cancers 2019, 11, 62. [Google Scholar] [CrossRef]
- Gennari, A.; Amadori, D.; Scarpi, E.; Farolfi, A.; Paradiso, A.; Mangia, A.; Biglia, N.; Gianni, L.; Tienghi, A.; Rocca, A. Impact of body mass index (BMI) on the prognosis of high-risk early breast cancer (EBC) patients treated with adjuvant chemotherapy. Breast Cancer Res. Treat. 2016, 159, 79–86. [Google Scholar] [CrossRef]
- Modi, N.D.; Tan, J.Q.E.; Rowland, A.; Koczwara, B.; Abuhelwa, A.Y.; Kichenadasse, G.; McKinnon, R.A.; Wiese, M.D.; Sorich, M.J.; Hopkins, A.M. The obesity paradox in early and advanced HER2 positive breast cancer: Pooled analysis of clinical trial data. NPJ Breast Cancer 2021, 7, 30. [Google Scholar] [CrossRef]
- Lohmann, A.E.; Soldera, S.V.; Pimentel, I.; Ribnikar, D.; Ennis, M.; Amir, E.; Goodwin, P.J. Association of Obesity With Breast Cancer Outcome in Relation to Cancer Subtypes: A Meta-Analysis. J. Natl. Cancer Inst. 2021, 113, 1465–1475. [Google Scholar] [CrossRef]
- Martel, S.; Poletto, E.; Ferreira, A.R.; Lambertini, M.; Sottotetti, F.; Bertolini, I.; Montemurro, F.; Bernardo, A.; Risi, E.; Zanardi, E. Impact of body mass index on the clinical outcomes of patients with HER2-positive metastatic breast cancer. Breast 2018, 37, 142–147. [Google Scholar] [CrossRef]
- Saleh, K.; Carton, M.; Dieras, V.; Heudel, P.-E.; Brain, E.; D’Hondt, V.; Mailliez, A.; Patsouris, A.; Mouret-Reynier, M.-A.; Goncalves, A.; et al. Impact of body mass index on overall survival in patients with metastatic breast cancer. Breast 2021, 55, 16–24. [Google Scholar] [CrossRef]
- Krasniqi, E.; Pizzuti, L.; Barchiesi, G.; Sergi, D.; Carpano, S.; Botti, C.; Kayal, R.; Sanguineti, G.; Marchetti, P.; Botticelli, A.; et al. Impact of BMI on HER2+ metastatic breast cancer patients treated with pertuzumab and/or trastuzumab emtansine. Real-world evidence. J. Cell. Physiol. 2020, 235, 7900–7910. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, L.; Bardia, A.; Rugo, H.S.; Carey, L.A.; Diéras, V.C.; Loibl, S.; Piccart, M.; Gianni, L.; Kalinsky, K.; O’Shaughnessy, J.; et al. The association of high body mass index with the safety and efficacy of sacituzumab govitecan in patients with metastatic triple-negative breast cancer from the ASCENT study. ESMO Open 2025, 10, 105294. [Google Scholar] [CrossRef] [PubMed]
- Roncato, R.; Peruzzi, E.; Gerratana, L.; Posocco, B.; Nuzzo, S.; Montico, M.; Orleni, M.; Corsetti, S.; Bartoletti, M.; Gagno, S.; et al. Clinical impact of body mass index on palbociclib treatment outcomes and effect on exposure. Biomed. Pharmacother. 2023, 164, 114906. [Google Scholar] [CrossRef]
- Simkens, L.H.; Koopman, M.; Mol, L.; Veldhuis, G.J.; Ten Bokkel Huinink, D.; Muller, E.W.; Derleyn, V.A.; Teerenstra, S.; Punt, C.J. Influence of body mass index on outcome in advanced colorectal cancer patients receiving chemotherapy with or without targeted therapy. Eur. J. Cancer 2011, 47, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e213520. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Alarfi, H.; Salamoon, M.; Kadri, M.; Alammar, M.; Haykal, M.A.; Alseoudi, A.; Youssef, L.A. The impact of baseline body mass index on clinical outcomes in metastatic breast cancer: A prospective study. BMC Res. Notes 2017, 10, 550. [Google Scholar] [CrossRef]
- Mazzarella, L.; Disalvatore, D.; Bagnardi, V.; Rotmensz, N.; Galbiati, D.; Caputo, S.; Curigliano, G.; Pelicci, P.G. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur. J. Cancer 2013, 49, 3588–3597. [Google Scholar] [CrossRef]
- Vici, P.; Pizzuti, L.; Natoli, C.; Gamucci, T.; Di Lauro, L.; Barba, M.; Sergi, D.; Botti, C.; Michelotti, A.; Moscetti, L.; et al. Triple positive breast cancer: A distinct subtype? Cancer Treat. Rev. 2015, 41, 69–76. [Google Scholar] [CrossRef]
- Ligorio, F.; Zambelli, L.; Fucà, G.; Lobefaro, R.; Santamaria, M.; Zattarin, E.; de Braud, F.; Vernieri, C. Prognostic impact of body mass index (BMI) in HER2+ breast cancer treated with anti-HER2 therapies: From preclinical rationale to clinical implications. Ther. Adv. Med. Oncol. 2022, 14, 17588359221079123. [Google Scholar] [CrossRef] [PubMed]
- Zhidkova, E.M.; Lylova, E.S.; Grigoreva, D.D.; Kirsanov, K.I.; Osipova, A.V.; Kulikov, E.P.; Mertsalov, S.A.; Belitsky, G.A.; Budunova, I.; Yakubovskaya, M.G.; et al. Nutritional Sensor REDD1 in Cancer and Inflammation: Friend or Foe? Int. J. Mol. Sci. 2022, 23, 9686. [Google Scholar] [CrossRef]
- Williamson, D.L.; Li, Z.; Tuder, R.M.; Feinstein, E.; Kimball, S.R.; Dungan, C.M. Altered nutrient response of mTORC1 as a result of changes in REDD1 expression: Effect of obesity vs. REDD1 deficiency. J. Appl. Physiol. 2014, 117, 246–256. [Google Scholar] [CrossRef]
- Lan, Y.C.; Chang, C.L.; Sung, M.T.; Yin, P.H.; Hsu, C.C.; Wang, K.C.; Lee, H.C.; Tseng, L.M.; Chi, C.W. Zoledronic acid-induced cytotoxicity through endoplasmic reticulum stress triggered REDD1-mTOR pathway in breast cancer cells. Anticancer Res. 2013, 33, 3807–3814. [Google Scholar]
- Yun, S.M.; Woo, S.H.; Oh, S.T.; Hong, S.E.; Choe, T.B.; Ye, S.K.; Kim, E.K.; Seong, M.K.; Kim, H.A.; Noh, W.C.; et al. Melatonin enhances arsenic trioxide-induced cell death via sustained upregulation of Redd1 expression in breast cancer cells. Mol. Cell. Endocrinol. 2016, 422, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Chang, B.; Sun, L.; Zhu, H.; Pang, L.; Tao, L.; Zou, H.; Du, J.; Dong, Y.; Qi, Y.; et al. REDD1 and p-AKT over-expression may predict poor prognosis in ovarian cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 5940–5949. [Google Scholar] [PubMed]
- Pinto, J.A.; Rolfo, C.; Raez, L.E.; Prado, A.; Araujo, J.M.; Bravo, L.; Fajardo, W.; Morante, Z.D.; Aguilar, A.; Neciosup, S.P.; et al. In silico evaluation of DNA Damage Inducible Transcript 4 gene (DDIT4) as prognostic biomarker in several malignancies. Sci. Rep. 2017, 7, 1526. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall Cohort (n = 169) | BMI < 30 (n = 109) | BMI ≥ 30 (n = 60) | p | ||||
|---|---|---|---|---|---|---|---|---|
| Age, median | 51 (min 26–max 84) | 49 (min 26–max 84) | 56 (min 28–max 78) | 0.019 1 | ||||
| Menopause status, n (%) | Premenopausal | 76 | 45% | 56 | 52% | 20 | 33% | 0.024 2 |
| Postmenopausal | 93 | 55% | 53 | 49% | 40 | 66% | ||
| Comorbidity, n (%) | Absent | 82 | 49% | 61 | 56% | 21 | 34% | 0.009 2 |
| Present | 87 | 51% | 48 | 44% | 39 | 64% | ||
| ECOG, n (%) | 0 | 129 | 76% | 90 | 83% | 39 | 64% | 0.010 2 |
| 1 | 40 | 24% | 19 | 18% | 21 | 34% | ||
| BMI kg/m2, median | 27.8 (min 19.7–max 46.8) | 26.1 (min 19.7–max 29.9) | 32.8 (min 30–max 46.8) | 0.000 1 | ||||
| Primary tumor laterality, n (%) | Right | 84 | 50% | 56 | 52% | 28 | 46% | 0.558 2 |
| Left | 85 | 50% | 53 | 49% | 32 | 52% | ||
| Histological subtype, n (%) | IDC | 140 | 83% | 93 | 86% | 47 | 77% | 0.365 2 |
| ILC | 7 | 4% | 4 | 4% | 3 | 5% | ||
| Mixed-other | 22 | 13% | 12 | 10% | 10 | 18% | ||
| ER, median | 40 (min 0–max 100) | 40 (min 0–max 100) | 25 (min 0–max 100) | 0.984 1 | ||||
| PR, median | 1 (min 0–max 100) | 1 (min 0–max 100) | 0 (min 0–max 95) | 0.638 1 | ||||
| Ki-67%, median | 35 (min 5–max 90) | 35 (min 5–max 90) | 40 (min 5–max 90) | 0.218 1 | ||||
| Grade, n (%) | 2 | 64 | 38% | 45 | 42% | 19 | 31% | 0.217 2 |
| 3 | 105 | 62% | 64 | 59% | 41 | 67% | ||
| Subtype, n (%) | HR-negative | 57 | 34% | 36 | 33% | 21 | 34% | 0.795 2 |
| HR-positive | 112 | 66% | 73 | 68% | 39 | 64% | ||
| Pattern of metastatic presentation, n (%) | De novo | 107 | 63% | 74 | 69% | 33 | 54% | 0.096 2 |
| Recurrent | 62 | 37% | 35 | 32% | 27 | 44% | ||
| Bone-only disease | 40 | 24% | 24 | 22% | 16 | 26% | 0.496 2 | |
| Visceral metastasis, n (%) | 97 | 57% | 60 | 56% | 37 | 61% | 0.405 2 | |
| Brain metastasis, n (%) | 25 | 15% | 16 | 15% | 9 | 15% | 0.955 2 | |
| First-line systemic therapy | THP | 100 | 59% | 64 | 59% | 36 | 59% | 0.982 2 |
| TH | 60 | 36% | 39 | 36% | 21 | 34% | ||
| T-DM1 | 9 | 5% | 6 | 6% | 3 | 5% | ||
| Current status | Alive | 106 | 63% | 74 | 69% | 32 | 52% | 0.061 2 |
| Deceased | 63 | 37% | 35 | 32% | 28 | 46% | ||
| Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Age | 0.99 (0.97–1.00) | 0.305 | |||
| Comorbidity | Absent | 0.76 (0.52–1.11) | 0.160 | 1.41 (0.96–2.06) | 0.083 |
| Present | |||||
| BMI | <30 | 1.24 (0.85–1.81) | 0.260 | ||
| ≥30 | |||||
| Menopause status | Premenopausal | 0.72 (0.50–1.05) | 0.095 | 1.24 (0.80–1.91) | 0.328 |
| Postmenopausal | |||||
| Grade | 2 | 0.95 (0.65–1.39) | 0.800 | ||
| 3 | |||||
| ER | 1.00 (0.99–1.00) | 0.839 | |||
| PR | 1.00 (0.99–1.00) | 0.747 | |||
| Ki-67 | 1.00 (0.99–1.01) | 0.125 | 1.01 (1.00–1.03) | 0.01 | |
| Pattern of metastatic presentation | De novo | 2.20 (1.51–3.19) | 0.000 | 2.28 (1.46–3.55) | 0.000 |
| Recurrent | |||||
| Subtype | HR-negative | 0.95 (0.64–1.40) | 0.807 | ||
| HR-positive | |||||
| Visceral metastasis | Absent | 1.16 (0.80–1.70) | 0.420 | ||
| Present | |||||
| Bone-only disease | Absent | 0.51 (1.20–3.20) | 0.007 | 0.60 (0.36–1.82) | 0.047 |
| Present | |||||
| Brain metastasis | Absent | 2.51 (1.57–3.99) | 0.000 | 2.22 (0.24–0.83) | 0.011 |
| Present | |||||
| Variables | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Age | 1.01 (0.98–1.02) | 0.416 | |||
| Comorbidity | Absent | 0.702 (0.42–1.15) | 0.165 | ||
| Present | |||||
| BMI | <30 | 1.53 (0.93–2.52) | 0.092 | 1.43 (0.84–2.45) | 0.181 |
| ≥30 | |||||
| Menopause status | Premenopausal | 0.79 (0.48–1.29) | 0.350 | ||
| Postmenopausal | |||||
| Grade | 2 | 1.11 (0.66–1.85) | 0.068 | ||
| 3 | |||||
| ER | 1.01 (0.99–1.00) | 0.439 | |||
| PR | 1.03 (0.99–1.00) | 0.902 | |||
| Ki-67 | 1.02 (1.01–1.03) | 0.000 | 1.01 (1.00–1.03) | 0.01 | |
| Pattern of metastatic presentation | De novo | 2.62 (1.59–4.32) | 0.000 | 1.79 (1.05–3.07) | 0.032 |
| Recurrent | |||||
| Subtype | HR-negative | 1.11 (0.66–1.85) | 0.682 | ||
| HR-positive | |||||
| Visceral metastasis | Absent | 1.16 (0.70–1.92) | 0.554 | ||
| Present | |||||
| Bone-only disease | Absent | 0.36 (1.31–5.85) | 0.007 | 0.51 (0.89–4.19) | 0.092 |
| Present | |||||
| Brain metastasis | Absent | 3.79 (2.16–6.65) | 0.000 | 2.23 (0.24–0.83) | 0.011 |
| Present | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birsin, Z.; Odabaşı Bükün, H.; Nazlı, İ.; Alkan, O.; Günaltılı, M.; Çerme, E.; Aliyev, V.; Cebeci, S.; Jeral, S.; Abbasov, H.; et al. Is Body Mass Index a Prognostic Factor in Metastatic HER2-Positive Breast Cancer? A Real-World Multicenter Study. Medicina 2025, 61, 1604. https://doi.org/10.3390/medicina61091604
Birsin Z, Odabaşı Bükün H, Nazlı İ, Alkan O, Günaltılı M, Çerme E, Aliyev V, Cebeci S, Jeral S, Abbasov H, et al. Is Body Mass Index a Prognostic Factor in Metastatic HER2-Positive Breast Cancer? A Real-World Multicenter Study. Medicina. 2025; 61(9):1604. https://doi.org/10.3390/medicina61091604
Chicago/Turabian StyleBirsin, Zeliha, Hülya Odabaşı Bükün, İsmail Nazlı, Onur Alkan, Murat Günaltılı, Emir Çerme, Vali Aliyev, Selin Cebeci, Seda Jeral, Hamza Abbasov, and et al. 2025. "Is Body Mass Index a Prognostic Factor in Metastatic HER2-Positive Breast Cancer? A Real-World Multicenter Study" Medicina 61, no. 9: 1604. https://doi.org/10.3390/medicina61091604
APA StyleBirsin, Z., Odabaşı Bükün, H., Nazlı, İ., Alkan, O., Günaltılı, M., Çerme, E., Aliyev, V., Cebeci, S., Jeral, S., Abbasov, H., Evrensel, T., Papila, Ç., Demirci, N. S., & Alan, Ö. (2025). Is Body Mass Index a Prognostic Factor in Metastatic HER2-Positive Breast Cancer? A Real-World Multicenter Study. Medicina, 61(9), 1604. https://doi.org/10.3390/medicina61091604






